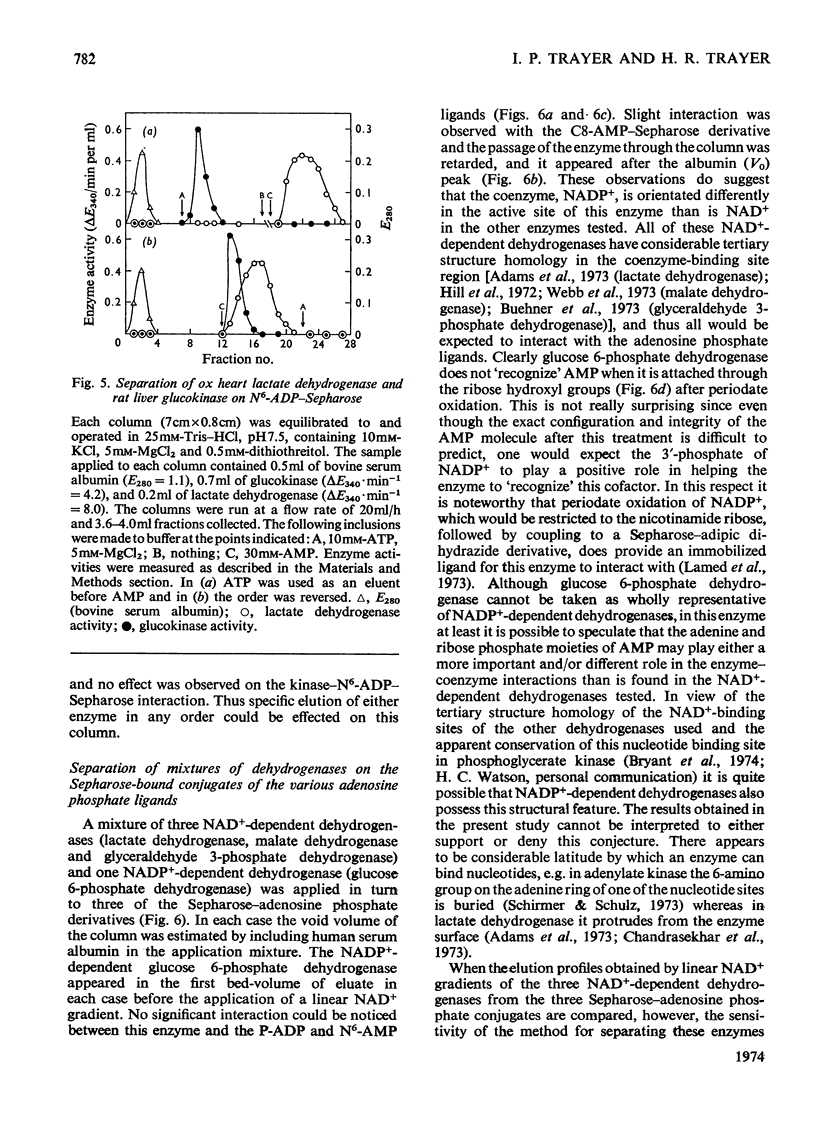
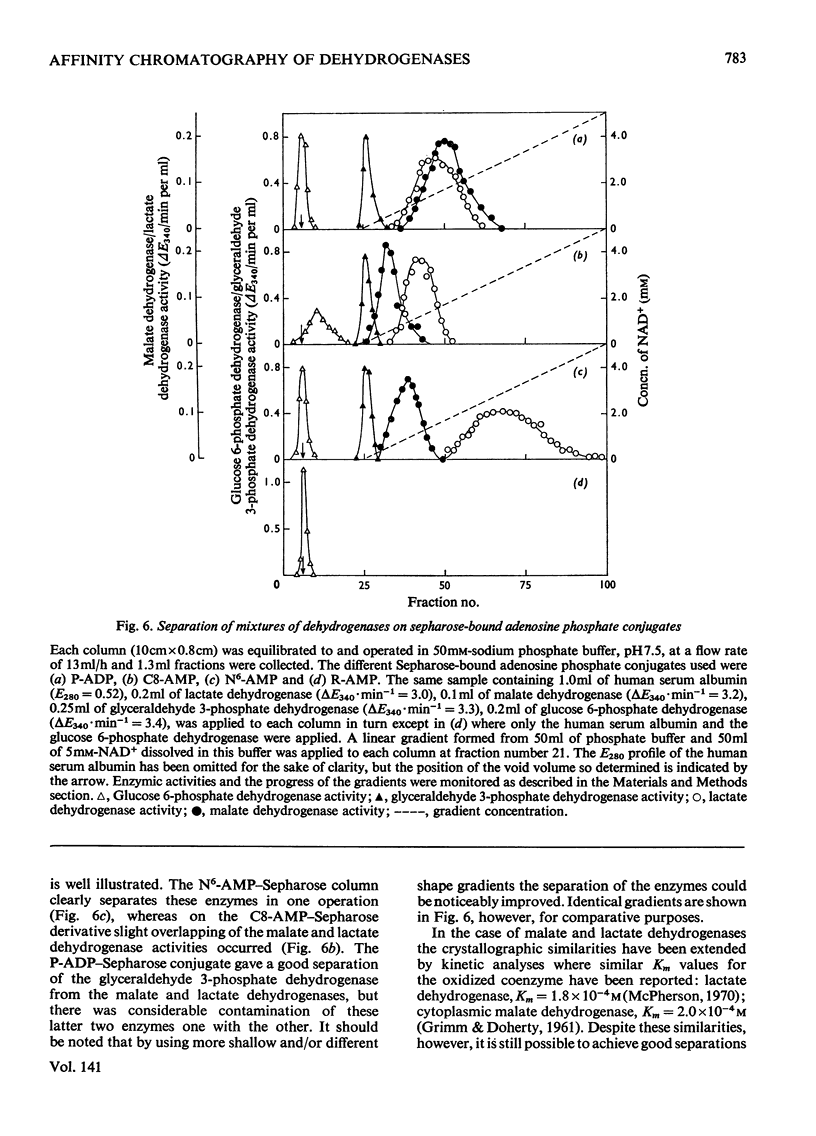
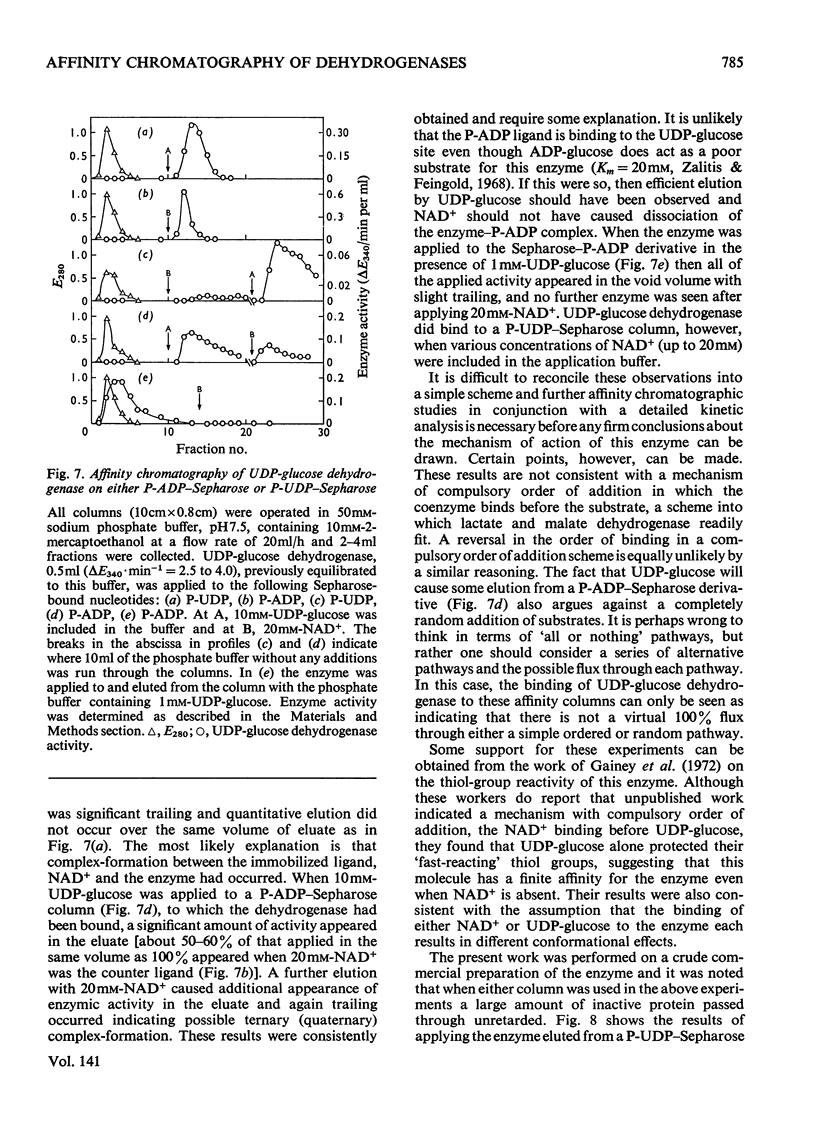
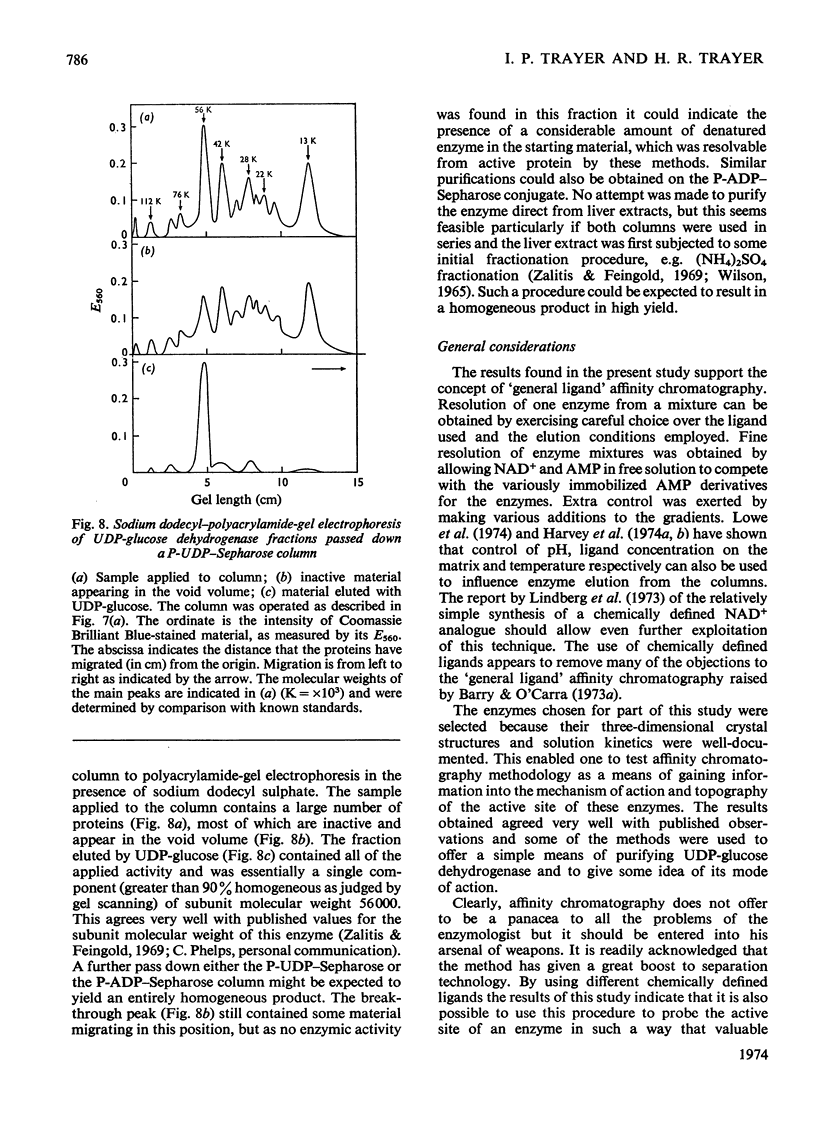
Abstract
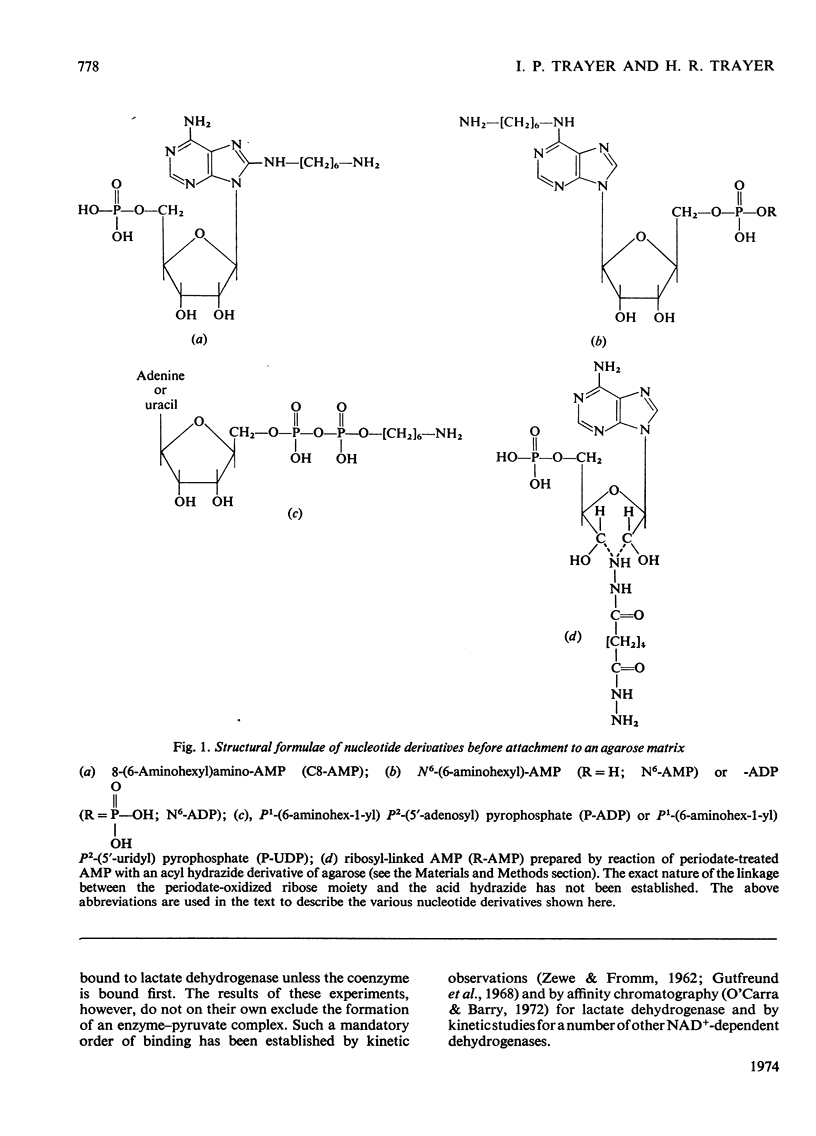
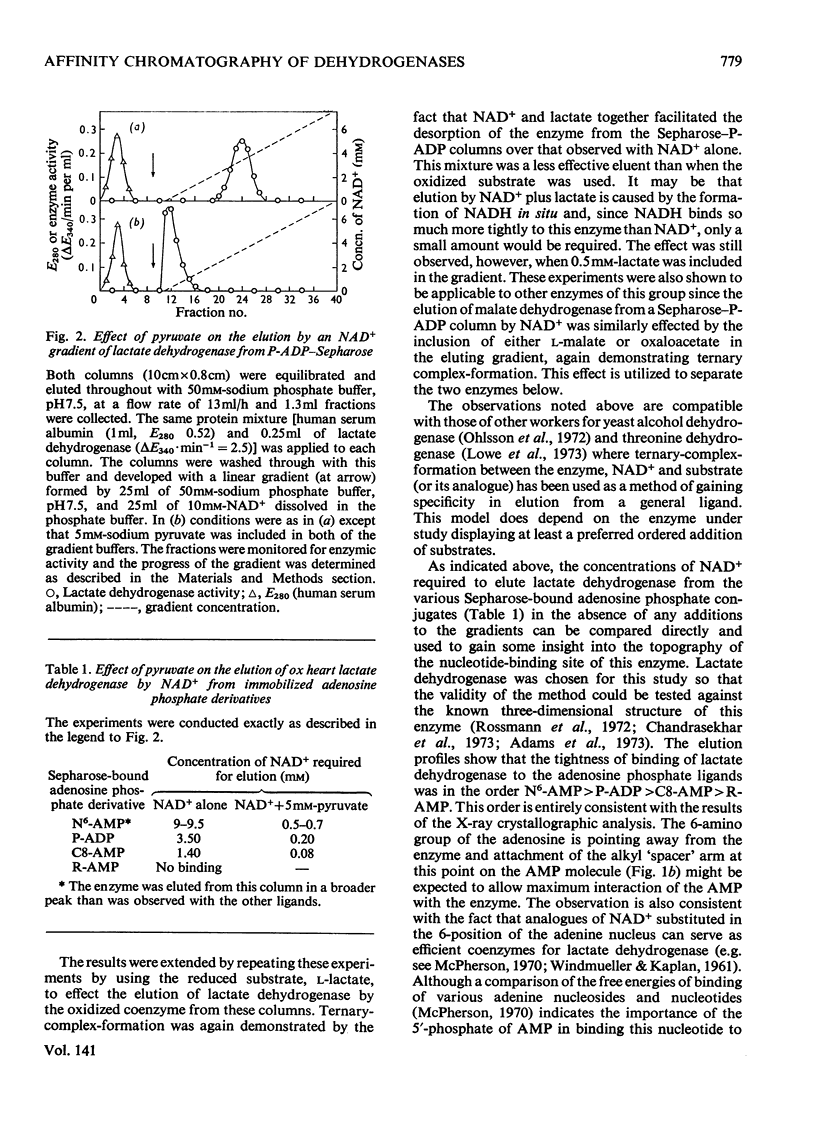
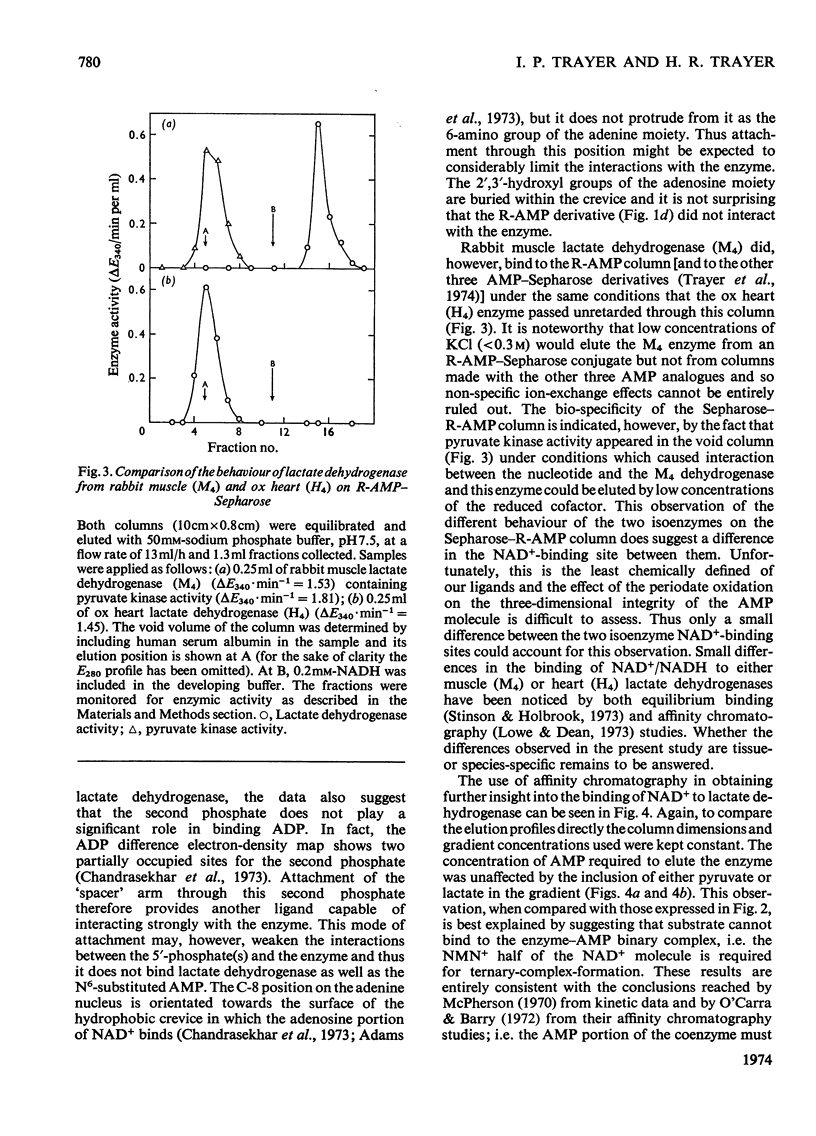
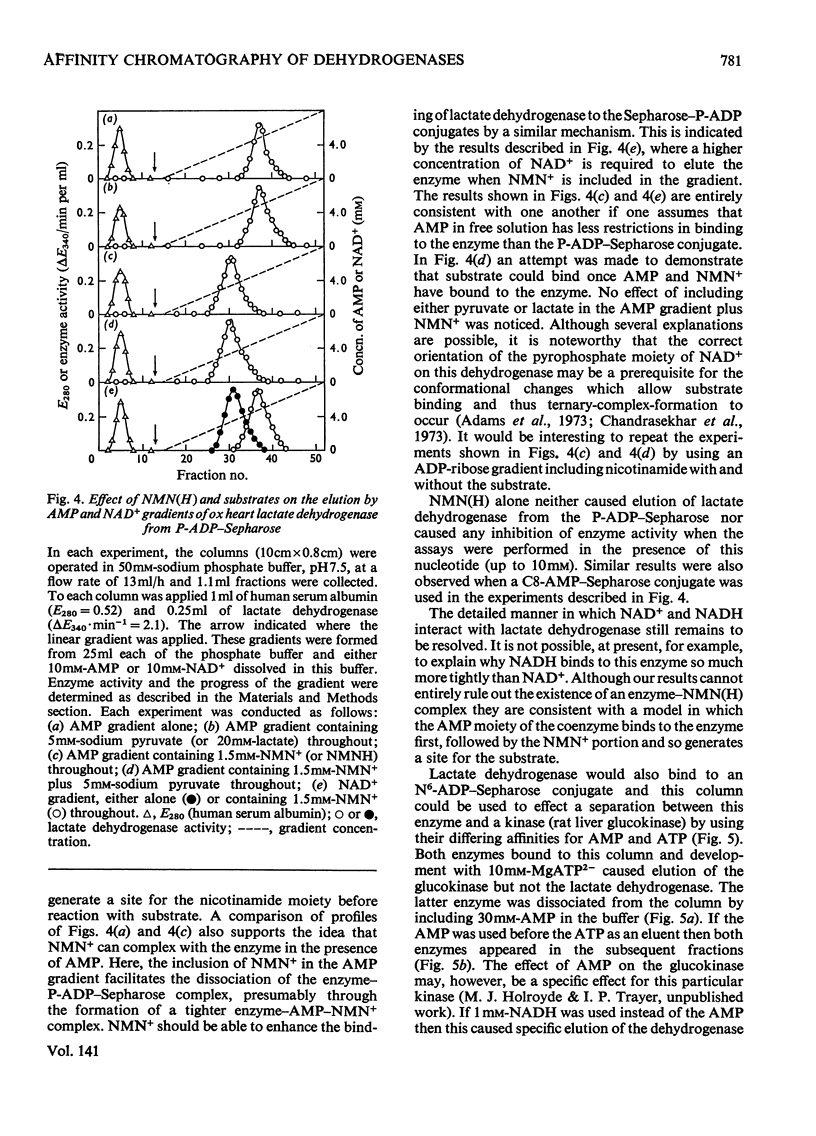
A series of chemically-defined adenosine phosphate ligands attached to Sepharose 4B were used as active-site probes in studying the interaction of enzymes with their coenzymes and substrates and to test the suitability of these matrices for `general ligand' affinity chromatography. Nicotinamide nucleotide-dependent dehydrogenases were used as models to test this methodology. Elution from these columns by NAD+ and/or AMP gradients (in the presence or the absence of substrates and/or nicotinamide mononucleotide) was consistent with: (1) the compulsory ordered addition of substrates to lactate and malate dehydrogenase; (2) the necessity for the NMN moiety of NAD+ to bind to these enzymes before the substrate; and illustrated: (3) that the binding of these two hydrogenases to these columns compared very well with the published three-dimensional models for these enzymes and (4) that separation of mixtures of dehydrogenases depended on the choice of matrix and displacing ion and whether any additions (e.g. substrates) were made to the gradients used. These techniques were used to purify UDP-glucose dehydrogenase from a crude starting material on a phosphate-linked UDP (or ADP) matrix. The binding of this enzyme to these two columns was not consistent with either an ordered or random addition of substrates and suggested a more complex mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Buehner M., Chandrasekhar K., Ford G. C., Hackert M. L., Liljas A., Rossmann M. G., Smiley I. E., Allison W. S., Everse J. Structure-function relationships in lactate dehydrogenase. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1968–1972. doi: 10.1073/pnas.70.7.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Barry S., O'Carra P. A simple general method for the preparation of "6-immobilized" analogues of AMP, ATP, NAD and of other adenine-containing compounds for affinity chromatography. FEBS Lett. 1973 Dec 1;37(2):134–139. doi: 10.1016/0014-5793(73)80442-9. [DOI] [PubMed] [Google Scholar]
- Barry S., O'Carra P. Affinity chromatography of nicotinamide-adenine dinucleotide-linked dehydrogenases on immobilized derivatives of the dinucleotide. Biochem J. 1973 Dec;135(4):595–607. doi: 10.1042/bj1350595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodelius P., Mosbach K. Separation of the isoenzymes of lactate dehydrogenase by affinity chromatography using an immobilized AMP-analogue. FEBS Lett. 1973 Sep 15;35(2):223–226. doi: 10.1016/0014-5793(73)80290-x. [DOI] [PubMed] [Google Scholar]
- Bryant T. N., Watson H. C., Wendell P. L. Structure of yeast phosphoglycerate kinase. Nature. 1974 Jan 4;247(5435):14–17. doi: 10.1038/247014a0. [DOI] [PubMed] [Google Scholar]
- Buehner M., Ford G. C., Moras D., Olsen K. W., Rossman M. G. D-glyceraldehyde-3-phosphate dehydrogenase: three-dimensional structure and evolutionary significance. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3052–3054. doi: 10.1073/pnas.70.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar K., McPherson A., Jr, Adams M. J., Rossmann M. G. Conformation of coenzyme fragments when bound to lactate dehydrogenase. J Mol Biol. 1973 Jun 5;76(4):503–518. doi: 10.1016/0022-2836(73)90488-9. [DOI] [PubMed] [Google Scholar]
- Collier R., Kohlhaw G. Affinity chromatography of transaminases. Anal Biochem. 1971 Jul;42(1):48–53. doi: 10.1016/0003-2697(71)90008-x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography of macromolecules. Adv Enzymol Relat Areas Mol Biol. 1972;36:29–89. doi: 10.1002/9780470122815.ch2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Everse J., Barnett R. E., Thorne C. J., Kaplan N. O. The formation of ternary complexes by diphosphopyridine nucleotide-dependent dehydrogenases. Arch Biochem Biophys. 1971 Apr;143(2):444–460. doi: 10.1016/0003-9861(71)90230-x. [DOI] [PubMed] [Google Scholar]
- GRIMM F. C., DOHERTY D. G. Properties of the two forms of malic dehydrogenase from beef heart. J Biol Chem. 1961 Jul;236:1980–1985. [PubMed] [Google Scholar]
- Gainey P. A., Pestell T. C., Phelps C. F. A study of the subunit structure and the thiol reactivity of bovine liver uridine diphosphate glucose dehydrogenase. Biochem J. 1972 Oct;129(4):821–830. doi: 10.1042/bj1290821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutfreund H., Cantwell R., McMurray C. H., Criddle R. S., Hathaway G. The kinetics of the reversible inhibition of heart lactate dehydrogenase through the formation of the enzyme-oxidized nicotinamide-adenine dinucleotide-pyruvate compounds. Biochem J. 1968 Feb;106(3):683–687. doi: 10.1042/bj1060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M. J., Lowe C. R., Craven D. B., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 2. Some parameters relating to the selection and concentration of the immobilised ligand. Eur J Biochem. 1974 Jan 16;41(2):335–340. doi: 10.1111/j.1432-1033.1974.tb03274.x. [DOI] [PubMed] [Google Scholar]
- Harvey M. J., Lowe C. R., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 5. Some applications of the influence of temperature on the binding of dehydrogenases and kinases. Eur J Biochem. 1974 Jan 16;41(2):353–357. doi: 10.1111/j.1432-1033.1974.tb03277.x. [DOI] [PubMed] [Google Scholar]
- Hill E., Tsernoglou D., Webb L., Banaszak L. J. Polypeptide conformation of cytoplasmic malate dehydrogenase from an electron density map at 3.0 angstrom resolution. J Mol Biol. 1972 Dec 30;72(3):577–589. doi: 10.1016/0022-2836(72)90176-3. [DOI] [PubMed] [Google Scholar]
- Lamed R., Levin Y., Wilchek M. Covalent coupling of nucleotides to agarose for affinity chromatography. Biochim Biophys Acta. 1973 Apr 28;304(2):231–235. doi: 10.1016/0304-4165(73)90239-0. [DOI] [PubMed] [Google Scholar]
- Lindberg M., Larsson P. O., Mosbach K. A new immobilized NAD+ analogue, its application in affinity chromatography and as a functioning coenzyme. Eur J Biochem. 1973 Dec 3;40(1):187–193. doi: 10.1111/j.1432-1033.1973.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Dean P. D. Affinity chromatography of lactate dehydrogenase on immobilized nucleotides. Biochem J. 1973 Jul;133(3):515–520. doi: 10.1042/bj1330515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Dean P. D.G. Affinity chromatography of enzymes on insolubilized cofactors. FEBS Lett. 1971 May 20;14(5):313–316. doi: 10.1016/0014-5793(71)80288-0. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Craven D. B., Kerfoot M. A., Hollows M. E., Dean P. D. The purification of nicotinamide nucleotide-dependent dehydrogenases on immobilized cofactors. Biochem J. 1973 Jul;133(3):507–513. doi: 10.1042/bj1330507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 4. Variation of the binding of dehydrogenases and kinases with pH. Eur J Biochem. 1974 Jan 16;41(2):347–351. doi: 10.1111/j.1432-1033.1974.tb03276.x. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Mosbach K., Dean P. D. Some applications of insolubilised cofactors to the purification of pyridine nucleotide-dependent dehydrogenases. Biochem Biophys Res Commun. 1972 Aug 21;48(4):1004–1010. doi: 10.1016/0006-291x(72)90708-5. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jr Interaction of lactate dehydrogenase with its coenzyme, nicotinamide-adenine dinucleotide. J Mol Biol. 1970 Jul 14;51(1):39–46. doi: 10.1016/0022-2836(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carra P., Barry S. Affinity chromatography of lactate dehydrogenase Model studies demonstrating the potential of the technique in the mechanistic investigation as well as in the purification of multi-substrate enzymes. FEBS Lett. 1972 Apr 1;21(3):281–285. doi: 10.1016/0014-5793(72)80183-2. [DOI] [PubMed] [Google Scholar]
- Ohlsson R., Brodelius P., Mosbach K. Affinity chromatography of enzymes on an AMP-analogue: Specific elution of dehydrogenases from a general ligand. FEBS Lett. 1972 Sep 15;25(2):234–238. doi: 10.1016/0014-5793(72)80492-7. [DOI] [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Adams M. J., Buehner M., Ford G. C., Hackert M. L., Lentz P. J., Jr, McPherson A., Jr, Schevitz R. W., Smiley I. E. Structural constraints of possible mechanisms of lactate dehydrogenase as shown by high resolution studies of the apoenzyme and a variety of enzyme complexes. Cold Spring Harb Symp Quant Biol. 1972;36:179–191. doi: 10.1101/sqb.1972.036.01.025. [DOI] [PubMed] [Google Scholar]
- SIEGEL J. M., MONTGOMERY G. A., BOCK R. M. Ultraviolet absorption spectra of DPN and analogs of DPN. Arch Biochem Biophys. 1959 Jun;82(2):288–299. doi: 10.1016/0003-9861(59)90124-9. [DOI] [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R., Small D. P., Bottomley R. C. Preparation of adenosine nucleotide derivatives suitable for affinity chromatography. Biochem J. 1974 Jun;139(3):609–623. doi: 10.1042/bj1390609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDMUELLER H. G., KAPLAN N. O. The preparation and properties of N-hydroxyethyl derivatives of adenosine, adenosine triphosphate, and nicotinamide adenine dinucleotide. J Biol Chem. 1961 Oct;236:2716–2726. [PubMed] [Google Scholar]
- Webb L. E., Hill E. J., Banaszak L. J. Conformation of nicotinamide adenine dinucleotide bound to cytoplasmic malate dehydrogenase. Biochemistry. 1973 Dec 4;12(25):5101–5109. doi: 10.1021/bi00749a013. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- ZEWE V., FROMM H. J. Kinetic studies of rabbit muscle lactate dehydrogenase. J Biol Chem. 1962 May;237:1668–1675. [PubMed] [Google Scholar]
- Zalitis J., Feingold D. S. Purification and properties of UDPG dehydrogenase from beef liver. Arch Biochem Biophys. 1969 Jul;132(2):457–465. doi: 10.1016/0003-9861(69)90389-0. [DOI] [PubMed] [Google Scholar]
- Zalitis J., Feingold D. S. The mechanism of action of UDPG dehydrogenase. Biochem Biophys Res Commun. 1968 Jun 10;31(5):693–698. doi: 10.1016/0006-291x(68)90617-7. [DOI] [PubMed] [Google Scholar]


