Abstract
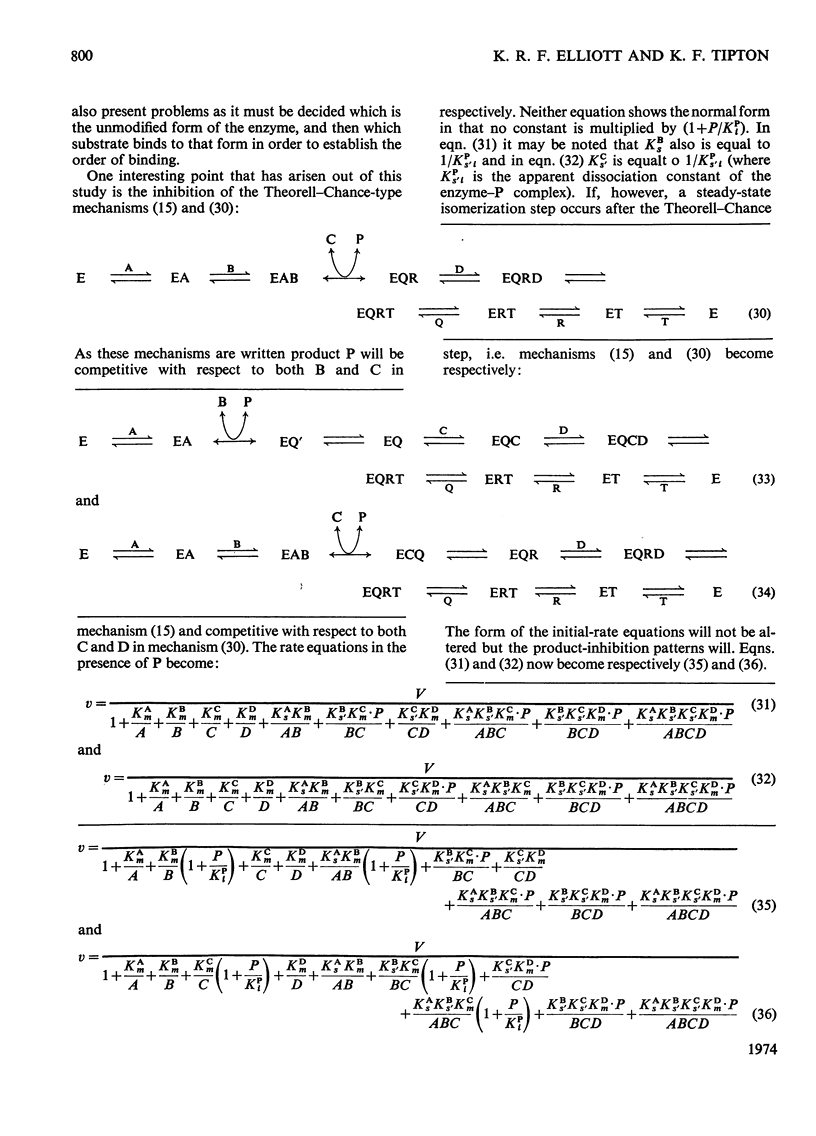
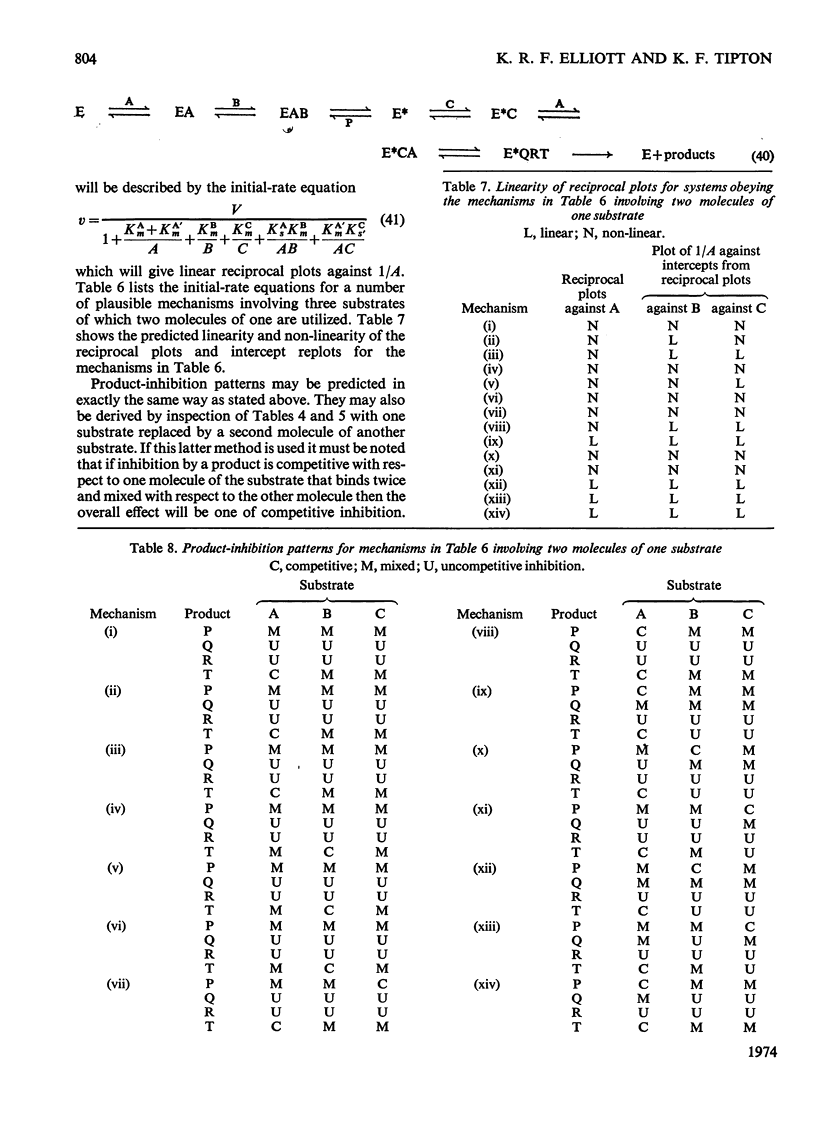
A treatment of kinetic data for enzyme mechanisms involving four substrates is described. The initial-rate equations and product-inhibition patterns for such mechanisms are presented. The treatment is extended to include analysis of enzyme mechanisms involving three substrates in which two molecules of one substrate are used.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- Dalziel K. The interpretation of kinetic data for enzyme-catalysed reactions involving three substrates. Biochem J. 1969 Sep;114(3):547–556. doi: 10.1042/bj1140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. R., Tipton K. F. Kinetic studies of bovine liver carbamoyl phosphate synthetase. Biochem J. 1974 Sep;141(3):807–816. doi: 10.1042/bj1410807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. R., Tipton K. F. Product inhibition studies on bovine liver carbamoyl phosphate synthetase. Biochem J. 1974 Sep;141(3):817–824. doi: 10.1042/bj1410817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. III. The order of substrate addition in the enzymatic reaction. J Biol Chem. 1959 Nov;234:2891–2896. [PubMed] [Google Scholar]
- Fromm H. J. The use of competitive inhibitors in studying the mechanism of action of some enzyme systems utilizing three substrates. Biochim Biophys Acta. 1967 Jul 11;139(2):221–230. doi: 10.1016/0005-2744(67)90026-5. [DOI] [PubMed] [Google Scholar]
- KNAPPE J., RINGELMANN E., LYNEN F. [On beta-hydroxy-beta-methylglutaryl reductase of yeast. On the biosynthesis of terpene. IX]. Biochem Z. 1959;332:195–213. [PubMed] [Google Scholar]
- LAGERKVIST U. Biosynthesis of guanosine 5'-phosphate. II. Amination of xanthosine 5'-phosphate by purified enzyme from pigeon liver. J Biol Chem. 1958 Jul;233(1):143–149. [PubMed] [Google Scholar]
- LAZZARINI R. A., ATKINSON D. E. A triphosphopyridine nucleotide-specific nitrite reductase from Escherichia coli. J Biol Chem. 1961 Dec;236:3330–3335. [PubMed] [Google Scholar]
- METZENBERG R. L., HALL L. M., MARSHALL M., COHEN P. P. Studies on the biosynthesis of carbamyl phosphate. J Biol Chem. 1957 Dec;229(2):1019–1025. [PubMed] [Google Scholar]
- Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Kinetic investigations with N-acetylglucosamine as the galactosyl group acceptor. J Biol Chem. 1971 Jun 25;246(12):3977–3984. [PubMed] [Google Scholar]
- PREISS J., HANDLER P. Biosynthesis of diphosphopyridine nucleotide. II. Enzymatic aspects. J Biol Chem. 1958 Aug;233(2):493–500. [PubMed] [Google Scholar]
- Palmer J. M., Wedding R. T. Purification and properties of succinyl-CoA synthetase from Jerusalem artichoke mitochondria. Biochim Biophys Acta. 1966 Jan 11;113(1):167–174. doi: 10.1016/s0926-6593(66)80131-5. [DOI] [PubMed] [Google Scholar]
- Pettersson G. Dalziel rate behaviour in ternary-complex mechanisms for enzyme reactions involving two substrates. Biochim Biophys Acta. 1972 Jul 13;276(1):1–11. doi: 10.1016/0005-2744(72)90002-2. [DOI] [PubMed] [Google Scholar]
- Plapp B. V. On calculation of rate and dissociation constants from kinetic constants for the Ordered Bi Bi mechanism of liver alcohol dehydrogenase. Arch Biochem Biophys. 1973 May;156(1):112–114. doi: 10.1016/0003-9861(73)90347-0. [DOI] [PubMed] [Google Scholar]
- Rudolph F. B., Fromm H. J. Kinetics of three substrate enzyme systems. Treatment of partially random mechanisms using equilibrium assumptions. J Theor Biol. 1973 May;39(2):363–371. doi: 10.1016/0022-5193(73)90105-7. [DOI] [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B. Formation of oxaloacetate from pyruvate and carbon dioxide. J Biol Chem. 1960 May;235:PC17–PC18. [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. Kinetic formulations for enzymic reactions involving two substrates. Can J Biochem Physiol. 1962 Jun;40:763–804. [PubMed] [Google Scholar]
- Warren G. B., Tipton K. F. Pig liver pyruvate carboxylase. The reaction pathway for the carboxylation of pyruvate. Biochem J. 1974 May;139(2):311–320. doi: 10.1042/bj1390311. [DOI] [PMC free article] [PubMed] [Google Scholar]


