Abstract
An experiment was conducted to evalute the effects of adding palm olein (POL), modified palm olein (high degree of acyl migration palm olein, H-AMD), and lard (total fatty acid saturation degree is similar to palm olein) to the diet of broilers. The study assessed production performance, fatty acid absorption, and abdominal fat deposition. A total of 100 one-week-old female broiler chicks were randomly assigned to three-tiered pens and fed five experimental diets. Enzymatic interesterification of POL causes acyl migration, transforming 1-palmitoyl-2,3-dioleoyl-sn-glycerol (sn-POO) and 1,3-dipalmitoyl-2-oleoyl-sn-glycerol (sn-POP) into 1,3-dioleoyl-2-palmitoyl-sn-glycerol (sn-OPO) and 1,2-dipalmitoyl-3-oleoyl-sn-glycerol (sn-PPO), which increases the saturated fatty acid content at the sn-2 position. Feeding broilers with this modified oil has improved the absorption effect of saturated fatty acids and increased the content of palmitic acid in abdominal tissue by 1.55%-1.69%. The impact on the content and positional distribution of fatty acids deposited in the body is limited. Low-density lipoprotein cholesterol (LDL-C) levels decreased by 34%, while high-density lipoprotein cholesterol (HDL-C) levels increased by 23%, resulting in a lower risk of atherosclerosis. No significant differences have been observed in carcass yield results of the POL and H-AMD groups. Compared with animal-derived oils such as lard which are also rich in saturated fatty acids at the sn-2 position, plant-derived oils such as POL and its modified products have a smaller effect on abdominal fat deposition.
Keywords: Enzymatic interesterification, Acyl migration, Abdomianl fat, Atherosclerosis
Introduction
With the increasing global demand for meat consumption, the production of commercial meat has been continuously increasing. The proportion of pork and beef consumption has decreased, while the demand for poultry meat has increased, accounting for one-third of total global meat production. Chicken meat, known for being a low-cost and high-quality source of protein, plays a crucial role in sustaining the global food supply. Among the total global meat trade volume, poultry meat has the highest export volume. Poultry production has become a significant economic driver for developed and developing regions (Archer & Sobotik, 2022; de Mesquita Souza Saraiva, Lim, do Monte, Givisiez, Alves, de Freitas Neto, et al., 2022). Dietary lipids provide at least twice the energy of an equivalent amount of carbohydrates and proteins. Therefore, the direct addition of oils to feed is a frequently used and pragmatic method to enhance the energy value of poultry feed.
Structured lipids refer to oils that have been redesigned in terms of fatty acid composition and position using methods such as chemical or enzyme-catalyzed reactions to meet specific nutritional or functional requirements (Zhou, Lee, Mao, Wang, & Zhang, 2022). The characteristic distribution of fatty acids in breast milk and some animal fats is that saturated fatty acids tend to be located at the sn-2 position, while unsaturated fatty acids are distributed at the sn-1 and sn-3 positions. This type of lipid has the characteristic of reducing fatty acid loss in feces and has a higher absorption rate. In infants, when using infant formula containing lard, the loss of palmitic acid in feces is reduced by eight times compared to random pig fat (Filer Jr, Mattson, & Fomon, 1969). Plant oils like palm oil tend to distribute saturated fatty acids at the sn-1 and sn-3 positions, making them highly valuable for structural redesign research.
The position distribution of fatty acids on triglycerides (TAG) plays a crucial role in nutrient metabolism. The prevalent view on the superiority of saturated fatty acids at the sn-2 position primarily revolves around the ease of calcium soap formation. Fat is predominantly absorbed in the small intestine (with 10%-30% of TAG undergoing pre-digestion in the stomach) (Ng, Chen, Ghazani, Wright, Marangoni, Goff, et al., 2019). Pancreatic lipases, which possess sn-1,3 specificity, functional as the key enzymes responsible for fat digestion. Consequently, pancreatic lipases hydrolyze TAG, yielding sn-2 monoacylglycerol (MAG) and free fatty acids (FFA). Saturated fatty acids, when present in the form of sn-2 MAG, exhibit a reduced propensity to form calcium soaps compared to their free form. This implies that the absorption of saturated fatty acids and calcium is unencumbered, and there may even be potential benefits in terms of improving the abundance of beneficial gut bacteria (Mattson & Volfenhein, 1962; Yaron, Shachar, Abramas, Riskin, Bader, Litmanovitz, et al., 2013). Additionally, another theoretical explanation proposes that the hydrolysis products of TAG, sn-2 MAG, and FFA, form mixed micelles with bile salts. These micelles, in turn, facilitate the absorption of fatty acids. Among them, compared with saturated fatty acids, which possess lower polarity, unsaturated fatty acids demonstrate a stronger capacity to form micelles. Therefore, the predominant distribution of saturated fatty acids at the sn-2 position encourages a higher proportion of free unsaturated fatty acids during hydrolysis, making it easier for micelles to form and facilitate fatty acid absorption (Baião & Lara, 2005).
The impact of modified fats on fat metabolism and deposition in broiler chickens is still not fully understood. As a result, this experiment's objective is to evaluate the effects of long-term feeding of structurally modified fats using palm oil on fat absorption and deposition in broiler chickens, as well as its effect on blood lipid levels, and carcass yield.
Materials and methods
Materials
Palm olein (POL; slip melting point: 24°C) was provided by Yihai Kerry Edible Oil Co., ltd. (Guangzhou, China), whereas the Lard was purchased from Huatai Edible Oil Co., ltd. (Qingdao, China). The high degree of acyl migration palm olein (H-AMD) was obtained by ester exchange of POL using a packed bed reactor with 85 g Lipozyme TL IM at 80°C for 30 minutes.
Chemical analysis
The composition of total fatty acid and fatty acid at the sn-2 position, TAG composition, and nuclear magnetic analysis of oils were performed according to the methods reported in our previous article (Ai, Lee, Xie, Tan, Lai, Li, et al., 2023).
Experimental design
One hundred one-week-old female broiler chicks from a commercial incubation facility were randomly assigned to three-tiered pens. Each pen had five cages and each cage had two animals. After pre-feeding for one week, the chicks were randomly divided into five experimental groups: CONTROL group, POL group, H-AMD group, LARD group, and soybean oil (SBO) group. The CONTROL group was fed with basic feed without fat addition. The POL group was fed with basic feed with palm oil addition. The H-AMD group was fed with basic feed with modified palm oil addition. The LARD group was fed with basic feed with lard addition. The SBO group was fed with basic feed with soybean oil addition. All fats were added to the basic feed at a ratio of 30g/kg. The formal feeding experiment began for 40 days, during which the animals had free access to food and water. All the procedures were approved by the Jinan University Animal Care and Use Committee.
Slaughtering and sampling
On the 39th day, cleared and weighed the remaining feed in the trough to initiate a 12-hour fasting period, with unrestricted access to water. After fasting, we randomly selected eight poultry specimens from each group collected blood samples, and used the heart puncture method to extract blood into heparinized tubes, which were immediately centrifuged to separate the plasma. The plasma samples were then stored at -20°C until further testing. After slaughtering the broilers and draining their blood, we dissected the heart, liver, spleen, stomach, abdominal fat, and ovaries divided the carcass, and separately weighed the skin, abdominal fat, breast muscle, and leg muscle, which were subsequently stored at -20°C for further analysis.
Measuring eviscerated weight, breast muscle weight, leg muscle weight, and abdominal weight while calculating the indexes of eviscerated rate, breast muscle rate, leg muscle rate and abdominal fat rate; eviscerated rate (%) = eviscerated weight/live weight×100; breast muscle yield (%) = ambilateral breast muscle weight/live weight×100; leg muscle yield (%) = ambilateral leg muscle weight/live weight×100; abdominal fat rate (%) = abdominal fat weight/eviscerated weight×100.
Lipid extraction
Adapt the Folch method to extract total lipids from the feces of the experimental chickens (Folch, Lees, & Sloane Stanley, 1957). 200 mg of fecal samples into small centrifuge tubes and 1 mL of physiological saline. After allowing the mixture to settle for 3 minutes, homogenized it for 20 seconds using a homogenizer. Next, add a chloroform-methanol mixture (2:1, volume ratio) to the tube and vigorously shake the mixture for 10 minutes to completely extract lipids. The mixture was then centrifuged at 3000 rpm for 10 minutes and carefully transferred the lower chloroform layer to another clean flask. Repeated this process three times and combined the chloroform layers from each repetition. After removing the solvent, the extracted lipid residue remained at the bottom of a round-bottom flask. To quantify the lipids, the internal standard docosanoic acid methyl ester and performed methylation.
For tissue lipid extraction, the previously stored tissues at -20°C to vacuum freeze-drying for 48 hours to remove moisture and obtain lyophilized samples. Following the method described by Kanjilal et al (Kanjilal, Kaki, Rao, Sugasini, Rao, Prasad, et al., 2013). homogenized 1 g of lyophilized tissue with 1 mL of 0.74% potassium chloride and 20 mL of a chloroform and methanol mixture (2:1, volume ratio) for 1 minute. shook the mixture overnight and then filtered it into another collection tube after thoroughly mixing it with 3 mL of 0.74% potassium chloride. Subsequently, washed the mixture with 3 mL of a chloroform/methanol/water mixture (3:48:47, volume ratio), and retained the chloroform layer for lipid analysis.
Blood chemistry
Plasma metabolites including triglycerides (TG), total cholesterol (TC), and fractions (high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C))were determined using an automatic analyzer with a commercial kit according to the manufacturing procedure.
Statistics and analysis
All analyses were performed in triplicate, and the results are reported as mean ± standard deviation. A one-way ANOVA was performed using SPSS 16 statistical software (SPSS Inc., Chicago, IL, USA), and differences were considered significant at p < 0.05, according to Duncan's Multiple Range Test.
Results
Fatty acids composition and positional distribution
Due to ester exchange rearranging fatty acyl groups on TAG, the total fatty acid composition of POL and H-AMD remains consistent; however, there are significant differences in fatty acids at the sn-2 position. In POL, palmitic acid content at the sn-2 position is 10.93%, whereas in H-AMD this proportion rises to 34.12% (Table 2), corresponding to a decrease in palmitic acid content at sn-1 and sn-3 positions. Compared with plant-derived oils such as POL and H-AMD, animal-derived oil lard has a similar ratio of saturated to unsaturated fatty acids, which is close to 1:1. However, while saturated fatty acids in POL and H-AMD are mainly palmitic acid, lard contains stearic acid in a proportion that is 3.7 times higher than them. The distribution of fatty acids at the sn-2 position among the three also varies greatly; saturated fatty acids in POL mainly exist at the sn-1,3 position, while H-AMD's saturated fatty acid content at the sn-2 position increases to about 40%, and that of Lard reaches as high as 68%.
Table 2.
Fatty acid compositions (%) of oil materials.
| Fatty acid | POL | H-AMD | LARD | |
|---|---|---|---|---|
| Total | C16:0 | 40.59±0.10 | 40.74±0.11 | 30.46±0.10 |
| C18:0 | 4.48±0.07 | 4.51±0.03 | 16.93±0.06 | |
| C18:1 | 44.26±0.11 | 44.14±0.13 | 40.38±0.17 | |
| C18:2 | 10.68±0.05 | 10.61±0.05 | 12.23±0.07 | |
| SFA | 45.06±0.08b | 45.25±0.08b | 47.38±0.10a | |
| UFA | 54.94±0.08a | 54.75±0.09a | 52.62±0.10b | |
| Sn-2 | C16:0 | 10.93±0.22 | 34.12±0.04 | 62.45±0.89 |
| C18:0 | 2.91±0.34 | 6.19±0.01 | 5.94±0.09 | |
| C18:1 | 69.67±0.76 | 47.80±0.04 | 23.02±1.45 | |
| C18:2 | 16.47±0.48 | 11.90±0.06 | 8.59±0.49 | |
| SFA | 13.84±0.28c | 40.31±0.03b | 68.39±0.97a | |
| UFA | 86.16±0.28a | 59.69±0.04b | 31.11±0.97c | |
POL, palm olein; H-AMD, high degree of acyl migration palm olein; LARD, lard oil; SFA, saturated fatty acid; UFA, unsaturated fatty acid.
Within a row, numbers with different superscripts differ statistically at p < 0.05.
Triglyceride composition
POO and PPO are the primary components of POL and H-AMD glycerides (>45%). The former primarily consists of sn-POO and sn-POP, while the latter, after modification, has increased sn-OPO and sn-PPO contents due to acyl migration. In contrast, Lard's TAG is primarily composed of POS and POO (48.49%) (Table 3), mainly consisting of sn-SPO and sn-OPO.
Table 3.
Main TAG compositions (area %) of palm olein, high degree of acyl migration palm olein, and lard oil.
| Main TAGs | POL | H-AMD | LARD |
|---|---|---|---|
| POP | 30.97±0.01a | 23.63±0.11b | 12.09±0.72c |
| POS | 5.48±0.48b | 4.36±0.60c | 20.56±0.26a |
| POO | 26.55±0.48b | 22.75±0.19c | 27.93±0.35a |
TAG, triglyceride; POL, palm olein; H-AMD, high degree of acyl migration palm olein; LARD, lard oil; POP, a TAG with one oleoyl and two palmitoyls; POS, a TAG with one palmitoyl and one oleoyl and one stearoyl; POO, a TAG with one palmitoyl and two oleoyls.
Within a row, numbers with different superscripts differ statistically at p < 0.05.
Stool fatty acid soaps
On the third day of feeding, the POL group had the highest content of fatty acid soap in feces (Table 4). Since the CONTROL group did not have any additional oil, its fatty acid resources mainly relied on corn and soybean meal from feed. Linoleic acid accounted for the highest proportion of fatty acid composition at 56.61%, followed by oleic acid at 24.58%. Therefore, in the CONTROL feeding group, fecal lipids were dominated by linoleic acid, and total fatty acid soap content was the lowest. Compared with the third day, fatty acid soap content in feces on the thirtieth day of feeding decreased.
Table 4.
Fatty acid soaps content of the stool on day 3 and day 30.
| Stool fatty acid soaps (mg/g dw stool) |
C16:0 | C18:0 | C18:1 | C18:2 | Total | |
|---|---|---|---|---|---|---|
| Day 3 | CONTROL | 5.21±0.01d | 1.90±0.02d | 6.56±0.04c | 13.03±0.08d | 26.70±0.12c |
| POL | 26.07±0.63a | 4.87±0.16b | 22.99±0.47a | 21.17±0.46a | 75.09±1.72a | |
| H-AMD | 20.50±0.45b | 3.92±0.07c | 16.73±0.41b | 16.39±0.47c | 57.53±1.39b | |
| LARD | 15.80±0.13c | 8.84±0.06a | 16.67±0.16b | 18.19±0.24b | 55.81±1.38b | |
| Day 30 | CONTROL | 3.43±0.04d | 1.36±0.22c | 4.21±0.07c | 7.13±0.08b | 16.13±0.17d |
| POL | 15.17±0.20a | 2.90±0.04b | 8.46±0.13a | 7.76±0.11ab | 34.28±0.46a | |
| H-AMD | 12.45±0.80b | 2.68±0.12b | 8.58±0.53a | 8.17±0.45a | 31.88±1.89b | |
| LARD | 7.82±0.11c | 6.07±0.08a | 7.08±0.10b | 7.11±0.07b | 28.08±0.31c | |
CONTROL, basal diet; POL, birds were fed basal diet but with palm olein; H-AMD, birds were fed basal diet but with high degree of acyl migration palm olein; LARD, birds were fed basal diet but with lard oil.
Within a row, numbers with different superscripts differ statistically at p < 0.05.
Carcass yield
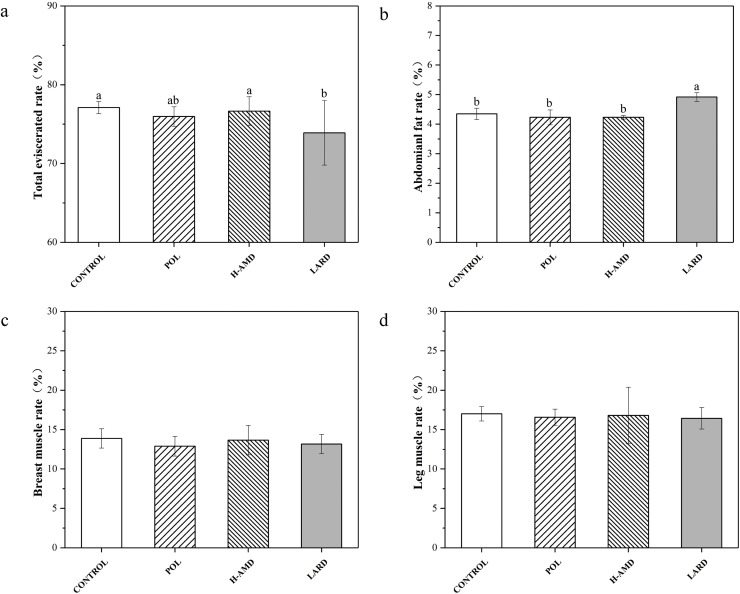
The carcass yield obtained from each experimental group (Fig. 1) shows that the total eviscerated rate of the H-AMD feeding group (76.66%) is slightly higher than that of the POL group (75.97%), but there is no significant difference in statistical data. Except for the total eviscerated rate of the lard group (73.90%), which is significantly lower (P<0.05), the results of other groups are between 75.97% and 77.10%. From the results of abdominal fat rate output, there was no significant difference (4.32%-4.42%) among all groups except for the lard group (P<0.05). In contrast, the abdominal fat rate of the lard group (4.92%) was significantly higher than that of other experimental groups. In addition, important indicators of broiler chickens, such as breast muscle rate and leg muscle rate, were examined, and there was no significant difference between the results of each oil-feeding group and the control group. The breast muscle rate ranged from 12.90% to 13.88%, and the leg muscle rate ranged from 16.43% to 17.01%.
Fig. 1.
Carcass parameters of broiler chickens fed different lipid sources.a,b (a The mean ± standard deviation with different letters denoting significant difference at p < 0.05 (n=8); b Abbreviations used: CONTROL, basal diet; POL, birds were fed basal diet but with palm olein; H-AMD, birds were fed basal diet but with high degree of acyl migration palm olein; LARD, birds were fed basal diet but with lard oil.).
Whole-body fatty acid profile
From Table 5, it is evident that, compared to the POL-fed group, the H-AMD group showed a significant increase in palmitic acid content in both skin and abdominal fat tissue (1.55%-1.69%). The H-AMD group exhibited smaller increases (<0.64%) or no significant differences in the fatty acid composition of breast and leg muscles. While the H-AMD group showed a marked increase in palmitic acid content, it also displayed a significant decrease in unsaturated fatty acids, such as oleic acid (2.29%-2.83%). The CONTROL group, which received no added oil, had the lowest linoleic acid content.
Table 5.
Whole-body fatty acid profile.
| Samples | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | |
|---|---|---|---|---|---|---|
| Breast muscle | CONTROL | 28.90±0.07a | 7.00±0.64a | 7.69±0.69b | 40.31±0.89a | 16.11±0.85c |
| POL | 28.71±0.17a | 4.73±0.13b | 8.81±0.31a | 39.65±0.78ab | 18.10±0.19b | |
| H-AMD | 28.70±0.37a | 5.02±0.30b | 7.97±0.34b | 39.25±0.85ab | 19.06±0.44a | |
| LARD | 28.48±0.33a | 4.90±0.16b | 9.28±0.21a | 38.77±0.15b | 18.78±0.31ab | |
| Leg muscle |
CONTROL | 28.96±0.11a | 8.32±0.04a | 6.86±0.94a | 41.93±1.95a | 13.95±0.95b |
| POL | 27.27±0.47c | 5.65±0.35c | 7.23±1.80a | 41.86±2.40a | 18.00±1.41a | |
| H-AMD | 27.91±0.23b | 6.17±0.32bc | 8.12±0.11a | 39.03±0.17b | 18.79±0.18a | |
| LARD | 28.05±0.28b | 6.59±0.55b | 6.69±0.11a | 41.00±0.58ab | 17.68±0.74a | |
| Skin | CONTROL | 28.11±0.74b | 8.30±0.33a | 5.62±0.20b | 42.74±0.16ab | 15.23±1.32b |
| POL | 27.47±0.28b | 7.21±0.49b | 4.86±0.21c | 43.65±0.84a | 16.82±1.13ab | |
| H-AMD | 29.16±0.13a | 6.67±0.71b | 5.90±0.09b | 41.17±0.73c | 17.10±0.77a | |
| LARD | 27.95±0.08b | 6.64±0.08b | 6.50±0.33a | 41.88±0.34bc | 17.03±0.16a | |
| Abdominal fat | CONTROL | 29.28±0.01a | 7.38±0.01a | 5.78±0.01b | 41.64±0.02c | 15.92±0.06b |
| POL | 27.57±0.02d | 6.84±0.04b | 5.04±0.01c | 43.85±0.05a | 16.72±0.01b | |
| H-AMD | 29.12±0.01b | 6.57±0.01c | 5.81±0.01b | 41.56±0.06c | 16.96±0.04a | |
| LARD | 28.22±0.01c | 6.81±0.04b | 6.08±0.02a | 42.29±0.06b | 16.61±0.01c |
CONTROL, basal diet; POL, birds were fed basal diet but with palm olein; H-AMD, birds were fed basal diet but with high degree of acyl migration palm olein; LARD, birds were fed basal diet but with lard oil.
Within a row, numbers with different superscripts differ statistically at p < 0.05.
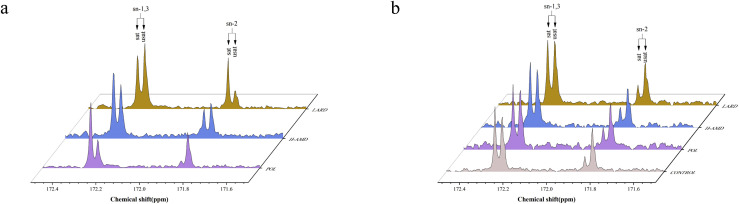
Positional distribution of fatty acids in triglycerides of abdominal fat
The distribution of fatty acids in abdominal fat is presented in Fig. 2. The nuclear magnetic resonance spectra illustrate the content of saturated and unsaturated fatty acids at the sn-1,3 and sn-2 positions of TAG. The fatty acid distribution in the original oils (POL, H-AMD, LARD) is shown in Fig. 2(a), where the saturated fatty acids content at the sn-2 position exhibits a gradient increase (integral values: 14%,41%,63%). Fig. 2(b) displays the fatty acid distribution in fat deposited in the abdominal region, where differences in saturated fatty acid content at the sn-2 position are relatively minor (integral values: 18%, 23%, 28%, 23%).
Fig. 2.
Distribution of Fatty acid of feed oil (a) and abdominal deposition fat (b).a (a Abbreviations used: sat, saturated fatty acid; usat, unsaturated fatty acid.).
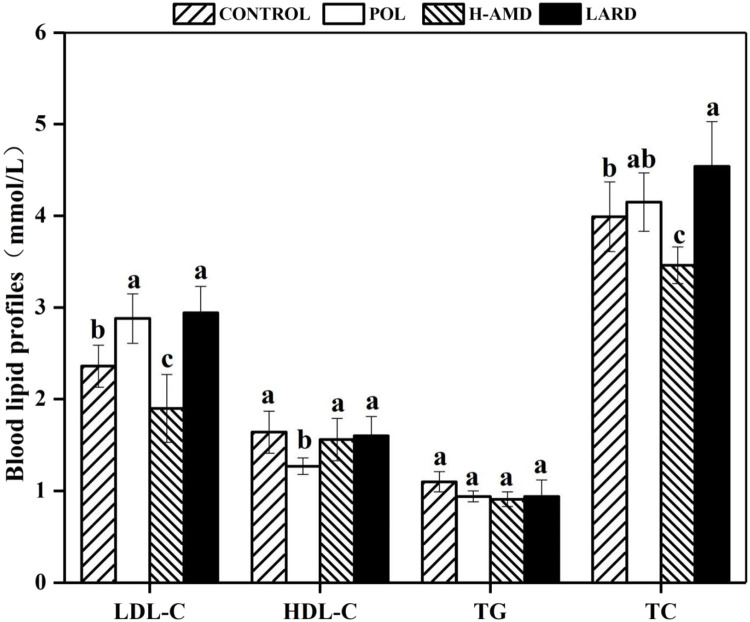
Fasting plasma lipid
The 12-hour fasting blood lipid levels of each experimental group were within the normal range (Fig. 3). Compared to the POL group, the H-AMD group exhibited more favorable blood lipid profiles. Specifically, the LDL-C level decreased by 34% (from 2.88 to 1.9 mmol/L), the HDL-C level increased by 23% (from 1.27 to 1.56 mmol/L), and triglyceride levels showed no significant differences among the groups. Total cholesterol levels decreased by 16.63% (from 4.15 to 3.46 mmol/L). The LARD group had the highest levels across all lipid variables.
Fig. 3.
Four indicators of fasting blood lipids.a,b (a The mean ± standard deviation with different letters denoting significant difference at p < 0.05 (n=8).b Abbreviations used: TC, total cholesterol; LDL-C; low-density lipoprotein cholesterol; HDL-C; high-density lipoprotein cholesterol; TG, triglyceride.).
Discussion
It is widely recognized that the distribution of saturated fatty acids at the sn-2 position of lipids is more favorable for lipid absorption compared to their distribution at the sn-1,3 positions. Based on this understanding, enzymatic interesterification was applied to palm oil, rich in saturated fatty acids, where 90% of saturated fatty acids are located at the sn-1,3 positions. The aim was to reposition the fatty acids on the glycerol backbone, allowing for a greater distribution of saturated fatty acids at the sn-2 position. Therefore, this study discusses the impact of sn-2 saturated fatty acid content on digestion, absorption, blood lipids, and abdominal fat deposition.
Through enzymatic interesterification of POL, the fatty acid positioning was rearranged to obtain a modified fat (H-AMD) with characteristics that resemble animal fats like lard, which tend to have a preference for distributing saturated fatty acids at the sn-2 position. The sn-2 saturated fatty acid content in H-AMD falls between that of POL and lard. Although both POL and H-AMD are predominantly composed of POP (a TAG with two palmitoyls and one oleoyl) and POO (a TAG with one palmitoyl and two oleoyls) triacylglycerols, there are differences in the amounts of these two TAG isomers between the two. In POL, sn-POO and sn-POP are the predominant isomers, while in H-AMD, sn-OPO and sn-PPO are the main isomers. This difference is a significant factor contributing to the approximately 30% disparity in the sn-2 palmitic acid content between the two. Feeding trials indicate that the modified POL (H-AMD) reduces the loss of fatty acids in feces, particularly palmitic acid. The improved absorption effects of the modified fat are more prominent in the early stage of feeding (day three) in female broilers. Many papers focusing on lipid absorption emphasize the importance of this TAG structure maintaining a high content of palmitic acid at the sn-2 position for infant nutrition absorption (Mu & Høy, 2004). However, there is limited emphasis on the importance of such lipid structures for gastrointestinal absorption in adults. Some studies have reported that adults are capable of effectively absorbing most dietary fatty acids, regardless of the positions of stearic acid and palmitic acid in TAGs (Berry, 2009; T. Wang, Wang, & Wang, 2016). Infants have low levels of enzyme activity, gastric and pancreatic secretions, bile salt concentration, and gastric motility at birth, and these factors may make them more reliant on such structured lipids (Gallier & Singh, 2020). The superior effect of H-AMD in improving lipid absorption in young broiler chickens may be attributed to these factors. Research has reported that, in chicks, lipase secretion levels increase by 20 times from day 4 to 21 post-hatching (Noy & Sklan, 1995). As a result, the advantage (enhanced absorption) brought about by differences in fatty acid structural distribution diminishes as the organism's ability to absorb fat improves.
H-AMD supplementation reduced fecal loss of palmitic acid, resulting in increased palmitic acid content in the body, particularly in abdominal and skin fat. Similar findings were reported by W. Smink et al (Smink, Gerrits, Hovenier, Geelen, Lobee, Verstegen, et al., 2008), where modified palm oil feeding significantly raised palmitic acid content in both breast muscle and abdomen fat (by 1.3%). In our study, the H-AMD group showed only minor increases (<0.64%) or no significant differences in fatty acid composition in breast and leg muscles. This may be due to the fact that intramuscular fat consists mainly of phospholipids, which are less affected by diet than storage fats like abdominal fat (Villaverde, Baucells, Cortinas, & Barroeta, 2006). The CONTROL group, relying on endogenous fatty acid synthesis without dietary fat supplementation, exhibited the lowest C18:2 content across tissues. This occurs because the primary fatty acids synthesized in the liver of chickens are C16:0, C16:1, C18:0, and C18:1, while polyunsaturated fatty acids (C18:2) rely more heavily on dietary intake.
Some opinions believe that “during absorption and transportation processes, no alterations occur in the composition of fatty acids. Therefore, there is a great similarity between the dietary fat fed to birds and the body fat that is deposited” (Baião & Lara, 2005). However, experimental results show that regardless of the addition of fat or the addition of animal-derived lard and plant-derived palm oil, or modified palm oil, it can only affect the changes in tissue fatty acid content to a certain extent. Compared to the CONTROL group, the differences in fatty acid content in the tissues of the other feeding groups (POL, H-AMD, LARD) were generally within 3%. This suggests that the content, type, and distribution of saturated fatty acids in dietary fat have a relatively small impact on the fatty acid composition of tissues. Even though the content of stearic acid in lard can be as high as 16.93%, the final content of this type of fatty acid in the deposited fat ranges from 6.08% to 9.28%. It indicates that the body can maintain fatty acid homeostasis in tissues to counteract the changes in fatty acid profiles caused by exogenous fatty acids.
Although the reconstitution of fatty acids in POL may have a limited impact on the final composition of deposited fatty acids in the body, does it affect the distribution of fatty acids in the deposited fat? Similar to the distribution of saturated fatty acids in POL and H-AMD? There is a hypothesis that due to the substrate specificity of lipases (gastric lipases, pancreatic lipases, and lipoprotein lipases all preferentially hydrolyze fatty acids at the sn-1,3 positions (Sivakanthan & Madhujith, 2020; Y. Wang, Zhang, Liu, Chang, Wei, Jin, et al., 2022)), 2-monoacylglycerol (2-MAG) produced after the hydrolysis of triacylglycerol (TAG) by lipases can be re-esterified into TAG and stored in the body, potentially leading to the inheritance of sn-2 fatty acids from dietary fats in tissues. Some studies have shown that at least the sn-2 position of dietary fats with higher levels of saturated fatty acids is preserved during the absorption by intestinal epithelial cells and re-esterification into TAG for packaging into chylomicrons for transport in the blood (Berry, Miller, & Sanders, 2007; Pufal, Quinlan, & Salter, 1995). Considering the distribution characteristics of sn-2 fatty acids in the deposited fat in the abdomen of this study, even though the sn-2 levels of saturated fatty acids in Lard and H-AMD are 54.55% and 26.47% higher, respectively, compared to POL, the differences in sn-2 saturated fatty acid levels in the final abdominal fat are not significant. We thought this may be related to the complex processes involved in the digestion, absorption, and deposition of dietary fats in adipose tissue. Since TAG cannot enter cells in a non-hydrolyzed form (Recazens, Mouisel, & Langin, 2021), dietary fats undergo numerous hydrolysis and re-esterification processes before they are deposited in adipose tissue (Giammanco, Cefalù, Noto, & Averna, 2015; Y. Wang, Zhang, et al., 2022). Some studies have shown that acyl migration accompanies hydrolysis, with 25% of 2-MAG being acyl transferred to 1(3)-MAG during the hydrolysis process. In addition, this might be related to the amount of 2-MAG provided by dietary fatty acids during the small intestinal digestion stage. Theoretically, one molecule of TAG is hydrolyzed to produce two molecules of FFA and one molecule of 2-MAG. However, in reality, based on numerous in vitro experimental results (including some of our undisclosed data), the amount of MAG produced during digestion is significantly lower than 50% of FFA content (Jimenez-Moya, Martin, Soler-Rivas, Barroeta, Tres, & Sala, 2021; Salvia-Trujillo, Verkempinck, Sun, Van Loey, Grauwet, & Hendrickx, 2017; Y. Wang, Cao, Liu, Chang, Wei, Jin, et al., 2022). Therefore, the supply of MAG from hydrolysis is insufficient to esterify the remaining FFA. However, when FFA supply exceeds MAG, TAG synthesis can occur through the α-glycerophosphate pathway (Karupaiah & Sundram, 2007). Some research reported this pathway is responsible for 20% of TAG synthesis during the fed state (Lehner, Kuksis, & Itabashi, 1993), indicating that the fatty acid composition at the sn-2 position in the synthesized TAG partially originates from FFA.
Considering the diversity of TAG synthesis pathways in the body, the distribution of fatty acid within adipose tissue is only minimally influenced by dietary fat. Therefore, despite considerable variations in the saturated fatty acid composition at the sn-2 position of dietary fats, this variation is not prominently reflected in the final deposition of adipose tissue. As demonstrated by Berry et al (Berry, Miller, & Sanders, 2007), in their study “The solid fat content of stearic acid-rich fats determines their postprandial effects”, randomized and non-randomized fats (original dietary oils) contained 22.81% and 3.1% stearic acid at the sn-2 position of triglycerides, respectively. However, in chylomicrons, these values decrease to 19.6% and 11.0%, respectively. This is only the first step of hydrolysis and esterification into the body, and the difference in sn-2 content has been reduced by 11.11%. Subsequently, the fats undergo further decomposition and synthesis in the liver and are then transported to the site of fat deposition (e.g., the abdomen), where they are ultimately deposited following hydrolysis by lipoprotein lipase and re-synthesis by hormone-sensitive lipase. Consequently, it may be challenging to achieve the expected results in certain studies aiming to customize fats with specific TAG structures and maintain fatty acids in specific positions after absorption.
The association between dietary fatty acids and cardiovascular disease risk has been extensively studied, and predictive equations on their blood lipid effects have been validated (Hooper, Martin, Jimoh, Kirk, Foster, & Abdelhamid, 2020; Mensink, Zock, Kester, & Katan, 2003; Ulbricht & Southgate, 1991). However, the interesterification does not alter the fatty acid composition, thus making it unable to predict the effects of ester-exchanged fats on blood lipids compared to natural fats. The results of this study on fasting blood lipids show that H-AMD has a lower potential for atherosclerosis compared to POL. There is no consensus on the effects of ester-exchanged modified fats on blood lipids. Some animal experimental studies suggest that palmitic acid and stearic acid distributed in the sn-2 position in triglycerides (TAG) are more likely to induce atherosclerosis compared to sn-1,3 positions (Berry, 2009). These effects may be due to the better absorption and lower chylomicron clearance of palmitic acid when esterified at the sn-2 position in the bloodstream. However, there are also studies with opposing views, suggesting that natural fats (with saturated fatty acids distributed at the sn-1,3 positions) are more likely to contribute to atherosclerosis (Kritchevsky, Tepper, Vesselinovitch, & Wissler, 1973; Michalski, Genot, Gayet, Lopez, Fine, Joffre, et al., 2013). Some studies have reported that interchanged fats show greater lipid-lowering effects (reduction in total cholesterol and low-density lipoprotein cholesterol) compared to mixed oils (Reena & Lokesh, 2007). There is also evidence that interchanged fats can reduce postprandial blood lipids (Sanders, Filippou, Berry, Baumgartner, & Mensink, 2011). However, human dietary intervention studies have not demonstrated any chronic effects of sn-positional composition or interesterification of palmitic acid-rich plant fats (whereby the proportion of palmitic acid in the sn-2 position is increased) on blood lipids or insulin sensitivity (Hall, Iqbal, Li, Gray, & Berry, 2017).
From the perspective of carcass yield, the type of dietary fat has limited influence on the breast and leg muscles, which are the main edible parts of broilers. Broilers fed with lard have a higher tendency to accumulate excessive fat in the abdominal region, which explains why they achieved the lowest eviscerated rate, as excessive fat deposition indirectly leads to a decline in meat production performance, no significant differences were observed among other experimental groups. From the perspective of poultry production, a high proportion of inedible abdominal fat is unfavorable for economic benefits. Regarding the differences in abdominal fat deposition caused by different dietary fat intake, some studies have reported that feeding oils rich in polyunsaturated fatty acids, such as sunflower seed oil and flaxseed oil, significantly reduces abdominal fat deposition compared to feeding butter, which is rich in saturated fat, due to enhanced beta-oxidation (Ferrini, Manzanilla, Menoyo, Esteve-Garcia, Baucells, & Barroeta, 2010). To further elucidate this issue, the results of broilers fed with soybean oil (SBO) are provided in the supplementary data (supplementary-Fig. 1). According to the results of this study, the abdominal fat percentage of broilers fed with lard is significantly higher than that of the SBO group. However, for oils such as POL and H-AMD, which have similar saturated fatty acid content to lard, there is no significant difference in abdominal fat deposition compared to the soybean oil-fed group. The conclusion that the high content of unsaturated fatty acids in soybean oil enhances beta-oxidation and reduces abdominal fat deposition does not adequately explain the results of feeding POL that have nearly 50% saturated fatty acid content (There are also articles reporting supporting evidence for this conclusion regarding the difference in abdominal fat deposition between palm oil and lard feeding (Ozung, 2020)). Previous studies have also focused more on comparing animal oils rich in saturated fatty acids (butter and lard) with plant oils rich in unsaturated fatty acids (soybean oil and flaxseed oil), and have included palm oil, which contains similar levels of saturated fat, to a lesser extent. It is speculated that saturated fatty acid content may not be the key factor in the difference in abdominal fat percentage between palm oil and lard-fed broilers. The results of the Gouk study indicate that the positional distribution of long-chain saturated fatty acids has a more profound effect on body fat accretion than the total content of saturated fatty acids (Gouk, Cheng, Mok, Ong, & Chuah, 2013).
To further investigate the results of abdominal fat rate, a new round of experimental research was conducted. Two types of oils, H-AMD-L and H-AMD-S, were derived from H-AMD using fractionation techniques. H-AMD-S contained a higher percentage of saturated fatty acids (61.18%) compared to Lard, with an increase of 13.79%. On the other hand, H-AMD-L had a higher content of unsaturated fatty acids (60.60%). H-AMD-S retained the PPP and PPO components from H-AMD, while H-AMD-L mainly retained the PPO and OPO components). The results of feeding experiments for abdominal fat rate can be found in supplementary data (Supplementary-Table 1, Table 2). In this study, there was no significant difference in abdominal fat rate among the three modified palm oils (H-AMD, H-AMD-L, H-AMD-S), but there was a trend of increased visceral fat rate compared to POL, mainly observed in H-AMD-L, although it was still lower than the results from the Lard feeding group. If saturated fatty acids were more likely to cause abdominal fat deposition, then H-AMD-S should have demonstrated the highest abdominal fat rate instead of Lard. POL and its modified products (H-AMD, H-AMD-L, H-AMD-S) had a relatively small impact on abdominal fat deposition compared to Lard. The saturated fatty acid content was not the determining factor for abdominal fat deposition, possibly due to inherent differences in the properties of animal and plant-derived oils. Similar studies have compared lard with hydrogenated vegetable shortening (which is rich in saturated fatty acids, but the specific content is unclear) and found that lard oil carries a higher risk of obesity and insulin resistance (Kubant, Poon, Sánchez-Hernández, Domenichiello, Huot, Pannia, et al., 2015). The result that plant-derived oils modified by enzymatic interesterification exhibit lower abdominal fat rates also demonstrates the advantage of such oils as substitutes for lard as raw materials for infant formula OPO. It also provides some reference for the design of future human milk fat substitutes. Not only do need to consider the composition of triglycerides (including fatty acid composition and positional distribution), but may also need to consider the influence of some inherent endogenous components of the substitute oils.
Table 1.
Ingredient and chemical composition of the basal diet (g/kg as fed basis).
| Ingredients | |
|---|---|
| Corn | 610.00 |
| Soybean meal | 200.00 |
| Wheat | 100.00 |
| Distillers' grains1 | 50.00 |
| Lysine | 1.80 |
| DL-methionine | 0.80 |
| Choline chloride | 1.50 |
| Mineral premix2 | 5.00 |
| Vitamin premix3 | 0.50 |
| Betaine | 0.40 |
| Sodium bicarbonate | 0.60 |
| Phytase | 0.10 |
| Sodium chloride | 3.00 |
| Dicalcium Phosphate | 8.00 |
| Zeolite powder | 18.30 |
The dietary distillers' grains are derived from corn.
Mineral premix provided per kg of complete diet: iron, 117 mg; copper, 12 mg; manganese, 125 mg; zinc, 80 mg; iodine, 0.3 mg; selenium, 0.13 mg.
Vitamin premix provided per kg of complete diet: vitamin A, 12,500 IU; vitamin D3, 3,500 IU; vitamin E, 30 IU; vitamin K3, 3 mg; vitamin B12, 0.04 mg; biotin, 0.6mg; folic acid, 1.25 mg; nicotinic acid, 50 mg; pantothenic acid, 10 mg; riboflavin, 8 mg.
Conclusion
The research findings revealed that POL when modified through enzymatic interesterification, exhibited an enhanced content of saturated fatty acids at the sn-2 position. Moreover, this modification led to a reduction in the loss of fatty acid soaps in feces. Consequently, the deposition of palmitic acid in abdominal adipose tissue increased, thereby reducing the risk of atherosclerosis. The homeostatic capability of the body limited the influence of dietary fats on the fatty acid composition, positional distribution, and carcass production of broiler chickens. Relative to saturated fatty acid derived from animal sources like lard oil, POL and modified POL displayed a diminished trend in abdominal fat deposition, showcasing their superiority as a source of OPO raw materials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Bureau of Science and Information of Guangzhou under grant 2024B03J00503, the National Natural Science Foundation of China under grant 32272341, the National Key Research and Development Program of China under grant 2023YFD2100401, the Opening Project of Hubei Key Laboratory of Lipid Chemistry and Nutrition of China under grant 202401, and the Department of Science and Technology of Guang-dong Province under the grant 2022B0202010003, are gratefully acknowledged.
Footnotes
To be revised to Poultry Science
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2024.104579.
Appendix. Supplementary materials
References
- Ai H., Lee Y.Y., Xie X., Tan C.P., Lai O.M., Li A., Wang Y., Zhang Z. Structured lipids produced from palm-olein oil by interesterification: A controllable lipase-catalyzed approach in a solvent-free system. Food Chem. 2023;412 doi: 10.1016/j.foodchem.2023.135558. [DOI] [PubMed] [Google Scholar]
- Archer G.S., Sobotik E.B. Evaluation of the timing of use of phosphatidic acid in the diet on growth performance and breast meat yield in broilers. Animals. 2022;12:3446. doi: 10.3390/ani12243446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baião N.C., Lara L. Oil and fat in broiler nutrition. Braz. J. Poultry Sci. 2005;7:129–141. [Google Scholar]
- Berry S.E. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: an overview and implications for cardiovascular disease. Nutr. Res. Rev. 2009;22:3–17. doi: 10.1017/S0954422409369267. [DOI] [PubMed] [Google Scholar]
- Berry S.E., Miller G.J., Sanders T.A. The solid fat content of stearic acid–rich fats determines their postprandial effects. Am. J. Clin. Nutr. 2007;85:1486–1494. doi: 10.1093/ajcn/85.6.1486. [DOI] [PubMed] [Google Scholar]
- de Mesquita Souza Saraiva M., Lim K., do Monte D.F.M., Givisiez P.E.N., Alves L.B.R., de Freitas Neto O.C., Kariuki S., Júnior A.B., de Oliveira C.J.B., Gebreyes W.A. Antimicrobial resistance in the globalized food chain: a one health perspective applied to the poultry industry. Brazilian J. Microbiol. 2022:1–22. doi: 10.1007/s42770-021-00635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini G., Manzanilla E., Menoyo D., Esteve-Garcia E., Baucells M., Barroeta A. Effects of dietary n-3 fatty acids in fat metabolism and thyroid hormone levels when compared to dietary saturated fatty acids in chickens. Livest. Sci. 2010;131:287–291. [Google Scholar]
- Filer Jr L., Mattson F., Fomon S. Triglyceride configuration and fat absorption by the human infant. J. Nutr. 1969;99:293–298. doi: 10.1093/jn/99.3.293. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gallier S., Singh H. Modification of milk fat globules during processing and gastrointestinal digestion. Dairy Fat Products Functionality: Fundamental Sci. Technol. 2020:133–152. [Google Scholar]
- Giammanco A., Cefalù A.B., Noto D., Averna M.R. The pathophysiology of intestinal lipoprotein production. Front. Physiol. 2015;6:61. doi: 10.3389/fphys.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouk S.W., Cheng S.F., Mok J.S.L., Ong A.S.H., Chuah C.H. Long-chain SFA at the sn-1, 3 positions of TAG reduce body fat deposition in C57BL/6 mice. Br. J. Nutrition. 2013;110:1987–1995. doi: 10.1017/S0007114513001475. [DOI] [PubMed] [Google Scholar]
- Hall W.L., Iqbal S., Li H., Gray R., Berry S.E. Modulation of postprandial lipaemia by a single meal containing a commonly consumed interesterified palmitic acid-rich fat blend compared to a non-interesterified equivalent. Eur. J. Nutr. 2017;56:2487–2495. doi: 10.1007/s00394-016-1284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper, L., Martin, N., Jimoh, O. F., Kirk, C., Foster, E., & Abdelhamid, A. S. (2020). Reduction in saturated fat intake for cardiovascular disease. Cochrane database of systematic reviews. [DOI] [PMC free article] [PubMed]
- Jimenez-Moya B., Martin D., Soler-Rivas C., Barroeta A.C., Tres A., Sala R. Acid versus crude oils for broiler chicken diets: in vitro lipid digestion and bioaccessibility. Anim. Feed. Sci. Technol. 2021;276 [Google Scholar]
- Kanjilal S., Kaki S.S., Rao B.V., Sugasini D., Rao Y.P., Prasad R.B., Lokesh B.R. Hypocholesterolemic effects of low calorie structured lipids on rats and rabbits fed on normal and atherogenic diet. Food Chem. 2013;136:259–265. doi: 10.1016/j.foodchem.2012.07.116. [DOI] [PubMed] [Google Scholar]
- Karupaiah T., Sundram K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutr. Metab. (Lond) 2007;4:16. doi: 10.1186/1743-7075-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S.A., Vesselinovitch D., Wissler R.W. Cholesterol vehicle in experimental atherosclerosis part 13. Randomized peanut oil. Atherosclerosis. 1973;17:225–243. doi: 10.1016/0021-9150(73)90090-7. [DOI] [PubMed] [Google Scholar]
- Kubant R., Poon A., Sánchez-Hernández D., Domenichiello A., Huot P., Pannia E., Cho C., Hunschede S., Bazinet R., Anderson G. A comparison of effects of lard and hydrogenated vegetable shortening on the development of high-fat diet-induced obesity in rats. Nutr. Diabetes. 2015;5:e188. doi: 10.1038/nutd.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner R., Kuksis A., Itabashi Y. Stereospecificity of monoacylglycerol and diacylglycerol acyltransferases from rat intestine as determined by chiral phase high-performance liquid chromatography. Lipids. 1993;28:29–34. doi: 10.1007/BF02536356. [DOI] [PubMed] [Google Scholar]
- Mattson F., Volfenhein R. Rearrangement of glyceride fatty acids during digestion and absorption. J. Biol. Chem. 1962;237:53–55. [PubMed] [Google Scholar]
- Mensink R.P., Zock P.L., Kester A.D., Katan M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Michalski M.C., Genot C., Gayet C., Lopez C., Fine F., Joffre F., Vendeuvre J.L., Bouvier J., Chardigny J.M., Raynal-Ljutovac K. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog. Lipid Res. 2013;52:354–373. doi: 10.1016/j.plipres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Mu H., Høy C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004;43:105–133. doi: 10.1016/s0163-7827(03)00050-x. [DOI] [PubMed] [Google Scholar]
- Ng N., Chen P.X., Ghazani S.M., Wright A.J., Marangoni A., Goff H.D., Joye I.J., Rogers M.A. Lipid digestion of oil-in-water emulsions stabilized with low molecular weight surfactants. Food Funct. 2019;10:8195–8207. doi: 10.1039/c9fo02210d. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Digestion and absorption in the young chick. Poult. Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- Ozung P. Comparative evaluation of palm oil and lard as partial replacement for maize in broiler chicken diets. EC Veterinary Sci. 2020;5:56–62. [Google Scholar]
- Pufal D.A., Quinlan P.T., Salter A.M. Effect of dietary triacylglycerol structure on lipoprotein metabolism: a comparison of the effects of dioleoylpalmitoylglycerol in which palmitate is esterified to the 2-or 1 (3)-position of the glycerol. Biochimica et Biophysica Acta (BBA)-Lipids Lipid Metabolism. 1995;1258:41–48. doi: 10.1016/0005-2760(95)00095-t. [DOI] [PubMed] [Google Scholar]
- Recazens E., Mouisel E., Langin D. Hormone-sensitive lipase: sixty years later. Prog. Lipid Res. 2021;82 doi: 10.1016/j.plipres.2020.101084. [DOI] [PubMed] [Google Scholar]
- Reena M.B., Lokesh B.R. Hypolipidemic effect of oils with balanced amounts of fatty acids obtained by blending and interesterification of coconut oil with rice bran oil or sesame oil. J. Agric. Food Chem. 2007;55:10461–10469. doi: 10.1021/jf0718042. [DOI] [PubMed] [Google Scholar]
- Salvia-Trujillo L., Verkempinck S., Sun L., Van Loey A., Grauwet T., Hendrickx M. Lipid digestion, micelle formation and carotenoid bioaccessibility kinetics: influence of emulsion droplet size. Food Chem. 2017;229:653–662. doi: 10.1016/j.foodchem.2017.02.146. [DOI] [PubMed] [Google Scholar]
- Sanders T.A., Filippou A., Berry S.E., Baumgartner S., Mensink R.P. Palmitic acid in the sn-2 position of triacylglycerols acutely influences postprandial lipid metabolism. Am. J. Clin. Nutr. 2011;94:1433–1441. doi: 10.3945/ajcn.111.017459. [DOI] [PubMed] [Google Scholar]
- Sivakanthan S., Madhujith T. Current trends in applications of enzymatic interesterification of fats and oils: A review. Lwt. 2020;132 [Google Scholar]
- Smink W., Gerrits W., Hovenier R., Geelen M., Lobee H., Verstegen M., Beynen A. Fatty acid digestion and deposition in broiler chickens fed diets containing either native or randomized palm oil. Poult. Sci. 2008;87:506–513. doi: 10.3382/ps.2007-00354. [DOI] [PubMed] [Google Scholar]
- Ulbricht T., Southgate D. Coronary heart disease: seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-m. [DOI] [PubMed] [Google Scholar]
- Villaverde C., Baucells M., Cortinas L., Barroeta A. Effects of dietary concentration and degree of polyunsaturation of dietary fat on endogenous synthesis and deposition of fatty acids in chickens. Br. Poult. Sci. 2006;47:173–179. doi: 10.1080/00071660600610898. [DOI] [PubMed] [Google Scholar]
- Wang T., Wang X., Wang X. Effects of lipid structure changed by interesterification on melting property and lipemia. Lipids. 2016;51:1115–1126. doi: 10.1007/s11745-016-4184-3. [DOI] [PubMed] [Google Scholar]
- Wang Y., Cao M., Liu R., Chang M., Wei W., Jin Q., Wang X. The enzymatic synthesis of EPA-rich medium-and long-chain triacylglycerol improves the digestion behavior of MCFA and EPA: evidence on in vitro digestion. Food Funct. 2022;13:131–142. doi: 10.1039/d1fo02795f. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang T., Liu R., Chang M., Wei W., Jin Q., Wang X. Reviews of medium-and long-chain triglyceride with respect to nutritional benefits and digestion and absorption behavior. Food Res. Int. 2022;155 doi: 10.1016/j.foodres.2022.111058. [DOI] [PubMed] [Google Scholar]
- Yaron S., Shachar D., Abramas L., Riskin A., Bader D., Litmanovitz I., Bar-Yoseph F., Cohen T., Levi L., Lifshitz Y. Effect of high β-palmitate content in infant formula on the intestinal microbiota of term infants. J. Pediatr. Gastroenterol. Nutr. 2013;56:376–381. doi: 10.1097/MPG.0b013e31827e1ee2. [DOI] [PubMed] [Google Scholar]
- Zhou J., Lee Y.Y., Mao Y., Wang Y., Zhang Z. Future of structured lipids: enzymatic synthesis and their new applications in food systems. Foods. 2022;11:2400. doi: 10.3390/foods11162400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.