Abstract
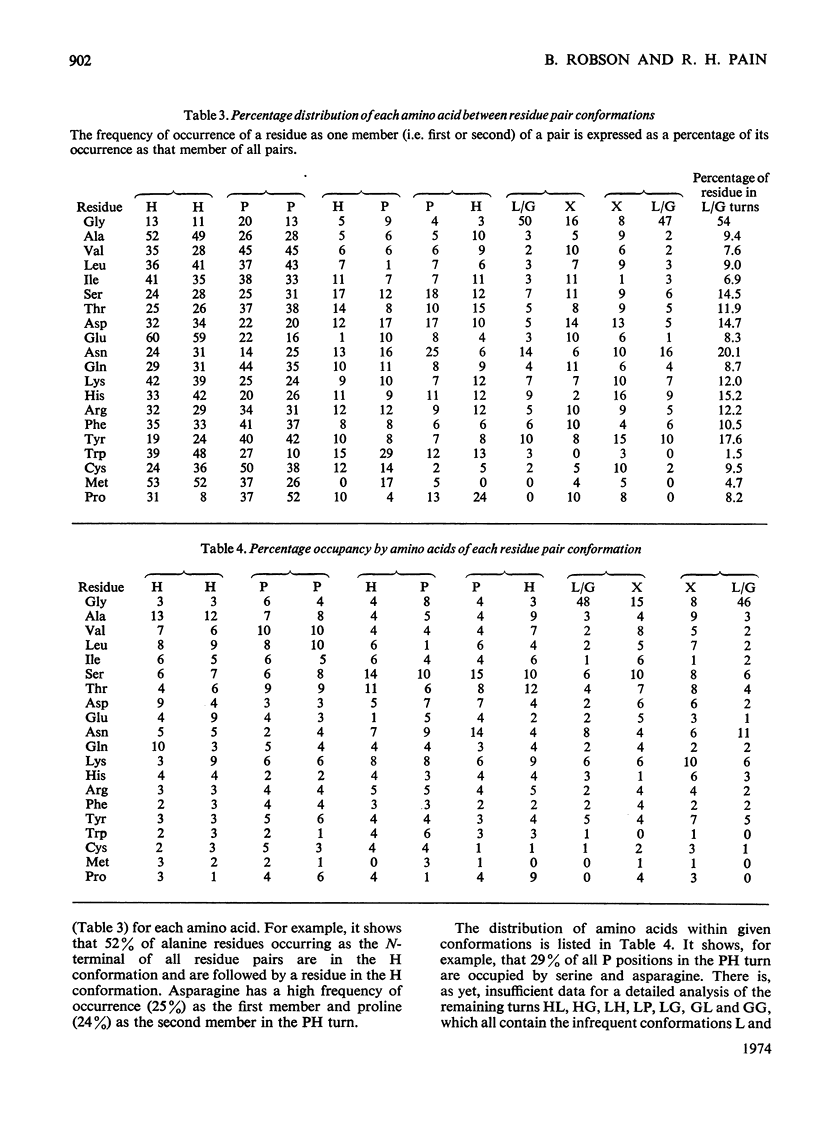
1. The residue pair is considered as the fundamental unit which differentiates α-helix, β-pleated sheet and the various turns and kink structures of the protein backbone. 2. The HPLG alphabet (Robson & Pain, 1974) is used to group pairs of residues, giving 16 possible conformational pairs, all of which are found with differing frequencies in the nine proteins examined. 3. The frequencies of occurrence of the 16 different types of turn or kink are analysed in relation to the constituent amino acids. Those containing the L or G conformation are of low frequency and are grouped for purposes of this analysis. 4. The distribution of amino acids within all the conformational pairs is non-random, with distinct preferences shown by certain residues. 5. All pairs containing an L or G conformation require the presence of a glycine or a proton-donor side chain. 6. The results are discussed in terms of the determination of these `random' structures by local interactions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Chain reversals in proteins. Biochim Biophys Acta. 1973 Apr 20;303(2):211–229. doi: 10.1016/0005-2795(73)90350-4. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Folding of polypeptide chains in proteins: a proposed mechanism for folding. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2293–2297. doi: 10.1073/pnas.68.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K. Logical analysis of the mechanism of protein folding. I. Predictions of helices, loops and beta-structures from primary structure. J Mol Biol. 1973 Apr 5;75(2):401–420. doi: 10.1016/0022-2836(73)90030-2. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in globular proteins. Development of a stereochemical alphabet on the basis of intra-residue information. Biochem J. 1974 Sep;141(3):869–882. doi: 10.1042/bj1410869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in proteins: possible implications for the mechanism of formation of helical regions. J Mol Biol. 1971 May 28;58(1):237–259. doi: 10.1016/0022-2836(71)90243-9. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An attempt to evaluate the influence of neighboring amino acids (n-1) and (n+1) on the backbone conformation of amino acid (n) in proteins. Use in predicting the three-dimensional structure of the polypeptide backbone of other proteins. J Mol Biol. 1973 Mar 25;75(1):13–31. doi: 10.1016/0022-2836(73)90526-3. [DOI] [PubMed] [Google Scholar]


