Abstract
The gut microbiome plays an important role in insect evolution and ecology. Bacteria support the host’s nutrition and defense and therefore play an important role in the fitness of the host. Halyomorpha halys is one of the most important invasive pest species in the world. Native to North-Eastern Asia, this Pentatomid bug has recently invaded North America and Europe, causing significant damage to agricultural production. Although an increasing number of studies investigated the biology of this pest species, little is known about the composition of its gut microbiota. Like many other Pentatomid species, H. halys harbors a primary symbiont called “Candidatus Pantoea carbekii,” which produces vitamins and essential amino acids for the host. However, information about the presence of other bacteria is currently lacking. Therefore, we investigated the gut microbiota of H. halys individuals, which were collected in the field across the year using a high-throughput 16S rRNA gene metabarcoding approach. Our results revealed 3309 different ASVs associated with H. halys, with Pantoea being the most abundant symbiont, present in almost all individuals. Additionally, many individuals harbor Commensalibacter, a genus of acetic acid bacterial symbionts. Besides these two predominant taxa, we show a high diversity of microorganisms associated with H. halys with seasonal fluctuations, highlighting a dynamic microbiota that might influence the biology of this species.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00248-024-02481-1.
Keywords: Gut microbiome, 16S rRNA gene, Pentatomidae, Pantoea, Commensalibacter
Introduction
Interactions between insects and microorganisms have been fundamental drivers of evolution and species diversification [1–3]. The symbiotic relationships range from mutualism to commensalism to parasitism, and the symbionts have significant impacts on the ecology of their insect hosts [3–5]. Symbionts fulfill many different functional roles for their host [6], like food digestion [5], production of vitamins and amino acids [7, 8], protection from harmful microorganisms [9], neutralization of plant defense chemicals [10, 11], or parasitoid resistance [12, 13]. Thus, the association of insects with symbionts allowed them to specialize in diets lacking essential nutrients, such as wood, blood, or plant sap [1]. This resulted in a dependency of insects on their symbionts, where the absence of a specific symbiont can cause slower development and growth, loss of fitness, sterility, higher mortality, and in the most extreme case the death of the insect [5]. Primary symbionts are passed on from mother to offspring via transovarian transmission during oogenesis [13], by egg smearing [14], or by depositing symbiont capsules on the egg masses [15]. While many symbionts are hosted in special cells, called bacteriocytes, which are often arranged in larger organs called bacteriomes [16], the gut of insects is an additional habitat where symbiotic organisms are frequently retained [5, 17, 18]. The gut microbiome can be influenced by the diet, as microorganisms can be acquired by the food intake [2, 17].
Heteropteran species are associated with a wide range of different bacteria [13, 14, 19, 20]. The genus Pantoea is a widespread symbiont in the family Pentatomidae. It was detected in several stink bug species like Acrosternum hilare, Nezara viridula, Murgantia histrionica, and Plautia stali [13] and plays an important role in the fixation of nitrogen [12]. Members of this genus can be found in the environment as free-living populations in soil, water, and plant material but are also associated with insects [17], where they synthesize amino acids and B vitamins and are involved in the metabolism of carbohydrates [7, 11]. Generally, stink bugs have a specialized tissue in the posterior region of the midgut where the symbionts are hosted and mainly one bacterial taxon is described, which differs for different hosts and clusters in phylogenetic analyses with Pantoea and Erwinia [13, 14, 21].
The Brown marmorated stink bug Halyomorpha halys is an invasive agricultural pest in North America and Europe originating in North-East Asia [22–24]. It is extremely polyphagous and feeds on more than 300 plants including some important agricultural crops [24–26]. Although in the last decade, H. halys became a well-studied insect [14, 23, 24, 27, 28], and the microbiomes of several different stink bug species were investigated [11, 29–31], currently, no study gives a general overview of the bacterial community in the gut of H. halys. Like most Pentatomid species, H. halys is associated with the primary symbiont “Candidatus Pantoea carbekii” (hereafter Pantoea carbekii) [13, 14]. This symbiont resides in the V4 region of the midgut and is transmitted maternally by smearing the gut fluids of the mother to the egg masses and is then acquired by feeding by the nymphs [14, 27]. Despite the polyphagous nature of H. halys, its diet lacks nutrients, such as essential amino acids and some vitamins, which are probably synthesized by Pantoea carbekii [27]. Therefore, the fitness of H. halys heavily relies on the presence of this symbiont. This was shown in laboratory experiments where inhibiting the symbiont transmission via surface sterilization or the application of micronutrient fertilizers with antimicrobial activity on the egg masses resulted in slower developmental time, lower survivorship, and the production of egg masses with fewer eggs and lower hatch rates [32, 33].
Even if previous studies showed the importance of the gut microbial community in the physiology, development, and behavior of insects [2, 34, 35] as well as nutritional metabolism and degradation of toxic compounds [11], studies on H. halys focused solely on the primary symbiont Pantoea carbekii [14, 27, 33]. Furthermore, seasonal changes in the gut microbiota were shown in different insects before, which might contribute to their biology, by improving survival during overwintering or tolerating heat stress [36–38]. To gain new information on the composition of the gut microbial community of H. halys, we characterized the microbial gut community of H. halys and investigated potential changes in the microbial composition across the year.
Methods
Samples, Sample Preparation, and Sequencing
We collected 126 individuals in the field in the years 2021–2023 in seven different locations in South Tyrol, Italy: Lana, Plaus, Appiano, Bolzano, Caldaro, Nalles, and at the Research Center Laimburg, Vadena. The locations have comparable climatic conditions, and all individuals were collected in or near apple orchards. The samples were collected using pheromone traps (rocket-shaped RESCUE! Trap, SERBIOS S.r.l., Badia Polesine, RO, Italy; PHEROCON BMSB DUAL Pheromone, Trécé Inc, Adair, OK, USA; Shindo Trap, CBC, Europe, S.r.l., Biogard division, Grassobbio, BG, Italy) or by collecting single adult individuals (Table S1, Table S2). The traps were controlled once a week, and living individuals were collected and stored in absolute ethanol at − 20 °C until dissection. The whole gut was dissected using sterilized scissors, tweezers, and a needle, and the gut was transferred to a 1.5-ml Eppendorf tube for DNA extraction with the Qiagen Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The DNA concentration was measured with the Qubit 4 Fluorometer and the 1X dsDNA High Sensitivity Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA), while the DNA purity was verified using the DS-11 FX + spectrophotometer (DeNovix Inc., Wilmington, DE, USA). After amplification of the V4 region of the 16S rRNA gene with the primer pair 515F–806R [39–41], amplicon 2 × 300 bp paired-end sequencing was performed on the Illumina MiSeq by a commercial provider (StarSeq GmbH, Mainz, Germany).
Data Analysis
The first and last 10 bp of each sequence and sequences shorter than 100 bp were removed using trim-galore (Version 0.6.10) [42, 43]. The quality was checked using FastQC (Version 0.11.9) [44]. QIIME2 (Version 2023.9) [45] and DADA2 [46] were used to denoise the sequences, truncate forward reads at 280 bp and reverse reads at 120 bp, and remove singletons. We classified the sequences using the Silva database (version 138.1) [47–49], and an amplicon sequence variant (ASV) table was generated using QIIME2 [45] (Table S3). All further analyses were performed on the genus level. All hits assigned to mitochondria, chloroplasts, eucaryotes, and archaea and hits with less than 30 reads were removed, which accounted for approximately 0.1% of the average number of reads per sample. All following analyses were performed in R (R version 4.2.0) [50]. A rarefaction curve was generated using the R package vegan [51]. Despite that all samples already reached a plateau at 2000 reads (Fig. S1), data were normalized using total sum standardization in vegan (decostand) [51] to account for possible biases introduced by the variations of the total number of reads between the samples (minimum 3431 reads; maximum 54,036 reads; Table S3). A linear model to show the correlation between Pantoea and Commensalibacter was generated using a Spearman correlation using the cor.test function in R [50]. A non-linear model was generated by using the package nlstools [52].
Alpha and beta diversity measures were all calculated using the package vegan in R [51]. Alpha diversity was calculated based on species richness, Shannon’s diversity index, and Pielou’s evenness. Differences between the sampling months were assessed using the Kruskal–Wallis test. For beta diversity analyses, we grouped the different months into seasons based on the H. halys life cycle and temperatures in the sampling region: winter (November, January; overwintering period), spring (February, March; first individuals become active after the overwintering period), early summer (May, June; overwintered individuals emerge, start feeding and oviposition), late summer (July, August; overwintered and first generation adults co-occur), and fall (September, October; first and possibly second generation adults feed and prepare for overwintering) [53, 54]. Furthermore, beta diversity was calculated to compare the different years. Therefore, months with sufficient sample sizes were chosen and compared (August and September of 2021 and 2022). The mean monthly temperature in the study region is: January 1.1 °C, February 4.1 °C, March 9.0 °C, April 13.1 °C, May 17.2 °C, June 20.9 °C, July 22.8 °C, August 22.3 °C, September 18.5 °C, October 12.7 °C, November 6.2 °C, and December 1.7 °C (https://wetter.provinz.bz.it/download-messdaten.asp). To display the differences in the microbial composition between the seasons, non-metric multi-dimensional scaling (NMDS) based on a Bray–Curtis dissimilarity matrix and Jaccard dissimilarity matrix was applied. Differences in the microbial community of individuals collected among different seasons were compared with a PERMANOVA, using the adonis package [55]. All figures were generated using the packages ggplot2 [56] and cowplot [57].
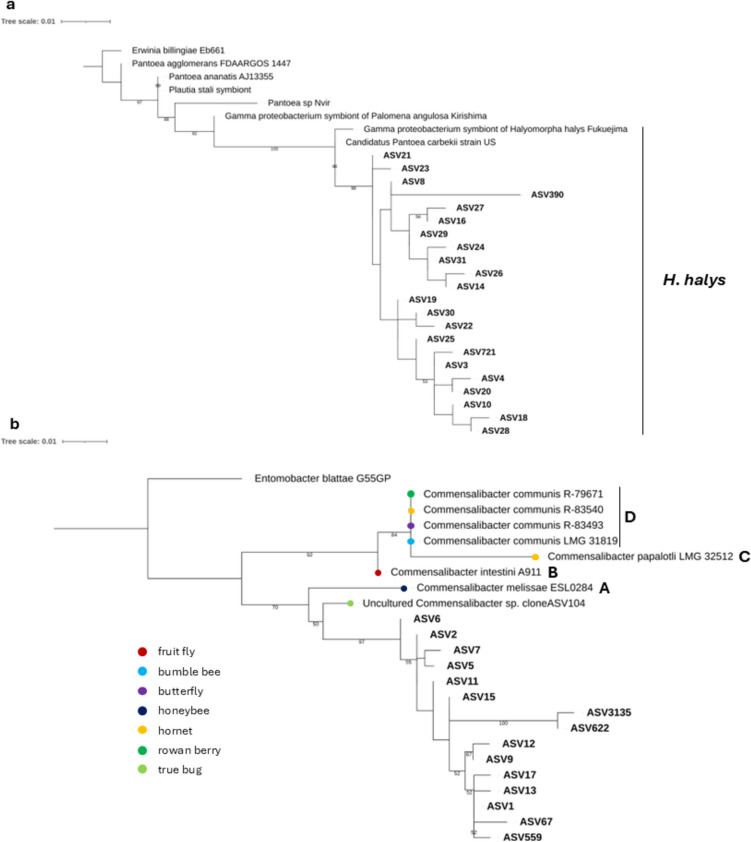
A phylogenetic analysis of ASVs classified as Pantoea (n = 22) and Commensalibacter (n = 15) was performed. The 16S rRNA gene sequences of Pantoea and Commensalibacter were selected based on literature and BLAST search (Table S4, Table S5). Erwinia billingiae Eb661 (NC_014306.1) and Entomobacter blattae G55GP (NZ_CP060244.1) were included as outgroups. Sequences were aligned using MAFFT v7.490 [58]. The alignment was manually edited to a final length of 274 positions. The maximum likelihood tree was calculated using IQ-TREE v1.6.12 [59] with a standard nonparametric bootstrap of 1000 replicates. Trees were visualized with iTOL [60].
Results
Sequencing of the 126 individuals yielded a total of 8,086,620 raw reads. After all filtering steps, the single individuals generated between 3431 and 54,036 reads, with an average of 19,929 reads (SD = 9147). The rarefaction curve reached a plateau before 2000 reads, and hence, all samples had sufficient sequencing depth (Fig. S1). All assigned reads were clustered to an average of 51.5 ASVs per individual (SD = 64.9). The highest number of ASVs found in a single individual was 303, whereas one individual harbored only 10 different ASVs (Table S3).
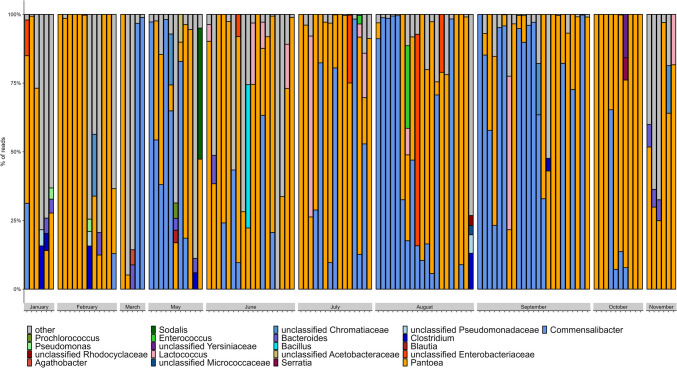
The most dominant ASVs were classified as Pantoea and Commensalibacter. Pantoea was detected in 122 out of 126 individuals (96.8%; Fig. 1). Thus, in four individuals, the primary symbiont was not present. The second most common genus was Commensalibacter (Acetobacteraceae), which was detected in 77 individuals (61.1%; Fig. 1). Overall, 45.6% of all reads were assigned to Pantoea and 35.0% to Commensalibacter. In both cases, numerous ASVs belonged to these taxa and 21 ASVs were classified as Pantoea and 15 as Commensalibacter. Interestingly, 19 of the 21 Pantoea and 11 of the 15 Commensalibacter variants were present in high abundance and multiple individuals. Individuals which harbored Pantoea had on average 13.1 ASVs belonging to Pantoea and 8.6 ASVs belonging to Commensalibacter which suggests that multiple strains of both taxa were present within single individuals.
Fig. 1.
The relative abundance of the different ASVs on individual levels, separated in the different months of their collection. “Other” refers to every ASV that accounts for < 5% of the average number of reads of each individual (1000 reads)
All other taxa were found less frequently. Lactococcus, the taxon with the third most common occurrence, accounted only for 1.24% of the reads. Similar, unclassified Enterobacteriaceae, Bacteroides, Clostridium, and Enterococcus were frequently present but also with abundances lower than 2% (Fig. S2). Other taxa we detected in low frequencies belonged to the family Acetobacteraceae and the genera Pseudomonas, Serratia, Sodalis, and Prochlorococcus.
Comparing the bacterial community of H. halys sampled in different months of the year, we found that Pantoea was the only bacterial genus detected in individuals from all ten investigated months (Fig. 2). The relative abundance of Commensalibacter was higher in the summer months (May to October) compared to the winter months (November to February), although not statistically significant (Kruskal–Wallis test, p = 0.173) (Fig. 1). A linear model showed a negative relation between Pantoea and Commensalibacter (Spearman’s correlation, R = − 0.52, p < 0.05), whereas a non-linear model showed an asymptotic behavior with Pantoea approaching 0 (Fig. S3). The number of individuals that harbored Commensalibacter always remained below 50% in winter and ranged from 0% in November to 50% in January (three individuals), whereas in February only 16% (two individuals) and in October 40% (four individuals) harbored this genus. From March to September, the number of infected individuals increased to 47% (seven individuals in July) up to 100% (eleven individuals in May). The relative abundance increased from 0% in November to < 10% in January to about 50% in March, May, August, and September.
Fig. 2.
The relative number of reads assigned to the different taxa by month. “Other” refers to every ASV that accounts for < 5% of the average number of reads of each individual (1000 reads)
The phylogenetic tree of Pantoea reveals that the sequences of Pantoea of H. halys form a clade distinct from Pantoea of other stink bugs (P. stali, N. viridula, and Palomena angulosa). Within this clade, Pantoea from Japan and the USA are separated from the ASVs identified in this study, which can be divided into two main subgroups (Fig. 3a). For Commensalibacter, the ASVs detected in this study form a clade that is distinct from Commensalibacter found in other insects. The closest strains were found in the true bug Probergrothius angolensis (MN514570.1) and the newly proposed species C. melissae sp. nov. ESL0284 isolated from honey bees [61] (Fig. 3b).
Fig. 3.
a Maximum likelihood tree of the 16S rRNA gene ASV sequences of Pantoea. Bootstrap values higher than 50 are reported on the tree branches. Accession numbers of the sequences used in this tree are reported in Table S4. b Maximum likelihood tree of the 16S rRNA gene ASV sequences of Commensalibacter. Bootstrap values higher than 50 are reported on the tree branches. The colored dots indicate the isolation source of Commensalibacter. Letters A, B, C, and D refer to Commensalibacter clusters defined based on the phylogenomic analysis of Botero et al. [61]. Accession numbers of the sequences used in this tree are reported in Table S5
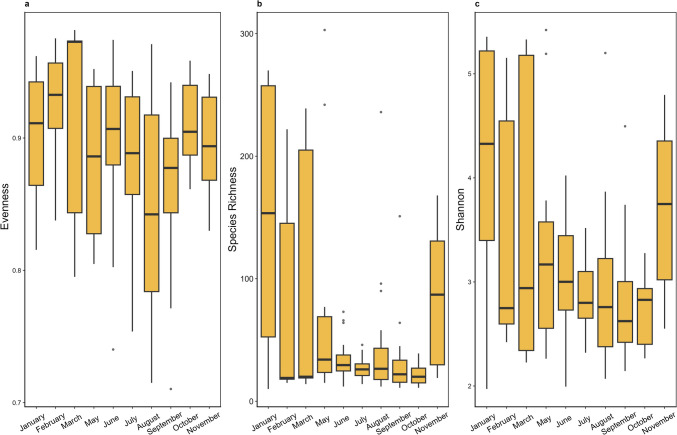
The alpha diversity measures species richness, Shannon diversity, and Pielou’s evenness did not show any significant differences among the different months (Kruskal–Wallis tests—species richness, p = 0.1209; Shannon diversity, p = 0.09605; Pielou’s evenness, p = 0.05593). However, there were fluctuations across the seasons with a higher alpha diversity in winter compared to summer (Fig. 4).
Fig. 4.
Boxplots of a Pielou’s evenness, b species richness, and c Shannon’s diversity indices in each month. The boxplots represent the median and interquartile range. The number of individuals analyzed in each month is January: 6, February: 12, March: 5, May: 11, June: 18, July: 15, August: 20, September: 23, October: 10, and November: 6
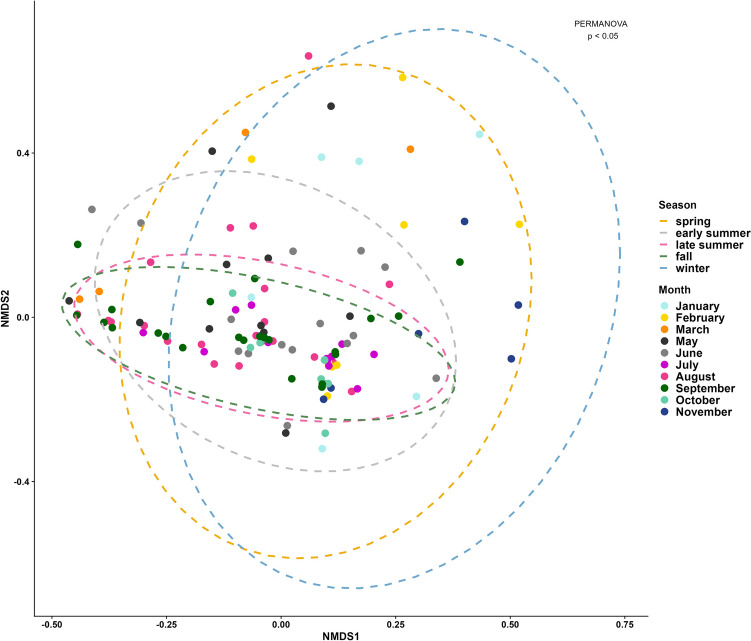
We grouped the individuals into five seasons: winter (November, January), spring (February, March), early summer (May, June), late summer (July, August), and fall (September, October). We used a NMDS based on Bray–Curtis and Jaccard dissimilarity matrices, and both revealed significant differences in the bacterial composition of the gut using PERMANOVA (for both, p < 0.05, 999 permutations) (Fig. 5, Fig. S4). The post hoc test of the Bray–Curtis dissimilarity revealed significant differences (p < 0.05) between individuals from late summer and spring, late summer and winter, spring and fall, winter and fall, and winter and early summer. In contrast, no differences were found between early summer and late summer (p = 0.656), early summer and fall (p = 0.337), early summer and spring (p = 0.463), late summer and fall (p = 0.665), and spring and winter (p = 0.585). NMDS analysis revealed that—although not completely segregated—winter and spring and late summer and fall clustered into significantly different groups (PERMANOVA, p < 0.05; Fig. 5), whereas early summer forms an additional cluster within the other two clusters. The NMDS plot based on Jaccard distances revealed significant differences between all groups (PERMANOVA, p < 0.05; Fig. S4), except early summer and late summer (p = 0.408), early summer and spring (p = 0.212), early summer and fall (p = 0.179), late summer and fall (p = 0.543), and spring and winter (p = 0.182). Moreover, the comparison between August and September of different years revealed significant differences between the years (PERMANOVA, p < 0.05).
Fig. 5.
NMDS based on Bray–Curtis dissimilarities. Each dot represents a single individual: the blue dotted lines indicate individuals sampled in the winter months (November, January), yellow indicates spring (February, March), gray indicates early summer (May, June), pink indicates late summer (July, August), and green indicates fall (September, October)
Discussion
The gut microbiome of insects plays a crucial role in the ecology of insects and adaptability to different environments [1, 6]. Although H. halys is one of the most important invasive pest species worldwide, research has to date focused mainly on its primary symbiont Pantoea carbekii. Furthermore, studies were mainly performed on laboratory-reared populations [27, 33]. Our study, for the first time, investigated the gut microbiota of individuals from natural populations. Overall, we found two main taxa: Pantoea and Commensalibacter which were abundant and in the majority of the individuals. Collections across the year allowed us to show that the gut microbiota has a clear seasonal pattern, with a lower relative abundance of Pantoea in summer compared to winter and a significant increase of Commensalibacter in summer. We found a higher alpha diversity in the winter months, and we show that the composition of the gut microbiota varies widely across the year.
The primary symbiont Pantoea was the most commonly found taxa, present in almost all individuals across the season. As shown in a previous study, Pantoea carbekii is the primary symbiont of H. halys and is essential for the fitness and survival of individuals [14, 27, 33]. Surprisingly, in this study, we found four individuals where no Pantoea reads were detected. We do not know if the individuals did not acquire the symbiont or only harbor the symbiont with very low abundance and whether the host suffered any fitness costs. Interestingly, almost all individuals harbor several Pantoea ASVs with up to 20 ASVs in one single individual, highlighting the co-presence of various Pantoea strains in most individuals.
We found some variation in the abundance of Pantoea across the seasons: In the winter months when H. halys is in reproductive diapause [26], the relative abundance of Pantoea is higher than in the summer months, when the insects are active. Environmental factors might play a role in the reduction of Pantoea. In the Pentatomid N. viridula, which is also associated with a Pantoea-like symbiont [62], a recent study showed that the symbiont titers decrease at higher temperatures in the laboratory [63]. This could indicate that the lower abundance of Pantoea in the summer months is influenced by the higher temperatures in the field. The phylogenetic analysis showed that Pantoea found in this study forms a clade with other Pantoea from H. halys. However, the branch support within this clade is relatively low due to the short length of the sequence and low resolution of the 16S rRNA gene. In the future, the use of one or more different markers should provide a better overview of possible different strains of Pantoea carbekii in H. halys.
The primary symbiont Pantoea carbekii is not the only predominant bacterial genus found in the gut microbiota of H. halys. An interesting finding of our study is that Commensalibacter occurs frequently, especially in the summer months where it is present in 50–100% of all individuals. In several individuals collected from May to September, almost all reads were assigned to this taxon. Similar to Pantoea, various ASVs classified as Commensalibacter co-occur within single individuals highlighting that if individuals are infected by this bacterium, they harbor a variety of different strains. The genus Commensalibacter belongs to the family Acetobacteraceae, which consists of seven species and was previously isolated from different insects having sugar-based diets that lack nitrogen [2, 64] as honey bees, fruit flies, butterflies, bumble bees, and hornets as well as other stink bugs as N. viridula [11, 61, 65]. Based on our phylogenetic analysis, the ASVs detected in this study were similar to C. melissae sp. nov. found in Western honey bees. Genomes of this species are characterized by a smaller size and a high number of species-specific gene clusters compared to the other Commensalibacter clusters. While genes for the utilization of glucose and other carbohydrates are missing, C. melissae still encodes genes for the synthesis of 11 amino acids, including eight essential ones, and several B vitamins. The ability to convert nitrate to nitrite and nitric oxide to nitrous oxide with a nitric oxide reductase might serve as a detoxification mechanism for survival in the microaerobic environment of the insect gut [61].
Generally, Acetobacteraceae can be isolated from plants, fruits, herbs, and flowers, and although they are involved in amino acid and carbohydrate metabolism, they do not seem to be fundamental for the survival of insects [64], which explains why we did not detect Commensalibacter in every individual. H. halys preferably feeds on the reproductive parts of plants [26, 66] and might encounter similar nutritional deficits common for a sugar-rich diet which might be compensated by this additional taxon. Most Hemiptera have an acidic anterior midgut region [67], and it was shown that Acetobacteraceae can tolerate sugar-rich and acidic environments. Furthermore, in the ant species Oecophylla smaragdina, it is expected that the higher consumption of carbohydrates results in higher concentrations of Acetobacteraceae [68]. We hypothesize that the environment in the gut of H. halys acts as a chemical filter against pathogenic microbes and supports the growth of Acetobacteraceae [69].
The low abundance of Commensalibacter in winter might be due to the fact that this symbiont is most probably acquired via the diet, and during the overwintering period, the bugs reduce or entirely stop feeding. This could also explain the varying frequency of Commensalibacter in H. halys individuals sampled in the summer months. While some individuals have extremely high frequencies of Commensalibacter, making up the majority of all assigned reads, other individuals have only a low number or no reads assigned to this taxon. In the mid of July, the first images are found in the study region [70]. If Commensalibacter is horizontally acquired by feeding, the newly developed young adults might have not acquired these bacteria yet, while the older, co-occurring individuals from the overwintered generation could feed on a broad variety of plants and hence harbor a high density of Commensalibacter. If and how Commensalibacter can stably colonize the gut of H. halys and its influence on the fitness of its host needs to be investigated in future studies.
Although the majority of the reads belonged to Pantoea and Commensalibacter, we detected many other taxa in lower abundance in different frequencies. Various taxa were already described in the literature as part of the gut microbiota of Pentatomids. For example, the Pentatomid N. viridula harbors Pantoea and Sodalis as symbionts as well as bacteria belonging to the genera Enterococcus, Serratia, and Bacillus in its gut [11, 30]. Similarly, the two-spotted stink bug Bathycoelia distincta, is frequently associated with Pantoea and Sodalis, whereas Pseudomonas, Serratia, Bacillus, and Lactococcus were detected in lower frequencies in this species [7]. A study on different true bug species detected a broad variety of different bacterial genera in the guts that varied widely in relative abundance in different species. Especially Commensalibacter, Pantoea, Enterobacteriaceae, Lactococcus, Enterococcus, Wolbachia, Rhizobiaceae, Serratia, and Pseudomonas were present in different frequencies in generally low abundances but have been described in other true bugs [71] suggesting that bacteria belonging to these genera are widespread in this insect family. While Yokenella is a genus frequently associated with Pentatomidae and Pentatomomorpha [7, 30, 71], we did not find this bacterium in H. halys.
Overall, the Shannon diversity in H. halys is lower compared to the median number of the Shannon diversity in other Hemiptera species [72]. However, we observed high fluctuations in species richness and Shannon diversity across the season. While individuals caught in January and November show a comparably high alpha diversity, single individuals still show low diversities. Individuals caught in the summer months show a lower alpha diversity than those collected during the winter months, which might be explained by a low alpha diversity of young adults that usually are found by the mid of July [70]. We expect that the short feeding period of young adults resulted in fewer bacteria acquired via the diet. In contrast, the higher alpha-diversities of H. halys individuals sampled in winter might result from a higher feeding activity before the overwintering phase, as the individuals form a fat body before overwintering [73]. Thus, an increased uptake of diverse plant material might result in a higher diversity of the microbial community in the gut. The observed seasonal fluctuation is in line with other studies that described seasonal differences in the alpha diversity in different insects. Larvae of Spodoptera frugiperda (Lepidoptera: Noctuidae) for instance have significant differences in the core community between the dry and rainy seasons [74], or honey bees, in which the microbiota changes mainly in the transition phases from summer to winter and from winter to spring [8].
For the stink bugs Piezodorus guildinii, Euschistus heros, and B. distincta, it was shown that the gut microbial community differs geographically [7, 31]. Here, we analyzed individuals from a single region, and therefore, future studies should investigate the microbial community of H. halys in multiple regions. Since invasive species can gain [75, 76] and lose [77] symbionts during the invasion process, it might be interesting to investigate if and how microbes influenced the invasion process of this pest species. Our data highlight a highly dynamic microbiome of H. halys which fluctuates across the season. Changes in beta diversity across the season were also shown in different species, like honeybees [38] and bark beetles [36]. To compare the stability of the microbial species across different years, we compared the beta diversity among the individuals sampled in August and September of 2021 and 2022 and found significant changes in the microbial community between individuals sampled in the different years. This indicates that the microbial composition is not stable and changes between different years. Given the polyphagous nature of H. halys, we suppose that differences in the availability of food sources across the seasons and differences in the vegetation between different years are one of the main drivers for the variability of the detected bacteria between individuals in the different seasons.
In summary, our study shows that H. halys is associated predominantly with two bacteria in the study region in Northern Italy. While the function of the primary symbiont Pantoea carbekii for host biology was investigated in previous studies, knowledge about the second co-occurring bacteria Commensalibacter is scarce. Moreover, we highlight a dynamic bacterial community across the season and between years. Further studies are needed to understand potential differences of the microbial community from different geographic areas. Especially the function of Commensalibacter and potential interactions between the two predominant symbionts and the effect of the changing gut community on the ecology of the host should be investigated.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Stefanie Fischnaller, Stefan Schwembacher, and Johann Schuler for providing the samples and Bastian Schauer for helpful discussions during data analysis.
Author Contribution
MF, EC, and HS designed the experiment. MF conducted the experiment, analyzed the data and wrote the first draft of the manuscript. MF, EC, HF, and HS edited, reviewed, and approved the final version.
Funding
This study was funded by internal funds of the Free University of Bozen-Bolzano to Hannes Schuler.
Data Availability
The data is available at the NCBI GenBank under the accession number PRJNA1145417.
Declarations
Ethics Approval
No approval was required.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cornwallis CK, Van ’T Padje A, Ellers J, et al (2023) Symbioses shape feeding niches and diversification across insects. Nat Ecol Evol 7:1022–10.1038/s41559-023-02058-0 [DOI] [PMC free article] [PubMed]
- 2.Engel P, Moran NA (2013) The gut microbiota of insects – diversity in structure and function. FEMS Microbiol Rev 37:699–735. 10.1111/1574-6976.12025 [DOI] [PubMed] [Google Scholar]
- 3.Moya A, Peretó J, Gil R, Latorre A (2008) Learning how to live together: genomic insights into prokaryote–animal symbioses. Nat Rev Genet 9:218–229. 10.1038/nrg2319 [DOI] [PubMed] [Google Scholar]
- 4.Feldhaar H (2011) Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36:533–543. 10.1111/j.1365-2311.2011.01318.x [Google Scholar]
- 5.Mondal S, Somani J, Roy S et al (2023) Insect microbial symbionts: ecology, interactions, and biological significance. Microorganisms 11:2665. 10.3390/microorganisms11112665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran NA, Ochman H, Hammer TJ (2019) Evolutionary and Ecological consequences of gut microbial communities. Annu Rev Ecol Evol Syst 50:451–475. 10.1146/annurev-ecolsys-110617-062453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fourie A, Venter SN, Slippers B, Fourie G (2023) Pantoea bathycoeliae sp. nov and Sodalis sp. are core gut microbiome symbionts of the two-spotted stink bug. Front Microbiol 14:1284397. 10.3389/fmicb.2023.1284397 [DOI] [PMC free article] [PubMed]
- 8.Li C, Tang M, Li X, Zhou X (2022) Community dynamics in structure and function of honey bee gut bacteria in response to winter dietary shift. mBio 13:e01131–22. 10.1128/mbio.01131-22 [DOI] [PMC free article] [PubMed]
- 9.Kaltenpoth M (2009) Actinobacteria as mutualists: general healthcare for insects? Trends Microbiol 17:529–535. 10.1016/j.tim.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 10.Ben-Yosef M, Pasternak Z, Jurkevitch E, Yuval B (2015) Symbiotic bacteria enable olive fly larvae to overcome host defences. R Soc Open Sci 2:150170. 10.1098/rsos.150170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coolen S, Rogowska-van Der Molen MA, Kwakernaak I, et al (2024) Microbiota of pest insect Nezara viridula mediate detoxification and plant defense repression. ISME J 18:wrae097. 10.1093/ismejo/wrae097 [DOI] [PMC free article] [PubMed]
- 12.Gurung K, Wertheim B, Falcao Salles J (2019) The microbiome of pest insects: it is not just bacteria. Entomol Exp Appl 167:156–170. 10.1111/eea.12768 [Google Scholar]
- 13.Shan H, Wu W, Sun Z et al (2021) The gut microbiota of the insect infraorder Pentatomomorpha (Hemiptera: Heteroptera) for the light of ecology and evolution. Microorganisms 9:464. 10.3390/microorganisms9020464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal R, Michel AP, Sabree ZL (2014) The crypt-dwelling primary bacterial symbiont of the polyphagous pentatomid pest Halyomorpha halys (Hemiptera: Pentatomidae). Environ Entomol 43:617–625. 10.1603/EN13341 [DOI] [PubMed] [Google Scholar]
- 15.Hosokawa T, Kikuchi Y, Nikoh N et al (2006) Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol 4:e337. 10.1371/journal.pbio.0040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luan J-B (2024) Insect bacteriocytes: adaptation, development, and evolution. Annu Rev Entomol 69:81–98. 10.1146/annurev-ento-010323-124159 [DOI] [PubMed] [Google Scholar]
- 17.Douglas AE (2015) Multiorganismal Insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34. 10.1146/annurev-ento-010814-020822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huerta-García A, Álvarez-Cervantes J (2024) The gut microbiota of insects: a potential source of bacteria and metabolites. Int J Trop Insect Sci 44:13–30. 10.1007/s42690-023-01147-8 [Google Scholar]
- 19.Sudakaran S, Kost C, Kaltenpoth M (2017) Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol 25:375–390. 10.1016/j.tim.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 20.Yang Z-W, Luo J-Y, Men Y et al (2023) Different roles of host and habitat in determining the microbial communities of plant-feeding true bugs. Microbiome 11:244. 10.1186/s40168-023-01702-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prado SS, Almeida RPP (2009) Phylogenetic placement of pentatomid stink bug gut symbionts. Curr Microbiol 58:64–69. 10.1007/s00284-008-9267-9 [DOI] [PubMed] [Google Scholar]
- 22.Arnold K (2009) Halyomorpha halys (Stål, 1855), eine für die europäischen Fauna neu nachgewiesene Wanzenart (Insecta: Heteroptera, Pentatomidae, Pentatominae, Cappaeini). Mitteilungen Thüring Entomol CV 16:19 [Google Scholar]
- 23.Haye T, Abdallah S, Gariepy T, Wyniger D (2014) Phenology, life table analysis and temperature requirements of the invasive brown marmorated stink bug, Halyomorpha halys, in Europe. J Pest Sci 87:407–418. 10.1007/s10340-014-0560-z [Google Scholar]
- 24.Leskey TC, Nielsen AL (2018) Impact of the invasive brown marmorated stink bug in North America and Europe: history, biology, ecology, and management. Annu Rev Entomol 63:599–618. 10.1146/annurev-ento-020117-043226 [DOI] [PubMed] [Google Scholar]
- 25.Kriticos DJ, Kean JM, Phillips CB et al (2017) The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J Pest Sci 90:1033–1043. 10.1007/s10340-017-0869-5 [Google Scholar]
- 26.Rice KB, Bergh CJ, Bergmann EJ et al (2014) Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J Integr Pest Manag 5:1–13. 10.1603/IPM14002 [Google Scholar]
- 27.Kenyon LJ, Meulia T, Sabree ZL (2015) Habitat visualization and genomic analysis of “Candidatus Pantoea carbekii”, the primary symbiont of the brown marmorated stink bug. Genome Biol Evol 7:620–635. 10.1093/gbe/evv006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maistrello L, Dioli P, Bariselli M et al (2016) Citizen science and early detection of invasive species: phenology of first occurrences of Halyomorpha halys in Southern Europe. Biol Invasions 18:3109–3116. 10.1007/s10530-016-1217-z [Google Scholar]
- 29.Fourie A, Venter SN, Slippers B, Fourie G (2022) A detection assay to identify alternative food sources of the two-spotted stink bug, Bathycoelia distincta (Hemiptera: Pentatomidae). J Econ Entomol 115:519–525. 10.1093/jee/toab256 [DOI] [PubMed] [Google Scholar]
- 30.Medina V, Sardoy PM, Soria M et al (2018) Characterized non-transient microbiota from stinkbug (Nezara viridula) midgut deactivates soybean chemical defenses. PLoS ONE 13:e0200161. 10.1371/journal.pone.0200161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moro MS, Wu X, Wei W et al (2021) Characterization and comparison of intestinal bacterial microbiomes of Euschistus heros and Piezodorus guildinii collected in Brazil and the United States. Front Microbiol 12:769965. 10.3389/fmicb.2021.769965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonella E, Orrù B, Alma A (2019) Egg masses treatment with micronutrient fertilizers has a suppressive effect on newly-emerged nymphs of the brown marmorated stink bug Halyomorpha halys. Entomol Gen 39:231–238. 10.1127/entomologia/2019/0819 [Google Scholar]
- 33.Taylor C, Coffey PL, DeLay BD, Dively GP (2014) The importance of gut symbionts in the development of the brown marmorated stink bug, Halyomorpha halys (Stål). PLoS ONE 9:e90312. 10.1371/journal.pone.0090312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang S, Kikuchi Y (2020) Impact of the insect gut microbiota on ecology, evolution, and industry. Curr Opin Insect Sci 41:33–39. 10.1016/j.cois.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 35.Motta EVS, Moran NA (2024) The honeybee microbiota and its impact on health and disease. Nat Rev Microbiol 22:122–137. 10.1038/s41579-023-00990-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moussa A, Nones S, Vannucchi PE et al (2024) The bacterial community of the European spruce bark beetle in space and time. Entomol Gen 44:211–222. 10.1127/entomologia/2023/2114 [Google Scholar]
- 37.Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266. 10.1146/annurev-ento-112408-085305 [DOI] [PubMed] [Google Scholar]
- 38.Almeida EL, Ribiere C, Frei W et al (2023) Geographical and seasonal analysis of the honeybee microbiome. Microb Ecol 85:765–778. 10.1007/s00248-022-01986-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apprill A, McNally S, Parsons R, Weber L (2015) Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat Microb Ecol 75:129–137. 10.3354/ame01753 [Google Scholar]
- 40.Parada AE, Needham DM, Fuhrman JA (2016) Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol 18:1403–1414. 10.1111/1462-2920.13023 [DOI] [PubMed] [Google Scholar]
- 41.Walters W, Hyde ER, Berg-Lyons D, et al (2016) Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1:e00009–15. 10.1128/mSystems.00009-15 [DOI] [PMC free article] [PubMed]
- 42.Krueger F, James F, Ewels P, et al (2023) Trim Galore, https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/. 10.5281/zenodo.7598955
- 43.Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17:10–12. 10.14806/ej.17.1.200
- 44.Andrews S, Lindenbaum P, Howard B, Ewels P (2019) FastQC, https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 45.Bolyen E, Rideout JR, Dillon MR et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glöckner FO, Yilmaz P, Quast C et al (2017) 25 years of serving the community with ribosomal RNA gene reference databases and tools. J Biotechnol 261:169–176. 10.1016/j.jbiotec.2017.06.1198 [DOI] [PubMed] [Google Scholar]
- 48.Quast C, Pruesse E, Yilmaz P et al (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yilmaz P, Parfrey LW, Yarza P et al (2014) The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res 42:D643–D648. 10.1093/nar/gkt1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R Core Team (2022) R: a language and environment for statistical computing
- 51.Oksanen J, Simpson GL, Blanchet GF, et al (2022) Vegan: community ecology package. R package version 2.6–4, https://CRAN.R-project.org/package=vegan.
- 52.Baty F, Ritz C, Charles S, et al (2015) A Toolbox for nonlinear regression in R : the package nlstools. J Stat Softw 66:. 10.18637/jss.v066.i05
- 53.Fischnaller S, Rottensteiner A (2020) Beobachtungen zur Phänologie der Marmorierten Baumwanze in Südtirol. Obstbau Weinbau Fachmag Beratungsrings 57:10–13 [Google Scholar]
- 54.Fischnaller S, Wolf M (2021) Der saisonale Zyklus von Halyomorpha halys in Südtirol. Heteropteron Mitteilungsblatt Arbeitsgruppe Mitteleur Heteropterologen 64:
- 55.Martinez Arbizu P (2020) pairwiseAdonis: pairwise multilevel comparison using adonis. R package version 0.4
- 56.Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New York. ISBN 978–3–319–24277–4, https://ggplot2.tidyverse.org.
- 57.Wilke CO (2023) cowplot: streamlined plot theme and plot annotations for “ggplot2”. R package version 1.1.2, https://wilkelab.org/cowplot/
- 58.Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Letunic I, Bork P (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296. 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Botero J, Sombolestani AS, Cnockaert M et al (2023) A phylogenomic and comparative genomic analysis of Commensalibacter, a versatile insect symbiont. Anim Microbiome 5:25. 10.1186/s42523-023-00248-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geerinck MWJ, Van Hee S, Gloder G et al (2022) Diversity and composition of the microbiome associated with eggs of the southern green stinkbug, Nezara viridula (Hemiptera: Pentatomidae). MicrobiologyOpen 11:e1337. 10.1002/mbo3.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kikuchi Y, Tada A, Musolin DL, et al (2016) Collapse of insect gut symbiosis under simulated climate change. mBio 7:e01578–16. 10.1128/mBio.01578-16 [DOI] [PMC free article] [PubMed]
- 64.Crotti E, Rizzi A, Chouaia B et al (2010) Acetic acid bacteria, newly emerging symbionts of insects. Appl Environ Microbiol 76:6963–6970. 10.1128/AEM.01336-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siozios S, Moran J, Chege M et al (2019) Complete reference genome assembly for Commensalibacter sp. strain AMU001, an acetic acid bacterium isolated from the gut of honey bees. Microbiol Resour Announc 8:e01459-e1518. 10.1128/MRA.01459-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haye T, Gariepy T, Hoelmer K et al (2015) Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: an increasing threat to field, fruit and vegetable crops worldwide. J Pest Sci 88:665–673. 10.1007/s10340-015-0670-2 [Google Scholar]
- 67.Holtof M, Lenaerts C, Cullen D, Vanden Broeck J (2019) Extracellular nutrient digestion and absorption in the insect gut. Cell Tissue Res 377:397–414. 10.1007/s00441-019-03031-9 [DOI] [PubMed] [Google Scholar]
- 68.Chua K-O, Song S-L, Yong H-S et al (2018) Microbial community composition reveals spatial variation and distinctive core microbiome of the weaver ant Oecophylla smaragdina in Malaysia. Sci Rep 8:10777. 10.1038/s41598-018-29159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tragust S, Herrmann C, Häfner J, et al (2020) Formicine ants swallow their highly acidic poison for gut microbial selection and control. eLife 9:e60287. 10.7554/eLife.60287 [DOI] [PMC free article] [PubMed]
- 70.Fischnaller S (2021) Saisonaler Zyklus der Marmorierten Baumwanze 2020 in Südtirol. Obstbau Weinbau Fachmag Beratungsrings 58:10–12 [Google Scholar]
- 71.Li G, Sun J, Meng Y et al (2022) The impact of environmental habitats and diets on the gut microbiota diversity of true bugs (Hemiptera: Heteroptera). Biology 11:1039. 10.3390/biology11071039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yun J-H, Roh SW, Whon TW et al (2014) Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl Environ Microbiol 80:5254–5264. 10.1128/AEM.01226-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee D-H, Leskey TC (2015) Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Bull Entomol Res 105:566–573. 10.1017/S0007485315000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higuita Palacio MF, Montoya OI, Saldamando CI, et al (2021) Dry and rainy seasons significantly alter the gut microbiome composition and reveal a key Enterococcus sp. (Lactobacillales: Enterococcaceae) core component in Spodoptera frugiperda (Lepidoptera: Noctuidae) Corn Strain From Northwestern Colombia. J Insect Sci 21:10. 10.1093/jisesa/ieab076 [DOI] [PMC free article] [PubMed]
- 75.Himler AG, Adachi-Hagimori T, Bergen JE et al (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332:254–256. 10.1126/science.1199410 [DOI] [PubMed] [Google Scholar]
- 76.Schuler H, Bertheau C, Egan SP et al (2013) Evidence for a recent horizontal transmission and spatial spread of Wolbachia from endemic Rhagoletis cerasi (Diptera: Tephritidae) to invasive Rhagoletis cingulata in Europe. Mol Ecol 22:4101–4111. 10.1111/mec.12362 [DOI] [PubMed] [Google Scholar]
- 77.Reuter M, Pedersen JS, Keller L (2005) Loss of Wolbachia infection during colonisation in the invasive Argentine ant Linepithema humile. Heredity 94:364–369. 10.1038/sj.hdy.6800601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available at the NCBI GenBank under the accession number PRJNA1145417.