Abstract
Following a gluten-free diet (GFD) is known as the main effective therapy available for celiac disease (CD) patients, which in some cases is not enough to heal all patients presentations completely. Accordingly, emerging researchers have focused on finding novel therapeutic/preventive strategies for this disorder. Moreover, previous studies have shown that celiac patients, especially untreated subjects, are at increased risk of developing viral and bacterial infections, which can become a challenge for the clinician. Viruses, such as Rotavirus, Reovirus, Adenovirus, Enterovirus, Rhinovirus, Astrovirus, Hepatitis virus, COVID-19, Norovirus, and Herpesvirus, have been related to CD pathogenesis. Therefore, clinicians need to pay more attention to evaluate CD patients’ viral infection history (especially nonresponders to the GFD), to look for effective preventive strategies and educate patients about important risk factors. In addition, there are still viruses whose role in CD pathogenesis has not been fully studied. In this review, current information on the association between CD and various viral infections was gathered to improve knowledge in this subject area and draw researchers’/clinicians’ attention to unstudied/less studied viruses in CD pathogenesis, which might guide future prevention approaches.
Keywords: Autoimmune, Celiac disease, Gluten-free diet, Management, Viral infections
Introduction
Celiac disease (CD) is a hereditary autoimmune disorder of the small intestine triggered by gluten ingestion [1]. Gluten is a digestion-resistant glutamine and proline-rich protein found in wheat, rye, and barley that enters the intestinal lumen in a semi-digested form and causes further damage to the intestine [2]. The prevalence of CD is about 1% in the general population [3]. This disorder affects women more than men and first-degree relatives of CD patients are at increased risk (4%–12%) for the disorder [3]. Celiac disease has a wide range of clinical manifestations including gastrointestinal symptoms (like diarrhea, bloating, weight loss, steatorrhea, etc.) and non-gastrointestinal symptoms (like fatigue, anemia, infertility, etc.) [4]. Human leukocyte antigen (HLA) DQ2 and DQ8 have a strong genetic association with CD; however, some other non-HLA genes also contribute to CD development [4]. Following the entry of semi-digested gluten peptides into the lamina propria of patients with CD, these peptides undergo deamidation by the enzyme tissue transglutaminase (tTG). This modified form of gluten is presented to antigen-presenting cells (APCs) expressing HLA-DQ2 or HLA-DQ8 on their surface. The interaction activates CD4 + T cells via the Th1 pathway, resulting in the production of pro-inflammatory cytokines. Additionally, CD8 + cytotoxic T cells are stimulated by interleukin-15 (IL-15), further contributing to the immune response. These pathological processes culminate in villous atrophy, crypt hyperplasia, and an increase in intraepithelial lymphocytes (IELs) [5–8].
Type 1 diabetes, Down syndrome, Turner and Williams syndromes, immunoglobulin A deficiency, etc., are known as CD-associated disorders [9]. Environmental factors, such as viral or bacterial infections, changes in the intestinal microbiome, infant gluten consumption, and breastfeeding, are also known as predisposing factors for CD development [10, 11]. Previous studies have shown that celiac patients, especially untreated subjects, are at increased risk of developing viral and bacterial infections [12, 13]. Viruses, such as Rotavirus, Reovirus, Adenovirus, Enterovirus, Rhinovirus, Astrovirus, Hepatitis virus, COVID-19, and Norovirus, have been related to CD pathogenesis. Viral infections have the potential to disrupt immunological tolerance, influencing the expression of both pro-inflammatory and immunosuppressive factors, and may initiate pathogenic processes [14]. Additionally, these infections promote the production of type 1 interferon (IFN), subsequently activating Th1 immunity in response to gluten [15–17].
In this review article, we have tried to gather current information about viral infections in celiac diseases, which can help researchers and physicians come up with new ideas for novel diagnostics and therapeutic/preventive panels for CD.
Virus infection and oral tolerance breakdown
The ingestion of harmless antigens, such as dietary proteins, usually leads to a state of immune nonresponsiveness known as oral tolerance. This process is intricately linked to how the gastrointestinal tract manages a diverse community of beneficial microbes. Maintaining this balance requires complex interactions between nonimmune cells and the gut-associated lymphoid tissue (GALT). These interactions help control inflammation in response to resident bacteria and food proteins, preventing tissue damage while allowing the immune system to effectively respond to potential pathogens. An imbalance in these regulatory roles may result in a breakdown of tolerance, which can manifest as inflammatory conditions, or inappropriate immune responses to benign food antigens, as observed in disorders like celiac disease and IgE-mediated food allergies [18] (Fig. 1).
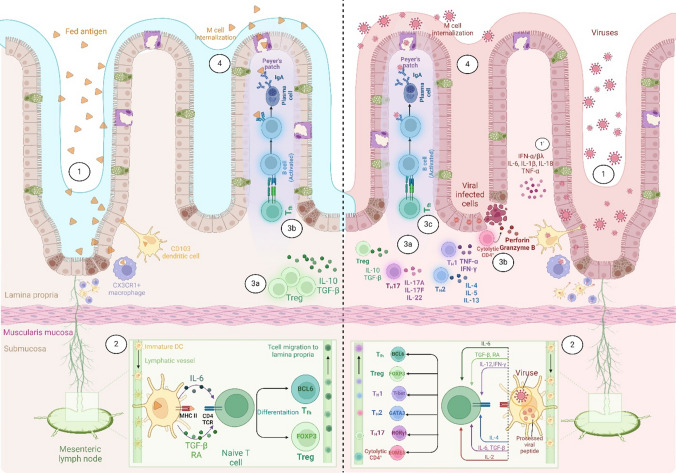
Fig. 1.
Comparative Overview of Oral Tolerance and Intestinal Immune Responses during Viral Infection. Left panel: 1) Antigens that are ingested make their way to the small intestine, where they are captured by CD103-expressing dendritic cells. At the same time, CXC3R1 + macrophages extend their dendrites through the intestinal epithelium to sample antigens from the lumen and transfer these antigens to CD103 + cells for transportation to the mesenteric lymph nodes (MLN). Particulate antigens are taken up by M cells positioned above Peyer’s patches [19, 20]. 2) Dendritic cells loaded with antigens then migrate to the mesenteric lymph nodes, where they present the antigens to naïve CD4+ T cells. During this process, DCs secrete transforming growth factor-beta (TGF-β) and retinoic acid (RA), which stimulate the expression of the Treg master transcription factor Foxp3 and equip newly differentiated Tregs with gut-homing receptors CCR9 and α4β7, they may secrete IL-6 to induce the expression of the Tfh master transcription factor BCL6. Subsequently, antigen-specific Tregs and Tfh cells return to the lamina propria of the small intestine. 3a) At this stage, Tregs can produce anti-inflammatory cytokines such as IL-10 and 3b) Tfh cells can activate B cells, converting them into plasma cells that produce IgA [21, 22]. 4) IgA can bind to and neutralize food allergens, thereby enabling oral tolerance to food [23]. Right panel: 1) The early detection of viruses by innate immune cells is facilitated by pattern recognition receptors, including intracellular sensors for DNA and RNA, 1`) which triggers an interferon and cytokine response that alerts neighboring cells and recruits leukocytes. 2) Dendritic cells in the lamina propria migrate to the mesenteric lymph nodes, where they present viral antigens to virus-specific T cells. 3a) Once activated, T cells travel to the intestinal tissue to promote antiviral immunity by secreting cytokines or through exerting cytotoxic effects. 3b) Cytotoxic CD 4 + T cells can lead to the destruction of infected cells by releasing perforin and granzyme B. 3c) B cells activated in Peyer’s patches undergo class switching to IgA and differentiate into plasma cells, supported by follicular T helper cells. 4) The intestinal plasma cells then produce secretory IgA, which serves to neutralize viruses present in the mucosal environment [24, 25].
Viral infections can disrupt this delicate balance. When a virus invades, epithelial and innate immune cells recognize it through pattern recognition receptors. This triggers signaling pathways that produce inflammatory mediators such as type I and III interferons, along with cytokines like IL-6, IL-1β, IL-18, and TNF-α. These mediators alert nearby cells to initiate antiviral defenses and recruit leukocytes to the site of infection [23]. Dendritic cells in the lamina propria capture viral particles and migrate to the mesenteric lymph nodes (MLN) to relay viral antigens to T cells. This activation leads to the differentiation of virus-specific T cells into memory and effector cells, which then home back to the intestinal tissues. Here, they can eliminate infected cells via cytotoxic mechanisms. Meanwhile, B cells, activated in areas like Peyer’s patches, produce virus-specific IgA antibodies, enhancing the immune response within the gut [24, 25] (Fig. 1). Importantly, the rapid immune response to viral infections can lead to an imbalance in the finely-tuned regulatory mechanisms of oral tolerance.
Virus infection and CD pathogenesis
As it was stated, oral tolerance to food antigens is primarily mediated by intestinal DCs that promote the activation of Treg responses. In individuals with CD, however, these DCs can instead initiate inflammatory responses characterized by CD4 + T cell activation against gluten. This alteration in DC function may be attributed to changes in the intestinal microenvironment, particularly the elevation of inflammatory cytokines such as type 1 interferons (IFNs). These findings suggest that viral infections within the intestinal tract may modulate immune responses to oral antigens, such as gluten, potentially exacerbating the development of CD [26, 27] (Fig. 2). For instance, reovirus is known to induce the production of type 1 IFNs [28]. Experimental studies in murine models demonstrate that administration of ovalbumin (OVA), as a model antigen, induces systemic tolerance characterized by the promotion of Tregs and a lack of OVA-specific Th1 cell responses [29, 30]. However, the oral inoculation of mice with reovirus strain T1L disrupts this oral tolerance, as evidenced by a reduction in Treg populations alongside an increase in OVA-specific Th1 cell responses. Furthermore, HLA-DQ8-transgenic mice inoculated with T1L and subsequently exposed to gliadin, a proteolytic fragment of gluten, develop antibodies specific to gluten and exhibit a delayed-type hypersensitivity (DTH) response, highlighting a failure to establish tolerance to gluten. Additionally, infection with T1L activates TG2, an enzyme that contributes to the immunopathogenesis of celiac disease [30]. Thus, viruses can elicit an inflammatory response to dietary gluten in celiac disease.
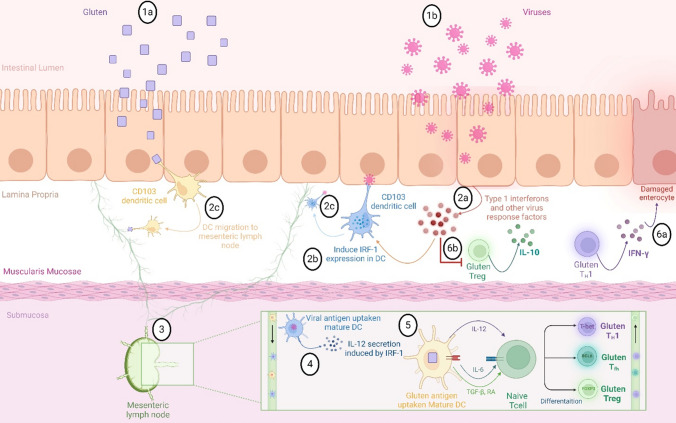
Fig. 2.
A Proposed Model for the Induction of Celiac Disease by Viruses. 1a) Upon entry, gluten antigens are captured by dendritic cells (DCs). 1b) Viruses, such as the T1L reovirus strain T1L, manipulate antiviral responses to establish persistent infections, leading to 2a) the release of type 1 interferons and other mediators that 2b) enhance the expression of interferon regulatory factor-1 (IRF-1) in dendritic cells within the lamina propria. 2c) Both DCs that have taken up gluten and those that have captured viral antigens migrate into the lymphatic vessels. 3) In this cytokine-rich inflammatory environment, antigen-laden dendritic cells travel to the mesenteric lymph nodes. 4) By promoting IRF-1 expression, virus-activated DCs secrete increased levels of IL-12. 5) The presence of IL-12 in the mesenteric lymph nodes activates gluten-specific inflammatory T cells (Th1). 6a These T cells, in turn, secrete IFN-γ, which damages enterocytes. 6b) Additionally, the type 1 interferons induced during T1L infection suppress regulatory T cells, promoting the expansion of Th1 immunity to gluten, a critical element in the pathogenesis of celiac disease [26]
Rotavirus and celiac disease
Rotavirus is a double-stranded RNA virus in the Reoviridae family, which is known to be a major cause of diarrheal diseases in children [31]. Currently, there are nine species of Rotavirus, referred to as Rotavirus group A to I [32]. Rotavirus has fecal–oral transmission pathways and Rotavirus A is known as the major cause of human infections [33, 34]. It infects enterocytes lining the small intestine and leads to gastroenteritis known as "stomach flu" [35]. Although Rotavirus was discovered in 1973 by Ruth Bishop and her colleagues, its impact on human health has been underestimated, especially in developing countries [36]. Studies have linked rotavirus infection, as an environmental modulator, to CD initiation or progression. In this regard, Stene et al. proposed that a high frequency of rotavirus infections may have a role in increasing the risk of CD development in genetically predisposed childhood who carried CD-related HLA risk alleles [37, 38]. Of interest, Zanoni et al. by screening a random peptide library found that in active CD a subset of anti-transglutaminase IgA antibodies recognized the rotavirus major neutralizing protein VP-7 and suggested a potential role of rotavirus infection in CD pathogenesis initiation [39]. VP-7 is a major glycoprotein of the rotavirus outer layer that elicits class I MHC-related cytotoxic T lymphocyte response.
Reovirus and celiac disease
Reovirus (respiratory and enteric orphan viruses) is a double-stranded RNA virus of the Reoviridae family and is thought to mainly infect adults [40, 41]. Reovirus has three serotypes including T1, T2, and T3 and its infection is usually mild or subclinical, so it does not cause significant disease in humans [42, 43]. Reovirus entrance is through the respiratory or fecal–oral route and one of its replication sites is the intestinal epithelium [44]. Recent studies have considered reoviruses to be an environmental modifier for CD development. In a study conducted by Bouziat et al., it has been suggested that this seemingly harmless virus can cause inappropriate immune activation and intestinal immune homeostasis disruption, leading to gluten intolerance and CD development [45]. In another study, by infecting mice with reovirus strains, it has been reported that the T1L strain of reovirus stimulates inflammatory signals as compared to mice infected with T3D-RV (a T3D reassortant harboring the S1 and L2 genetic segments of T1L) [46]. Their data suggested that reoviral infection may trigger CD in susceptible patients.
Adenovirus and celiac disease
Adenovirus is a double-stranded DNA virus of the Adenoviridae family with a prevalence of around 14% in young children under the age of three. Infection with adenovirus is associated with respiratory (usually) or gastrointestinal (occasionally) problems and many patients are asymptomatic and their infections are self-limiting [47, 48]. Adenoviruses are transmitted via fecal–oral route, respiratory droplets, touching contaminated surfaces, etc. There are more than 50 different adenoviral serotypes in humans (Human adenovirus 1–57; HAdV-1 to HAdV-57), divided into seven different species (Human adenovirus A, B, C, D, E, F, and G) [49, 50]. Some adenovirus serotypes, such as HAdV-40 and HAdV-41, are linked to gastroenteritis in children [51]. Studies also have identified infection with Adenovirus type 12 (Adl2), which is commonly isolated from the intestine, as a trigger for CD development [52]. In this regard, it has been reported that there is a molecular mimicry between alpha-gliadin protein and the early region Elb protein of human Adl2 [53]. Moreover, a significant increase in the anti-Ad12 antibody titers was observed among CD patients relative to controls [53, 54]. Lähdeaho et al. also showed higher IgG antibody levels against nonhomologous Ad40 E1b peptide in celiac and dermatitis herpetiformis patients in comparison with controls [55]. However, later studies did not confirm the role of adenovirus in increasing the risk of CD. For instance, Mahon et al. performed a PCR method for finding adenovirus 12 DNA encoding the E1B-58 kDa in small intestinal biopsy samples of patients with CD, ruling out the link between persistent adenovirus 12 infection and CD pathogenesis [56]. Tarish et al. also found that the level of anti-adenovirus IgG did not increase in CD patients and was not correlated with IFN-α level in their studied subjects. Accordingly, they concluded that CD may not be caused by past adenovirus infection [57].
Enterovirus and celiac disease
Enterovirus is a single-stranded positive-sense RNA virus of the Picornaviridae family known as a major cause of human infectious illnesses. It mainly affects individuals younger than 20 years, especially infants and children under 4 years [58]. Enterovirus includes ten species of enteroviruses (A to J) and three rhinovirus species (A to C) [59]. Enteroviruses are frequently found in an infected person’s respiratory secretions and stool and are spread through fecal–oral and respiratory routes or contaminated objects [59]. Enterovirus infection clinical outcomes vary from mild respiratory sickness to myocarditis and severe neonatal sepsis-like disease [60]. Studies linked Enterovirus A and B infection to CD development [61]. For instance, Lindfors et al. in their metagenomic study showed that repeated exposure to enterovirus along with higher gluten consumption increased the risk of CD autoimmunity in genetically susceptible children between 1 and 2 years of age [62]. Kahrs et al. also in their longitudinal study reported an association between a higher prevalence of enterovirus during early childhood and later onset of CD [61]. Likewise, a recent study conducted by Oikarinen et al. suggested that enterovirus infection may trigger subsequent intolerance to gluten and is associated with CD development [63]. It is noteworthy that an association between enterovirus infection during pregnancy and subsequent CD development in childhood has not been proven [64].
It has been explained that enterovirus infection is associated with lower Foxp3 expression in Tregs (as an important factor for Tregs development) along with increased Th1 immunity results in autoimmune response induction [65].
Moreover, Chen et al. study showed that the frequencies of Th17 cells (which are important in CD pathogenesis) were significantly increased in peripheral blood samples of children infected with enterovirus relative to controls and suggested that the Th17 lineage may be considered an important mediator during this viral infection [66]. Decreased activation of CCL22 (C–C Motif Chemokine Ligand 22), which is associated with Th2 deviation, is also reported in enterovirus infection [65].
Rhinovirus and celiac disease
Rhinovirus is another single-stranded positive-sense RNA virus of the Picornaviridae family that belongs to the Enterovirus genus [67]. Rhinovirus is divided into three categories (RV-A, B, and C) based on their surface glycoproteins and its infection is known as the most important cause of the common colds in children and adults [68]. Rhinovirus transmission occurs by aerosol routes or direct contact [69, 70]. It is known that rhinoviruses replicate in the respiratory tract at relatively low temperatures and due to the high temperature of the gastrointestinal tract and the presence of gastric acid in that area, they do have not the ability to replicate there [71]. However, there is evidence suggesting that many viral respiratory infections can cause gastrointestinal symptoms in children [72]. In this regard, Simre et al. found that rhinoviruses were slightly more common in nasal swab samples of children with CD than controls, but not in their fecal samples. Based on the possibility of certain rhinovirus species, such as species C, replicating at higher temperatures and the expression of main rhinovirus receptor intercellular adhesion molecules-1 (ICAM-1) in the intestinal mucosa in CD, they suggested that rhinovirus infection by increasing the expression of ICAM-1 can cause leukocyte (including gluten-specific T cells) migration toward infection site, thus triggering an immune response to gluten leads to CD [73]. Ruohtula et al. showed that rhinovirus infection in infants before the age of one year is associated with increased Foxp3 expression in Treg cells and decreased Th1- and Th2-related cytokines, which suggested upregulation of immunoregulatory mechanisms after rhinovirus infection [65].
Astrovirus and celiac disease
Astrovirus is a positive-sense, single-stranded RNA virus (like the Picornaviridae) of the Astroviridae family [74]. Human astroviruses are classified into eight serotypes and infection with this virus generally causes acute gastroenteritis most commonly in children (usually under 2 years), which is self-limited and is accompanied by watery diarrhea, nausea, fever, anorexia, etc. [75–77]. In a study conducted by Mousavi Nasab et al. in 2020, human astrovirus was the most frequently detected virus in Iranian children with gastrointestinal infections [78]. In another study by Sayed Othma et al. in 2022, astrovirus was among the most frequently identified viruses in the stool samples of children with gastroenteritis, which was mostly reported in warm weather [79]. Astrovirus is transmitted fecal–orally and through contaminated food, water, and surfaces [80]. Interestingly, Smits et al. using (real-time) PCR method found a strain closely related to Astrovirus VA1 in the stool of a 4-year-old male with new onset CD [81].
Hepatitis viruses and celiac disease
Liver disorders, such as autoimmune liver disease, viral hepatitis, and nonalcoholic fatty liver disease (NAFLD), are known as CD-associated disorders [82]. There are five major hepatitis viruses (A, B, C, D, and E), each of which is in a distinct viral family that affects the liver and causes inflammation. HAV and HEV are spread through the fecal–oral route and can cause acute infections that are generally cleared by the host immune system [83]. HBV (transmitted through sexual activity, contaminated injecting equipment sharing, etc.) and HCV (transmitted through blood products) are known as the major causes of chronic hepatitis that can progress to hepatocellular carcinoma [83]. HDV, which is transmitted through contact with contaminated blood or other body fluids, is dependent on HBV infection for replication and can cause acute or chronic liver disease [83]. It has been hypothesized that HCV and HBV may be associated with gluten intolerance and the development of CD [84].
Hepatitis B (HBV) is a double-stranded DNA virus and a member of the Hepadnaviridae family [85]. There are little data about the link between HBV infection and CD and their relationship seems to be controversial. Previous studies pointed out a lower HBV vaccination response in CD patients than in the general population [86–89]. This unresponsiveness to HBV vaccination can be linked to HLA molecules, including HLA-B8, DR3, and DQ2 homozygotes and defects in their normal immune response [88, 89]. According to the Noh et al. study, unresponsiveness to the HBV vaccine was observed among adults who were HLA-DQ2 positive (homozygous or heterozygous) [89]. On the contrary, Nemes et al. and Ertem et al. found similar seroconversion after hepatitis B vaccination in patients who were vaccinated after adherence to the dietary treatment and healthy controls [90, 91]. Ertekin et al. also found significantly higher anti-HB titers in CD patients under GFD than in CD subjects without GFD adherence [92]. These studies suggested the effect of patients’ gluten intake at the time of vaccination on the vaccine-induced immune response [93]. These hypotheses are still debated and due to the representation of serious problems following hepatitis B vaccination unresponsiveness, new vaccination strategies like the use of boosters and/or higher doses of HBV vaccine were proposed for this group of patients [93].
Hepatitis C (HCV) is a positive-strand RNA virus belonging to the Flaviviridae family [94]. The presence of the HLA-DQ2 haplotype in subjects with HCV can be considered an effective factor in CD and HCV co-occurrence [95]. Furthermore, interferon therapy, particularly with IFN-α, is a recognized therapeutic strategy for chronic HCV infection. However, this treatment can potentially induce or exacerbate autoimmune disorders and has been implicated in the onset of CD [95]. This phenomenon is attributed to an imbalance in Th1/Th2 cell responses, which is crucial for the activation of CD and can be triggered by IFN-α [96].
Marconcini et al. in their retrospective study reported a higher prevalence of anti-gliadin antibodies among HCV subjects related to controls, which was not statistically significant [97]. Ruggeri et al. also observed positive anti-endomysial antibodies (AEA) in 2% of HCV patients (who had positive tTG antibody results) [98]. However, some studies denied the association between these two diseases or the increased prevalence of CD in patients with HCV. For instance, in their study on a group of CD patients with HCV, Garg et al. found that while these two disorders can occur simultaneously, they are not causally linked [95]. Garbovan et al. in their study concluded that the prevalence of CD in chronic HCV patients has decreased over time [99]. Hernandez et al. study also did not show an increased incidence of CD in patients with HCV infection [100].
Coronavirus disease and celiac disease
Coronavirus disease (COVID-19) is a positive-sense single-stranded RNA virus of Coronaviridae family [101]. Coronavirus causes mild to fatal respiratory tract infections and also can infect the gastrointestinal tract through angiotensin-converting enzyme 2 (ACE2) receptor [102]. ACE2 is a receptor that is expressed on pneumocytes and other cells like absorptive enterocytes and its expression is reduced following villous atrophy, which happens in CD [103]. It affects both children and adults, but its clinical symptoms are milder in children [104]. COVID-19 modes of transmission include airborne, respiratory droplets, and fecal–oral routes [105]. Lebwohl et al. in their cohort study conducted in Sweden showed that there was no significant increase in the risk of severe disease outcomes, hospitalization, or death attributed to COVID-19 in CD patients [106]. Asri et al. also in their study compared CD and COVID-19 patients and concluded that the high level of anti-inflammatory mediators in active CD patients may protect them from developing severe COVID-19 [13]. In their questionnaire-based study conducted via telephone and email with 101 CeD patients in Turkey, Gokden et al. found that patients on a gluten-free diet did not face any additional risk of COVID-19 susceptibility compared to the non-CD population [107]. A small cohort study conducted by Italian researchers on 21 patients with refractory celiac disease (who have persistent or recurrent symptoms despite strict GFD adherence for more than 12 months) reported no cases of COVID-19 among them [108]. An interesting hypothesis in this regard is the protective role of HLA-DQ2/DQ8 haplotype (as CD predisposing factors) against COVID-19 infection [109]. On the contrary, the role of COVID-19 in disrupting gut integrity has been suggested as an effective factor in the development of celiac disease [110]. Cakir et al. found that the pandemic COVID-19 period is associated with an increased number of CD patients per year [110]. Similarly, Trovato et al. reported that previous COVID-19 exposure may lead to the development of celiac disease in genetically predisposed subjects [111].
Norovirus and celiac disease
Norovirus has a single-stranded positive-sense RNA and belongs to the Caliciviridae family [112]. It is a highly contagious virus, transmitted through direct person-to-person contact, contaminated food, surfaces, etc. [113]. Norovirus is distributed throughout the small intestine and is known as one of the most common causes of gastroenteritis [114]. Its human strain is classified into three genogroups (GI, GII, and GIV) and can infect all age groups, but older people and immunocompromised patients have high risks of a severe form of the infection [115–117]. Although previous studies did not evaluate the potential association between the norovirus and CD, some studies reported its accompaniance with enteropathy [118]. Woodward et al. detected norovirus in fecal samples of common variable immunodeficiency (CVID) patients with malabsorption, who had duodenal villous atrophy [119]. It has also been reported that norovirus can break oral tolerance and promote pro-inflammatory T cell responses to dietary antigens in murine models resulting in food intolerance [120]. However, Simre and co-workers in their prospective study on stool and nasal swab samples of children with CD (before the diagnosis) and their matched controls, showed no marked difference in terms of infection with viruses like norovirus between their studied groups [73].
Herpesvirus and celiac disease
The Herpesviridae family consists of large double-stranded DNA viruses that cause various diseases in humans and animals [121]. This family is divided into three subfamilies: Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae. Alphaherpesvirinae includes herpes simplex virus (HSV)-1 (HHV-1), HSV-2 (HHV-2), and varicella-zoster virus (VZV or HHV-3). Betaherpesvirinae features human cytomegalovirus (HCMV or HHV-5) and human herpesviruses 6A, 6B (HHV-6A, HHV-6B), and 7 (HHV-7). The Gammaherpesvirinae subfamily comprises Epstein–Barr virus (EBV or HHV-4) and Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV-8) [122]. Cytomegalovirus and herpes simplex virus are the two main herpesviruses that can cause ulcerative disease in the gastrointestinal tract. While this disease can affect healthy individuals, it is more prevalent and severe in immunocompromised patients [123]. Research involving animal models has demonstrated an association between infections caused by gastric herpesviruses and the symptoms of functional gastrointestinal disorders (FGIDs). Furthermore, herpesviruses are posited to contribute to the development of fibromyalgia (FM), a chronic pain condition frequently observed in conjunction with FGIDs [124]. Human herpesviruses rank among the most prevalent infectious agents in the gastrointestinal tract and have been implicated in oncogenesis as well as a range of gastrointestinal disorders [125]
Clinical observations indicate a heightened risk of herpesviruses in patients with CD, although comprehensive studies are sparse [126]. Chatterjee et al. [127] investigated the risk of herpes zoster (HZ) in adults with CD, focusing on those aged under and over 50. A retrospective cohort design was used, analyzing data from patients with CD and matched controls over ten years. Results indicate no significant increase in HZ risk for those under 50, while those aged 50 and above showed a modestly higher risk. The findings suggest that routine early vaccination for HZ in CD patients is not warranted, recommending vaccination starting at age 50 instead. Ludvigsson et al. [128] investigated the association between CD and an increased risk of HZ. Analyzing data from 29,064 CD patients and 144,342 matched controls in Sweden, researchers found that 0.53% of CD patients developed HZ compared to 0.35% of controls. The hazard ratio indicated a 1.62-fold increased risk of HZ in CD patients, particularly notable in those aged 60 and older. Overall, CD was linked to a higher risk of HZ, although the absolute risk remains low.
A study involving 21 patients found that tissue transglutaminase antibody levels increased in two patients (9.5%) during CMV infection, while no such increase was observed in control patients who tested negative for CMV via PCR and IgG [129]. Jansen et al. examined the relationship between CMV and TG2A in 4,420 children, revealing that those with extremely high TG2A levels (ten times the normal range) were less frequently infected with CMV, suggesting a potential protective effect.
A 24-year-old woman presented with severe symptoms including watery diarrhea, weight loss, and electrolyte imbalances. Diagnostic tests revealed celiac disease, characterized by flat mucosa and increased intraepithelial lymphocytes, alongside HSV esophagitis. This case is notable for the acute presentation of celiac crisis, likely triggered by her HSV infection, which led to life-threatening malnutrition [130].
While emerging studies indicate a potential link between herpesviruses and celiac disease, further research is necessary to clarify the nature of this association and its implications for clinical management and treatment strategies.
Preventable viral infections by vaccination
Vaccine effectiveness in the general population has demonstrated high efficacy in both clinical trials and real-world settings, as evidenced by various statistical models [131]. Chodick et al. [132] reported a lower vaccine effectiveness in immunosuppressed patients, with rates of 71% compared to 90% in the general population. While the importance of vaccinations in the prevention of infectious diseases is well established, the immunogenicity of vaccines in individuals with CD remains uncertain [131].
Most studies examining the efficacy and immunogenicity of vaccines in individuals with CD have predominantly focused on the immunological response to the HBV vaccine, with limited data available regarding other vaccinations [133]. A meta-analysis published in 2015 showed that patients with CD have a statistically significant lower rate of protective HBsAb titer compared to non-affected controls [134]. These data were confirmed by similar results found in other studies [90, 135–137]. Consequently, a large number of patients with CD may be nonresponders to HBV vaccination [133]. However, Zingone et al. [138] assessed HBsAg and HBsAb titers in three groups of 163 celiac disease (CD) patients—group A (57 gluten-exposed), group B (46 gluten-free), and group C (60 infants)—along with 48 healthy controls (group D). The study found that all CD patient groups exhibited an inadequate response to hepatitis B vaccination compared to controls.
Sari et al. [139] investigated the immunogenicity of the inactivated HAV vaccine in 33 children with CD compared to a sex- and age-matched control group of 66 healthy children. They evaluated seroconversion rates at 1 and 7 months postvaccination and found that the immune responses were comparable, with nonresponder rates of 21.2% for CD patients and 22.6% for controls after 1 month, and 3% vs. 1.6% after 7 months. In contrast, a study by Urganci et al. [140] involving 16 pediatric CD patients showed a significantly lower immunological response compared to 50 healthy controls over a 7-year follow-up, with nonresponder rates of 75% versus 100%.
Vaarala et al. [141] conducted a nationwide cohort study to investigate the impact of rotavirus vaccination on the risk of CD in Finnish children born between 2009 and 2010. By comparing vaccinated and unvaccinated children, the researchers aimed to assess whether vaccination influences the incidence of this autoimmune condition. The study found no significant difference in the risk of CD between the two groups during a follow-up period of 4–6 years. These findings indicate that oral rotavirus vaccination is safe for individuals at risk for CD. Moreover, Hemming-Harlo et al. reported that rotavirus vaccination can reduce CD autoimmunity risk in childhood and adolescence [142].
Studies investigating the immune response to COVID-19 vaccines among patients with CD who received viral vector or mRNA formulations have indicated that the humoral responses targeting the spike protein of SARS-CoV-2 are comparable to those observed in healthy controls [143, 144]. In this regard a retrospective cohort study assessed the effectiveness of the BNT162b2 mRNA COVID-19 vaccine in preventing SARS-CoV-2 infection in patients with CD. The study involved 5,381 vaccinated CD patients and 14,939 matched controls. The analysis revealed no significant difference in the risk of breakthrough infections between the two groups. These findings indicate that COVID-19 vaccination is effective for patients with CD, showing effectiveness similar to that of the general population [145].
In summary, while vaccination is critical in preventing infectious diseases, the varied immune responses observed among patients with CD highlight the necessity for further research to determine the immunogenicity of vaccines beyond HBV, ensuring optimal protection for this population.
Conclusion
In the present study, we tried to discusses current information on the association between CD and various viral infections. The high susceptibility of CD patients to several serious infections underscores the importance of such studies. Although strict adherence to a gluten-free diet is usually helpful for improving CD patients’ symptoms, some subjects do not respond completely to the diet, that sometimes need immunosuppressive therapy to relieve their complications. Given that different viral infections can trigger or worsen gastrointestinal symptoms in these patients, our study serves as a catalyst for the healthcare system to focus more on assessing the viral infection history of CD patients (particularly those who do not respond to the gluten-free diet). This enhances the search for effective preventive strategies and promotes patient education regarding important risk factors. Adopting appropriate treatment for suspected viral infections may allow physicians to minimize the virus’s impact on disease outcomes. Moreover, further research on the possible role of unstudied/less studied viruses in CD pathogenesis might guide future approaches to prevention.
Acknowledgements
This study was supported by the Celiac Disease and Gluten-Related Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author contributions
N.A. and M.R.N were involved in conceptualization. Investigation was done by N.A. and S.M. M.R.N. and S.R.M helped in project administration. Supervision was done by M.R.N. M.J. helped in visualization. N.A., S.M., and M.J. contributed to writing—original draft. M.R.N. and M.R.T. contributed to writing—review and editing.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419–26. [DOI] [PubMed] [Google Scholar]
- 2.Kagnoff MF. Overview and pathogenesis of celiac disease. Gastroenterology. 2005;128(4):S10–8. [DOI] [PubMed] [Google Scholar]
- 3.Adams F. The extant works of aretaeus: the cappadocian. Vol. 27. Sydenham Society; 1856.
- 4.N Marsh M, W Johnson M, Rostami K. Mucosal histopathology in celiac disease: a rebuttal of Oberhuber’s sub-division of Marsh III. Gastroenterol Hepatol Bed Bench. 2015 Spring;8(2):99–109. PubMed PMID: 25926934; eng. [PMC free article] [PubMed]
- 5.Urubkov S, Khovanskaya S, Smirnov S. The content of certain minerals and trace elements in products made from amaranth and buckwheat flour for children with gluten intolerance. Boпpocы дeтcкoй диeтoлoгии. 2020;18(5):49–53. [Google Scholar]
- 6.Hörnell A, Lagström H, Lande B, et al. Breastfeeding, introduction of other foods and effects on health: a systematic literature review for the 5th Nordic Nutrition Recommendations. Food Nutr Res. 2013;57(1):20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plugis NM, Khosla C. Therapeutic approaches for celiac disease. Best Pract Res Clin Gastroenterol. 2015;29(3):503–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asri N, Rostami-Nejad M. The Facts of Celiac Disease; A Comprehensive Review. Int J Celiac Disease. 2019;7(2):48–52. [Google Scholar]
- 9.Parzanese I, Qehajaj D, Patrinicola F, et al. Celiac disease: from pathophysiology to treatment. World J Gastrointest Pathophysiol. 2017;8(2):27–38. 10.4291/wjgp.v8.i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137(6):1912–33. [DOI] [PubMed] [Google Scholar]
- 11.Lewis D, Haridy J, Newnham ED. Testing for coeliac Disease. Australian Prescriber. 2017;40(3):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasbarrini G, Dionisi T, Corazza GR, et al. COVID-19 in celiac disease: a multicentric retrospective cohort study. Eur Rev Med Pharmacol Sci. 2021;25(12):4400–4. 10.26355/eurrev_202106_26150. [DOI] [PubMed] [Google Scholar]
- 13.Asri N, Nazemalhosseini Mojarad E, Mirjalali H, et al. Toward finding the difference between untreated celiac disease and COVID-19 infected patients in terms of CD4, CD25 (IL-2 Rα), FOXP3 and IL-6 expressions as genes affecting immune homeostasis. BMC Gastroenterol. 2021;21(1):462–462. 10.1186/s12876-021-02056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarczak J, Kaba J, Reczyńska D, et al. Impaired expression of cytokines as a result of viral infections with an emphasis on small ruminant lentivirus infection in goats. Viruses. 2016;8(7):186. 10.3390/v8070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takayama S, Iwaki K, Nishida Y, et al. Effects of oral administration of interferon-alpha on antibody production in mice with induced tolerance. J Interferon Cytokine Res. 1999;19(8):895–900. [DOI] [PubMed] [Google Scholar]
- 16.Cammarota G, Cuoco L, Cianci R, et al. Onset of coeliac disease during treatment with interferon for chronic hepatitis C. The Lancet. 2000;356(9240):1494–5. [DOI] [PubMed] [Google Scholar]
- 17.Brown JJ, Jabri B, Dermody TS. A viral trigger for celiac disease. PLoS Pathog. 2018;14(9): e1007181. 10.1371/journal.ppat.1007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinthrajah RS, Hernandez JD, Boyd SD, et al. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. 2016;137(4):984–97. 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tordesillas L, Berin MC. Mechanisms of oral tolerance. Clin Rev Allergy Immunol. 2018;55(2):107–17. 10.1007/s12016-018-8680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezende RM, Weiner HL. Oral tolerance: an updated review. Immunol Lett. 2022;245:29–37. 10.1016/j.imlet.2022.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Lee GR. Molecular mechanisms of T helper cell differentiation and functional specialization. Immune Netw. 2023;23(1): e4. 10.4110/in.2023.23.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Sherman A, Liao G, et al. Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev. 2013;65(6):759–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berin MC. Mucosal antibodies in the regulation of tolerance and allergy to foods. Semin Immunopathol. 2012;34(5):633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockhart A, Mucida D, Parsa R. Immunity to enteric viruses. Immunity. 2022;55(5):800–18. 10.1016/j.immuni.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4⁺ T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136–48. 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JJ, Jabri B, Dermody TS. A viral trigger for celiac disease. PLoS Pathog. 2018;14(9): e1007181. 10.1371/journal.ppat.1007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rostami Nejad M, Ishaq S, Al Dulaimi D, et al. The role of infectious mediators and gut microbiome in the pathogenesis of celiac disease. Arch Iranian Med. 2015;18(4):244–9. [PubMed] [Google Scholar]
- 28.Lai MH, Joklik WK. The induction of interferon by temperature-sensitive mutants of reovirus, UV-irradiated reovirus, and subviral reovirus particles. Virology. 1973;51(1):191–204. 10.1016/0042-6822(73)90379-6. [DOI] [PubMed] [Google Scholar]
- 29.Murphy K, Weaver C. Janeway’s immunobiology. Garland science; 2016.
- 30.Bouziat R, Hinterleitner R, Brown JJ, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356(6333):44–50. 10.1126/science.aah5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aly M, Al Khairy A, Al Johani S, et al. Unusual rotavirus genotypes among children with acute diarrhea in Saudi Arabia. BMC Infectious Diseases. 2015;15(1):192. 10.1186/s12879-015-0923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barros BdCVd, Chagas EN, Bezerra LW, et al. Rotavirus A in wild and domestic animals from areas with environmental degradation in the Brazilian Amazon. Plos One. 2018;13(12):e0209005. 10.1371/journal.pone.0209005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136(6):1939–51. 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angel J, Franco MA, Greenberg HB. Rotaviruses. In: Mahy BWJ, Van Regenmortel MHV, editors. Encyclopedia of Virology. 3rd ed. Oxford: Academic Press; 2008. p. 507–13. [Google Scholar]
- 35.Lundgren O, Svensson L. Pathogenesis of rotavirus diarrhea. Microbes Infect. 2001;3(13):1145–56. 10.1016/s1286-4579(01)01475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishop R. Discovery of rotavirus: implications for child health. J Gastroenterol Hepatol. 2009;24(s3):S81–5. 10.1111/j.1440-1746.2009.06076.x. [DOI] [PubMed] [Google Scholar]
- 37.Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101(10):2333–40. 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 38.Rostami-Nejad M, Rostami K, Sanaei M, et al. Rotavirus and coeliac autoimmunity among adults with non-specific gastrointestinal symptoms. Saudi Medical J. 2010;31(8):891–4. [PubMed] [Google Scholar]
- 39.Zanoni G, Navone R, Lunardi C, et al. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006;3(9):e358–e358. 10.1371/journal.pmed.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roldão A, Silva AC, Mellado MCM, et al. 1.47 - Viruses and Virus-Like Particles in Biotechnology: Fundamentals and Applications. In: Moo-Young M, editor. Comprehensive Biotechnology (Second Edition). Burlington: Academic Press; 2011. p. 625–649.
- 41.Tai JH, Williams JV, Edwards KM, et al. Prevalence of reovirus-specific antibodies in young children in nashville. Tennessee J Infect Dis. 2005;191(8):1221–4. 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapikian AZ, Shope RE. Rotaviruses, Reoviruses, Coltiviruses, and Orbiviruses. In: Baron S, editor. Medical Microbiology. Galveston (TX): University of Texas Medical Branch at Galveston. Copyright © 1996, The University of Texas Medical Branch at Galveston.; 1996. [PubMed]
- 43.Phillips MB, Stuart JD, Rodríguez Stewart RM, et al. Current understanding of reovirus oncolysis mechanisms. Oncolytic Virother. 2018;7:53–63. 10.2147/OV.S143808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soleimanjahi H, Heydarabadi FH. Reovirus and Rotaviruses: Basic General, Molecular, Clinical, and Application Features. Reference Module in Biomedical Sciences: Elsevier; 2021.
- 45.Bouziat R, Hinterleitner R, Brown JJ, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356(6333):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown JJ, Short SP, Stencel-Baerenwald J, et al. Reovirus-induced apoptosis in the intestine limits establishment of enteric infection. J Virol. 2018;92(10):e02062-e2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young J-AH, Weisdorf DJ. 312–Infections in Recipients of Hematopoietic Stem Cell Transplants. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Eighth Edition). Philadelphia: W.B. Saunders; 2015. p. 3425–3439.e5.
- 48.Usman N, Suarez M. Adenoviruses. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 49.Kajon AE, Weinberg JB, Spindler KR. Adenoviruses☆. Reference Module in Biomedical Sciences: Elsevier; 2019.
- 50.O’Shea H, Blacklaws BA, Collins PJ, et al. Viruses Associated With Foodborne Infections. Reference Module in Life Sciences: Elsevier; 2019.
- 51.Sanaei Dashti A, Ghahremani P, Hashempoor T, et al. Molecular epidemiology of enteric adenovirus gastroenteritis in under-five-year-old children in Iran. Gastroenterol Res Pract. 2016;2016:2045697–2045697. 10.1155/2016/2045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gujral N, Freeman HJ, Thomson ABR. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18(42):6036–59. 10.3748/wjg.v18.i42.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kagnoff MF, Paterson YJ, Kumar PJ, et al. Evidence for the role of a human intestinal adenovirus in the pathogenesis of coeliac disease. Gut. 1987;28(8):995–1001. 10.1136/gut.28.8.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tye-Din JA, Galipeau HJ, Agardh D. Celiac disease: a review of current concepts in pathogenesis, prevention, and novel therapies [review]. Frontiers Pediatrics. 2018;21:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lähdeaho ML, Parkkonen P, Reunala T, et al. Antibodies to E1b protein-derived peptides of enteric adenovirus type 40 are associated with celiac disease and dermatitis herpetiformis. Clin Immunol Immunopathol. 1993;69(3):300–5. 10.1006/clin.1993.1184. [DOI] [PubMed] [Google Scholar]
- 56.Mahon J, Blair G, Wood G, et al. Is persistent adenovirus 12 infection involved in coeliac disease? A search for viral DNA using the polymerase chain reaction. Gut. 1991;32(10):1114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarish HR, Hameed WS, Abdul-Mehdi RJ, et al. Role of previous adenovirus infection and its association with IFN-α in occurrence of celiac disease in Iraqi patients. J Med Sci Clin Res. 2016;4:10326–30. [Google Scholar]
- 58.Reale A, Trevisan M, Alvisi G, et al. The silent enemy: Celiac disease goes viral. J Cell Physiol. 2018;233(4):2693–4. [DOI] [PubMed] [Google Scholar]
- 59.Nikonov OS, Chernykh ES, Garber MB, et al. Enteroviruses: classification, diseases they cause, and approaches to development of antiviral drugs. Biochemistry (Mosc). 2017;82(13):1615–31. 10.1134/S0006297917130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M, Wang H, Tang J, et al. Clinical characteristics of severe neonatal enterovirus infection: a systematic review. BMC Pediatr. 2021;21(1):127–127. 10.1186/s12887-021-02599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kahrs C, Chuda K, Tapia G, et al. Enterovirus as trigger of coeliac disease: nested case-control study within prospective birth cohort. BMJ. 2019. 10.1136/bmj.l231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindfors K, Lin J, Lee HS, et al. Metagenomics of the faecal virome indicate a cumulative effect of enterovirus and gluten amount on the risk of coeliac disease autoimmunity in genetically at risk children: the TEDDY study. Gut. 2020;69(8):1416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oikarinen M, Puustinen L, Lehtonen J, et al. Enterovirus infections are associated with the development of celiac disease in a birth cohort study. Front Immunol. 2020;11: 604529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlsson AK, Lindberg BA, Bredberg AC, et al. Enterovirus infection during pregnancy is not a risk factor for celiac disease in the offspring. J Pediatr Gastroenterol Nutr. 2002;35(5):649–52. [DOI] [PubMed] [Google Scholar]
- 65.Ruohtula T, Kondrashova A, Lehtonen J, et al. Immunomodulatory effects of rhinovirus and enterovirus infections during the first year of life. Front Immunol. 2020;11: 567046. 10.3389/fimmu.2020.567046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J, Tong J, Liu H, et al. Increased frequency of Th17 cells in the peripheral blood of children infected with enterovirus 71. J Med Virol. 2012;84(5):763–7. 10.1002/jmv.23254. [DOI] [PubMed] [Google Scholar]
- 67.Palmenberg AC, Gern JE. Classification and evolution of human rhinoviruses. Methods Mol Biol. 2015;1221:1–10. 10.1007/978-1-4939-1571-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner RB. Rhinovirus: more than just a common cold virus. J Infect Dis. 2007;195(6):765–6. 10.1086/511829. [DOI] [PubMed] [Google Scholar]
- 69.Bochkov YA, Gern JE. Rhinoviruses and their receptors: implications for allergic disease. Curr Allergy Asthma Rep. 2016;16(4):30–30. 10.1007/s11882-016-0608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leung NHL. Transmissibility and transmission of respiratory viruses. Nature Rev Microbiology. 2021;19(8):528–45. 10.1038/s41579-021-00535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhinoviruses WB. In: Heggenhougen HK, editor. International encyclopedia of public health. Oxford: Academic Press; 2008. p. 577–81. [Google Scholar]
- 72.Simre K, Uibo O, Peet A, et al. Exploring the risk factors for differences in the cumulative incidence of coeliac disease in two neighboring countries: the prospective DIABIMMUNE study. Digestive Liver Disease. 2016;48(11):1296–301. 10.1016/j.dld.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 73.Simre K, Uibo O, Peet A, et al. Early-life exposure to common virus infections did not differ between coeliac disease patients and controls. Acta paediatrica (Oslo, Norway : 1992). 2019 Sep;108(9):1709–1716. 10.1111/apa.14791 [DOI] [PubMed]
- 74.Dubois H, van Loo G, Wullaert A. Chapter Three - Nucleic Acid Induced Interferon and Inflammasome Responses in Regulating Host Defense to Gastrointestinal Viruses. In: Vanpouille-Box C, Galluzzi L, editors. International Review of Cell and Molecular Biology. Vol. 345: Academic Press; 2019. p. 137–171. [DOI] [PMC free article] [PubMed]
- 75.Esona MD, Gautam R, Chhabra P, et al. Gastrointestinal Tract Infections: Viruses. Reference Module in Biomedical Sciences: Elsevier; 2021.
- 76.Shrestha SK, Shrestha J, Andreassen AK, et al. Genetic diversity of astrovirus in children from a birth cohort in Nepal [Original Research]. Frontiers Microbiology. 2021. 10.3389/fmicb.2020.588707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dolin R, Treanor JJ. 179 - Astroviruses and Picobirnaviruses. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: W.B. Saunders; 2015. p. 2128- 2130.e1. [Google Scholar]
- 78.Mousavi Nasab SD, Zali F, Kaghazian H, et al. Prevalence of astrovirus, adenovirus, and sapovirus infections among Iranian children with acute gastroenteritis. Gastroenterol Hepatol Bed Bench. 2020 Winter;13(Suppl1):S122-s127 [PMC free article] [PubMed]
- 79.Othma AAS, Gomaa HEE, El Anany MG, et al. Use of multiplex PCR in diagnosis of childhood acute viral diarrhoea caused by rotavirus, norovirus, astrovirus and adenovirus in Upper Egypt. Egyptian J Med Human Genetics. 2022;23(1):40. 10.1186/s43042-022-00261-5. [Google Scholar]
- 80.Moser L, Schultz-Cherry S. Astroviruses. Encyclopedia of Virology. 2008:204–210. 10.1016/B978-012374410-4.00348-4
- 81.Smits SL, van Leeuwen M, van der Eijk AA, et al. Human astrovirus infection in a patient with new-onset celiac disease. J Clin Microbiol. 2010;48(9):3416–8. 10.1128/jcm.01164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubio-Tapia A, Murray JA. Liver involvement in celiac disease. Minerva Med. 2008;99(6):595–604. [PMC free article] [PubMed] [Google Scholar]
- 83.Louten J. Chapter 12 - hepatitis viruses. In: Louten J, editor. Essential human virology. Boston: Academic Press; 2016. p. 213–33. [Google Scholar]
- 84.Algam S, Mohamed MS, Abdelrahman H, et al. Study of association between celiac disease and hepatitis c infection in sudanese patients. J Microbiology Laboratory Sci. 2019;1:1–6. [Google Scholar]
- 85.Wachtman L, Mansfield K, et al. Chapter 1 - Viral Diseases of Nonhuman Primates. In: Abee CR, Mansfield K, Tardif S, et al., editors. Nonhuman Primates in biomedical research. 2nd ed. Boston: Academic Press; 2012. p. 1–104. [Google Scholar]
- 86.Vitaliti G, Praticò A, Cimino C, et al. Hepatitis B vaccine in celiac disease: Yesterday, today and tomorrow. World J Gastroenterology : WJG. 2013. 10.3748/wjg.v19.i6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park SD, Markowitz J, Pettei M, et al. Failure to respond to hepatitis B vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr. 2007;44(4):431–5. 10.1097/MPG.0b013e3180320654. [DOI] [PubMed] [Google Scholar]
- 88.Ahishali E, Boztas G, Akyuz F, et al. Response to hepatitis B vaccination in patients with celiac disease. Dig Dis Sci. 2008;53(8):2156–9. 10.1007/s10620-007-0128-3. [DOI] [PubMed] [Google Scholar]
- 89.Noh KW, Poland GA, Murray JA. Hepatitis B vaccine nonresponse and celiac disease. Am J Gastroenterol. 2003;98(10):2289–92. 10.1111/j.1572-0241.2003.07701.x.PubMedPMID:14572581;eng. [DOI] [PubMed] [Google Scholar]
- 90.Nemes E, Lefler E, Szegedi L, et al. Gluten intake interferes with the humoral immune response to recombinant hepatitis B vaccine in patients with celiac disease. Pediatrics. 2008;121(6):e1570–6. 10.1542/peds.2007-2446.PubMedPMID:18519462;eng. [DOI] [PubMed] [Google Scholar]
- 91.Ertem D, Gonen I, Tanidir C, et al. The response to hepatitis B vaccine: does it differ in celiac disease? Eur J Gastroenterol Hepatol. 2010;22(7):787–93. 10.1097/MEG.0b013e32832e9d41.PubMedPMID:19584738;eng. [DOI] [PubMed] [Google Scholar]
- 92.Ertekin V, Tosun MS, Selimoglu MA. Is there need for a new hepatitıs B vaccine schedule for children with celiac disease? Hepat Mon. 2011;11(8):634–7. 10.5812/kowsar.1735143x.715.PubMedPMID:22140387;eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Filippelli M, Lionetti E, Pulvirenti A, et al. New approaches in hepatitis B vaccination for celiac disease. Immunotherapy. 2014;6(8):945–52. 10.2217/imt.14.64. [DOI] [PubMed] [Google Scholar]
- 94.Mulrooney-Cousins PM, Michalak TI. Chapter 6 - Molecular Testing in Hepatitis Virus Related Disease. In: Coleman WB, Tsongalis GJ, editors. Diagnostic Molecular Pathology: Academic Press; 2017. p. 63–73.
- 95.Casella G, Viganò D, Romano Settanni C, et al. Association between celiac disease and chronic hepatitis C. Gastroenterol Hepatol Bed Bench. 2016 Summer;9(3):153–157. PubMed PMID: 27458507; eng. [PMC free article] [PubMed]
- 96.Singh A, Zaeri N, Ho IK. Onset of celiac disease after treatment of chronic hepatitis c with interferon based triple therapy. Case Rep Hepatology. 2015. 10.1155/2015/763497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marconcini ML, Fayad L, Shiozawa MBC, et al. Autoantibody profile in individuals with chronic hepatitis C. Rev Soc Bras Med Trop. 2013;46:147–53. [DOI] [PubMed] [Google Scholar]
- 98.Ruggeri C, La Masa AT, Rudi S, et al. Celiac disease and non-organ-specific autoantibodies in patients with chronic hepatitis C virus infection. Dig Dis Sci. 2008;53(8):2151–5. 10.1007/s10620-007-0146-1.PubMedPMID:18231858;eng. [DOI] [PubMed] [Google Scholar]
- 99.Cristina Garbovan E, Aldea C, Sur G, et al. Celiac Disease and Chronic Hepatitis C Virus Infection – Prevalence Studies. Int J Celiac Dis. 2022;7(2):46–7. [Google Scholar]
- 100.Hernandez L, Johnson TC, Naiyer AJ, et al. Chronic hepatitis C virus and celiac disease, is there an association? Dig Dis Sci. 2008;53(1):256–61. 10.1007/s10620-007-9851-z. [DOI] [PubMed] [Google Scholar]
- 101.Alexandersen S, Chamings A, Bhatta TR. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat Commun. 2020;11(1):6059. 10.1038/s41467-020-19883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhong P, Xu J, Yang D, et al. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduction Targeted Ther. 2020;5(1):256. 10.1038/s41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elli L, Facciotti F, Lombardo V, et al. Impact of a SARS-COV-2 Infection in patients with celiac Disease. 2020.
- 104.Hasnat F, Noman F, Moben AL, et al. Difference in clinical patterns between COVID-19 affected children and adults. Mymensingh Med J : MMJ. 2021;30(4):1093–9. [PubMed] [Google Scholar]
- 105.Mukhra R, Krishan K, Kanchan T. Possible modes of transmission of Novel coronavirus SARS-CoV-2: a review. Acta Biomedica Atenei Parmensis. 2020;91(3):e2020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lebwohl B, Larsson E, Söderling J, et al. Risk of severe Covid-19 in patients with celiac disease: a population-based cohort study. Clin Epidemiol. 2021;13:121–30. 10.2147/CLEP.S294391.PubMedPMID:33628059;eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gokden Y, Hot S, Adas M, et al. Celiac disease and COVID-19 pandemic: should we worry? Acta Gastro-Enterologica Belgica. 2020;83(4):517–25. [PubMed] [Google Scholar]
- 108.Elli L, Scaramella L, Lombardo V, et al. Refractory celiac disease and COVID-19 outbreak: findings from a high incidence scenario in Northern Italy. Clin Res Hepatol Gastroenterol. 2020;44(5):e115–20. 10.1016/j.clinre.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greco N, Meacci A, Mora B, et al. Coeliac disease in the COVID-19 pandemic: does HLA have a protective effect? Ann Med. 2022;54(1):617–21. 10.1080/07853890.2022.2039955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cakir M, Guven B, Issi F, et al. New-onset celiac disease in children during COVID-19 pandemic. Acta Paediatr. 2022;111(2):383–8. 10.1111/apa.16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trovato CM, Montuori M, Cucchiara S, et al. ESPGHAN ‘biopsy-sparing’ guidelines for celiac disease in children with low antitransglutaminase during COVID-19. Eur J Gastroenterol Hepatol. 2020;32(12):1523–6. 10.1097/meg.0000000000001924. [DOI] [PubMed] [Google Scholar]
- 112.Capece G, Gignac E. Norovirus. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 113.Barclay L, Park GW, Vega E, et al. Infection control for norovirus. Clin Microbiol Infect. 2014;20(8):731–40. 10.1111/1469-0691.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med. 2012;367(22):2126–32. 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y, Hall AJ, Kirk MD. Norovirus disease in older adults living in long-term care facilities: strategies for management. Curr Geriatr Rep. 2017;6(1):26–33. 10.1007/s13670-017-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361(18):1776–85. 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bok K, Prevots DR, Binder AM, et al. Epidemiology of Norovirus Infection Among Immunocompromised Patients at a Tertiary Care Research Hospital, 2010–2013. Open Forum Infectious Dis. 2016. 10.1093/ofid/ofw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Filyk HA, Osborne LC. The multibiome: the intestinal ecosystem’s influence on immune homeostasis, health, and disease. EBioMedicine. 2016;13:46–54. 10.1016/j.ebiom.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Woodward JM, Gkrania-Klotsas E, Cordero-Ng AY, et al. The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. Am J Gastroenterol. 2015;110(2):320–7. 10.1038/ajg.2014.432. [DOI] [PubMed] [Google Scholar]
- 120.Bouziat R, Biering SB, Kouame E, et al. Murine norovirus infection induces TH1 inflammatory responses to dietary antigens. Cell Host Microbe. 2018;24(5):677-688.e5. 10.1016/j.chom.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weller SK, Coen DM. Herpes simplex viruses: mechanisms of DNA replication. Cold Spring Harb Perspect Biol. 2012;4(9): a013011. 10.1101/cshperspect.a013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luczkowiak J, Álvarez M, Sebastián-Martín A, et al. Chapter 4 - DNA-Dependent DNA Polymerases as Drug Targets in Herpesviruses and Poxviruses. In: Gupta SP, editor. Viral Polymerases: Academic Press; 2019. p. 95–134.
- 123.Goodgame RW. Viral infections of the gastrointestinal tract. Curr Gastroenterol Rep. 1999;1(4):292–300. 10.1007/s11894-999-0112-5.PubMedPMID:10980963;eng. [DOI] [PubMed] [Google Scholar]
- 124.Duffy C, Pridgen WL, Whitley RJ. Gastric herpes simplex virus type 1 infection is associated with functional gastrointestinal disorders in the presence and absence of comorbid fibromyalgia: a pilot case-control study. Infection. 2022;50(5):1303–11. 10.1007/s15010-022-01823-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sarshari B, Mohebbi SR, Ravanshad M, et al. Detection and quantification of Epstein-Barr virus, cytomegalovirus, and human herpesvirus-6 in stomach frozen tissue of chronic gastritis and gastric cancer patients. Microbiology Immunol. 2022;66(8):379–85. 10.1111/1348-0421.13013. [DOI] [PubMed] [Google Scholar]
- 126.Chatterjee A, Chittajallu V, Ford A, et al. Increased risk of herpes zoster infection in patients with celiac disease 50 Years old and older. Dig Dis Sci. 2024;69(8):2922–6. 10.1007/s10620-024-08487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chatterjee A, Chittajallu V, Ford A, et al. Increased risk of herpes zoster infection in patients with celiac disease 50 Years old and older. Digestive Diseases Sci. 2024;69(8):2922–6. 10.1007/s10620-024-08487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ludvigsson JF, Choung RS, Marietta EV, et al. Increased risk of herpes zoster in patients with coeliac disease - nationwide cohort study. Scandinavian J Public Health. 2018;46(8):859–66. 10.1177/1403494817714713. [DOI] [PubMed] [Google Scholar]
- 129.Sarmiento L, Galvan JA, Cabrera-Rode E, et al. Type 1 diabetes associated and tissue transglutaminase autoantibodies in patients without type 1 diabetes and coeliac disease with confirmed viral infections. J Med Virol. 2012;84(7):1049–53. 10.1002/jmv.23305. [DOI] [PubMed] [Google Scholar]
- 130.Chen A, Linz CM, Tsay JL, et al. Celiac crisis associated with herpes simplex virus esophagitis. ACG Case Rep J. 2016;3(4): e159. 10.14309/crj.2016.132.PubMedPMID:27921058;PubMedCentralPMCID:PMCPMC5126499.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Passanisi S, Dipasquale V, Romano C. Vaccinations and immune response in celiac disease. Vaccines. 2020. 10.3390/vaccines8020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clinical Infectious Dis. 2022;74(3):472–8. 10.1093/cid/ciab438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scarmozzino R, Zanoni G, Arcolaci A, et al. Vaccine efficacy and safety in patients with celiac disease. Vaccines. 2024;12(12):1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Opri R, Veneri D, Mengoli C, et al. Immune response to Hepatitis B vaccine in patients with celiac disease: a systematic review and meta-analysis. Human Vaccines Immunother. 2015;11(12):2800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leonardi S, Spina M, Spicuzza L, et al. Hepatitis B vaccination failure in celiac disease: is there a need to reassess current immunization strategies? Vaccine. 2009;27(43):6030–3. [DOI] [PubMed] [Google Scholar]
- 136.Ertem D, Gonen I, Tanidir C, et al. The response to hepatitis B vaccine: does it differ in celiac disease? Eur J Gastroenterology Hepatology. 2010;22(7):787–93. [DOI] [PubMed] [Google Scholar]
- 137.Balamtekın N, Uslu N, Baysoy G, et al. Responsiveness of children with celiac disease to different hepatitis B vaccination protocols. Turkish J Gastroenterology: Off J Turkish Soc Gastroenterology. 2011;22(1):27–31. [DOI] [PubMed] [Google Scholar]
- 138.Zingone F, Capone P, Tortora R, et al. Role of gluten intake at the time of hepatitis B virus vaccination in the immune response of celiac patients. Clin Vaccine Immunol : CVI. 2013;20(5):660–2. 10.1128/cvi.00729-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sari S, Dalgic B, Basturk B, et al. Immunogenicity of hepatitis a vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr. 2011;53(5):532–5. 10.1097/MPG.0b013e318223b3ed. [DOI] [PubMed] [Google Scholar]
- 140.Urganci N, Kalyoncu D. Response to hepatitis A and B vaccination in pediatric patients with celiac disease. J Pediatric Gastroenterology Nutr. 2013;56(4):408–11. [DOI] [PubMed] [Google Scholar]
- 141.Vaarala O, Jokinen J, Lahdenkari M, et al. Rotavirus Vaccination and the Risk of Celiac Disease or Type 1 Diabetes in Finnish Children at Early Life. The Pediatric Infectious Disease Journal. 2017;36(7). [DOI] [PubMed]
- 142.Hemming-Harlo M, Lähdeaho M-L, Mäki M, et al. Rotavirus vaccination does not increase type 1 diabetes and may decrease celiac disease in children and adolescents. Pediatr Infect Dis J. 2019;38(5):539–41. [DOI] [PubMed] [Google Scholar]
- 143.Ibsen JH, Chopra A, Vaage EB, et al. Immune responses to SARS-CoV-2 vaccines in celiac disease. Scand J Gastroenterol. 2023;58(2):142–7. 10.1080/00365521.2022.2114809. [DOI] [PubMed] [Google Scholar]
- 144.Scalvini D, Schiepatti A, Maimaris S, et al. Humoral immunogenicity of COVID-19 vaccines in patients with coeliac disease and other noncoeliac enteropathies compared to healthy controls. Eur J Gastroenterol Hepatol. 2023;35(2):167–73. 10.1097/meg.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 145.Ben-Tov A, Lebwohl B, Banon T, et al. BNT162b2 mRNA COVID-19 vaccine effectiveness in patients with coeliac disease autoimmunity: real-world data from mass vaccination campaign. Viruses. 2023;15(9):1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.