Abstract
This study aimed to investigate the relationship between unintentional weight loss and 30-day mortality in sepsis patients in the intensive care unit (ICU). A retrospective cohort study sepsis patients in the ICU was conducted using data from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, involving 1842 sepsis patients in the ICU. We utilized multivariate Cox regression analysis to evaluate the association between unintentional weight loss and the risk of 30-day mortality. In addition, we conducted stratified and interaction analyses to determine the consistency of this association across various demographic and clinical subgroups. Out of the 1842 patients, 19.2% (354) died within 30 days. The fully adjusted multivariate Cox regression model revealed that for every one-unit decrease in body weight, the risk of death increased by 58% (hazard ratio [HR] = 1.58; 95% confidence interval [CI] = 1.20–2.07). Unintentional weight loss was found to be positively correlated with 30-day mortality. Subgroup analysis yielded consistent results across all groups. Unintentional weight loss was positively associated with a greater risk of mortality in critically ill patients with sepsis in the ICU.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83260-3.
Keywords: Unintentional weight loss, Sepsis, 30-day mortality, Retrospective cohort study
Subject terms: Medical research, Risk factors
Introduction
Sepsis is a complex disease triggered by infection, characterized by a range of pathologic, physiological, and biochemical abnormalities, posing a serious challenge to global health systems. It constitutes a significant healthcare burden globally, both in high-income and low- and middle-income countries1–4, and poses a major threat to the lives of patients in intensive care units5. In the United States, sepsis hospitalization rates even exceed those of stroke and myocardial infarction6. The high cost of its treatment accounts for more than one-third of all fatal hospitalizations7. The latest comprehensive analysis predicts that approximately 5.3 million people worldwide die from sepsis each year3, highlighting the profound impact of sepsis on global health. Despite significant advances in the field of sepsis pathophysiology, therapeutic strategies and epidemiological research, sepsis mortality remains high8. One study showed that in an analysis of 300 sepsis deaths conducted in six hospitals in the United States, in 88% of cases, the outcome was unavoidable even with access to better quality medical interventions9. These statistics emphasize the severity of sepsis and the complexity of its treatment, as well as the urgency of reducing its mortality rate.
In this context, identifying potential risk factors for sepsis becomes particularly important. Studies have shown that cancer patients with involuntary weight loss are more likely to develop sepsis after elective surgery10. Involuntary weight loss may be one of the risk factors for mortality in sepsis patients, and this kind of weight loss is usually closely related to malnutrition, physiological changes, and metabolic disorders. In the general population, underweight, overweight, or obese individuals have a higher risk of death compared to those with normal weight11–14. However, in acute and critical illnesses, overweight and obese patients often exhibit higher survival rates15–18, a phenomenon known as the "obesity paradox19–21." The applicability of this paradox in sepsis patients and its relationship with involuntary weight loss is not clear and remains controversial. A population-based Mendelian randomization study from Norway suggests that an increase in genetically predicted body mass index (BMI) is associated with increased incidence and mortality of bacterial sepsis, challenging the traditional view that a higher BMI may have a protective effect on patients with severe infectious diseases22. This finding suggests that weight management in sepsis patients may be more complex than the simple BMI indicator, especially when considering the case of involuntary weight loss.
Given that most studies primarily assess patients’ mortality risk based on BMI, this approach may overlook the potential impact of involuntary weight loss on the mortality rates of patients across different weight groups. Therefore, this study aims to fill this research gap by thoroughly exploring the clinical significance of involuntary weight loss in sepsis patients and how it affects the 30-day mortality rate of patients in different weight groups, thereby providing more precise guidance for clinical treatment.
Materials and methods
Database introduction
The data for our study were sourced from the open-source clinical database MIMIC-IV, an updated version of its predecessor, MIMIC-III. The MIMIC-IV encompasses a comprehensive dataset of more than 50,000 patients admitted to the ICU at Beth Israel Deaconess Medical Center spanning the period from 2008 to 201923. The principal investigator, Weide Lin, secured access to the database (Certification Number: 62407435) following the successful completion of online examinations and the execution of a data use agreement. The institutional review boards of both Beth Israel Deaconess Medical Center and MIT Affiliates endorsed the creation of the MIMIC-IV database. Given the anonymous nature of the database, informed consent was not needed. The Ethics Committee of the First Hospital of Putian City granted a waiver of review for this study, with the ethical approval number being 2024-115.
Selection of the study population
Patients who were eligible for our study were diagnosed with sepsis, which we defined according to the third international consensus definition as a life-threatening organ dysfunction caused by a dysregulated host response to infection5. Specifically, organ dysfunction was indicated by a sequential organ failure assessment (SOFA) score increase of two or more points24,25. The MIMIC-IV database encompasses a total of 73,181 patients, 50,920 of whom were initially admitted to the ICU. Our study focused on 20,054 adult sepsis patients aged 18 years or older from the MIMIC-IV database. Of these patients, 6930 were admitted to the ICU. We excluded 5088 patients due to pregnancy, neoplasia, not meeting the Sepsis-3.0 diagnostic criteria, absence of a documented weight loss variable, or ICU admissions lasting less than 24 h. For patients with multiple ICU admissions, we considered only the data from their first hospital and ICU admission. The final study cohort comprised 1842 patients with sepsis. A flowchart detailing the patient selection process is depicted in Fig. 1.
Fig. 1.
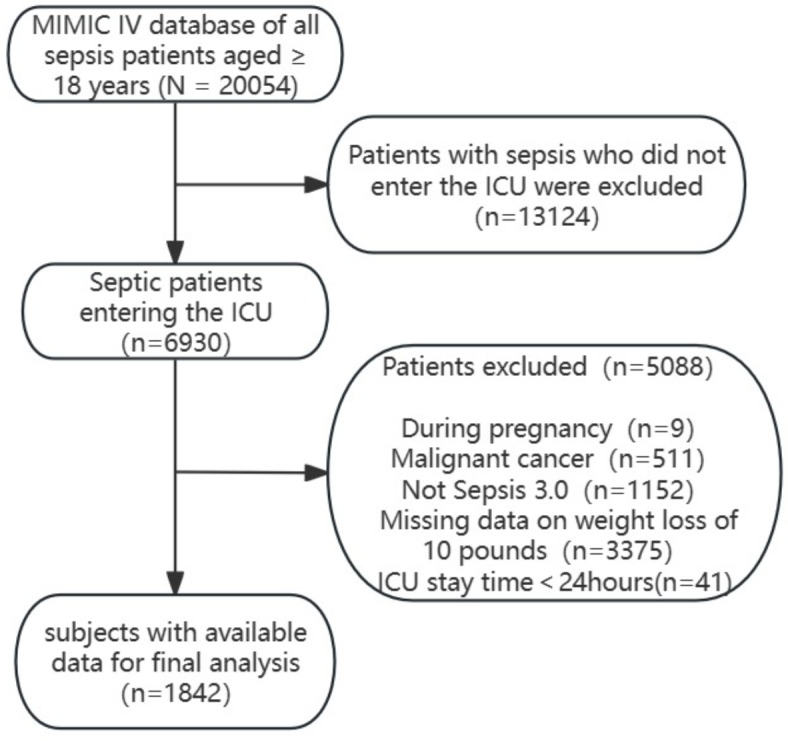
Schematic representation of the participant selection process and distribution of participant groups.
Unintentional weight loss
In the MIMIC-IV database, code 225,124 is used to identify and document unintentional weight loss > 4.54 kg. The determination of this weight loss is based on the process of collecting medical history by asking patients if they have experienced significant involuntary weight loss in the short term, rather than based on a specific time frame. If patients report such weight loss, this information is recorded in the medical records, typically reflected in the admission history or family health assessment section of the patient’s chart.
Variable extraction and outcome
Data extraction was performed utilizing PostgreSQL (version 13.9). The collected data included demographic details, vital sign measurements, laboratory test results, and critical illness scores. Baseline data were specifically gathered within the initial 24-h period following ICU admission. The covariates considered in our analysis were age, sex, race, insurance status, weight, heart rate, mean arterial pressure (MAP), white blood cell (WBC) count, glucose levels, hemoglobin (Hb), albumin (Alb), serum creatinine (Scr), total bilirubin (TBIL), Charlson Comorbidity Index (CCI), acute physiology score III (APS III) and SOFA score. The primary study outcome was 30-day mortality.
Statistical analyses
Categorical variables are expressed as proportions (%) and were tested with a chi-square test or Fisher’s exact test. Continuous variables with a normal distribution are depicted as the mean ± standard deviation (SD) and were compared using Student’s t test or one-way ANOVA. For continuous variables not conforming to a normal distribution, the data are shown as the median and interquartile range (IQR), and the Kruskal‒Wallis H test was used for statistical analysis. Furthermore, 19.06% of the data for the covariate total bilirubin were missing, and we addressed this by utilizing simple mean imputation. For albumin, 35.83% of the data were missing, and we managed this by categorizing albumin into subgroups based on the available data (< 30, ≥ 30, missing). The remaining covariates had minimal missing data, ranging from 0.054 to 7.33%, which did not warrant the use of interpolation methods.
Multivariable Cox regression analyses were adopted to assess the independent association between unintentional weight loss and 30-day mortality. An extended Cox model approach was used for different covariates adjusted models. Model I adjusted for covariates with a change in effect size of more than 10%; Model II, in addition to Model I, adjusted for covariates with a p-value of less than 0.1 in the univariate analysis; Model III, in addition to Model II, adjusted for covariates with known clinical significance as reported previously26. The specific covariates included in each model are as follows: the crude model was unadjusted; Model 1 was adjusted for WBC, Hb, and Alb; Model 2 was further adjusted for age, glucose, TBIL, CCI, APS III, and SOFA on top of Model 1; Model 3 further included sex, race, insurance status, heart rate, MAP, and Scr in addition to the variables in Model 2.
To bolster the robustness of our findings, we utilized Cox regression models to conduct interaction and subgroup analyses. The data were stratified based on various factors, including sex (female and male), race (white, African American, and other), insurance type (Medicare, Medicaid, and other), age (younger than 65 years and older), weight (less than 70.85 kg, between 70.85 and 89.80 kg, and greater than 89.80 kg), CCI score (less than 3, 3 to 6, and greater than 6), APS III score (less than 45, 45 to 65, and greater than 65), and SOFA score (2 to 3 and greater than 3).
All the statistical analyses were conducted using R software version 4.4.2 and Free Statistics software version 1.9.2. A two-tailed test was applied, and a p value less than 0.05 was considered to indicate statistical significance.
Results
Participants and demographic characteristics
This study included 1842 eligible participants, all aged 18 years or older. The overall 30-day mortality rate within this population was 19.2%. Table 1 presents the stratified clinical and biochemical characteristics of the study cohort based on the occurrence of unintentional weight loss. The average age of the participants was 66.2 years, with a standard deviation of 17.5 years, and 54.3% (1000 individuals) were male. Those who experienced this degree of weight loss had significantly lower body weights and decreased levels of albumin, hemoglobin, creatinine, and total bilirubin. In terms of the distribution of infection sites, patients with unintentional weight loss are more commonly associated with pulmonary and gastrointestinal infections, while infections in the urinary tract and other areas are relatively less frequent. Moreover, this subgroup had a notably higher 30-day mortality rate. No other significant differences were observed in the baseline characteristics.
Table 1.
Baseline characteristics of participants.
| Variables | ALL patients | Unintentional Weight Loss | P value | |
|---|---|---|---|---|
| (n = 1842) | No (n = 1585) | Yes (n = 257) | ||
| Age(years) | 66.2 ± 17.5 | 66.3 ± 17.6 | 65.6 ± 17.1 | 0.537 |
| Gender, n(%) | 0.037 | |||
| Female | 842 (45.7) | 740 (46.7) | 102 (39.7) | |
| Male | 1000 (54.3) | 845 (53.3) | 155 (60.3) | |
| Race/Ethnicity,n(%) | 0.551 | |||
| White | 1312 (71.2) | 1131 (71.4) | 181 (70.4) | |
| African Americans | 135 (7.3) | 112 (7.1) | 23 (8.9) | |
| Other | 395 (21.4) | 342 (21.6) | 53 (20.6) | |
| Insurance,n (%) | 0.13 | |||
| Medicare | 898 (48.8) | 787 (49.7) | 111 (43.2) | |
| Medicaid | 138 (7.5) | 119 (7.5) | 19 (7.4) | |
| Other | 806 (43.8) | 679 (42.8) | 127 (49.4) | |
| Weight(kg) | < 0.001 | |||
| < 50 | 63 (3.5) | 36 (2.3) | 27 (10.6) | |
| 50–100 | 1342 (73.8) | 1143 (73.1) | 199 (78) | |
| > 100 | 413 (22.7) | 384 (24.6) | 29 (11.4) | |
| Laboratory metries | ||||
| Heart rate(bpm) | 91.1 ± 16.9 | 91.0 ± 16.6 | 91.8 ± 18.7 | 0.506 |
| MAP(mmHg) | 77.3 ± 10.2 | 77.4 ± 10.1 | 76.6 ± 11.0 | 0.252 |
| WBC(× 109/L) | 15.4 (10.3, 21.8) | 15.5 (10.6, 21.9) | 15.1 (9.0, 21.4) | 0.183 |
| Glucose(mmol/L) | 7.9 ± 3.0 | 7.9 ± 3.0 | 7.6 ± 2.7 | 0.148 |
| Hb(g/L) | 9.9 ± 2.0 | 10.1 ± 2.0 | 9.0 ± 1.9 | < 0.001 |
| Alb(g/L) | < 0.001 | |||
| < 30 | 602 (32.7) | 482 (30.4) | 120 (46.7) | |
| ≥ 30 | 580 (31.5) | 520 (32.8) | 60 (23.3) | |
| NA* | 660 (35.8) | 583 (36.8) | 77 (30) | |
| Scr(mg/dL) | 1.4 (1.0, 2.4) | 1.4 (1.0, 2.4) | 1.3 (0.9, 2.2) | 0.016 |
| TBIL(mg/dL) | 1.4 (0.6, 2.7) | 1.5 (0.6, 2.7) | 1.1 (0.5, 2.7) | 0.009 |
| CCI | 5.0 (4.0, 7.0) | 5.0 (4.0, 7.0) | 6.0 (4.0, 8.0) | 0.581 |
| APSIII | 60.6 ± 25.4 | 60.2 ± 25.4 | 63.1 ± 25.5 | 0.091 |
| SOFA | 3.8 ± 2.0 | 3.8 ± 2.0 | 3.6 ± 1.6 | 0.071 |
| 30-Day Mortality,n(%) | < 0.001 | |||
| No | 1488 (80.8) | 1303 (82.2) | 185 (72) | |
| Yes | 354 (19.2) | 282 (17.8) | 72 (28) | |
| Septic Shock | 0.273 | |||
| No | 1349 (73.2) | 1168 (73.7) | 181 (70.4) | |
| Yes | 493 (26.8) | 417 (26.3) | 76 (29.6) | |
| Source of Sepsis | 0.026 | |||
| Urinary Tract | 347 (18.8) | 310 (19.6) | 37 (14.4) | |
| Lungs | 300 (16.3) | 254 (16) | 46 (17.9) | |
| Gastrointestinal Tract | 113 (6.1) | 91 (5.7) | 22 (8.6) | |
| Others | 136 (7.4) | 125 (7.9) | 11 (4.3) | |
| NA* | 946 (51.4) | 805 (50.8) | 141 (54.9) | |
| Pathogens Causing Sepsis | 0.082 | |||
| Escherichia Coli | 114 (6.2) | 107 (6.8) | 7 (2.7) | |
| Streptococcus | 93 (5.0) | 77 (4.9) | 16 (6.2) | |
| Clostridium Difficile | 61 (3.3) | 49 (3.1) | 12 (4.7) | |
| Staphylococcus Aureus | 96 (5.2) | 86 (5.4) | 10 (3.9) | |
| Others | 47 (2.6) | 39 (2.5) | 8 (3.1) | |
| NA* | 1431 (77.7) | 1227 (77.4) | 204 (79.4) | |
Continuous variables are presented as the mean ± SD or median (quartile), while categorical variables are presented as absolute numbers (percentages).
MBP, mean blood pressure; WBC, white blood cell; Hb, hemoglobin; Alb, albumin; NA*, missing values; Scr, serum creatinine; TBIL, total bilirubin; CCI, Charlson comorbidity index; APSIII, acute physiology score III; SOFA, sequential organ failure assessment.
Associations between unintentional weight loss and 30-day mortality
Univariate regression analyses were conducted to ascertain the factors correlated with 30-day mortality, as detailed in Table 2. The analysis revealed that age, race, weight, glucose levels, Hb, Alb, TBIL, CCI, APS III, SOFA score and unintentional weight loss were significant predictors of 30-day mortality (all P < 0.05). The K‒M curve demonstrated a significant correlation between unintentional weight loss and the risk of death within 30 days (Fig. 2).
Table 2.
Univariate analysis of risk factors associated with 30-day mortality in patients with sepsis.
| Variables | HR(95%CI) | P value |
|---|---|---|
| Age(years) | 1.02 (1.01,1.03) | < 0.001 |
| Gender,(Male vs Female) | 0.9919 (0.8047,1.2225) | 0.939 |
| Race/Ethnicity | ||
| White | Ref | |
| African Americans | 0.53 (0.31,0.89) | 0.017 |
| Other | 0.9904 (0.7691,1.2754) | 0.94 |
| Insurance | ||
| Medicare | Ref | |
| Medicaid | 0.76 (0.49,1.18) | 0.221 |
| Other | 0.87 (0.7,1.08) | 0.199 |
| Weight(kg) | ||
| < 50 | Ref | |
| 50–100 | 0.53 (0.34,0.84) | 0.007 |
| > 100 | 0.48 (0.29,0.79) | 0.004 |
| Laboratory metries | ||
| Heart rate | 0.9999 (0.9938,1.0061) | 0.985 |
| MAP | 0.9923 (0.9825,1.0022) | 0.128 |
| WBC | 1.0039 (0.9941,1.0137) | 0.437 |
| Glucose | 1.03 (1.0,1.07) | 0.044 |
| Hb | 0.92 (0.87,0.96) | < 0.001 |
| Alb(g/L) | ||
| < 30 | Ref | |
| ≥ 30 | 0.63 (0.49,0.82) | < 0.001 |
| NA* | 0.69 (0.54,0.88) | 0.003 |
| Scr | 1.03 (0.98,1.08) | 0.201 |
| TBIL | 1.03 (1.02,1.04) | < 0.001 |
| CCI | 1.19 (1.15,1.24) | < 0.001 |
| APSIII | 1.03 (1.02,1.03) | < 0.001 |
| SOFA | 1.12 (1.07,1.17) | < 0.001 |
| Unintentional Weight Loss,(Yes vs No) | 1.67 (1.29,2.17) | < 0.001 |
HR, hazard ratio; CI, confidence interval; Ref, reference; HR, heart rate; MAP, mean arterial pressure; WBC, white blood cell; Hb, hemoglobin; Alb, albumin; NA*, missing values; Scr, serum creatinine; TBIL, total bilirubin; CCI, Charlson comorbidity index; APSIII, acute physiology score III; SOFA, sequential organ failure assessment.
Fig. 2.
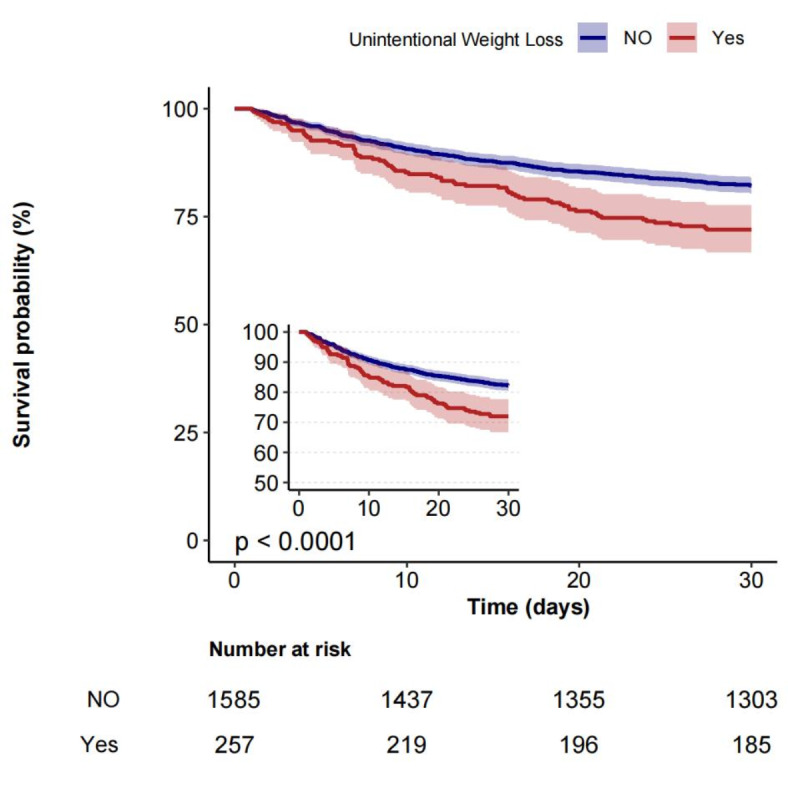
Kaplan–Meier analysis of Unintentional weight loss in patients with sepsis.
According to the extended multivariate Cox models (Table 3), the hazard ratios associated with unintentional weight loss prior to admission were found to be consistent (HR range 1.47–1.67, p < 0.05 for all). This significant association was observed in both unadjusted and adjusted models.In the unadjusted model, for every one-unit decrease in body weight, the risk of death increased by 67% (Crude model, HR = 1.67; 95% CI: 1.29–2.17; p < 0.001). According to the fully adjusted Model 3, for every one-unit decrease in body weight, the risk of death increased by 58% (Model 3, HR = 1.58; 95% CI: 1.20–2.07; p = 0.001).
Table 3.
Relationships between unintentional weight loss and 30-day mortality in different models.
| Variable | Crude model | Model I | Model II | Model III | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Unintentional weight loss (No) | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | ||||
| Unintentional weight loss (Yes) | 1.67 (1.29–2.17) | < 0.001 | 1.47 (1.13–1.92) | 0.004 | 1.61 (1.23–2.10) | 0.001 | 1.58 (1.20–2.07) | 0.001 |
The crude model was not adjusted.
Model 1 was adjusted for WBC + Hb + Alb.
Model 2 was adjusted for Model 1 + age + glucose + TBIL + CCI + APSIII + SOFA.
Model 3 was adjusted for Model 2 + sex + race + insurance + heart rate + MAP + Scr.
HR, hazard ratio; CI, confidence interval; Ref, reference; WBC, white blood cell; Hb, hemoglobin; Alb, albumin; TBIL, total bilirubin; CCI, Charlson comorbidity index; APSIII, acute physiology score III; SOFA, sequential organ failure assessment; MAP, mean arterial pressure; Scr, serum creatinine.
Subgroup analyses
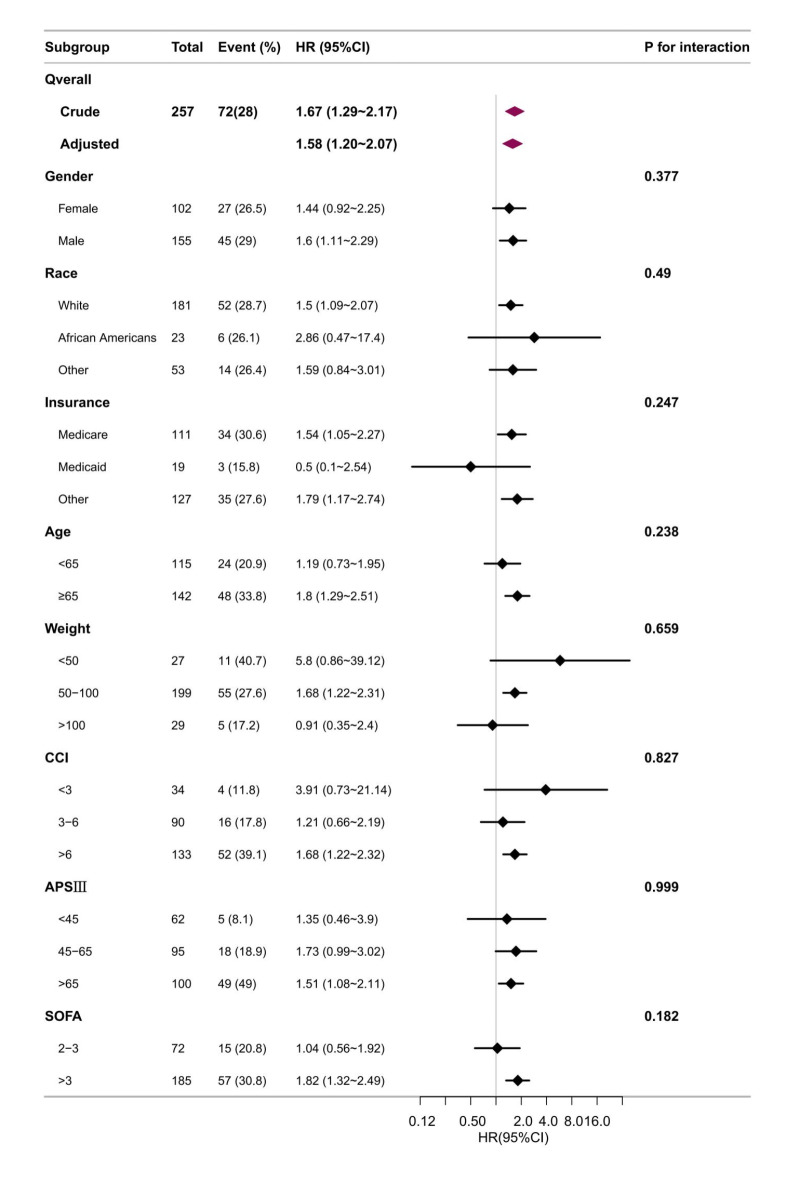
In this study, we carried out stratified and interaction analyses to determine whether the correlation between unintentional weight loss and 30-day mortality held true across various subgroups, as depicted in Fig. 3. The analysis yielded consistent findings when stratified by sex (female and male), race (white, African American, and other), insurance type (Medicare, Medicaid, and other), age (younger than 65 and 65 years old or older), weight (younger than 50 kg, between 50 and 100 kg, and older than 100 kg), CCI score (less than 3, 3 to 6, and older than 6), APS III score (less than 45, 45 to 65, and older than 65), and SOFA score (2 to 3 and older than 3).
Fig. 3.
The forest plots of sub-group analysis.
Figure 3 illustrates that unintentional weight loss is linked to a heightened risk of 30-day mortality across several participant subgroups, with males (HR: 1.60, 95% CI: 1.11–2.29), whites (HR: 1.50, 95% CI: 1.09–2.07), Medicare recipients (HR: 1.54, 95% CI: 1.05–2.27), individuals aged 65 years and older (HR: 1.80, 95% CI: 1.29–2.51), those with weights between 50 and 100 kg (HR: 1.68, 95% CI: 1.22–2.31), patients with a CCI score above 6 (HR: 1.68, 95% CI: 1.22–2.32), those with an APS III score (below 45, 45 to 65, and above 65) above 65 (HR: 1.51, 95% CI: 1.08–2.11), and those with a SOFA score above 3 (HR: 1.82, 95% CI: 1.32–2.49) showing significant associations. Nevertheless, the impact of unintentional weight loss on 30-day mortality was consistent across subgroups. Stratified analyses for effect modification did not yield statistically significant interactions (all p values for interaction > 0.05).
Discussion
This retrospective cohort study utilizing the MIMIC-IV database represents the exploratory study to establish a positive correlation between unintentional weight loss among critically ill sepsis patients in the ICU and the 30-day mortality rate. Our findings revealed that unintentional weight loss of this magnitude is an independent risk factor associated with a 58% increase in the risk of death within 30 days post-ICU admission. These results were consistent across all analyzed subgroups, with no significant interaction effects observed.
Through a comprehensive review of the literature, we identified key factors that are strongly associated with a poor prognosis for sepsis patients, including low body mass index27 and malnutrition28. Nutritional intake is an important factor affecting weight change29, This may include the intake of calories, proteins, fats, carbohydrates, vitamins, and minerals. A clinical randomized trial study pointed out that increasing the intake of low-fat plant-based foods while reducing high-fat and animal-based foods is associated with weight loss30. Other studies have shown that a high-protein diet combined with meal replacements can improve long-term nutritional status and is associated with weight reduction31. Insufficient or unbalanced nutritional intake may lead to weight loss, which may be related to our study results. Secondly, short-term fluctuations in fluid intake may temporarily affect weight measurements. We have observed that involuntary weight loss is a common clinical characteristic of these factors, and to our knowledge, this study may be the first to explore the association between involuntary weight loss and sepsis. It is currently unclear whether unintentional weight loss serves as a marker of overall poor health, malnutrition, and multiple disease burdens. A study of patients with disseminated tumors noted that unintentional weight loss was an independent predictor of increased risk of complications and death after planned surgery in these patients10. In this study, involuntary weight loss was also found to be an independent risk factor for 30-day mortality in sepsis patients, highlighting the importance of involuntary weight loss as a clinical indicator.
Adipose tissue is known to confer a range of protective effects due to its role in the storage of catabolic products and its capacity to modulate inflammatory responses32. Typically, patients with a lower BMI are thought to have insufficient fat reserves, which may diminish their resistance to severe illnesses33–35. Oh et al.36 have shown that septic patients who are underweight or malnourished tend to be at greater risk of death; conversely, extra body weight or adipose tissue may be associated with lower mortality, a phenomenon known as the “obesity paradox” in a practical clinical setting. There is also evidence that mortality in obese or overweight septic patients is negatively correlated with body weight and significantly positively correlated with body weight in low-body-weight patients37–39. A study published in the JACC presented analogous results40. A Mendelian randomization study involving body mass index and the risk of death in BSI patients noted that genetically predicted increases in BMI were associated with increased morbidity and mortality in bacterial sepsis patients. Weng et al.41 reported that underweight, low normal weight and abdominal obesity were associated with an increased risk of sepsis-related death in the next 10 years. These findings contradict the obesity paradox, so research on the “obesity paradox” is controversial. It is worth noting that research to date has primarily focused on the impact of body weight and nutritional status on the prognosis of sepsis patients, but has often overlooked the impact of involuntary weight loss in patients of different weight categories on prognosis. This change in body weight may be a key variable that has an important influence on the pathophysiology of ICU sepsis patients and modulates their pathological processes. This study grouped patients by body weight and found that involuntary weight loss is an independent predictive factor affecting the prognosis of sepsis patients in all weight groups. Our findings emphasize the importance of assessing involuntary weight loss in sepsis patients.
Our findings have been validated and further expanded upon in the context of the MIMIC-IV database. Reduced nutritional reserves and an altered immune response may be a possible mechanism underlying the association between unintentional weight loss and an increased risk of sepsis-related mortality. Future research needs to further explore these complex relationships and potential mechanisms to determine the role of involuntary weight loss in the pathophysiology of sepsis, and to explore how to incorporate the assessment of involuntary weight loss into the clinical management of sepsis patients, as well as how early identification and timely intervention can optimize the prognosis and outcomes for these patients.
Although this study provides some valuable insights, we are well aware of its limitations. Firstly, as a retrospective study, we may not be able to fully control or exclude all unmeasured or unknown potential confounding factors, such as nutritional intake, fluid intake, etc., which may have affected the results of the study. Second, this study was unable to identify a specific time frame for unintentional weight loss. Additionally, whether the weight distribution observed in this study differs in regions outside the United States, and whether the research findings are applicable to populations with different regional backgrounds, remain questions that require further investigation. Finally, this study did not provide direct evidence of a causal relationship between unintentional weight loss and sepsis, which needs to be explored in depth in future prospective studies.
Conclusion
In this expansive population-based cohort study encompassing 1,842 United States patients aged 18 years and older admitted to the ICU for sepsis, a significant positive correlation was identified between unintentional weight loss and the risk of 30-day mortality, irrespective of the patients’ weight status. With each unit of weight loss, there was a 58% increase in the risk of mortality within the 30-day postadmission period.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to all the participants, staff members, and our esteemed colleagues in the study for their exceptional contributions to this research endeavor.
Author contributions
WL contributed to the study’s conception and design, statistical analysis, and data interpretation, as well as the drafting and revision of the manuscript. RL and JC were involved in data acquisition and revising the data analysis and interpretation. YY, SH, SL and JL participated in the study’s conception and design, data interpretation, and manuscript revisions. BL was involved in the study’s conception and design, data interpretation, and manuscript revisions and supervised the entire study. All authors have read and approved the final manuscript for publication.
Funding
This study was conducted without the support of any specific grant from funding agencies, whether from the public sector, commercial entities, or nonprofit organizations.
Data availability
All the datasets utilized in the current study are freely accessible in the MIMIC-IV v2.2 database (https://mimic.physionet.org/).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weng, L. et al. Sepsis-related mortality in China: A descriptive analysis. Intensive Care Med.44(7), 1071–1080. 10.1007/s00134-018-5203-z (2018). [DOI] [PubMed] [Google Scholar]
- 2.Reinhart, K. et al. Recognizing sepsis as a global health priority—A WHO resolution. N. Engl. J. Med.377(5), 414–417. 10.1056/NEJMp1707170 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann, C. et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med.193(3), 259–272. 10.1164/rccm.201504-0781OC (2016). [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi, L. U., Bierrenbach, A. L., Toscano, C. M., Schettino, G. P. & Azevedo, L. C. Sepsis-related deaths in Brazil: An analysis of the national mortality registry from 2002 to 2010. Crit. Care.18(6), 608. 10.1186/s13054-014-0608-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA315(8), 801. 10.1001/jama.2016.0287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour, C. W. et al. Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. Am. J. Respir. Crit. Care Med.186(12), 1264–1271. 10.1164/rccm.201204-0713OC (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee, C. et al. Incidence and trends of sepsis in us hospitals using clinical vs claims data, 2009–2014. JAMA318(13), 1241. 10.1001/jama.2017.13836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecconi, M., Evans, L., Levy, M. & Rhodes, A. Sepsis and septic shock. Lancet392(10141), 75–87. 10.1016/S0140-6736(18)30696-2 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Rhee, C. et al. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw. Open.2(2), e187571. 10.1001/jamanetworkopen.2018.7571 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thirunavukarasu, P., Sanghera, S., Singla, S., Attwood, K. & Nurkin, S. Pre-operative unintentional weight loss as a risk factor for surgical outcomes after elective surgery in patients with disseminated cancer. Int. J. Surg.18, 7–13. 10.1016/j.ijsu.2015.03.021 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Flegal, K. M., Kit, B. K., Orpana, H. & Graubard, B. I. Association of all-cause mortality with overweight and obesity using standard body mass index categories. JAMA309(1), 71–82. 10.1001/jama.2012.113905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Angelantonio, E. et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet388(10046), 776–786. 10.1016/S0140-6736(16)30175-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun, Y. Q. et al. Body mass index and all cause mortality in HUNT and UK Biobank studies: Linear and non-linear mendelian randomisation analyses. BMJ26, 1042. 10.1136/bmj.l1042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scribani, M. et al. Sex-specific associations between body mass index and death before life expectancy: A comparative study from the USA and Sweden. Global Health Action.12(1), 1580973. 10.1080/16549716.2019.1580973 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepper, D. J. et al. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Crit Care.20(1), 181. 10.1186/s13054-016-1360-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang, S. et al. The role of increased body mass index in outcomes of sepsis: A systematic review and meta-analysis. BMC Anesthesiol.17(1), 118. 10.1186/s12871-017-0405-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atamna, A., Elis, A., Gilady, E., Gitter-Azulay, L. & Bishara, J. How obesity impacts outcomes of infectious diseases. Eur. J. Clin. Microbiol. Infect Dis.36(3), 585–591. 10.1007/s10096-016-2835-1 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Keller, K. et al. Survival benefit of obese patients with pulmonary embolism. Mayo Clin. Proc.94(10), 1960–1973. 10.1016/j.mayocp.2019.04.035 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Afzal, S., Tybjærg-Hansen, A., Jensen, G. B. & Nordestgaard, B. G. Change in body mass index associated with lowest mortality in Denmark, 1976–2013. JAMA315(18), 1989. 10.1001/jama.2016.4666 (2016). [DOI] [PubMed] [Google Scholar]
- 20.McAuley, P. A. et al. Exercise capacity and the obesity paradox in heart failure: The FIT (henry ford exercise testing) project. Mayo Clin. Proc.93(6), 701–708. 10.1016/j.mayocp.2018.01.026 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Pandey, A., Patel, K. V. & Lavie, C. J. Obesity, central adiposity, and fitness: Understanding the obesity paradox in the context of other cardiometabolic parameters. Mayo Clin. Proc.93(6), 676–678. 10.1016/j.mayocp.2018.04.015 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Rogne, T. et al. Body mass index and risk of dying from a bloodstream infection: A Mendelian randomization study. PLoS Med.17(11), e1003413. 10.1371/journal.pmed.1003413 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data.10(1), 1. 10.1038/s41597-022-01899-x (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komorowski, M., Celi, L. A., Badawi, O., Gordon, A. C. & Faisal, A. A. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med.24(11), 1716–1720. 10.1038/s41591-018-0213-5 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA.315(8), 801. 10.1001/jama.2016.0287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang, G., Gao, Y., Liu, K. & Wang, X. Serum potassium in elderly heart failure patients as a predictor of readmission within 1 year. Heart Vessels38, 507–516 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Shimizu, R., Nakanishi, N., Ishihara, M., Oto, J. & Kotani, J. Utility of lean body mass equations and body mass index for predicting outcomes in critically ill adults with sepsis: A retrospective study. Diseases12(2), 30. 10.3390/diseases12020030 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson, M. K. et al. The relationship among obesity, nutritional status, and mortality in the critically Ill*. Crit. Care Med.43(1), 87–100. 10.1097/CCM.0000000000000602 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Keller, H. et al. Weight loss and weight gain: multi-level determinants associated with resident 3-month weight change. [DOI] [PubMed]
- 30.Crosby, L. et al. Changes in food and nutrient intake and diet quality on a low-fat vegan diet are associated with changes in body weight, body composition, and insulin sensitivity in overweight adults: A randomized clinical trial. J. Acad. Nutr. Diet.122, 1922-1939.e0 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Röhling, M. et al. Effects of a protein-rich, low-glycaemic meal replacement on changes in dietary intake and body weight following a weight-management intervention—The ACOORH trial. Nutrients13, 376 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zampieri, F. G., Jacob, V., Barbeiro, H. V., Pinheiro Da Silva, F. & Souza, H. P. D. Influence of body mass index on inflammatory profile at admission in critically ill septic patients. Int. J. Inflamm.10.1155/2015/734857 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barreira, T. V. et al. anthropometric correlates of total body fat, abdominal adiposity, and cardiovascular disease risk factors in a biracial sample of men and women. Mayo Clinic Proc.87(5), 452–460. 10.1016/j.mayocp.2011.12.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagawa, M., Kerr, D., Uchida, H. & Binns, C. W. Differences in the relationship between BMI and percentage body fat between Japanese and Australian-Caucasian young men. Br. J. Nutr.95(5), 1002–1007. 10.1079/BJN20061745 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Arita, Y. et al. Paradoxical decrease of an adipose-specific protein, adiponectin in Obesity. Biochem. Biophys. Res. Commun.257(1), 79–83. 10.1006/bbrc.1999.0255 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Oh, T. K. & Song, I. A. The association of body mass index and waist circumference with sepsis-related mortality in South Korea. Diagnostics14(6), 574. 10.3390/diagnostics14060574 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao, L., Liu, J. J., Fan, Q. C., Ling, L. T. & Ding, H. B. Association of obesity and mortality in sepsis patients: A meta-analysis from observational evidence. Heliyon9(9), e19556. 10.1016/j.heliyon.2023.e19556 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, N., Tian, L., Zhou, Q., Miao, Y. & Ma, H. The association between body mass index and mortality in septic older adults. Geriatr. Nurs.54, 199–204. 10.1016/j.gerinurse.2023.10.003 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Lebovitz, S. et al. The relationship between body mass index and in-hospital mortality in bacteremic sepsis. JCM12(11), 3848. 10.3390/jcm12113848 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue, Y. et al. Risk and protective factors related to mortality from pneumonia among middleaged and elderly community residents: The JACC study. J. Epidemiol.17(6), 194–202. 10.2188/jea.17.194 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weng, L. et al. Body-mass index and long-term risk of sepsis-related mortality: a population-based cohort study of 0.5 million Chinese adults. Crit. Care24(1), 534. 10.1186/s13054-020-03229-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the datasets utilized in the current study are freely accessible in the MIMIC-IV v2.2 database (https://mimic.physionet.org/).