Abstract
This study aimed to compare computed tomography (CT) findings between basaloid lung squamous cell carcinoma (SCC) and non-basaloid SCC. From July 2003 to April 2021, 39 patients with surgically proven basaloid SCC were identified. For comparison, 161 patients with surgically proven non-basaloid SCC from June 2018 to January 2019 were selected consecutively. Clinical features, demographic characteristics, and CT findings were compared using chi-square test or Fisher’s exact test except for differences in means for which Student’s t-test was used. Additionally, Mantel-Haenszel test was performed to control the confounding of the presence of cavitation between basaloid and non-basaloid SCCs with tumors stratified by clinical T staging. Compared with patients with non-basaloid SCC, patients with basaloid SCC had significantly (p < 0.001) more frequent respiratory symptoms at the time of presentation. Regarding CT findings, endobronchial tumor growth and obstructive pneumonia or atelectasis were significantly (p = 0.028 and p = 0.028, respectively) more common in basaloid SCC than in non-basaloid SCC. Compared with non-basaloid SCC, cavitation was absent (p = 0.005) and internal profuse necrosis was significantly (p = 0.022) less frequent in basaloid SCC. Furthermore, presence of cavitation consistently showed significant difference after the tumors stratified based on clinical T staging (p = 0.015). Basaloid SCC had some CT findings different from non-basaloid SCC. Basaloid SCC showed more frequent endobronchial tumor growth with obstructive pneumonia or atelectasis. Internal profuse necrosis was less common, and cavitation was absent in basaloid SCC compared to non-basaloid SCC.
Keywords: Lung neoplasms, Squamous cell carcinoma, Computed tomography
Subject terms: Lung cancer, Non-small-cell lung cancer
Introduction
In 1992, Brambrilla et al.1reported basaloid carcinoma as a distinct form of lung cancer with unique histopathologic features and poor prognosis. Distinguishing histopathologic features were small cells with prominent nucleoli, scanty cytoplasm, high mitotic rate, and a lobular growth pattern with peripheral palisading1. Previously in 2004 World Health Organization (WHO) classification of tumors of the lungs, basaloid carcinoma was classified as a variant of large cell carcinoma in its pure form or as a variant of squamous cell carcinoma (SCC) when areas of squamous differentiation were present2,3. However, immunohistochemical expression of squamous markers was found in the basaloid carcinoma4–6. As a result, in the 2015 WHO classification of tumors of the lungs, basaloid SCC of the lung was re-categorized as a subtype of SCC6. This categorization was maintained in the latest 2021 WHO classification of tumors of the lungs7. Currently, SCC, not otherwise specified (NOS) of the lung consists of three subtypes: keratinizing, nonkeratinizing, and basaloid.
Basaloid SCC is a rare subtype of SCCs, with reported prevalence of 3.9 to 5.2% among all lung squamous carcinomas8. Whether basaloid SCC has worse prognosis than non-basaloid SCC remains controversial. In 2008, Moro-Sibilot et al.3 compared clinical features and survival of 90 patients with lung carcinoma having a basaloid pattern and 1,328 patients other non-small cell lung carcinomas (NSCLCs) and found that basaloid pattern could indicate a poor prognosis in NSCLC, especially in stage I and II patients. In contrast, Kim et al.9 reported that those with basaloid SCC did not have a worse prognosis than those with other NSCLCs or require a different treatment modality. In both studies by Moro-Sibilot et al.3 and Kim et al.9, the fifth edition of American Joint Committee on Cancer(AJCC) tumor, node and metastasis (TNM) staging system was used. Another study on clinical features of 22 basaloid SCCs by Wang et al.10, in which the seventh edition of AJCC TNM staging system was used, demonstrated that basaloid SCC had similar clinical features without showing a significant difference in survival rate compared to poorly differentiated SCC. Furthermore, a recent population-based analysis by Yuan et al.8, using the eighth edition of AJCC TNM staging system, reported that basaloid SCC had significantly better survival than non-basaloid SCC, large cell carcinoma, and lung adenocarcinoma. Thus, clinical characteristics and prognosis of basaloid SCCs remain unclear.
Nevertheless, basaloid SCC holds unique status among lung cancers. It has unique clinical and prognostic features different from non-basaloid SCC, large cell carcinoma, and lung adenocarcinoma8. Furthermore, a molecular study has revealed that basaloid SCC has different mRNA expression profile from non-basaloid SCC, constituting a distinct molecular pathobiology5,11. This characteristic molecular profile may contribute to its intrinsic resistance to cytotoxic chemotherapy and help us develop targeted therapies.
Several studies have reported prognosis and clinicopathological findings of basaloid SCC1,3,5,8–10,12. However, imaging findings on basaloid SCC of the lung remain largely unreported. In 2020, one of our authors reported CT findings of two cases of basaloid SCC, where tumors appeared as well-defined round, lobulated lesion with prominent intratumor necrosis, with one case showing cavitation13. Previously, Kim et al.14 reported CT findings of basaloid SCC in 12 patients where tumors presented as solitary nodule or mass without internal profuse necrosis or cavitation. However, studies comparing CT features of basaloid and non-basaloid SCC have not been reported yet. Therefore, the objective of this study was to compare CT findings of basaloid and non-basaloid SCCs.
Results
Baseline clinical characteristics
There was no statistically significant difference in baseline clinical characteristics except for the presence of respiratory symptoms (cough, sputum, hemoptysis, dyspnea, and chest pain or discomfort) between patients with basaloid SCCs and those with non-basaloid SCCs (Table 1). Patients with basaloid SCCs were more frequently symptomatic at the time of presentation than patients with non-basaloid SCCs (56.4% vs. 27.3%, p < 0.001).
Table 1.
Baseline clinical characteristics of patients with basaloid and non-basaloid SCCs.
| Characteristic | Basaloid (n = 39) | Non-basaloid (n = 161) | p value |
|---|---|---|---|
| Age (years)a | 66.38 ± 9.357 | 66.3 ± 8.828 | 0.957 |
| Sex (M: F) | 37:2 | 148:13 | 0.74 |
| Smoking history | 0.633 | ||
| Current smoker | 13 (33.3) | 53 (32.9) | |
| Ex-smoker | 24 (61.5) | 92 (57.1) | |
| Never smoker | 2 (5.1) | 16 (9.9) | |
| Smoking duration (pack-years)a | 37.077 ± 16.6388 | 36.292 ± 23.9652 | 0.811 |
| Symptomsb | 0.001 | ||
| Yes | 22 (56.4) | 44 (27.3) | |
| No | 17 (43.6) | 117 (72.7) |
Unless otherwise indicated, data are given as number of patients with percentages in parentheses. aData are given as mean ± standard deviation. bIncluded symptoms are cough, sputum, hemoptysis, dyspnea, and chest pain or discomfort. A p value < 0.05 was considered to be significant.
Clinical T and N staging
There was no statistically significant difference in clinical T or N staging between patients with basaloid SCCs and those with non-basaloid SCCs (Table 2).
Table 2.
Clinical T and N staging of patients with basaloid and non-basaloid SCCs.
| Staging | Basaloid (n = 39) | Non-basaloid (n = 161) | p value |
|---|---|---|---|
| cT stage | 0.418 | ||
| T1a | 1 (2.6) | 1 (0.6) | |
| T1b | 8 (20.5) | 20 (12.4) | |
| T1c | 5 (12.8) | 31 (19.3) | |
| T2a | 12 (30.8) | 44 (27.3) | |
| T2b | 5 (12.8) | 15 (9.3) | |
| T3 | 4 (10.3) | 35 (21.7) | |
| T4 | 4 (10.3) | 15 (9.3) | |
| cN stage | 0.818 | ||
| N0 | 30 (76.9) | 123 (76.4) | |
| N1 | 6 (15.4) | 22 (13.7) | |
| N2 | 2 (5.1) | 4 (2.5) | |
| N3 | 1 (2.6) | 2 (1.2) |
Data are given as number of patients with percentages in parentheses. A p value < 0.05 was considered to be significant.
CT features
Initial CT features of patients with basaloid and non-basaloid SCCs are summarized in Table 3. Mean tumor size, mean attenuation value in unenhanced and contrast-enhanced CT, mean net enhancement, tumor margin, tumor shape, tumor distribution, presence of calcification, pleural tagging, fissural or pleural retraction, pleural effusion, pericardial effusion, and underlying emphysema were not statistically significant between those with basaloid SCCs and those with non-basaloid SCCs. Endobronchial tumor growth was more frequent in basaloid SCCs than in non-basaloid SCCs (46.2% vs. 28.0%, p = 0.028). Obstructive pneumonia or atelectasis was more common in basaloid SCCs than in non-basaloid SCCs (41.0% vs. 23.6%, p = 0.028) (Fig. 1). Cavitation was not observed in any basaloid SCCs whereas 15.5% of non-basaloid SCCs showed cavitation (p = 0.005) (Fig. 2). Internal profuse necrosis was less frequently observed in basaloid SCCs than in non-basaloid SCCs (10.3% vs. 28.6%, p = 0.022). Air bronchogram was more frequently observed in basaloid SCCs than in non-basaloid SCCs (17.9% vs. 5.6%, p = 0.011).
Table 3.
CT characteristics of patients with basaloid and non-basaloid SCCs.
| Characteristic | Basaloid (n = 39) | Non-basaloid (n = 161) | p value |
|---|---|---|---|
| Tumor size (mm)a | 34.987 ± 18.1393 | 38.018 ± 18.1098 | 0.350 |
| Pre-contrast CT value (HU)a | 29.194 ± 12.2769 | 26.771 ± 11.9599 | 0.282 |
| Post-contrast CT value (HU)a | 59.308 ± 20.1206 | 57.067 ± 19.6877 | 0.526 |
| Net enhancement value (HU)a | 29.702 ± 17.0234 | 29.850 ± 17.1349 | 0.963 |
| Margin | 0.739 | ||
| Smooth | 0 (0) | 2 (1.2) | |
| Spiculated/Lobulated | 36 (92.3) | 144 (89.4) | |
| Not assessable | 3 (7.7) | 15 (9.3) | |
| Shape | 0.839 | ||
| Round | 0 (0) | 1 (0.6) | |
| Lobular/Irregular | 36 (92.3) | 145 (90.1) | |
| Not assessable | 3 (7.7) | 15 (9.3) | |
| Distribution | 0.256 | ||
| Central | 25 (64.1) | 87 (54.0) | |
| Peripheral | 14 (35.9) | 74 (46.0) | |
| Endobronchial tumor growth | 0.028 | ||
| No | 21 (53.8) | 116 (72.0) | |
| Yes | 18 (46.2) | 45 (28.0) | |
| Cavitation | 0.005 | ||
| No | 39 (100) | 136 (84.5) | |
| Yes | 0 (0) | 25 (15.5) | |
| Internal profuse necrosis | 0.022 | ||
| No | 35 (89.7) | 115 (71.4) | |
| Yes | 4 (10.3) | 46 (28.6) | |
| Calcification | 0.058 | ||
| No | 33 (84.6) | 151 (93.8) | |
| Yes | 6 (15.4) | 10 (6.2) | |
| Air bronchogram | 0.011 | ||
| No | 32 (82.1) | 152 (94.4) | |
| Yes | 7 (17.9) | 9 (5.6) | |
| Pleural tagging | 0.668 | ||
| No | 26 (66.7) | 113 (70.2) | |
| Yes | 13 (33.3) | 48 (29.8) | |
| Fissural / Pleural retraction | 0.907 | ||
| No | 33 (84.6) | 135 (8.9) | |
| Yes | 6 (15.4) | 26 (16.1) | |
| Pleural Effusion | 0.098 | ||
| No | 37 (94.9) | 160 (99.4) | |
| Yes | 2 (5.1) | 1 (0.6) | |
| Pericardial effusion | |||
| No | 39 (100) | 161 (100) | |
| Yes | 0 (0) | 0 (0) | |
| Obstructive pneumonia or atelectasis | 0.028 | ||
| No | 23 (59.0) | 123 (76.4) | |
| Yes | 16 (41.0) | 38 (23.6) | |
| Emphysema | 0.208 | ||
| No | 24 (61.5) | 81 (50.3) | |
| Yes | 15 (38.5) | 80 (49.7) | |
Unless otherwise indicated, data are given as number of patients with percentages in parentheses.
a Data are given as mean ± standard deviation. A p value < 0.05 was considered to be significant.
Fig. 1.
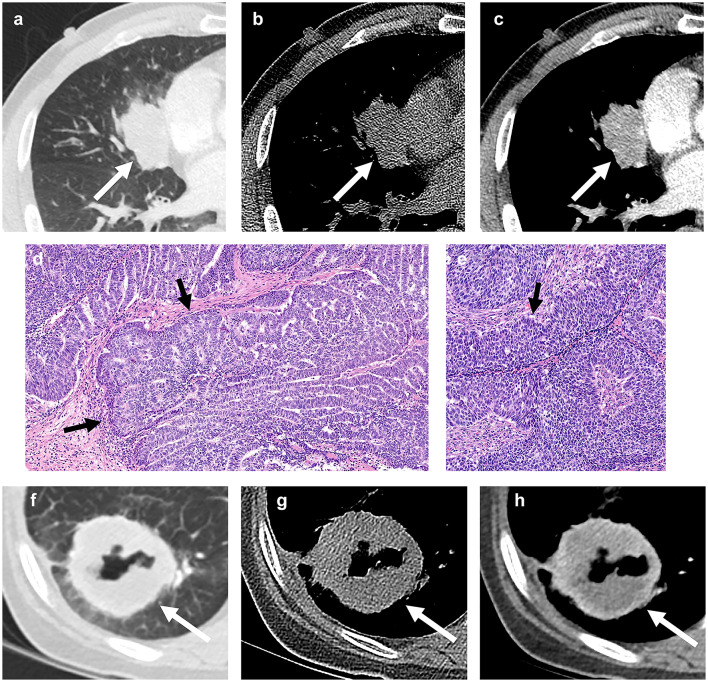
A 74-year-old man with basaloid squamous cell carcinoma of the lung. (a) A lung window setting of computed tomography (CT) scan showing mass obstructing right upper lobar bronchus (arrow) causing distal atelectasis. (b) and (c) Unenhanced (b) and enhanced (c) scans showing moderate enhancement of the mass (arrow). (d) and (e) Photomicrograph images obtained at medium power magnification (haematoxylin and eosin stain) showing nests of tumor cells with peripheral palisading (arrow).
Fig. 2.
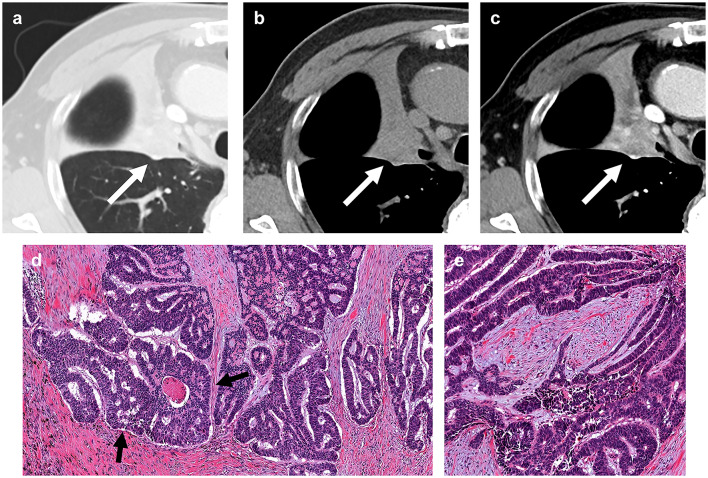
A 60-year-old man with basaloid squamous cell carcinoma of lung. (a) A lung window setting computed tomography (CT) scan showing a lobulated mass (arrow) in right middle lobe. (b) and (c) Unenhanced (b) and enhanced (c) scans showing mild enhancement of the mass (arrows). (d) and (e) Photomicrograph images obtained at medium power magnification (haematoxylin and eosin stain) showing nests of tumor cells with peripheral palisading (arrow). A 67-year-old woman with non-basaloid squamous cell carcinoma of the lung. (f) A lung window setting CT showing a mass with cavitation (arrow) in the right lower lobe. (g) and (h) Unenhanced (g) and enhanced CT (h) showing cavitation and internal profuse necrosis.
Considering that tumor size can be associated with cavitation in SCC, presence of cavitation in patients with basaloid or non-basaloid SCCs was stratified by clinical T staging (Table 4)15. Afterwards, Mantel-Haenszel test of conditional independence was performed, yielding a p-value of 0.015 (Table 5). Thus, there was a significant difference in presence of cavitation between basaloid and non-basaloid SCCs regardless of the tumor size.
Table 4.
Presence of cavitation in patients with basaloid and non-basaloid SCCs, stratified by clinical T staging.
| cT stage | Cavitation | Basaloid | Non-basaloid | Total |
|---|---|---|---|---|
| T1a | No | 1 | 1 | 2 |
| Yes | 0 | 0 | 0 | |
| Total | 1 | 1 | 2 | |
| T1b | No | 8 | 18 | 26 |
| Yes | 0 | 2 | 2 | |
| Total | 8 | 20 | 28 | |
| T1c | No | 5 | 25 | 30 |
| Yes | 0 | 6 | 6 | |
| Total | 5 | 31 | 36 | |
| T2a | No | 12 | 40 | 52 |
| Yes | 0 | 4 | 4 | |
| Total | 12 | 44 | 56 | |
| T2b | No | 5 | 9 | 14 |
| Yes | 0 | 6 | 6 | |
| Total | 5 | 15 | 20 | |
| T3 | No | 4 | 32 | 36 |
| Yes | 0 | 3 | 3 | |
| Total | 4 | 35 | 39 | |
| T4 | No | 4 | 11 | 15 |
| Yes | 0 | 4 | 4 | |
| Total | 4 | 15 | 19 | |
| Total | No | 39 | 136 | 175 |
| Yes | 0 | 25 | 25 | |
| Total | 30 | 161 | 200 |
Data are given as number of patients.
Table 5.
Mantel-Haenszel test of conditional independence for presence of cavitation in patients with basaloid and non-basaloid SCCs, stratified by clinical T staging.
| Chi-Squared | Degree of freedom | p value | |
|---|---|---|---|
| Mantel-Haenszel | 5.917 | 1 | 0.015 |
Additionally, considering prior report suggesting that SCCs with cavitation showed more frequent peripheral location, presence of cavitation in patients with basaloid or non-basaloid SCCs was stratified by tumor distribution (Table 6)15. Subsequently, Mantel-Haenszel test of conditional independence was conducted, yielding a p-value of 0.026 (Table 7). Thus, there was a significant difference in presence of cavitation between basaloid and non-basaloid SCCs, independent of tumor distribution.
Table 6.
Presence of cavitation in patients with basaloid and non-basaloid SCCs, stratified by tumor distribution.
| Distribution | Cavitation | Basaloid | Non-basaloid | Total |
|---|---|---|---|---|
| Central | No | 25 | 82 | 107 |
| Yes | 0 | 5 | 5 | |
| Total | 25 | 87 | 112 | |
| Peripheral | No | 14 | 54 | 68 |
| Yes | 0 | 20 | 20 | |
| Total | 14 | 74 | 88 | |
| Total | No | 39 | 136 | 175 |
| Yes | 0 | 25 | 25 | |
| Total | 39 | 161 | 200 |
Data are given as number of patients.
Table 7.
Mantel-Haenszel test of conditional independence for presence of cavitation in patients with basaloid and non-basaloid SCCs, stratified by tumor distribution.
| Chi-Squared | Degree of freedom | p value | |
|---|---|---|---|
| Mantel-Haenszel | 4.928 | 1 | 0.026 |
Discussion
Basaloid SCC holds distinct status among SCCs. It shows unique histopathologic and clinical features. However, little is known concerning its radiologic features. In previous study by Kim et al.14on CT findings of basaloid SCCs in 12 patients, cavitation was absent in all 12 cases. This could be a unique radiologic feature of basaloid SCC considering that cavitation is found in approximately 10 to 20% of lung cancers and is more common in SCC16–19. For further investigation, we conducted a comparison study with larger sample size. To our knowledge, this is the first study in English literature to compare CT findings of basaloid SCCs and non-basaloid SCCs of the lung.
In our study, cavitation was seen in 15.5% of non-basaloid SCC, which corresponded to known prevalence of cavitation in lung cancers. However, none of the basaloid SCCs showed cavitation, in concordance with our previous study14. Furthermore, in our study, basaloid SCCs showed less frequent internal profuse necrosis on CT than non-basaloid SCCs.
Cavitation in primary lung cancer is usually caused by drainage of the necrotic substance through the bronchial tree20. There are several proposed mechanisms of tumor necrosis. The most commonly accepted theory is that bronchial obstruction and vessel invasion of the tumor can cause ischemia, which results in necrosis of the tumor. When tumor cells invade the vessel wall, they gain access to perivascular spaces. Tumor cells can then spread interstitially or along alveolar spaces through pores of Kohn. Consequently, pulmonary architecture within the tumor is destroyed and central avascular necrosis occurs15,16,19. Histopathologically, basaloid SCC is characterized by small cells showing lobular growth pattern with peripheral palisading1. Basaloid SCC may show centrilobular foci of necrosis under microscopic examination1,12,21. However, we believe that the histopathologic characteristic of basaloid SCC makes it unlikely for basaloid SCC to show prominent necrosis that is readily evident on CT. Our theory is that palisading by radially arranged cells at the periphery of lobules could act as a physical barrier that prevents vascular infiltration of the tumor. Thus, it would be less likely for large area of necrosis to develop in basaloid SCC. Another histological characteristic of basaloid SCC is its growth from the bronchial lining in an invasive finger-like fashion1. We postulate that this could lead to gradual narrowing rather than abrupt obstruction of the bronchi, making basaloid SCC less vulnerable to ischemia and necrosis. Consequently, we believe that such histopathologic characteristics would have led to absence of cavitation in basaloid SCC in our study.
In concordance with our study, majority of basaloid SCCs in previous literatures presented as nodules or masses without cavitation3,14,22–24. Yet, some exceptions exist. In 2012, Yamada et al.25reported an unusual case of basaloid SCC with central cavitation. In their study, the tumor showed no evidence of vascular invasion or demonstrable foci of comedo-type tumor necrosis. Instead, coagulative necrosis of pre-existing alveolar wall was found at the cancer-cavity junction. Yamada et al. explained this histopathological finding using unidirectional check-valve mechanism. They then proposed a mechanism of thin-walled cavity formation in lung adenocarcinoma and SCC. According to their theory, tumor growth in the bronchiolar wall might have acted as a unidirectional check-valve, causing air trapping and subsequent rupture of the alveoli fused into a cavity25–27. In 2020, Kim et al.13 reported CT findings of two cases of basaloid SCC, one of which presented a well-defined nodule with cavitation located at periphery of left upper lobe. Upon pathologic examination, the tumor did not show lymphovascular or perineural invasion. We believe that the same theory of cavity formation by unidirectional check-valve mechanism can be applied to this case.
Another notable CT feature of basaloid SCC was that endobronchial tumor growth was more frequently observed in basaloid SCC than in non-basaloid SCC. We believe this might have led to more frequent obstructive pneumonia or atelectasis in patients with basaloid SCC as well as more frequent respiratory symptoms at the time of presentation.
By far, this is the first study on radiologic findings of basaloid SCC to compare differences between CT features of basaloid SCCs and non-basaloid SCCs. However, this study has several limitations. First, our study had a retrospective design. In addition, the two patient groups were chosen from different time periods. Patients with basaloid SCCs were selected from July 2003 to April 2021, whereas patients with non-basaloid SCCs were selected from June 2018 to January 2019. Due to this heterogeneity in time period, comparison of patient prognosis and survival was inadequate. Second, our study was a single-center study with a small sample size of basaloid SCC owing to the rarity of this subtype among SCCs of the lung. To validate our results, further studies with larger sample size and multi-center comparison would be necessary. Lastly, while we have proposed a hypothesis regarding the absence of cavitation in basaloid SCCs, this could not be histopathologically confirmed, as our study primarily focused on the CT image findings. Further studies with more detailed analysis of histopathologic features would be necessary to validate this hypothesis.
Conclusion
In conclusion, basaloid SCCs of lung showed several distinct CT findings compared to non-basaloid SCCs of lung. Basaloid SCCs showed more frequent endobronchial tumor growth and obstructive pneumonia or atelectasis on CT. Furthermore, basaloid SCCs presented with less frequent internal profuse necrosis without cavitation in any tumors.
Materials and methods
Patient selection
The institutional review board (IRB) of Samsung Medical Center approved this retrospective study, and the requirement for patient informed consent to use clinical data was waived (IRB file number, 2021-05-062). The study was conducted under the principles of the Declaration of Helsinki. We searched medical records from July 2003 to April 2021 and found 1,350 patients who received lobectomy or pneumonectomy of lung for SCCs at BLINDED, a tertiary referral hospital located in Seoul, South Korea. We used “basaloid feature” or “basaloid squamous cell carcinoma” to search for papers in all electronic medical records and found 39 (0.7%) out of 1350 patients (from July 2003 to April 2021), including 12 patients with basaloid SCC used in previous study by Kim et al.14. All samples were reviewed according to the latest WHO classification by a pulmonary pathologist. They were confirmed to have basaloid SCC. From the same group of patients with SCCs, 161 patients without “basaloid feature” or “basaloid squamous cell carcinoma” were selected consecutively from June 2018 to January 2019.
Clinical and pathological data
Clinical data of patients were reviewed retrospectively for the following variables: age, sex, smoking history, and the presence of respiratory symptoms at the time of presentation. Included symptoms were cough, sputum, hemoptysis, dyspnea, and chest pain or discomfort. Histopathologic reports were recorded for all 200 patients. Resected tumors were reported using the latest edition of WHO classification available at the time of surgery. A pathologist with experience in lung cancer (BLINDED) reviewed all the slides to confirm the diagnosis.
Image acquisition
Initial CT images obtained prior to any treatment or biopsy were used for the analysis. CT studies were performed using various helical CT scanners (mostly 16- to 64- multidetector row CT scanners) from several vendors. Scanning parameters were: 120 kVp and 170–200 mA under automatic exposure control, a beam width of 10–20 mm, and rotation time of 0.3–0.4 s. Image data were reconstructed using standard soft-tissue algorithms. Data were reformatted with a section thickness of 1–2.5 mm for transverse images. Unenhanced and enhanced CT scans were both obtained. Intravenous contrast medium was administered to all patients. The interval between initiation of contrast injection and scanning was 45 s. Reconstructed images were then sent directly to a Picture Archiving and Communicating System (PACS; GE Healthcare Integrated Imaging Solution).
Image analysis
All CT images were interpreted on a 2000 × 2000 PACS monitor in both mediastinal (width, 400 HU; level, 20 HU) and lung (width, 1,500 HU; level − 700 HU) window settings. CT findings were analyzed by three radiologists with 2, 5, and 15 years of experience, respectively (BLINDED). Decisions were reached with consensus. The following findings were evaluated: tumor size, attenuation value (in Hounsfield units) in unenhanced and contrast-enhanced CT, net enhancement (attenuation difference between contrast-enhanced and unenhanced images), tumor margin, tumor shape, tumor distribution, and presence of endobronchial tumor growth, cavitation, internal profuse necrosis, calcification, air-bronchogram, pleural tagging, fissural or pleural retraction, pleural effusion, pericardial effusion, obstructive pneumonia or atelectasis, and underlying emphysema. Tumor size was determined as the largest diameter on transverse image. Attenuation of the tumor was measured by region of interest covering more than half of the tumor diameter. Tumor distribution was determined by dividing the lung into inner and outer regions at the midline on CT images. Tumors with center located medial to the midline were considered central. Those with center located lateral to the midline were considered peripheral. All tumors were staged based on the eighth edition of the AJCC TNM staging system for NSCLC published in 201728.
Statistical analysis
Clinicopatholologic and CT features of patients with basaloid and non-basaloid SCCs were compared using the chi-square test or Fisher’s exact test except for differences in means, for which Student’s t-test was used. Mantel-Haenszel test was performed to compare the presence of cavitation between basaloid and non-basaloid squamous cell carcinomas with tumors stratified by clinical T staging and tumor distribution, respectively. A p-value of less than 0.05 was considered significant.
Abbreviations
- CT
Computed tomography
- SCC
Squamous cell carcinoma
- NSCLC
Non-small cell lung carcinoma
- WHO
World Health Organization
Author contributions
Y.S. contributed to data curation, formal analysis, investigation and writing of the original draft. Y.K.C. contributed to conceptualization, data curation, formal analysis, investigation, methodology, project administration, and writing of the original draft. C.H.K. contributed to data curation, investigation. C.H.K, M.J.C, T.J.K., and H.J.Y. edited the manuscript. J.H. and J.H.L. provided resources for the research. All authors reviewed the manuscript.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brambilla, E. et al. Basal cell (basaloid) carcinoma of the lung: a new morphologic and phenotypic entity with separate prognostic significance. Hum. Pathol.23, 993–1003. 10.1016/0046-8177(92)90260-a (1992). [DOI] [PubMed] [Google Scholar]
- 2.Beasley, M. B., Brambilla, E. & Travis, W. D. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol.40, 90–97. 10.1053/j.ro.2005.01.001 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Moro-Sibilot, D. et al. Lung carcinomas with a basaloid pattern: a study of 90 cases focusing on their poor prognosis. Eur. Respir J.31, 854–859. 10.1183/09031936.00058507 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Sturm, N. et al. Thyroid transcription factor 1 and cytokeratins 1, 5, 10, 14 (34betaE12) expression in basaloid and large-cell neuroendocrine carcinomas of the lung. Hum. Pathol.32, 918–925. 10.1053/hupa.2001.27110 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Brambilla, C. et al. Lung squamous cell carcinomas with basaloid histology represent a specific molecular entity. Clin. Cancer Res.20, 5777–5786. 10.1158/1078-0432.CCR-14-0459 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Travis, W. D. et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol.10, 1243–1260. 10.1097/JTO.0000000000000630 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Nicholson, A. G. et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J. Thorac. Oncol.17, 362–387. 10.1016/j.jtho.2021.11.003 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Yuan, G. et al. Clinical characteristics and prognosis of basaloid squamous cell carcinoma of the lung: a population-based analysis. PeerJ7, e6724. 10.7717/peerj.6724 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, D. J., Kim, K. D., Shin, D. H., Ro, J. Y. & Chung, K. Y. Basaloid carcinoma of the lung: a really dismal histologic variant? Ann. Thorac. Surg.76, 1833–1837. 10.1016/s0003-4975(03)01296-7 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Wang, L. C. et al. Analysis on the clinical features of 22 basaloid squamous cell carcinoma of the lung. J. Cardiothorac. Surg.6, 10. 10.1186/1749-8090-6-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyhanian, K. et al. Neuroendocrine differentiation distinguishes basaloid variant of lung squamous cell carcinoma. Diagn. Pathol.17, 46. 10.1186/s13000-022-01223-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moro, D. et al. Basaloid bronchial carcinoma. A histologic group with a poor prognosis. Cancer73, 2734–2739. https://doi.org/10.1002/1097-0142(19940601)73:11<2734::aid-cncr2820731114>3.0.co;2-4 (1994). [DOI] [PubMed]
- 13.Kim, J. H., Yoon, H. J., Lee, E. & Kim, E. J. Basaloid Squamous Cell Carcinoma of the lung: two case reports with CT imaging findings. Taehan Yongsang Uihakhoe Chi. 81, 746–752. 10.3348/jksr.2020.81.3.746 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, C. H. et al. CT findings of basaloid squamous cell carcinoma of the lung in 12 patients: a distinct category of squamous cell carcinoma in 2015 WHO classification of lung tumors. Med. (Baltim).101, e29197. 10.1097/MD.0000000000029197 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolodziejski, L. S. et al. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma50, 66–73 (2003). [PubMed] [Google Scholar]
- 16.Chaudhuri, M. R. Primary pulmonary cavitating carcinomas. Thorax28, 354–366. 10.1136/thx.28.3.354 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouroux, J., Padovani, B., Elkaim, D. & Richelme, H. Should cavitated bronchopulmonary cancers be considered a separate entity? Ann. Thorac. Surg.61, 530–532. 10.1016/0003-4975(95)00973-6 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Theros, E. G. Caldwell Lecture: varying manifestation of peripheral pulmonary neoplasms: a radiologic-pathologic correlative study. AJR Am J Roentgenol 128, 893–914. (1976). 10.2214/ajr.128.6.893 (1977). [DOI] [PubMed]
- 19.Onn, A. et al. Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome. Radiology237, 342–347. 10.1148/radiol.2371041650 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Hansell, D. M. et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology246, 697–722. 10.1148/radiol.2462070712 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Travis, W. D., Colby, T. V., Corrin, B., Shimosato, Y. & Brambilla, E. Histological Typing of Lung and Pleural Tumours. International Histological Classification of Tumours 3rd edn (Springer Science & Business Media, 2012). [Google Scholar]
- 22.Ito, K. et al. [Basaloid squamous cell carcinoma;report of a case]. Kyobu Geka. 72, 641–643 (2019). [PubMed] [Google Scholar]
- 23.Abugroun, A., Ahmed, F. & Espina, T. D. Altamirano Ufion, A. Basaloid squamous cell carcinoma of the lung Associated with syndrome of inappropriate antidiuretic hormone secretion. World J. Oncol.8, 188–190. 10.14740/wjon1065w (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagakawa, H. et al. Basaloid squamous-cell carcinoma of the lung in a young woman. Int. J. Clin. Oncol.11, 66–68. 10.1007/s10147-005-0533-6 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Yamada, S. et al. Basaloid carcinoma of the lung associated with central cavitation: a unique surgical case focusing on cytological and immunohistochemical findings. Diagn. Pathol.7, 175. 10.1186/1746-1596-7-175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue, X. et al. Comparative study of solitary thin-walled cavity lung cancer with computed tomography and pathological findings. Lung Cancer. 78, 45–50. 10.1016/j.lungcan.2012.06.004 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Kunihiro, Y. et al. High-resolution CT findings of primary lung cancer with cavitation: a comparison between adenocarcinoma and squamous cell carcinoma. Clin. Radiol.71, 1126–1131. 10.1016/j.crad.2016.06.110 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Amin, M. B. et al. AJCC Cancer Staging Manual 8th edn (Springer International Publishing, 2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.