Abstract
This study aimed to identify imaging risk factors for spinal cord injury without radiologic abnormalities (SCIWORA) in children. We retrospectively analyzed the medical records and magnetic resonance imaging (MRI) findings of children with SCIWORA admitted to our hospital between January 1, 2012, and September 30, 2022. Univariate and binary logistic regression analyses were used to evaluate the prognostic impact of various factors including MRI type, maximum cross-sectional area of spinal cord injury, injury length, injury signal intensity ratio. The American Spinal Injury Association (ASIA) impairment scale (AIS) was used to assess neurological improvement of spinal cord injury. A total of 39 patients met the inclusion criteria, comprising three males and 36 females aged 6.38 ± 1.7 years. The injuries were predominantly dance-related (25 patients), followed by car accidents (4 patients), and falls/sprains (10 patients). Seventeen patients showed improvement in the AIS grade, while 22 did not. Binary logistic regression analysis revealed that the maximum cross-sectional area and length of the damaged segment were significant prognostic factors. Receiver operating characteristic curve analysis demonstrated an area under the curve (AUC) of 0.91, with a maximum cutoff value, sensitivity, and specificity of 0.12, 81.80%, and 88.20%, respectively, for the maximum damage cross-sectional area. For the damaged segment length, the AUC, maximum cutoff value, sensitivity, and specificity were 0.78, 6.50, 50%, and 41%, respectively. Whole-spine MRI plays a crucial role in the diagnosis and prognosis of pediatric patients with SCIWORA. The cross-sectional area and length of spinal cord injury are risk factors for poor prognosis.
Level of evidence: IV
Subject terms: Risk factors, Paediatric research, Diseases, Trauma
Introduction
Spinal cord injury without radiologic abnormalities (SCIWORA) was first described by Pang and Wilberger1. It refers to a spinal cord injury in which the clinical symptoms of spinal cord injury are present despite the absence of fracture or dislocation on radiography, CT, or other imaging examinations. SCIWORA primarily affects the cervical spine, resulting in cord contusion and axonal injury. SCIWORA can occur in children and adults but is more common in children than in adults because of the congenital soft tissue elasticity of the spinal cord and the immature vertebral body. The mechanism of injury in SCIWORA is related to hyperextension and flexion, which results in transient vertebral dislocation or distraction. This condition commonly occurs in children less than 8 years of age as a result of increased laxity and elasticity of the developing spine. Jianmin reported that dancing and taekwondo are risk factors associated with SCIWORA in Chinese children2. Ren et al.3 found a correlation between thoracic SCIWORA in children and back flexion in dance practice. Domestic scholars have summarized the imaging characteristics and pathogenesis of spinal cord injury without fracture and dislocation or explored the relationship between clinical drug therapy, operation timing, rehabilitation training, and prognosis, but have not analyzed the correlation between imaging findings and prognosis2–5. This study aimed to analyze the role and potential factors of spinal/spinal cord magnetic resonance Imaging (MRI) in the diagnosis and prognosis of SCIWORA.
Methods
Patient population
A total of 39 patients with SCIWORA were admitted to our hospital between January 1, 2012, and June 30, 2022. These patients included 36 females and three males, aged 6.38 ± 1.7 years. Medical records and MRI results of these patients were retrospectively analyzed. The inclusion criterion was patients with SCIWORA, with complete imaging data including spinal/spinal cord magnetic resonance imaging (MRI) results. Patients with spinal fractures, spinal cord tumors, tuberculosis, infectious diseases, incomplete medical records, or lost to follow-up were excluded. Clinical data included the MRI type, maximum cross-sectional area of the spinal cord injury, injury length, and injury signal intensity ratio. This study was approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University (Approved Number is 2024-Yan-131). The informed consent of patients’ guardians was obtained. All methods were performed in accordance with in accordance with the Declaration of Helsinki. Clinical data approval for publication was granted by patients’ guardians and data was anonymized.
MRI examination
MRI examinations were performed using a SIGNA 3.0T (General Electric, Boston, MA, USA) superconducting MR Imaging system with a whole spinal coil. Conventional transverse- and sagittal-plane scans were performed. Specific scanning sequences and parameters for cross-sectional (T1) and sagittal plane (T2) imaging are detailed below:
Cross-sectional (T1) scanning parameters: Sequence: FSE T1WI; TR: 648 ms; TE: Minimum Full; Slice Thickness: 6.5 mm; Slice Gap: 1 mm; Field of View: 20 × 20 cm; Matrix: 256 × 200; Number of Excitations: 1;
Cross-sectional (T2) scanning parameters: Sequence: FSE T2WI; TR: 5443 ms TE: 85 ms; Slice Thickness: 6.5 mm; Slice Gap: 1 mm; Field of View: 20 × 20 cm Matrix: 256 × 200; Number of Excitations: 1.5;
Sagittal plane (T1) scanning parameters: Sequence: FSE T1WI; TR: 665 ms; TE: Minimum Full; Slice Thickness: 3 mm; Slice Gap: 0.3 mm; Field of View: 24 × 24 cm; Matrix: 320 × 224; Number of Excitations: 1.5;
Sagittal plane (T2) scanning parameters: Sequence: FSE T2WI; TR: 2800 ms; TE: 85 ms; Slice Thickness: 3 mm; Slice Gap: 0.3 mm; Field of View: 24 × 24 cm; Matrix: 320 × 224; Number of Excitations: 1
Standardized MRI protocols were used to ensure consistent image quality and facilitate accurate diagnosis and prognostic evaluation in patients with SCIWORA.
Outcome evaluation
The clinical manifestations observed in these patients included reduced muscle strength in both lower limbs to varying degrees, accompanied by urinary and bowel incontinence.
The American Spinal Injury Association (ASIA) impairment scale (AIS) was used to assess neurological improvement6, referencing the International Standards for Neurological Classification of Spinal Cord Injury7,8. Patients who showed an improvement of at least one AIS grade during the 6-month follow-up period after trauma were categorized into the improvement group, while those with no improvement were included in the non-improvement group.
Statistical analysis
Statistical analyses were performed using SPSS software (Version 26.0, IBM, USA) for univariate and binary multifactor logistic regression analyses. Statistical significance was set at P < 0.05.
Results
The patient population comprised 3 males and 36 females, indicating a significant female predominance (36/39; 92.31%). Four patients were 1–3 years old, 11 patients were 4–6 years old, and 24 patients were 7–9 years old.
Injuries in 25 patients were attributed to dance-related accidents, four patients were in car accidents, and 10 had falls and sprains. The thoracic vertebra was the most frequently injured site, affecting 36/39 (92.31%) patients. The most common locations of thoracic injury occurred between T4 and T11. Lumbar injuries were present in nine patients (23.08%), while cervical injuries were less common, occurring in only four patients (10.26%).
Based on the AIS classification6, there were 20 patients categorized as AIS Class A 5 as AIS Class B, 4 as AIS Class C, and 10 as AIS Class D.
The high utilization rates of steroid therapy (94.87%) and nutritional neurological drug treatment (97.44%) were observed across all AIS grades.
Table 1 presents all the above clinical characteristics, information clinical manifestations, and demographic characteristics of the patients.
Table 1.
Baseline characteristics of patients.
| AIS grading | A (N = 20) | B (N = 4) | C (N = 4) | D (N = 11) | Overall (N = 39) |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 2 (10.00%) | 1 (25.00%) | 0 (0.00%) | 0 (0.00%) | 3 (7.69%) |
| Female | 18 (90.00%) | 3 (75.00%) | 4 (100.00%) | 11 (100.00%) | 36 (92.31%) |
| Age | |||||
| 0–3 years | 3 (15.00%) | 1 (25.00%) | 0 (0.00%) | 0 (0.00%) | 4 (10.26%) |
| 4–6 years | 3 (15.00%) | 1 (25.00%) | 4 (100.00%) | 2 (18.18%) | 10 (25.64%) |
| 7–9 years | 14 (70.00%) | 2 (50.00%) | 0 (0.00%) | 9 (81.82%) | 25 (64.10%) |
| Etiology | |||||
| Car accident | 2 (10.00%) | 1 (25.00%) | 1 (25.00%) | 0 (0.00%) | 4 (10.26%) |
| Fall or sprain | 6 (30.00%) | 0 (0.00%) | 2 (50.00%) | 3 (27.27%) | 11 (28.21%) |
| Dance | 12 (60.00%) | 3 (75.00%) | 1 (25.00%) | 8 (72.73%) | 24 (61.54%) |
| Treatment methods | |||||
| Mannitol | 13 (65.00%) | 0 (0.00%) | 3 (75.00%) | 3 (27.27%) | 19 (48.72%) |
| Steroid therapy | 18 (90.00%) | 4 (100.00%) | 4 (100.00%) | 11 (100.00%) | 37 (94.87%) |
| Nutritional neurological drug treatment | 20 (100.00%) | 4 (100.00%) | 4 (100.00%) | 10 (90.91%) | 38 (97.44%) |
| Injury length | |||||
| Less than or equal to 5 vertebrae | 7 (35.00%) | 2 (50.00%) | 3 (75.00%) | 9 (81.82%) | 21 (53.8%) |
| 5–10 vertebrae | 10 (50.00%) | 2 (50.00%) | 1 (25.00%) | 2 (18.18%) | 15 (38.46%) |
| Greater than or equal to 10 vertebrae | 2 (10.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (5.13%) |
| Clinical presentation | |||||
| Lower back pain | 2 (10.00%) | 0 (0.00%) | 0 (0.00%) | 3 (27.27%) | 5 (12.82%) |
| Loss of movement in both lower limb | 11 (55.00%) | 0 (0.00%) | 3 (75.00%) | 3 (27.27%) | 17 (43.59%) |
| Weakness in both lower limbs | 7 (35.00%) | 3 (75.00%) | 0 (0.00%) | 4 (36.36%) | 14 (35.90%) |
| Sensory disturbance in both lower limbs | 12 (60.00%) | 1 (25.00%) | 0 (0.00%) | 1 (9.09%) | 14 (35.90%) |
| Accompanied by difficulty in urination | 0 (0.00%) | 1 (25.00%) | 1 (25.00%) | 5 (45.45%) | 7 (17.95%) |
| Associated malformations | |||||
| Occult spinal dysraphism | 9 (45.00%) | 2 (50.00%) | 2 (50.00%) | 2 (18.18%) | 15 (38.46%) |
| Spondylolysis | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (2.56%) |
| Sacralization of L1 | 0 (0.00%) | 1 (25.00%) | 0 (0.00%) | 0 (0.00%) | 1 (2.56%) |
| Spinal cord signal changes | |||||
| Edema | 17 (85.00%) | 4 (100.00%) | 4 (100.00%) | 11 (100.00%) | 36 (92.31%) |
| Hemorrhage | 3 (15.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 3 (7.69%) |
| Mixed | 2 (10.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (5.13%) |
| Normal | 2 (10.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (5.13%) |
MRI results
All patients underwent spinal/spinal cord MRI within 1 week of trauma. Among the patients with serious clinical manifestations after injury, 31 underwent MRI examination on the first or second days after injury, and 5 underwent MRI examination on the third day after injury. Three patients with relatively mild clinical manifestations were examined 4 or 5 days after injury.
MRI revealed abnormal spinal cord signals, including spinal cord edema (characterized by a low signal on T1WI and a high signal on T2WI and bleeding (high signal on T1WI). Specifically, 34 patients showed edema, 6 exhibited bleeding, 6 had a mixed presentation, and 1 appeared normal.
The results of the MRI were interpreted by two experienced radiologists who measured and averaged the length and cross-sectional area of spinal cord injury. The spinal cord injury segments involved the cervical pulp in 4 patients, thoracic pulp in 36 patients, and lumbar pulp in 9 patients. The length of the injury was less than or equal to 5 vertebral cones in 26 patients, 5–10 cones in 16 patients, and ≥ 10 cones in 4 patients. Additional findings included 18 patients with recessive spina bifida, one patient with pedicle isthmus nonunion, and two patients with sacral lumbarization (Figs. 1, 2 and 3).
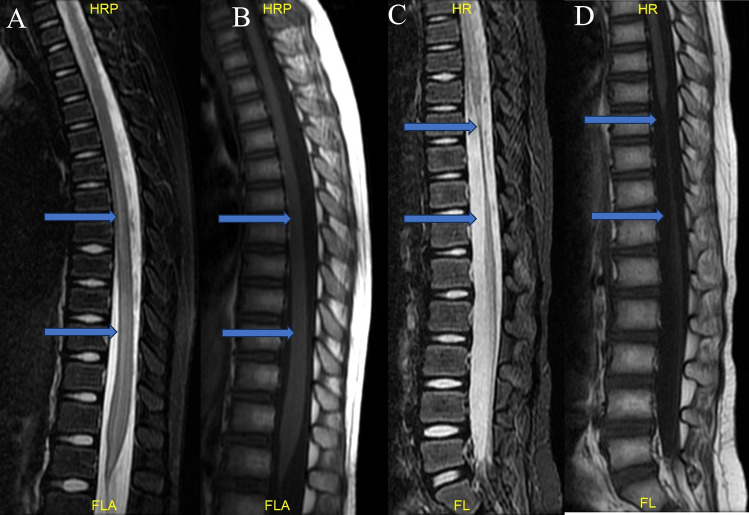
Fig. 1.
Six-year-old female patient with weakness in both lower extremities for 5 h. (A) (T2WI) and (B) (T1WI) spinal MRIs performed at the time of injury showed long segment spinal edema below T5. (C,D) shows that the spinal cord was significantly atrophied below T8 during the 6-month follow-up.
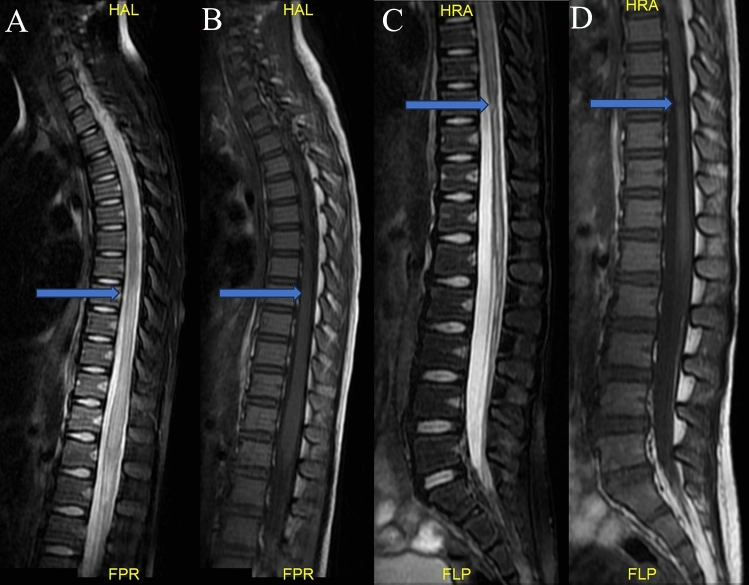
Fig. 2.
Seven-year-old female who suffered from weakness in both lower limbs for 4 h after accidentally falling while dancing. (A) (T2WI) and (B) (T1WI) show spinal MRI performed after the injury and long-level spinal edema below T7. (C,D) show spinal cord atrophy below T9, and the central canal was enlarged at the 6-month follow-up.
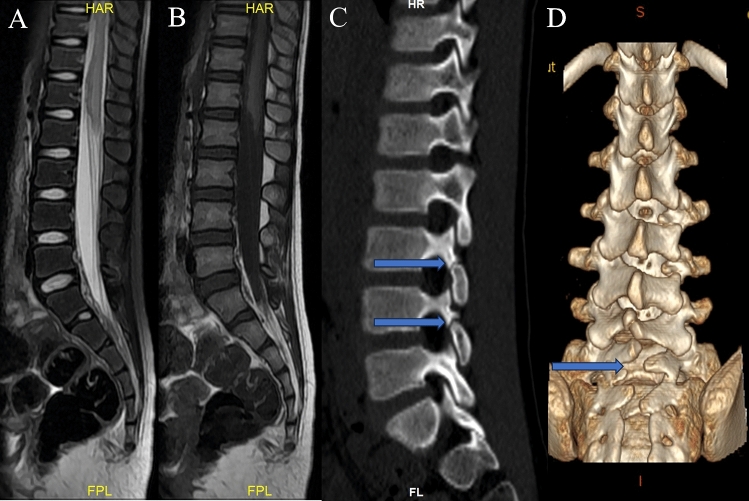
Fig. 3.
Seven-year-old female patient with disappeared sensorimotor activity for 12 h in both lower limbs after performing a back bend. (A) (T2WI) and (B) (T1WI) spinal MRI performed when the injury occurred and conical edema was accompanied by slight bleeding. (C) shows a disjointed isthmus of the vertebral arch at L3-4, and (D) shows L5-S2 recessive spina bifida.
Follow-up
Of the 39 patients with follow-up data, 17 (43.59%) belonged to the AIS improvement group and 22 (56.41%) were in the non-improvement group.
Univariate logistic analysis
Univariate logistic analysis showed that, in the week after injury, the signal intensity of edema in the injured area and the ratio of damaged to normal signal intensity were not significant factors in predicting prognosis (P > 0.01). However, the maximum cross-sectional area of the injury and the length of the injured segment were significantly correlated with the prognosis of spinal cord function, with a statistical significance of P ≤ 0.05 (Table 2).
Table 2.
Univariate logistic analysis.
| Independent variable | Improvement group (n = 17) | Unimproved group (n = 22) | P value |
|---|---|---|---|
| Age (year) | 6.88 ± 0.29 | 5.98 ± 0.48 | 0.36 |
| Sex: male | 0 | 3 | 0.24 |
| Sex: female | 17 | 19 | 0.24 |
| Signal intensity of edema in the injured area | 711.56 ± 118.91 | 776.36 ± 111.61 | 0.48 |
| Signal strength in normal area | 481.24 ± 78.02 | 505.72 ± 76.94 | 0.75 |
| Damage/normal ratio | 1.49 ± 0.05 | 1.63 ± 0.10 | 0.41 |
| Maximum cross-sectional area of damage (cm2) | 0.76 ± 0.01 | 0.18 ± 0.03 | < 0.001a |
| Damaged segment length (vertebral body) | 4.12 ± 0.44 | 6.82 ± 0.68 | 0.003a |
aP < 0.05, statistically significant.
Multivariate logistic regression analysis
Using the MRI spinal cord injury plane within 1 week of injury as the independent variable and the improvement in AIS classification as the dependent variable, the results of multivariate logistic regression analysis showed that the relevant factors affecting the improvement in AIS classification were the maximum cross-sectional area of the spinal cord injury and the length of the injured segment (P < 0.05) (Table 3).
Table 3.
Multivariate logistic regression analysis.
| Item | Regression coefficient B | Standard error | Wald value | P value | OR value | 95% CI |
|---|---|---|---|---|---|---|
| Maximum cross-sectional area of damage (cm2) | − 45.46 | 15.79 | 8.29 | 0.004a | 0.00 | 0.00 |
| Damaged segment length (vertebral body) | − 0.61 | 0.30 | 3.98 | 0.046a | 0.544 | 0.3–0.99 |
aP < 0.05 was statistically significant.
ROC curve analysis
The maximum cross-sectional area of the spinal cord injury and the length of the injured segment were used to plot the Receiver operating characteristic ROC curve. The area under the curve (AUC) of the maximum cross-sectional area was 0.91 (95% CI 0.81–1.0, sensitivity 81.80%, specificity 88.20%, P < 0.001). The length of the damaged segment AUC was 0.78 (95% CI 0.64–0.93, sensitivity 50%, specificity 94.10%, P < 0.001). The two variables had significant predictive value for the improvement of the AIS classification (Table 4, Fig. 4).
Table 4.
ROC curve analysis.
| Factor class | AUC | 95% CI | Sensibility (%) | Specificity (%) | P value |
|---|---|---|---|---|---|
| Maximum cross-sectional area of damage (cm2) | 0.91 | 0.81–1.00 | 81.80 | 88.20 | < 0.001a |
| Damaged segment length (vertebral body) | 0.78 | 0.64–0.93 | 50.00 | 94.10 | < 0.001a |
aP < 0.05 was statistically significant.
Fig. 4.
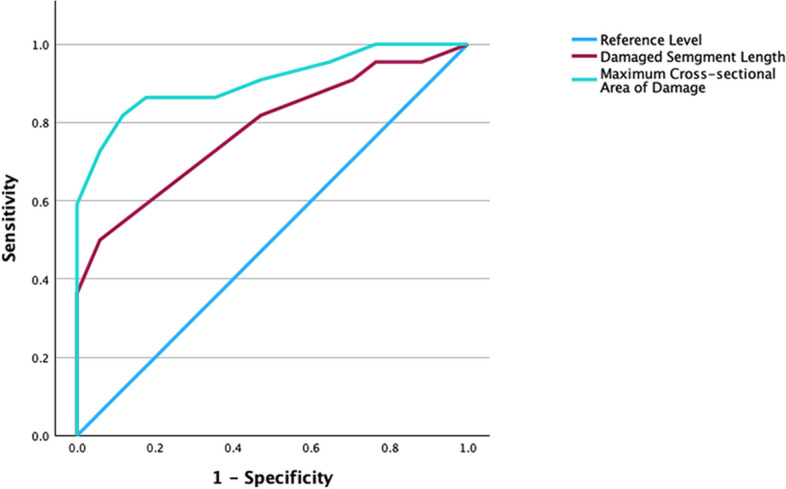
ROC curve analysis of the patients.
Discussion
In this study, we demonstrated that MRI plays a crucial role in the diagnosis and prognosis of pediatric SCIWORA. The cross-sectional area and length of the spinal cord injury are risk factors for poor prognosis. We observed 30 patients with dance-related injuries, 12 with falls, and 5 with injuries related to car accidents. The majority of injuries occurred among patients in the 7–9 years age bracket (64.10%). This age group is typically active and participates in higher-risk activities, such as dance and sports, which aligns with the etiology data showing a high incidence of dance-related injuries (61.54%). This observation underscores the importance of targeted interventions in these activities, potentially focusing on enhanced training techniques and safety measures to mitigate risk. Among the dance-related injuries, the main clinical symptoms observed were sudden lumbar pain, lower-limb weakness, and dysuria, often accompanied by numbness and other lower-limb symptoms. These symptoms typically appear immediately or shortly after engaging in dance movements involving the lower back. Our findings align with Liang’s2 observations and may be attributed to the trend of children initiating dance or taekwondo training at a young age in China. Carroll et al.9 reported on the treatment of 433 pediatric patients with SCIWORA, with exercise-related injuries being the most common mechanism, followed by falls and motor vehicle accidents. In China, patients with SCIWORA frequently experience injuries to the thoracic and thoracolumbar segments, whereas in Western countries, such injuries are predominantly caused by motor vehicle accidents. Clinical symptoms resulting from car accidents and falls often correlate with the site and severity of the injury, with varying degrees of delayed-onset limb movement disorders and defecation difficulties.
The mechanism of injury in SCIWORA is closely linked to hyperextension and flexion, which leads to transient vertebral dislocation or stretching. This phenomenon predominantly affects children under the age of 8 years owing to the unique anatomical and biomechanical properties of their immature spines, which typically do not achieve full anatomical maturity until around 8 years of age10,11. In this study, four patients were 1–3 years old, 11 were 4–6 years old, and 24 were 7–9 years old, consistent with those previously reported. The overwhelming predominance of females (92.31%) in this study points towards a potential gender-specific vulnerability in the context of spinal cord injuries without fractures or dislocations. It suggests that female children might have distinct anatomical, biomechanical, or hormonal characteristics that predispose them to such injuries, or that they are more likely to be involved in activities leading to these conditions. SCIWORA typically manifests clinical symptoms within 48 h of the inciting event. Minor injuries may result in transient numbness, paresthesia, or paralysis, whereas more severe injuries can lead to various degrees of neurological impairment12.
Research indicates that SCIWORA is associated with the relaxation and increased elasticity of the developing spine, resulting in overactivity relative to the spinal cord, ultimately leading to stretching and transectional injuries. Several anatomical features contribute to this vulnerability in children. Ligaments and joint capsules are more elastic, articular processes tend to be horizontal, intervertebral discs and disc annulus cavities have a higher water content, and endplates are more biologically active and vascular-rich. Additionally, the uncus process is more prone to anterior wedging in children compared to adults4,13. These factors collectively render children’s spines inherently malleable, allowing the vertebral bodies to shift without fracture, whereas closed spinal cord injuries remain susceptible to injury1,13.
In addition, in this study, thirty-one patients (79.49%) with serious clinical manifestations underwent MRI examination on the first or second days after injury, five (12.82%) underwent MRI examination on the third day, and only three (7.69%) underwent MRI examination on the fourth day or fifth day. The patients included underwent initial examinations in clusters. A retrospective analysis revealed that MRI features within 1 week after injury commonly showed spinal cord edema as the primary change, sometimes accompanied by bleeding, with a broad abnormal signal range indicating the extent of spinal cord injury.
Among the patients analyzed, the average injury spanned approximately 16 vertebral segments, with injuries to the thoracic 10–12 segments being the most frequent, as reported by Tong8. Ischemic injury is a significant factor in dance-related spinal cord injuries. MRI findings in children with posterior lower back spinal cord injuries often indicate abnormal signals at T9, T10, and T11, suggesting a correlation between the injury sites and the most stressed lumbar movement sites, possibly due to spinal cord ischemia resulting from excessive dorsal extension. These findings are consistent with the observed clinical symptoms. The detection of conditions like occult spinal dysraphism in 38.46% of the cases, confirmed through imaging, underscores the importance of comprehensive diagnostic assessments of pediatric patients presenting with spinal injuries. These findings advocate for routine screening for congenital anomalies in children with spinal cord injuries, which could significantly impact the management strategies and improve prognostic accuracy.
Furthermore, binary logistic regression analysis revealed that the maximum cross-sectional area and length of the damaged segment were significant prognostic factors. The current study identified correlations between the maximum cross-sectional area of spinal cord injury, the length of the injured segment, and clinical prognosis. ROC curve analysis had significant predictive value for the improvement of AIS classification. Larger cross-sectional areas of injury and longer injured segments were associated with worse prognoses. Six months post-injury, varying degrees of spinal cord atrophy were observed, with earlier and more severe atrophy. Notably, patients classified as The American Spinal Injury Association (ASIA) impairment scale (AIS) grade A exhibited severe atrophy below the site of injury. David reported14 that factors contributing to recovery from spinal cord injury without fracture and dislocation included age, accident type, and the absence of initial MRI lesions. These findings underscore the importance of early assessment and intervention to predict and manage outcomes in patients with SCIWORA.
Most studies have demonstrated the relatively accurate predictive capability of MRI in assessing the prognosis of SCIWORA patients5,15. Guidelines emphasize the significance of abnormal intramedullary signals and lesion length in the subacute stage for predicting the prognosis of acute hyperextension spinal cord injury in children. Friegang reported that SCIWORA warrants exclusion, necessitating a complete spinal MRI to rule out structural and potentially serious causes of nerve injury16. High T2-weighted signal intensity in MRI and its extent have a discernible impact on the prognosis of spinal cord injury17. In this study, a prolonged T2 signal on MRI was closely correlated with the influence of maximum cross-sectional area and segment length on the prognosis of spinal cord injury. Additionally, patients with SCIWORA who exhibited bleeding manifestations tended to experience more severe nerve damage and poorer prognoses18. Injury severity is a pivotal factor in prognosis and is closely associated with injury grade19. Hamilton and Myles20 assessed the recovery potential of incomplete injury and noted that patients with mild-to-moderate injury often exhibited good functional recovery potential and were likely to regain full function, whereas the prognosis for severe injury was less optimistic. All patients were advised to remain at the hospital for medical care. Patients who are treated with methylprednisolone and/or dexamethasone are generally discharged from the hospital immediately after neurological status improves, according to local practice and guidelines21. The high utilization rates of steroid therapy (94.87%) and nutritional neurological drug treatment (97.44%) across all AIS grades reflect a consensus in the acute management of pediatric spinal cord injuries. Clinicians should recognize that SCIWORA often results from high-energy trauma, with a significant proportion of non-spinal injuries22,23.
Although the ASIA classification serves as an effective predictor of neurological prognosis, its application may be limited in children due to cognitive and expressive constraints24. MRI evaluation is simple, feasible, and independent of the patient’s subjective influence, and holds great significance for clinical prognosis. It can predict the neurological prognosis of children in the early stages of injury and facilitate the development of personalized rehabilitation treatments and guidance.
This study had some limitations. First, this was a retrospective study conducted at a single center. A prospective study may be needed to increase statistical analysis. Second, there was no control group in this study, and a control group is needed in the future. Third, our study focused only on MRI findings related to clinical outcomes. Clinical outcomes are related to many factors. This may have led to bias in the conclusion. Fourth, this study did not identify risk factors for SCIWORA; risk factors for injury should be further explored in the future.
In conclusion, the cross-sectional area and length of the spinal cord injury indicated by MRI are risk factors for prognosis. Doctors should pay close attention to patients with the aforementioned injuries.
Acknowledgements
We would like to express our thanks to all staff in our radiology department for assisting with medical record data collection.
Author contributions
CW conceived the study, participated in its design, and drafted the manuscript. JYH helped in collecting the clinical data and help drafted the manuscript, draw the figures and all authors read and approved the final manuscript.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pang, D. & Wilberger, J. E. Jr. Spinal cord injury without radiographic abnormalities in children. J. Neurosurg.57, 114–129 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Liang, J., Wang, L., Hao, X., Wang, G. & Wu, X. Risk factors and prognosis of spinal cord injury without radiological abnormality in children in China. BMC Musculoskelet. Disord.23, 428 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren, J. et al. Pediatric thoracic SCIWORA after back bend during dance practice: A retrospective case series and analysis of trauma mechanisms. Childs Nerv. Syst.33, 1191–1198 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Koestner, A. J. & Hoak, S. J. Spinal cord injury without radiographic abnormality (SCIWORA) in children. J. Trauma Nurs.8, 101–108 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Zeitoun, D., El Hajj, F., Sariali, E., Catonné, Y. & Pascal-Moussellard, H. Evaluation of spinal cord compression and hyperintense intramedullary lesions on T2-weighted sequences in patients with cervical spondylotic myelopathy using flexion-extension MRI protocol. Spine J.15, 668–674 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Roberts, T. T., Leonard, G. R. & Cepela, D. J. Classifications in brief: American Spinal Injury Association (ASIA) impairment scale. Clin. Orthop. Relat. Res.475, 1499–1504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirshblum, S., Snider, B., Rupp, R. & Read, M. S. Updates of the international standards for neurologic classification of spinal cord injury: 2015 and 2019. Phys. Med. Rehabil. Clin. N. Am.31, 319–330 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Tong, A. N. et al. Ischemic damage may play an important role in spinal cord injury during dancing. Spinal Cord58, 1310–1316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll, T. et al. Spinal cord injuries without radiologic abnormality in children: A systematic review. Spinal Cord53, 842–848 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Alexander, M. S. & Alexander, C. J. Recommendations for discussing sexuality after spinal cord injury/dysfunction in children, adolescents, and adults. J. Spinal Cord Med.30(Suppl 1), S65-70 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson, C. J., Vogel, L. C., Chlan, K. M., Betz, R. R. & McDonald, C. M. Depression in adults who sustained spinal cord injuries as children or adolescents. J. Spinal Cord Med.30(Suppl 1), S76-82 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darain, H. et al. Epidemiology, clinical features and consequences of spinal cord injury in children. J. Coll. Physicians Surg. Pak.28, 532–535 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Kriss, V. M. & Kriss, T. C. SCIWORA (spinal cord injury without radiographic abnormality) in infants and children. Clin. Pediatr. (Phila)35, 119–124 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Brauge, D. et al. Multicenter study of 37 pediatric patients with SCIWORA or other spinal cord injury without associated bone lesion. Orthop. Traumatol Surg. Res.106, 167–171 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Farrell, C. A., Hannon, M. & Lee, L. K. Pediatric spinal cord injury without radiographic abnormality in the era of advanced imaging. Curr. Opin. Pediatr.29, 286–290 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Freigang, V. et al. Management and mid-term outcome after “real SCIWORA” in children and adolescents. Glob. Spine J.12, 1208–1213 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, Y. et al. Acute hyperextension myelopathy in children: Radiographic predictors of clinical improvement. Spinal Cord60, 498–503 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Kumar, Y. & Hayashi, D. Role of magnetic resonance imaging in acute spinal trauma: A pictorial review. BMC Musculoskelet. Disord.17, 310 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang, D. Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurgery55, 1325–1342 (2004) (discussion 1342–1343). [DOI] [PubMed] [Google Scholar]
- 20.Hamilton, M. G. & Myles, S. T. Pediatric spinal injury: Review of 61 deaths. J. Neurosurg.77, 705–708 (1992). [DOI] [PubMed] [Google Scholar]
- 21.Zou, Z. et al. Pediatric spinal cord injury with radiographic abnormality: The Beijing experience. Spine J.23, 403–411 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Piatt, J. H. Jr. Pediatric spinal injury in the US: Epidemiology and disparities. J. Neurosurg. Pediatr.16, 463–471 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Knox, J. Epidemiology of spinal cord injury without radiographic abnormality in children: A nationwide perspective. J. Child. Orthop.10, 255–260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuszynski, M. H. et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Clinical trial inclusion/exclusion criteria and ethics. Spinal Cord45, 222–231 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.