Abstract
Cytokine storm syndromes such as hemophagocytic lymphohistiocytosis (HLH), Adult-onset Still’s disease (AOSD), and COVID-19 cytokine storm (CCS) are characterized by markedly elevated inflammatory cytokines. However clinical measurement of serum cytokines is not widely available. This study examined the clinical utility of C-reactive protein (CRP) and ferritin, two inexpensive and widely available inflammatory markers, for distinguishing HLH from AOSD and CCS. This single centre retrospective study included 44 secondary HLH patients, 14 AOSD patients, and 13 CCS patients. Baseline CRP and ferritin measured within 72 h of diagnosis and before administration of corticosteroids or other anti-inflammatory therapies were analyzed. The median CRP in HLH patients was lower than AOSD (71 mg/L vs. 172 mg/L, p < 0.001) and CCS (71 mg/L vs. 121 mg/L, p = 0.0095) patients. Serum ferritin levels were lower in CCS compared to HLH (1,386 µg/L vs. 29,019 µg/L, p < 0.001) and AOSD (11,359 µg/L vs. 29,019 µg/L, p = 0.035). A CRP < 130 mg/L when combined with an HScore > 136 improves the specificity of HScore alone for HLH from 85.2 to 96.3%. Adding CRP < 130 mg/L to ferritin > 15,254 µg/L increases specificity for HLH from 88.9 to 100%. This study demonstrates that median CRP is lower in HLH than in AOSD and CCS, and median ferritin is lower in CCS than in HLH or AOSD. This study demonstrates the clinical utility of these widely available inflammatory markers for distinguishing between different cytokine storm syndromes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82760-6.
Keywords: HLH, Malignancy-associated HLH, Hemophagocytic syndrome, Soluble interleukin-2 receptor, Ferritin
Subject terms: Cytotoxic T cells, Bone marrow, Cytokines, Interferons, Interleukins, Tumour-necrosis factors
Introduction
The emergence of cytokine storm syndrome (CCS) as a key cause of morbidity and mortality during the COVID-19 pandemic generated immense interest and research into maladaptive inflammatory cytokine responses in human disease1–3. One of the earliest comparisons of CCS was to hemophagocytic lymphohistiocytosis (HLH)4, a conceptual analogy that drove much of the key research regarding the immunology of COVID-19 infection5. These investigations highlighted similarities and differences between CCS and HLH. While both are characterized by pathological immune activation leading to multi-organ damage, CCS is driven largely by interleukin (IL)-66,7, whereas the interferon-γ (IFN-γ)-CXCL-9 axis plays a more central role in the pathophysiology of HLH8,9.
A key objective in the early research on CCS was to determine if analogous immunosuppressive treatment strategies in HLH would apply to CCS. For decades, virus-driven HLH such as Epstein-Barr virus (EBV)-HLH has been treated successfully with corticosteroids and etoposide chemotherapy, even in the absence of effective antiviral therapy10. Likewise, immunosuppressive therapies targeting hypercytokinemia, including corticosteroids, tocilizumab (IL-6 inhibitor) and baricitinib (JAK inhibitor) provided modest reductions in mortality for patients with severe COVID-19 well before the advent of effective anti-virals11–14.
The insights gained from studying CCS has spurred research into other, more rare inflammatory conditions. A particularly urgent problem is how patients with different cytokine storm syndromes can be diagnosed and treated in a timely manner. While CCS is easily recognized in that patients have acute COVID-19 infection, HLH can be challenging to differentiate from other inflammatory syndromes. One such syndrome is adult-onset Still’s disease (AOSD), which, like HLH, presents with fever and extreme hyperferritinemia. However, the IL-1/IL-18 and IL-6 cytokine axes appear to play a more prominent role in AOSD than in HLH15. Moreover, a subset of AOSD patients can present with particularly severe illness known as catastrophic adult-onset Still’s disease (cAOSD) and these patients can be particularly difficult to distinguish from HLH16.
Another challenge posed by cytokine storm syndromes is the disconnect between research-based cytokine/chemokine assays and clinically available tests. Real time, clinically validated serum cytokine and chemokine measurement is not available in most centers. As a case in point, we recently demonstrated that C-reactive protein (CRP) and soluble CD25 (sCD25) are useful in differentiating AOSD from HLH in patients with extreme hyperferritinemia17. However, this finding has limited transferability to centers with sCD25 testing. Even in our own center, sCD25 is only routinely sent in patients with suspected HLH, and is often not done for patients with other cytokine storm syndromes, such as CCS.
Simple, clinically available biomarkers for diagnosing and distinguishing between cytokine storm syndromes are urgently needed. CRP is produced by hepatocytes mainly in response to IL-6, but also IL-1 and tumour necrosis factor (TNF)18. Large studies have shown that CRP predicts need for mechanical ventilation and mortality in CCS19. Serum ferritin is a marker of macrophage activity and is markedly elevated in most patients with HLH and AOSD20,21. Although C-reactive protein and ferritin are routinely measured in many centers in patients with inflammatory conditions, little is known about the diagnostic utility of these ubiquitous and inexpensive biomarker in distinguishing between cytokine storm syndromes. We therefore conducted a single-center retrospective study to examine the utility of CRP and ferritin in distinguishing between CCS, AOSD, and HLH in adults.
Methods
Patients: HLH, AOSD, and CCS
Institutional ethics approval (H22-00445-A003) was obtained through the University of British Columbia Clinical Research Ethics Board. All methods were performed in accordance with the relevant guidelines and regulations. This was a retrospective single center study. All patients enrolled in the study were from the Vancouver Coastal Health region hospitals between January 1st, 2000, to June 28th, 2023.
Inclusion criteria for the HLH group included adults (≥ 18 years old) diagnosed with secondary HLH by the treating Hematologist based on HLH-2004 and/or H-score diagnostic criteria (“total HLH” group) defined as H-score > 169. The etiology of secondary HLH subgroups is listed on Supplementary Table 1.
Inclusion criteria for the AOSD group included adults with a diagnosis of AOSD admitted to hospital with AOSD flare, based on Yamaguchi criteria (see Supplementary Table 2). MS scores were also calculated for AOSD patients using Wang’s cutoff of > -1.08 for MAS/HLH in AOSD (see Supplementary Table 2)22,23. All of the AOSD patients had an MS Score < -1.08 in keeping with AOSD without MAS.
Inclusion criteria for the CCS group included patients admitted to the Intensive Care Unit diagnosed based on a known diagnosis of COVID-19 with marked immune dysregulation clinically and biochemically, including fever, hyperferritinemia, coagulopathy, and elevated CRP > 50, ferritin > 500. Patients were excluded if they had received tocilizumab prior to collection of laboratory samples.
Ferritin and CRP levels were collected for each patient if available, and values were included in analysis only if drawn within 72 h of the time of HLH diagnosis, and prior to the initiation of disease-targeted therapies including steroids. HScore was calculated from data collected but bone marrow biopsy results were not taken into consideration when calculating an HScore given that this data was not available for all patients and was not available for patients with CCS or AOSD who did not meet an indication for bone marrow biopsy. As such, a cutoff H-score of > 134 was used (usual cutoff is 169 and marrow features are ascribed 35 points). The utility of the HScore without bone marrow biopsy results was shown by Nyvlt et al.24.
Statistical analysis
Descriptive statistics
Descriptive statistics were computed to summarize the patient demographics and clinical characteristics. Median and interquartile ranges (IQR) were reported for continuous variables, while frequencies and percentages were used for categorical variables. These statistics were presented for the overall study population and stratified by diagnosis (HLH, AOSD, CCS).
Logistic regression analysis
Binary logistic regression was performed to assess the predictive value of selected biomarkers (CRP, ferritin, and HScore) for the diagnosis of HLH. The model’s goodness-of-fit and the significance of individual predictors were evaluated, with odds ratios (OR) and 95% confidence intervals (CI) reported for significant associations.
Kruskal-Wallis Test and Dunn’s Post-Hoc Analysis
Due to the non-parametric nature of the biomarker data, the Kruskal-Wallis test was employed to detect differences in median values across the three diagnostic groups. Significant findings from the Kruskal-Wallis test led to subsequent pairwise comparisons using Dunn’s post-hoc test with Bonferroni correction to adjust for multiple comparisons. This approach enabled the identification of specific groups between which the biomarkers significantly differed.
ROC curve analysis
Receiver Operating Characteristic (ROC) curve analysis was conducted for CRP, ferritin, HScore, and combined biomarkers to evaluate their diagnostic performance in distinguishing HLH from other conditions. The Area Under the Curve (AUC) was calculated, along with sensitivity and specificity values at optimal cutoff points, to assess the accuracy of each biomarker.
Software.
All analyses were carried out using R statistical software (version 4.3.2). The dplyr and gtsummary packages were utilized for data manipulation and descriptive statistics, pROC for ROC curve analysis, and dunn.test for post-hoc comparisons. Results were visualized using ggplot2 and ggsignif for enhanced graphical presentations.
Results
Seventy one patients were included in the study. Table 1 summarizes the demographic characteristics, clinical features, and laboratory data of the 44 patients in the adult HLH group, 14 patients in the AOSD comparison group, and 13 patients in the CCS comparison group (Table 1).
Table 1.
Summary of patient demographics and collected laboratory values.
| Characteristic | AOSD, N = 141 | CCS, N = 131 | HLH, N = 441 |
|---|---|---|---|
| Sex | |||
| Female | 11 (79%) | 7 (54%) | 23 (52%) |
| Male | 3 (21%) | 6 (46%) | 21 (48%) |
| Age (years) | 42 (34, 66) | 70 (49, 78) | 57 (44, 68) |
| CRP (mg/L) | 172 (137, 223) | 121 (95, 172) | 71 (37, 104) |
| Ferritin (ug/L) | 11,359 (6,000, 14,951) | 1,386 (969, 2,335) | 29,019 (8,888, 56,289) |
| WBC (x109/L) | 10 (7, 14) | 10 (7, 11) | 3 (1, 8) |
| Hgb (g/L) | 104 (84, 121) | 121 (117, 135) | 85 (77, 94) |
| Plt (x109/L) | 239 (209, 343) | 239 (188, 246) | 54 (31, 90) |
| ALT (IU/L) | 35 (16, 62) | 116 (90, 176) | 105 (69, 264) |
| AST (IU/L) | 71 (27, 103) | 102 (46, 146) | 223 (77, 508) |
| LDH (IU/L) | 555 (288, 838) | 410 (348, 548) | 1,453 (527, 3,323) |
| Bilirubin (umol/L) | 6 (5, 7) | 21 (7, 23) | 21 (12, 97) |
| Triglycerides (mmol/L) | 2.02 (1.86, 2.77) | 2.01 (1.18, 2.24) | 3.17 (2.24, 4.61) |
| Fibrinogen (g/L) | 4.15 (3.30, 5.14) | 6.00 (6.00, 6.60) | 2.20 (1.30, 3.35) |
| HScore | 119 (86, 143) | 87 (33, 112) | 190 (158, 219) |
1n (%); Median (IQR).
29 patients either passed away prior to ICU admission or had code status precluding ICU admission.
CRP is significantly lower in HLH compared to AOSD and CCS
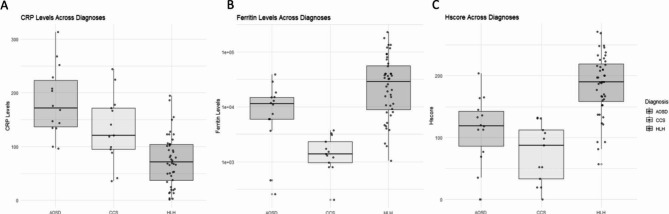
Median CRP values in total HLH were 71 mg/L (IQR 37–104), compared to 172 mg/L in the AOSD group (IQR 137–223, p < 0.001), and 121 mg/L in the CCS group (IQR 92–172, p = 0.0095) (Fig. 1A).
Fig. 1.
Comparison of CRP, Ferritin, and HScore in patients with HLH, AOSD, and CCS. (A) Median CRP was significantly lower in HLH (71, IQR 37–104) compared to AOSD (172, IQR 137, 223, p < 0.001) and CCS (121, IQR 97–172, p = 0.0095). (B) Median serum ferritin levels were significantly lower in CCS (1,386.0 ug/L, IQR 969–2335) compared to HLH (29,019 ug/L, IQR 8,888 − 56,289, p < 0.001) and AOSD (11,359 ug/L, IQR 6,000–14,951, p = 0.014). Median serum ferritin levels were also significantly lower in AOSD compared to HLH (p = 0.0352). (C) Median HScore in HLH was 190 (IQR 158–219) compared to 119 in AOSD (IQR 86–143, p < 0.001) and 87 in CCS (IQR 33–112, p < 0.001).
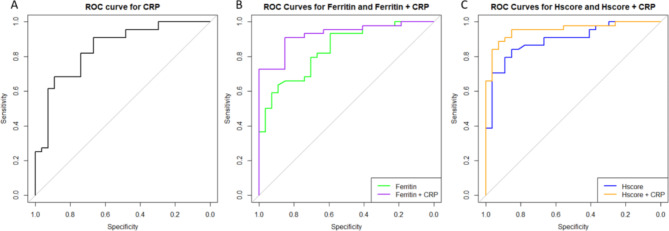
Given that total HLH CRP values were significantly lower than CRP levels compared to AOSD and CCS, a Receiver Operating Characteristics (ROC) curve was performed for total HLH cases, AOSD, and CCS (Fig. 2A). This demonstrated an Area Under the Curve (AUC) of 0.845, indicating a good diagnostic performance in diagnosing HLH, with a sensitivity of 90.9% and a specificity of 66.7% for CRP values less than 130 mg/L, as calculated by Youden’s index.
Fig. 2.
ROC curves for all patients (HLH, CCS, and AOSD). (A) ROC curve for CRP alone demonstrates an AUC of 0.845, indicating a good diagnostic performance in diagnosing HLH, with a sensitivity of 90.9% and a specificity of 66.7% for CRP values less than 130 mg/L, as calculated by Youden’s index. (B) The addition of CRP to ferritin improves the AUC from 0.842 to 0.927 with an increased sensitivity of 90.9% (improved from 63.6% for ferritin alone) and a specificity of 85.2% (88.9% with ferritin alone). (C) The addition of CRP to HScore improves the AUC from 0.894 to 0.954 and results in an increased sensitivity of 88.6% (from 84.1% for HScore alone) and increased specificity from 85.2–92.6%.
Ferritin is significantly higher in HLH compared to AOSD and CCS
Median serum ferritin levels were significantly lower in CCS (Med = 1,386.0 ug/L, IQR 969–2335) compared to HLH (Med = 29,019 ug/L, IQR 8,888 − 56,289, p < 0.001) and AOSD (Med = 11,359 ug/L, IQR 6,000–14,951, p = 0.014). Median serum ferritin levels were also significantly lower in AOSD compared to HLH (p = 0.0352) (Fig. 1B). A ROC curve was done for ferritin levels in total HLH patients, which demonstrated an AUC of 0.842 for ferritin levels greater than 15,254 ug/mL, with a sensitivity of 63.6% and specificity of 88.9%, as calculated by Youden’s index, indicating a fair diagnostic performance for HLH (Fig. 2B).
HScore was significantly higher in HLH compared to AOSD and CCS. In the original HScore study, the best cutoff was determined to be a score of 169, which corresponded to a sensitivity of 93%, a specificity of 86%, and accurate classification of 90% of the patients25. However, since bone marrow biopsies were not done in the CCS patients, and rarely done in AOSD patients, we subtracted the 35 points for a bone marrow biopsy showing hemophagocytosis and used an HScore cutoff of > 134 as positive. The median HScore in HLH patients was 190 (IQR 158–219) compared to 119 in AOSD (IQR 86–143, p < 0.001) and 87 in CCS (IQR 33–112, p < 0.001) (Fig. 1C). A ROC curve demonstrated an AUC of 0.894 for HScore > 134 with a sensitivity of 84.1% and a specificity of 85.2% (Fig. 2C).
Addition of CRP improves AUC and specificity of the HScore and ferritin
ROC curves were created to examine the usefulness of CRP in improving sensitivity and specificity of HScore and ferritin in the diagnosis of HLH.
The addition of CRP to ferritin improves the AUC from 0.842 to 0.927 with an increased sensitivity of 90.9% (improved from 63.6% for ferritin alone) and a specificity of 85.2% (88.9% with ferritin alone) (Fig. 2B).
The addition of CRP to HScore improves the AUC from 0.894 to 0.954 and results in an increased sensitivity of 88.6% (vs. 84.1% for HScore alone) and increased specificity from 85.2 to 92.6% (Fig. 2C).
Given that probability thresholds generated by combined ROC curves is challenging to use in a clinical setting, we calculated specificity and sensitivity of combining CRP < 130 mg/L and HScore > 134 as well as CRP < 130 mg/L and Ferritin > 15,254 ug/L (cut-offs suggested by the individual ROC analyses). Combining CRP and HScore cut-offs results in a sensitivity of 77.3% and a specificity of 96.3%. Combining CRP and ferritin cut-offs results in sensitivity of 59.1% and a specificity of 100% for the diagnosis of HLH.
We also examined the value of CRP in HLH patients vs. AOSD alone (not including CCS patients) since these two syndromes are often particularly difficult to distinguish in clinical setting. We found that the addition of CRP improves specificity for the diagnosis of HLH. AUC for CRP alone is 0.845 with a sensitivity of 90.9% and specificity of 81.8% for CRP < 130 mg/L. Comparably, AUC for HScore in this subset analysis was 0.852 with a sensitivity of 79.5% and specificity of 81.8% for HScore > 148. When CRP is combined with HScore, AUC improves to 0.959 with sensitivity of 86.4% and specificity of > 99% for the combined ROC curve (Fig. 3).
Fig. 3.
ROC curve for patients with HLH and AOSD only (CCS patients excluded). AUC for CRP alone is 0.845 with a sensitivity of 90.9% and specificity of 81.8% for CRP < 130 mg/L (red). AUC for HScore in this subset analysis was 0.852 with a sensitivity of 79.5% and specificity of 81.8% for HScore > 148 (blue). When CRP is combined with HScore, AUC improves to 0.959 with sensitivity of 86.4% and specificity of > 99% for the combined ROC curve (green).
Comparison of CRP and ferritin in HLH subgroups
Supplementary Fig. 1 shows the difference between HLH subgroups by etiology, including pediatric vs. adult HLH. Among HLH subtypes, CRP was significantly lower in infection-associated HLH (IAHS) (Mdn = 49.0 mg/L, p = 0.01) and unspecified hemophagocytic syndrome group (UHS) (Mdn = 76.4 mg/L, p = 0.025) compared to AOSD. CRP levels were also significantly lower in the IAHS group than in CCS (p = 0.036, Mdn 121.0). However, CRP in the malignancy-associated HLH (MAHS) was not significantly different from AOSD or CCS.
Discussion
Although CRP is widely available and commonly used in evaluating patients with inflammatory diseases, there are very little published data examining its value in distinguishing between different cytokine storm syndromes. We recently demonstrated, using the same cohort as the present study, that CRP and sCD25 combined have high utility in distinguishing between AOSD and HLH17. In that study, CRP > 130 mg/L and sCD25 < 3900 U/mL distinguish AOSD from HLH with an area under the curve (AUC) of 0.94 (95% confidence interval 0.93–0.97) and sensitivity 91%, specificity 93%. However, the transferability of those findings to centers without ready access to sCD25 assays is limited. When the HScore was developed, cytokine and cytokine receptors were excluded from analysis because the investigators wished to focus on widely available clinical and laboratory markers. CRP is nearly universally available, and the present study shows that adding CRP to the HScore is superior to HScore alone in distinguishing between AOSD, CCS and HLH.
The results of this study have important implications for existing HLH and AOSD diagnostic criteria. In HLH-2004, which was initially designed for use in pediatric HLH patients, the cut-off value for ferritin is 500 µg/L, which is much too low to be of diagnostic value in adults. In our study, the median ferritin value was 29,019 µg/L (IQR 8888−56,289), which is in keeping with a wealth of other studies over the past decade demonstrating that ferritin is typically extremely elevated in adult HLH, and further, that extreme hyperferritinemia alone is not specific for adult HLH26–28. Likewise, while markedly elevated CRP and ferritin are well described in AOSD, neither of these inflammatory markers is incorporated in the Yamaguchi or Fautrel criteria for diagnosis on AOSD29,30.
The median CRP levels in our HLH cohort (71 mg/L) are consistent with CRP values reported by Zhou and colleagues, who reported a median CRP of 75.3 mg/L in adult patients with HLH31. Biologically, the lower level of CRP in HLH may be explained by the previously described difference in cytokine profiles between these disorders. IL-6 has been found to be the major cytokine that induces CRP gene expression through activation of transcription factor STAT318. In AOSD, there is an overproduction of IL-1, followed by a cytokine storm involving IL-6, IL-18, and TNF32–34. As such, it is not surprising that AOSD is associated with significant elevations in CRP, and the use of anakinra, an IL-1 inhibitor, has indeed been effective in the treatment of AOSD35,36. While the data comparing IL-6 in HLH to other hyperinflammatory syndromes is limited, the pattern of moderately elevated CRP levels in our study is consistent with previous studies demonstrating that IL-6 is significantly less elevated in HLH compared to other hyperinflammatory syndrome mimics37,38.
When analyzing CRP levels in HLH based on triggering etiology, we found that these trends are maintained in most HLH subgroups, except for MAHS (Supplemental Fig. 1). Keeping in mind our limited sample size in these subgroups, this is not unexpected as CRP has been found to be elevated in many malignancies39. In our study, the majority of IAHS cases were triggered by EBV. This is consistent with literature showing that levels of CRP in viral infections are less pronounced when compared to bacterial infections due to interferon-mediated suppression of CRP by infections40.
Other studies have examined combinations of inflammatory biomarkers for diagnosis of HLH23,35. However, none of them included CRP. Lachmann et al. recently demonstrated that serum ferritin greater than 3000ug/L, combined with fever greater than 38 °C and an HScore of 168 improves the sensitivity and specificity of HLH diagnosis in critically ill hyperferritinemic patients41. Similarly, findings from the Optimized HLH Inflammatory (OHI) index for malignancies found that combining optimized sCD25 (greater than 3900 U/mL) and ferritin (greater than 1000 ug/L) levels increased the sensitivity and specificity for diagnosing HLH than either biomarker alone21. However, sCD25 can take days to result and is not readily available in many centres. This study demonstrates that CRP, which is widely and rapidly available in most centers, can have additional value when combined with ferritin and HScore in differentiating HLH from other cytokine storms.
In conclusion, our research demonstrates that CRP is significantly lower in HLH compared to AOSD or CCS, and that combining CRP with ferritin or HScore, leads to improved diagnostic specifity of HLH. While these results must be interpreted within the usual limitations of a retrospective, single center study, they provide proof of principle that simple, readily available biomarkers have utility in distinguishing between cytokine storm syndromes. Larger multi-center studies examining a broader range of cytokine storm syndromes will be of interest.
Limitations and future directions
The present study has several limitations. First, as this was a retrospective study, there is a possibility of selection bias amongst study participants. AOSD and CCS groups were chosen as comparators given the high degree of clinical and laboratory overlap with HLH, which often pose diagnostic dilemma at our center. Second, the number of patients in the AOSD and CCS groups, as well as the HLH subgroups was small, which could have led to an overestimation of effect size, and limited the generalization of our results. These small patient samples, however, reflect the rarity of these conditions, and future studies are needed to ensure reproducibility of our findings. Third, much remains to be understood about the role of CRP in inflammatory pathways. Future studies directly correlating CRP and IL-6 levels in cytokine storm syndromes would be of value to confirm whether CRP can be used reliably as a surrogate marker of IL-6 activity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
M.G., C.S., L.Y.C., and A.S. wrote the main manuscript textM.G. and C.S. prepared Figs. 1, 2 and 3; Table 1 M.G. and C.S. performed statistics on the final data L.Y.C. and A.S. conceived the main project idea and conceptual frameworkAll authors reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Informed consent and approval
Due to the retrospective nature of the study, the University of British Columbia Clinical Research Ethics Board waived the need of obtaining informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mariam Goubran and Caroline Spaner contributed equally.
Contributor Information
Luke Y. C. Chen, Email: lchen2@bccancer.bc.ca
Audi Setiadi, Email: audi.setiadi@cw.bc.ca.
References
- 1.England, J. T. et al. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev.45, 100707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fajgenbaum, D. C. & June, C. H. Cytokine Storm. N Engl J Med.383(23), 2255–2273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cron, R. Q., Goyal, G. & Chatham, W. W. Cytokine Storm Syndrome. Annual Review of Medicine.74(1), 321–337 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Mehta, P. et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet.395(10229), 1033–1034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature (2020). [DOI] [PMC free article] [PubMed]
- 6.Chen, L. Y. C., Hoiland, R. L., Stukas, S., Wellington, C. L. & Sekhon, M. S. Confronting the controversy: Interleukin-6 and the COVID-19 cytokine storm syndrome. Eur. Respirat. J.56(4), 2003006 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElvaney, O. J., Curley, G. F., Rose-John, S. & McElvaney, N. G. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respirat. Med.9(6), 643–654 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan, M. B., Hildeman, D., Kappler, J. & Marrack, P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood104(3), 735–743 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Locatelli, F. et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N. Engl. J. Med.382(19), 1811–1822 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Spaner, C., Goubran, M., Setiadi, A. & Chen, L. Y. C. COVID-19, haemophagocytic lymphohistiocytosis, and infection-induced cytokine storm syndromes. Lancet Infect. Dis.22(7), 937–938 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afra, K., Chen, L. Y. C. & Sweet, D. Tocilizumab for hospitalized patients with COVID-19. CMAJ.193(15), E521 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Investigators, R.-C. et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N. Engl. J. Med.384(16), 1491–1502 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abani, O. et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet397(10285), 1637–1645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer, A., Prinz, C., Fichtner, F., Fischer, A. L., Thieme, V., Grundeis, F., et al. Janus kinase inhibitors for the treatment of COVID-19. Cochrane Database Syst. Rev.6(6), Cd015209 (2022). [DOI] [PMC free article] [PubMed]
- 15.Ruscitti, P., Cantarini, L., Nigrovic, P. A., McGonagle, D. & Giacomelli, R. Recent advances and evolving concepts in Still’s disease. Nat. Rev. Rheumatol. (2024). [DOI] [PubMed]
- 16.Wahbi, A. et al. Catastrophic adult-onset Still’s disease as a distinct life-threatening clinical subset: Case–control study with dimension reduction analysis. Arthritis Res. Therapy23(1), 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckett, M., Spaner, C., Goubran, M., Wade, J., Avina-Zubieta, J. A., Setiadi, A., et al. CRP and sCD25 help distinguish between adult-onset Still’s disease and HLH. Eur. J. Haematol. (2024). [DOI] [PubMed]
- 18.Plebani, M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin. Chem. Lab. Med. (2023). [DOI] [PubMed]
- 19.Knight, S. R. et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ.370, m3339 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen, L. A. et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood.116(9), 1574–1584 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Zoref-Lorenz, A. et al. An improved index for diagnosis and mortality prediction in malignancy-associated hemophagocytic lymphohistiocytosis. Blood.139(7), 1098–1110 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minoia, F. et al. Development and initial validation of the MS score for diagnosis of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann Rheum Dis.78(10), 1357–1362 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Wang, R. et al. Application of MS score in macrophage activation syndrome patients associated with adult onset Still’s disease. Annals of the Rheumatic Diseases.80(9), e145 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Nyvlt P, Schuster FS, Ihlow J, Heeren P, Spies C, Hiesgen J, et al. Value of hemophagocytosis in the diagnosis of hemophagocytic lymphohistiocytosis in critically ill patients. Eur J Haematol. 2024. [DOI] [PubMed]
- 25.Fardet, L. et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol.66(9), 2613–2620 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Allen, C. E., Yu, X., Kozinetz, C. A. & McClain, K. L. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer50(6), 1227–1235 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Schram, A. M. et al. Marked hyperferritinemia does not predict for HLH in the adult population. Blood125(10), 1548–1552 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Chen, L. Y. C., Hayden, A. & Mattman, A. Extreme hyperferritinaemia, soluble interleukin-2 receptor, and haemophagocytic lymphohistiocytosis. Br. J. Haematol.185(3), 605–606 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi, M. et al. Preliminary criteria for classification of adult Still’s disease. J. Rheumatol.19(3), 424–430 (1992). [PubMed] [Google Scholar]
- 30.Lebrun, D. et al. Validation of the Fautrel classification criteria for adult-onset Still’s disease. Semin. Arthritis Rheumatism47(4), 578–585 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Zhou, M. et al. Clinical features and outcomes in secondary adult hemophagocytic lymphohistiocytosis. QJM111(1), 23–31 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Choi, J. H. et al. Serum cytokine profiles in patients with adult onset Still’s disease. J. Rheumatol.30(11), 2422–2427 (2003). [PubMed] [Google Scholar]
- 33.Mitrovic, S., Feist, E. & Fautrel, B. Adult-Onset Still’s Disease. Periodic and Non-Periodic Fevers 93–132 (Springer International Publishing; 2019).
- 34.Zhang, M., Xie, M., Wang, Y., Li, J. & Zhou, J. Combination value of biomarkers in discriminating adult onset Still’s disease and sepsis. Wien Klin Wochenschr.133(3–4), 118–122 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Giampietro, C. et al. Anakinra in adult-onset Still’s disease: Long-term treatment in patients resistant to conventional therapy. Arthritis Care Res. (Hoboken).65(5), 822–826 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Giacomelli, R. et al. The treatment of adult-onset Still’s disease with anakinra, a recombinant human IL-1 receptor antagonist: A systematic review of literature. Clin. Exp. Rheumatol.39(1), 187–195 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Xu, X. J., Tang, Y. M., Song, H., Yang, S. L., Xu, W. Q., Zhao, N., et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J. Pediatrics160(6), 984–90 e1 (2012). [DOI] [PubMed]
- 38.Lin, H. et al. IFN-gamma signature in the plasma proteome distinguishes pediatric hemophagocytic lymphohistiocytosis from sepsis and SIRS. Blood Adv.5(17), 3457–3467 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart, P. C., Rajab, I. M., Alebraheem, M. & Potempa, L. A. C-reactive protein and cancer-diagnostic and therapeutic insights. Front Immunol.11, 595835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enocsson, H. et al. Interferon-alpha mediates suppression of C-reactive protein: Explanation for muted C-reactive protein response in lupus flares?. Arthritis Rheum.60(12), 3755–3760 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Lachmann, G. et al. Hyperferritinemia in critically ill patients. Criti. Care Med.48(4), 459–465 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.