Abstract
Diquat (DQ) is a non-selective, fast-acting herbicide that is extensively used in aquatic systems. DQ has been registered as the substitute for paraquat due to its lower toxicity. However, the widespread presence of DQ in aquatic systems can pose an ecological burden on aquatic organisms. In addition, DQ can degrade into its metabolites, diquat-monopyridone (DQ-M) and diquat-dipyridone (DQ-D) in the environment, while the ecological risks of the metabolites remain uncertain. Herein, the aquatic ecological risks of DQ and its metabolites were compared using zebrafish as model non-target organisms. Results indicated that DQ and its metabolites did not induce significant acute toxicity to zebrafish embryos at environmentally relevant levels. However, exposure to DQ and DQ-D resulted in oxidative stress in zebrafish larvae. DQ treatment led to increased levels of reactive oxygen species (ROS), malondialdehyde (MDA), and glutathione (GSH) in the larvae, while DQ-D increased internal MDA and GSH levels. Moreover, the activities of the antioxidative enzymes, superoxide dismutase (SOD) and catalase (CAT) were significantly suppressed by DQ and DQ-D. Besides, the expression levels of oxidative stress-related genes (Mn-SOD, CAT, and GPX) were disturbed accordingly after DQ and DQ-D treatments. These findings highlighted the importance of a more comprehensive understanding of the ecological risks of agrochemical substitutions as well as agrochemical metabolites. Such knowledge is crucial for significant improvements in agrochemical regulation and policy-making in the future.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82905-7.
Keywords: Diquat, Pesticide metabolites, Oxidative stress, Ecological risk, Zebrafish
Subject terms: Environmental sciences, Hydrology
Introduction
Diquat (DQ) is a fast-acting, non-selective herbicide widely used for controlling vegetation in both terrestrial and aquatic environments1. DQ has been widely used throughout the world due to its excellent herbicidal performance2. DQ has gained popularity due to its efficacy in weed control and its lower toxicity compared to paraquat, leading to its replacement and widespread use as an agricultural and household herbicide3,4. As one of the few herbicides registered for direct application in aquatic systems, DQ inevitably ends up in aquatic ecosystems by transfer processes such as run-off, drainage, and leaching, after repeated applications near or in aquatic environments4,5. Previous study has reported that the detected concentrations of DQ in 114 surface water samples ranged from 0.002 to 0.038 mg/L6. Environmental levels of DQ, after applications in agricultural fields or water bodies to control weed growth usually remain between 0.1 and 1.0 mg/L and often exceed recommended limits due to misuse or improper application practices7. Besides, DQ has also been found to be persistent in the water with a dissipation time (DT50) of 1000 days8. In some cases, the DQ concentrations may maintain stable in the hydrosphere for a long period of time. In addition, DQ residues in aquatic systems tended to accumulate in weeds within a range of 0.6–2.4 mg/g (dry weight)9. Consequently, the presence of DQ residues in aquatic environments would most likely pose an ecological burden to aquatic organisms10.
Despite the lower toxicity compared to paraquat, DQ would still pose unexpected adverse effects on the aquatic ecosystem. Sanchez et al. reported that hepatic enzymes responsible for xenobiotic metabolism in three-spined stickleback (Gasterosteus aculeatus L.) were altered after treatments with DQ of 222 and 444 µg/L11. DQ also induced disturbance in the protein profiles in the liver of rainbow trout (Oncorhynchus mykiss)12. It was reported that DQ significantly inhibited the growth of adult freshwater snails (Lymnaea stagnalis) and impaired the development of their offspring at the concentration of 222.2 µg/L13. DQ could also disrupt the normal developmental stages of Xenopus metamorphosis, resulting in reduced fore and hind limb lengths14. In addition, Wang et al. reported that DQ would disrupt the behavior of zebrafish (Danio rerio) larvae15. In general, the complex exposure to DQ induced intricate response patterns in aquatic organisms and led to more uncertainties and conflicts regarding its ecotoxicity.
Pesticide residues in the environment are subsequently broken down into various metabolites by a variety of processes, including hydrolysis, photolysis, oxidation, and biodegradation16. Interestingly, some metabolites of pesticides are more abundant in the environment than their parent compounds17. More worryingly, pesticide metabolites tend to exhibit increased environmental mobility and persistence with the occurrence of degradation, which in return would result in more potent adverse effects on human beings and ecosystems compared to their parent compounds18,19. Zhang et al. reported that the hydroxylated metabolite of chlorothalonil induced more potent toxicity and endocrine disrupting effects19. Ji et al. also compared the endocrine-disrupting effects of four commonly used pesticides (benalaxyl, fenoxaprop-ethyl, malathion, and pyriproxyfen) and their 21 metabolites and found that about half of the metabolites exhibited stronger endocrine-disrupting effects16. Unfortunately, the risk assessments of the agrochemical metabolites are still given too little attention in the registration and authorization of agrochemicals, making the risks associated with agrochemical metabolites an emerging issue and a scientific blind spot. To data, only a few studies have reported the presence of DQ metabolites, and the detection of DQ metabolites in tissues revealed that diquat-monopyridone (DQ-M) and diquat-dipyridone (DQ-D) were the main metabolites in biological materials20,21. Unfortunately, few studies have addressed the toxicity of DQ-M and DQ-D, despite the prevalence of adverse effects induced by DQ. Due to the extensive use as a contact herbicide for aquatic weeds control, there is a high probability that aquatic organisms will be exposed to DQ and its metabolites22. Therefore, there is an urgent need to conduct the risk assessments of DQ and its metabolites in aquatic organisms.
Zebrafish has been widely used as a vertebrate model to assess the adverse effects of contaminants due to its unique advantages in high throughput screening, high fecundity, short reproductive cycle, rapid development, transparency during embryonic stages, orthologous genes with human beings, etc23. In addition, the Organization for Economic Cooperation and Development (OECD) has achieved great progress in standardizing test guidelines for the zebrafish embryo toxicity (FET) test (OECD 203, OECD 236)24. In addition to the primary developmental endpoints, external contaminants would induce more profound adverse damage in organisms. Exposure to xenobiotics or toxic environmental contaminants may induce an imbalance between reactive oxygen species (ROS) generation and antioxidant defenses, resulting in oxidative damage in organisms25. Therefore, the assessment of oxidative stress has become an important endpoint in aquatic toxicology26.
Herein, valuable morphometric endpoints (body length, heart rate, mortality, and malformation rate) at different stages along zebrafish embryo development as well as additional types of data on the molecular and biochemical responses (contents of oxidative damage biomarkers, activities of enzymatic antioxidants, and expression levels of related genes) to DQ and its metabolites (DQ-M and DQ-D) were collected to compare their aquatic toxicity. These findings may contribute to a more comprehensive understanding of the potential ecological risks of DQ and its metabolites to aquatic organisms and provide essential data to guide the scientific use of DQ.
Materials and methods
Materials and reagents
Diquat (DQ, CAS#: 85-00-7, > 95.0% purity), diquat-monopyridone (DQ-M, CAS#: 54016-01-2, > 95.0% purity), and diquat-dipyridone (DQ-D, CAS#: 35022-72-1, > 95.0% purity) were purchased from Toronto Research Chemicals Co., Ltd (Canada). Diquat-D4 (DQ-D4, CAS#: 347841-65-0, > 95.0% purity) was purchased from Tianjin Alta Technology Co., Ltd (China). Detailed information regarding DQ, DQ-M, DQ-D, and DQ-D4 are listed in Table S1. DQ, DQ-M, and DQ-D were dissolved in Milli-Q water with a stock concentration of 10−1 M and then diluted to working concentrations for subsequent assays.
Fish handling and exposure
The adult-wild type AB zebrafish used in the present study were purchased from the Institute of Hydrobiology, Chinese Academy of Sciences. Zebrafish husbandry, management, embryo collection, and all experimental protocols (developmental toxicity parameter statistics, oxidative damage biomarkers measurements, enzymatic antioxidants assessments, oxidative damage-related genes transcription evaluations) were in strict accordance with the ARRIVE Essential 10 guidelines and approved by the Institutional Animal Care and Use Committee of Zhejiang Shuren University. The authors confirm that all methods were performed in accordance with the relevant guidelines and regulations. To distinguish the toxic effects between DQ and its metabolites, twenty zebrafish embryos (0.5-1.0 hpf) were randomly divided into 24-well plates containing 2 mL exposure solutions (3 replicates). The exposure concentrations were Hank’s embryo culture media (negative control), and three environmentally relevant concentrations for DQ, DQ-M, and DQ-D (10−7–10−5 M)6,9. The exposure solutions were renewed on a daily basis, followed by a semi-static scenario to prevent concentration fluctuations27. The hatching of the embryos was recorded on a daily basis, commencing at 48 hpf, with observations being documented at 24 h intervals. At the end of exposure period (96 hpf), the developmental toxicity parameters including survival rates, deformity rates, body length, and heart rate were calculated. Following the exposure period, the larvae were anesthetized using MS-222 and flash-frozen in liquid nitrogen for subsequent assays.
Verification of exposure concentration
In order to circumvent fluctuations in exposure concentrations, exposure solutions in all experimental groups were collected for verification of exposure concentration every 24 h following renewal of the water. The verification of the exposure concentrations of the target chemicals was conducted using ultraperformance liquid chromatography (UPLC) in tandem with a micromass triple quadrupole detector (MS/MS) according to previous studies20,28. Briefly, 1 mL of the exposure solutions, collected at different time points (0 h, 24 h, 48 h, and 72 h) were spiked with 20 µL internal standard (500 ng/mL) and underwent centrifugation at 14,000 rpm for 20 min. Then, the supernatants were filtered through 0.22 μm aqueous filter membrane prior to analysis. The supernatants were subjected to quantify target compounds using Agilent 1290 UPLC-MS/MS (Agilent, USA). The MS/MS was equipped with an electrospray ionization source (ESI) operating in the positive-ion mode. The column employed was an UPLC Hilic Z column (2.1 mm × 100 mm, 2.7 μm, Agilent, USA) and maintained at a temperature of 35 °C. The mobile phase comprised a solution of acetonitrile and water with the addition of 20 mM ammonium formate as well as 0.1% formic acid. The gradient elution was conducted as follows: 80% organic phase (0–1.0 min), 80–73% organic phase (1.0–3.0 min), 73-80% organic phase (3.0–4.0 min), 80% organic phase (4.0–5.0 min). An injection volume of 2 mL was employed with a flow rate of 0.3 mL/min. The temperature of the source gas (nitrogen) was 350 °C with a flow rate of 8 L/min; the sheath gas temperature was 380 °C with a flow rate of 12 L/min; the nebulizer pressure was set at 55 psi; the capillary voltage was 1.0 kV. The samples were analyzed using full scan and product ion monitoring mode with a multiple reaction monitoring (MRM) method used for quantification. The acquisition of data was controlled using the Agilent Masshunter Software. The limit of detection (LOD) for DQ, DQ-M, and DQ-D were 0.3, 0.3, and 0.9 ng/mL, respectively. The optimized mass parameters for the determination of DQ, DQ-M, DQ-D, and DQ-D4 in positive ion mode are shown in Table S2.
Contents of oxidative damage biomarkers
The embryonic levels of ROS, malondialdehyde (MDA), and glutathione (GSH) were analyzed to reveal the oxidative stress to zebrafish according to our previous study29. The levels of ROS were quantified using the Reactive Oxygen Species Assay Kit (BOXBIO, China). One hundred zebrafish larvae were pooled and homogenized in PBS buffer (500 µL, equal to 0.1 g/mL) and subsequently subjected to a two-step centrifugation process. The sediment was resuspended in the DCFH-DA probe (1 µmol/L) and incubated in the dark at 37 °C prior to measurement with a microplate reader (excitation 488 nm, emission 525 nm). The internal MDA levels were quantified using the Malondialdehyde Assay Kit (BOXBIO, China). Briefly, two hundred zebrafish larvae were pooled and homogenized with MDA Extracting Buffer (1 mL, equal to 0.1 g/mL) in an ice bath. The supernatant was mixed with the Working Solution and incubated in a boiling water bath for 1 h. Then, the mixture was cooled in an ice bath and the supernatant was collected in order to record absorption values at 450 nm, 532 nm, and 600 nm. The GSH levels in zebrafish larvae were analyzed using the Reduced Glutathione Content Assay Kit (BOXBIO, China). Generally, one hundred zebrafish larvae were pooled and homogenized in the Extracting Buffer (500 µL, equal to 0.1 g/mL) and the supernatant was collected and incubated at room temperature for 2 min prior to measuring the absorption values at 412 nm. The total protein contents in zebrafish larvae were initially quantified using Enhanced BCA Protein Kit (BEYOTIME, China) to normalize the actual levels of these oxidative damage biomarkers.
Activities of enzymatic antioxidants
The enzyme activities of superoxide dismutase (SOD) and catalase (CAT) were analyzed to evaluate the neutralizing effects of harmful free radicals in zebrafish larvae according to our previous study29. The enzyme activity of SOD was measured using the Superoxide Dismutase Activity Assay Kit (BOXBIO, China). Four hundred zebrafish larvae were pooled and homogenized in the SOD Extracting Buffer (100 µL, equal to 2 g/mL) in an ice bath. The supernatant was collected and incubated at room temperature for 30 min prior to measuring the absorption values at 560 nm. The enzyme activity of CAT was measured using the Catalase Activity Assay Kit (BOXBIO, China). Briefly, two hundred zebrafish larvae were pooled and homogenized with the CAT Extracting Buffer (1 mL, equal to 0.1 g/mL) in an ice bath. The supernatant was collected and mixed with 10 µL Crude Enzyme Solution and 190 µL CAT Working Solution prior to measuring the absorption values at 240 nm at 5 s and 65 s, respectively. All results of the enzyme activities were also normalized by the total protein contents.
Transcription of oxidative damage-related genes
The expression levels of oxidative damage-related genes in zebrafish larvae were evaluated to reveal the underlying mechanism. Briefly, twenty zebrafish larvae from different groups were homogenized with the TRIzol Reagent (Life Technologies, USA) for total mRNA extraction. The mRNA was used for cDNA reverse transcription using the PrimeScript RT Master Mix (Takara, Japan). The qRT-PCR procedure was conducted using the Quantstudio Real-time PCR System (ThermoFisher, USA) with a final volume of 10 µL. The thermal cycling procedure was set as follows: 37 °C for 15 min and 98 °C for 5 min; 40 cycles of 95 °C for 60 s, 95 °C for 15 s, 60 °C for 60 s. The relative expression levels were normalized using the 2–∆∆Ct method with β-actin as the reference gene30. The primer sequences of the target genes are listed in Table S3.
Statistical analysis
The data were analyzed using two sample t-test; normality tests were assessed using Shapiro-Wilk test; tests for equal variance were conducted using Levene test; post-hoc analysis were conducted using Tukey HSD test with Origin 8.0 software (OriginLab Corporation, USA). Results are presented as the mean ± standard deviation (SD) from 3 independent experiments. The levels of significance were set at *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Quantification of DQ, DQ-M, and DQ-D in the exposure solutions
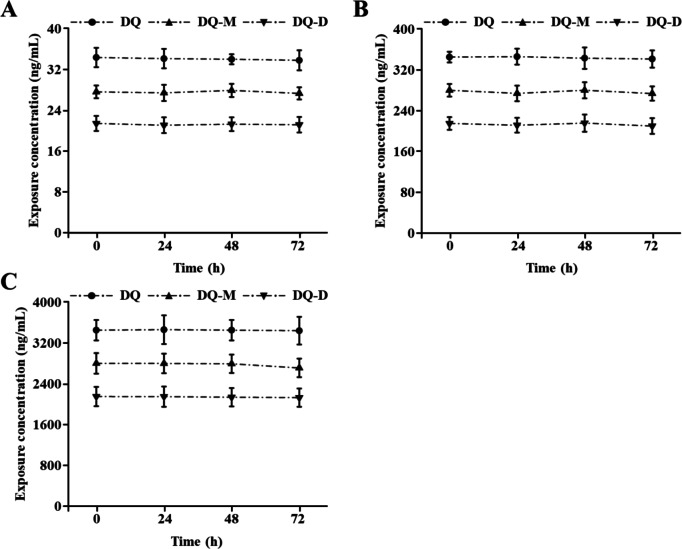
Generally, no significant fluctuation was observed in the measured concentrations of DQ, DQ-M, or DQ-D in the water samples compare to the nominal concentrations. As shown in Fig. 1, the initial mean concentrations of DQ, DQ-M, and DQ-D were in accord with the nominal concentrations, which were 33.73 ± 2.89, 340.05 ± 17.35, 3422.10 ± 261.21 ng/mL for DQ (equal to 9.8 × 10−8 M, 9.9 × 10−7 M, 1.0 × 10−5 M), 27.61 ± 1.25, 278.13 ± 13.36, 2783.33 ± 201.76 ng/mL for DQ-M (equal to 9.9 × 10−8 M, 1.0 × 10−6 M, 1.0 × 10−5 M), and 21.02 ± 1.87, 212.22 ± 16.45, 2129.20 ± 173.42 ng/mL for DQ-D (equal to 9.8 × 10−8 M, 9.9 × 10−7 M, 9.9 × 10−6 M). The mean concentrations of DQ in the water samples during the exposure period were 32.78–33.98 ng/mL, 329.34-336.91 ng/mL, and 3283.60-3381.68 ng/mL, respectively. For DQ-M, the mean concentrations in the water samples during the exposure period were 26.78–26.92, 268.20-275.85, and 2666.90-2751.76 ng/mL, respectively. As for DQ-D, the exposure concentrations also remained constant with the nominal concentrations, which were 20.88–21.13 ng/mL, 206.32-215.94 ng/mL, and 2071.33-2162.91 ng/mL, respectively.
Fig. 1.
The contents of the target compounds in the exposure medium at each time point within 72 h after DQ, DQ-M, and DQ-D violation (change half of the exposure solution every 24 h). (A) The exposure concentration of 10− 7 M DQ, DQ-M, and DQ-D exposure solution. (B) The exposure concentration of 10− 6 M DQ, DQ-M, and DQ-D exposure solution. (C) The exposure concentration of 10− 5 M DQ, DQ-M, and DQ-D exposure solution. Results were shown as mean ± standard deviation (SD) of at least three independent experiments (N = 9 samples).
Developmental toxicity to zebrafish
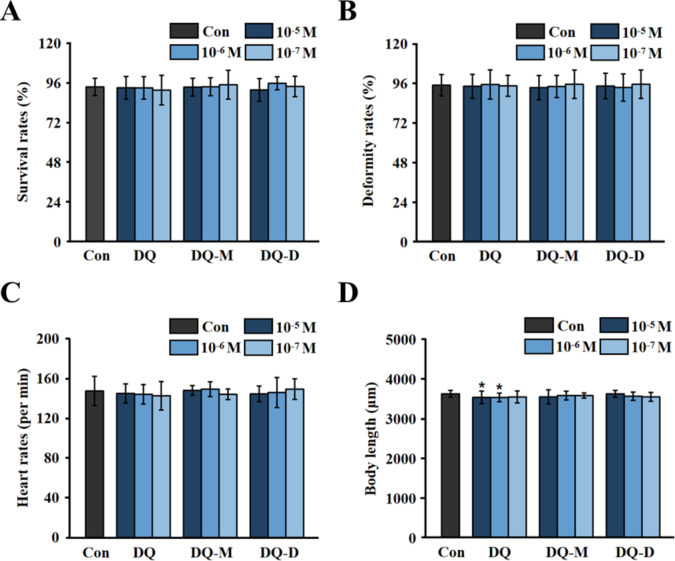
There may not be much of a difference in the developmental toxicity to zebrafish embryos induced by DQ, DQ-M, or DQ-D at the tested concentrations. As shown in Fig. 2, no significant difference was observed in the survival rates, deformity rates, or heart rates of zebrafish larvae after 96 h exposure. However, DQ significantly suppressed the body length of zebrafish larvae at the concentrations of 10− 5 and 10− 6 M.
Fig. 2.
DQ, DQ-M, and DQ-D induced developmental toxicity to zebrafish embryos. (A) Survival rates of zebrafish larvae after 96 h exposure to different concentrations of DQ, DQ-M, and DQ-D (N = 60 samples). (B) Deformity rates of zebrafish larvae after 96 h exposure to different concentrations of DQ, DQ-M, and DQ-D (N = 60 samples). (C) Heart rates of zebrafish larvae after 96 h exposure to different concentrations of DQ, DQ-M, and DQ-D (N = 15 samples). (D) Body length of larval zebrafish after 96 h exposure to different concentrations of DQ, DQ-M, and DQ-D (N = 15 samples). Results were shown as mean ± standard deviation (SD) of at least three independent experiments (*P < 0.05, **P < 0.01 and ***P < 0.001).
Levels of oxidative damage biomarkers
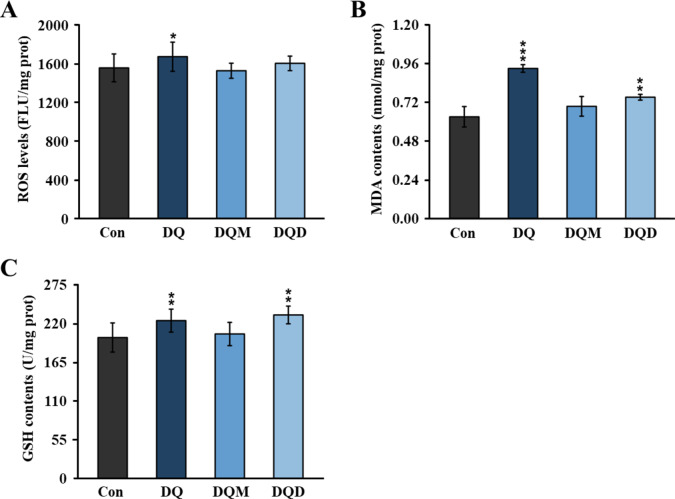
DQ, DQ-M, and DQ-D differed in the generation of oxidative damage biomarkers in zebrafish larvae. The ROS levels in larvae exposed to DQ were 1.07-fold higher than that in the control group, but DQ-M and DQ-D had no significant effects on the internal ROS levels (Fig. 3A). DQ and DQ-D posed similar effects on MDA and GSH levels. The internal MDA levels in the DQ and DQ-D treated groups were 1.48- and 1.19-fold higher than the control group (Fig. 3B). As to GSH levels, DQ and DQ-D promoted GSH production by 12.96% and 16.02%, respectively (Fig. 3C). However, no significant difference was observed in the MDA and GSH levels of DQ-M treated larvae.
Fig. 3.
Contents of oxidative damage biomarkers in zebrafish larvae after 96 h exposure to 10− 6 M DQ, DQ-M, and DQ-D. (A) Concentration of ROS in zebrafish larvae after 96 h exposure to 10− 6 M DQ, DQ-M, and DQ-D. (B) Concentration of MDA in zebrafish larvae after 96 h exposure to 10− 6 M DQ, DQ-M, and DQ-D. (C) GSH levels in zebrafish larvae after 96 h exposure to 10− 6 M DQ, DQ-M, and DQ-D. Results were shown as the mean ± standard deviation (SD) of at least three independent experiments (N = 9 samples, *P < 0.05, **P < 0.01 and ***P < 0.001).
Activities of enzymatic antioxidants
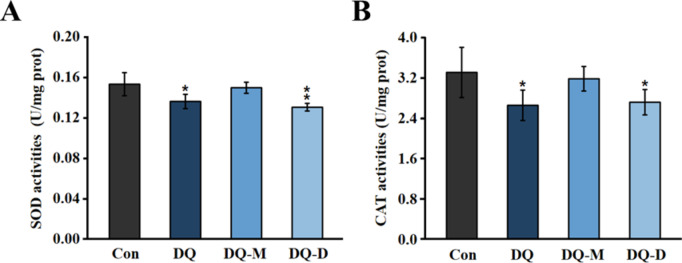
DQ and DQ-D also altered the activities of enzymatic antioxidants except for DQ-M (Fig. 4). For SOD activities, DQ and DQ-D inhibited the enzyme activities by 11.15% and 14.86%, respectively. DQ and DQ-D treatments also significantly suppressed the enzyme activity of CAT in zebrafish larvae, which were 19.70% and 17.88% lower compared with that of the control group, respectively.
Fig. 4.
Activities of enzymatic antioxidants in larval zebrafish after 96 h exposure to 10− 6 M DQ, DQ-M, and DQ-D. (A) Enzyme activities of SOD in larval zebrafish after 96 h exposure to 10− 6 M DQ, DQ-M, and DQ-D. (B) Enzyme activities of CAT in larval zebrafish after 96 h exposure to 10− 6 M DQ, DQ-M, and DQ-D. Results were shown as the mean ± standard deviation (SD) of at least three independent experiments (N = 9 samples, *P < 0.05, **P < 0.01 and ***P < 0.001).
Expression levels of oxidative stress-related genes
The expression levels of oxidative stress-related genes were analyzed to monitor the response at transcriptional level in zebrafish larvae after exposure to the target chemicals. Generally, DQ and DQ-D significantly altered the transcription of oxidative stress-related genes, while DQ-M did not (Fig. 5). Specifically, DQ and DQ-D enhanced the transcription of GPX by 1.19- and 1.12-fold, respectively. The expression of CAT and Mn-SOD in zebrafish larvae were significantly inhibited with DQ treatment, which were 81.32% and 88.48%, respectively, of the control group. DQ-D also decreased the expression of CAT and Mn-SOD by 13.09% and 15.21%, respectively. However, none of the target chemicals significantly disturbed the transcription of GPX1a and Cu/Zn-SOD within the test concentrations.
Fig. 5.
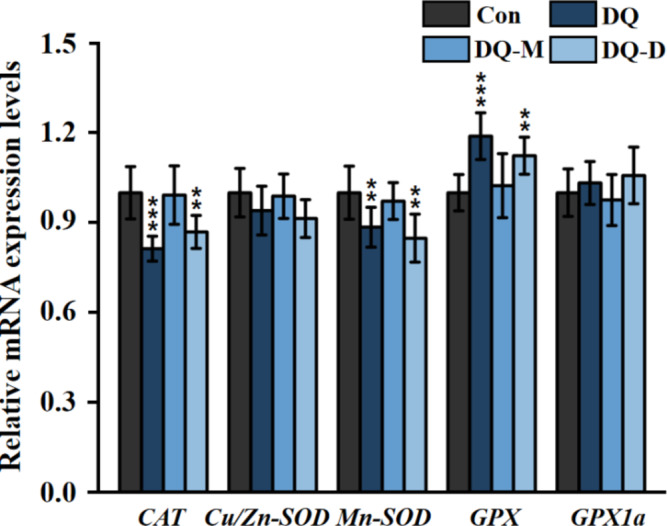
Expression levels of oxidative stress-related genes in larval zebrafish after 96 h exposure to 10− 6 M DQ, DQ-M, and DQ-D. Results were shown as the mean ± standard deviation (SD) of at least three independent experiments (N = 15 samples, *P < 0.05, **P < 0.01 and ***P < 0.001).
Discussion
The massive use of agrochemicals worldwide has posed serious threat to human beings and ecosystems31. As a non-selective, fast-acting herbicide, DQ is one of the few herbicides registered for direct application to aquatic systems, resulting in its inevitable presence in aquatic ecosystems through various transfer processes after repeated applications4,5. Previous studies also have proved that DQ might maintain an integrated state in the hydrosphere for long periods and tend to accumulate in aquatic organisms8,9. Given these, the environmental levels of DQ in aquatic systems would be quite high and would most likely to become an ecological burden to aquatic organisms10. Besides, DQ undergoes degradation via abiotic processes (including chemical and photochemical reactions) and biotic transformation processes (mediated by microorganisms, plants, or animals)32. Sufficient researches have demonstrated that the metabolites of pesticides would pose more potent adverse effect than the parent compounds16–19. It is therefore of the utmost importance that scientists fully understand the potential risks posed by DQ and its metabolites to aquatic organisms.
DQ has been widely used as a substitution for paraquat. Substitution, whose actual ecological risks are yet uncertain, also poses risks when replacing a well-investigated agrochemical. The urgency of recognizing the profound impacts of agrochemicals in the environment has aggravated as researchers have issued warnings about the adverse outcomes by applying certain agrochemicals. As an aquatic herbicide, the potential risks posed by DQ to non-target aquatic organisms are considerable33. The determined 96 h LC50 value of DQ for rainbow trout (Oncorhynchus mykiss) larvae (85 days post-hatch) was 9.8 mg/L12. Similarly, the 96 h LC50 value of DQ for common carp larvae (Cyprinus carpio L.) was 50 mg/L34. Leblanc reported that the 24 h and 96 h LC50 values of DQ for mosquitofish were even up to 723 and 289 mg/L, respectively35. As a substitution for paraquat, DQ is known to be significantly less toxic3. The 96 h LC50 of paraquat for Mesopotamichthys sharpeyi and catfish (Clarias gariepinus) were 1.49 and 1.75 mg/L, respectively36,37. In the current study, apart from the suppression of body length by DQ at 10− 5 M (equivalent to 3.44 mg/L), no notable toxicity to zebrafish embryos was observed after exposure to DQ and its metabolites at environmentally relevant levels. However, many pesticides currently being used have uncertain effects (sub-lethal effects and conventional apical endpoints) on aquatic organisms compared to those with well-established toxicity profiles. As a low-toxicity substitution for paraquat, DQ has been widely applied in aquatic systems. However, the sub-lethal effects induced by DQ and its metabolite on aquatic organisms call for further and more detailed investigation.
Oxidative stress, resulting from the imbalanced generation of ROS following exposure to contaminants, has become an important issue in aquatic toxicology38. ROS are produced by the physiological metabolism of organisms and play a vital role in regulating physiological functions39. ROS levels typically increase sharply under environmental stress and abnormal ROS levels are closely associated with pathologic changes such as inflammation and cell death29,39. Herein, ROS levels in zebrafish larvae were significantly promoted after DQ exposure, suggesting DQ-induced oxidative stress and the possibility of significant embryonic damage. Free radical attacks on membrane phospholipids caused by excessive ROS generation can lead to the production of MDA40. In the present study, the increased MDA levels induced by DQ and DQ-D treatments hinted the possibility of oxidative stress. Moreover, the higher MDA levels induced by DQ were in accordance with the higher internal ROS levels. The abnormal generation of ROS induced by contaminants could subsequently be eliminated by antioxidant systems in organisms, including non-enzyme inhibitors (GSH) and antioxidant enzymes (e.g. SOD and CAT)41. GSH is known to play key role in the detoxification process by forming water-soluble substances with contaminants to be excreted from the body42. Therefore, GSH depletion is investigated as an indicator of oxidative stress. Herein, DQ and DQ-D significantly increased embryonic GSH levels. Pesticides such as diazinon and diuron have also been reported to increase GSH levels in zebrafish embryos and larvae43. As a free radical scavenger, GSH serves as the first line of defense against toxic chemicals and counteracts the effects of oxidative stress44. The increased GSH levels implied that DQ and DQ-D caused oxidative damage to zebrafish and activated the antioxidant defense system. However, GSH levels might decrease in certain situations after exposure to contaminants. For example, long-term exposure to fluindapyr significantly down-regulated GSH levels in earthworms29. GSH levels in zebrafish increased with exposure to low concentrations of microplastics (0.1 and 1 mg/L), but decreased with exposure to higher concentration (10 mg/L)45. These might be attributed to the suppression on detoxification process. SOD and CAT are also vital to eliminate excess ROS41. SOD effectively sustains the oxidative balance by converting superoxide radicals to H2O2, while CAT subsequently catalyzes H2O2 to water and oxygen to protect against the oxidative damage from ROS26,45. Results showed that the enzyme activities of SOD and CAT were significantly suppressed by DQ and DQ-D. The reduction in SOD and CAT activities suggested the compromised protective system of zebrafish larvae and the increasing susceptibility of zebrafish larvae to DQ and DQ-D-induced oxidative damage.
Evaluating the transcription of oxidative stress-related genes can help to reflect the antioxidant capacity of zebrafish. Cu/Zn-SOD and Mn-SOD encode two types of superoxide dismutase in eukaryocytes38. Herein, the enzyme activity of SOD is the integrated activity of two enzymes. The downregulated expressions of Mn-SOD and CAT were in consistence with the suppressed enzyme activities of SOD and CAT. These findings suggested that SOD and CAT in zebrafish larvae might be impaired by DQ and DQ-D-induced oxidative damage, and the down-regulated expression of the corresponding encoding genes could account for the suppressed enzyme activities. Peroxidases (GPX, GPX1a) are a family of cytosolic and mitochondrial isoenzymes responsible for the reduction of fatty acid hydroperoxides and H2O2 by using of glutathione46. It has been speculated that the up-regulation of GPX was probably to activate antioxidant activities to eliminate free radicals induced by DQ and DQ-D.
The profits of global agrochemical application come at the cost of their ubiquitous presence in the environment. Abiotic and biotic transformations efficiently eliminate agrochemicals from the environment but also trigger potentially hazardous metabolites32. Previous studies have reported the presence of various pesticide metabolites in aquatic systems with some metabolites exhibiting more frequent detection than their parent compounds47. As the global consumption of agrochemicals is anticipated to keep increasing, the issue of the ecological risks of agrochemical metabolites remains important. For instance, it has been reported that the metabolite (4-hydroxychlorothalonil) of the fungicide chlorothalonil could induce higher lethal effects to zebrafish embryos and had more complex endocrine-disrupting effects19. In addition, Ji et al. reported that about half of the tested pesticide metabolites exhibited stronger endocrine-disrupting effects than their corresponding parent compounds16. In the present study, DQ and its metabolites did not induce significant acute toxicity to zebrafish embryos, but evident oxidative stress was observed after exposure to DQ and DQ-D. Oxidative stress is closely involved in developmental toxicity during zebrafish embryogenesis48. Therefore, the aquatic herbicide DQ and the metabolite DQ-D would inevitably pose ecological risks to aquatic organisms. Though, DQ-D-induced sub-lethal effects were to some extent lower than those induced by DQ. However, pesticide metabolites can achieve higher concentrations due to the bioaccumulation process in the terrestrial food chain. Therefore, even though the initial effects of pesticide metabolites are typically lowered, they may still be a highly relevant issue of concern49.
Conclusion
Overall, our study demonstrated that despite the low acute toxicity as paraquat substitution, DQ and its metabolite (DQ-D) still posed oxidative stress to zebrafish larvae by increasing oxidative contents and suppressing antioxidative enzymes, which would induce unavoidable ecological risks to non-target aquatic organisms. Data presented here remind us that there are still room for considerable perfection in agrochemical regulation and related policy-making.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Natural Science Foundation of Zhejiang Province (LQ22B070007), the Youth Foundation of National Natural Science Foundation of China (22106142).
Author contributions
Lanxin Shi: Investigation, Visualization; Xinru Wang: Investigation, Methodology; Yaoyao Dai: Investigation, Simulation and Calculation; Wendong Zhou: Investigation, Visualization; Shenggan Wu: Methodology; Bo Shao: Methodology; Gorettie Nsubuga Nabanoga: Writing-review and editing; Chenyang Ji: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing-original draft, Writing-review and editing; Meirong Zhao: Funding acquisition, Resources, Supervision.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Magalhães, N., Carvalho, F. & Dinis-Oliveira, R. Human and experimental toxicology of diquat poisoning: Toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum. Exp. Toxicol.37, 1131–1160 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Feng, D., Fu, L., Du, X. & Yao, L. Acute diquat poisoning causes rhabdomyolysis: a case report and literature review. Am. J. Med. Sci.364, 1 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Choi, S., Park, Y. & Koh, H. NF-κB/p53‐activated inflammatory response involves in diquat-induced mitochondrial dysfunction and apoptosis. Environ. Toxicol.33, 1005–1018 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Pateiro-Moure, M., Arias-Estévez, M. & Simal-Gándara, J. Competitive and non-competitive adsorption/desorption of paraquat, diquat and difenzoquat in vineyard-devoted soils. J. Hazard. Mater.178, 194–201 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Siemering, G., Hayworth, J. & Greenfield, B. Assessment of potential aquatic herbicide impacts to California aquatic ecosystems. Arch. Environ. Contam. Toxicol.55, 415–431 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Oh, J., Lee, J., Lee, S. & Shin, H. Ultra-trace level determination of diquat and paraquat residues in surface and drinking water using ion-pair liquid chromatography with tandem mass spectrometry: A comparison of direct injection and solid-phase extraction methods. J. Sep. Sci.37, 2900–2910 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Ritter, A., Shaw, J., Williams, W. & Travis, K. Characterizing aquatic ecological risks from pesticides using a diquat dibromide case study. I. Probabilistic exposure estimates. Environ. Toxicol. Chem.19, 749–759 (2000). [Google Scholar]
- 8.European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance diquat. EFSA J.13 (11), 4308 (2015). [Google Scholar]
- 9.Emmett, K. Final Risk Assessment for Diquat Bromide (Washington State Department of Ecology, 2002).
- 10.Xiao, Y. et al. Metabolomics analysis of the potential toxicological mechanisms of diquat dibromide herbicide in adult zebrafish (Danio rerio) liver. Fish. Physiol. Biochem.48, 1039–1055 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Sanchez, W., Palluel, O., Lagadic, L., Aït-Aïssa, S. & Porcher, J. Biochemical effects of nonylphenol polyethoxylate adjuvant, diquat herbicide and their mixture on the three-spined stickleback (Gasterosteus aculeatus L.). Mar. Environ. Res.1, 29–33 (2006). [DOI] [PubMed] [Google Scholar]
- 12.McCuaig, L., Martyniuk, C. & Marlatt, V. Morphometric and proteomic responses of early-life stage rainbow trout (Oncorhynchus mykiss) to the aquatic herbicide diquat dibromide. Aquat. Toxicol.222, 105446 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Coutellec, M., Delous, G., Cravedi, J. & Lagadic, L. Effects of the mixture of diquat and a nonylphenol polyethoxylate adjuvant on fecundity and progeny early performances of the pond snail Lymnaea stagnalis in laboratory bioassays and microcosms. Chemosphere73, 326–336 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Babalola, O. & van Wyk, H. Exposure Impacts of Diquat dibromide herbicide formulation on amphibian larval development. Heliyon7, e06700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, X., Souders, C., Zhao, Y. & Martyniuk, C. Mitochondrial bioenergetics and locomotor activity are altered in zebrafish (Danio rerio) after exposure to the bipyridylium herbicide diquat. Toxicol. Lett.283, 13–20 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Ji, C. et al. The potential endocrine disruption of pesticide transformation products (TPs): The blind spot of pesticide risk assessment. Environ. Int.137, 105490 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Berton, T. et al. Development of an analytical strategy based on LC-MS/MS for the measurement of different classes of pesticides and theirs metabolites in meconium: application and characterisation of foetal exposure in France. Environ. Res.132, 311–320 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Lu, M. et al. Endocrine disrupting potential of fipronil and its metabolite in reporter gene assays. Chemosphere120, 246–251 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Zhang, Q. et al. The identification of the metabolites of chlorothalonil in zebrafish (Danio rerio) and their embryo toxicity and endocrine effects at environmentally relevant levels. Environ. Pollut.218, 8–15 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Fuke, C. et al. Analysis of paraquat, diquat and two diquat metabolites in biological materials by high-performance liquid chromatography. Leg. Med.4, 156–163 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Nakajima, M. et al. Purification and characterization of diquat (1,1’-ethylene-2, 2’-dipyridylium)-metabolizing enzyme from paraquat-resistant rat liver cytosol. Toxicology154, 55–66 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Jones, G. & Vale, J. Mechanisms of toxicity, clinical features, and management of diquat poisoning: A review. Toxicol. Clin. Toxicol.38, 123–128 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Wang, Q. et al. Identification of apoptosis and macrophage migration events in paraquat-induced oxidative stress using a zebrafish model. Life Sci.157, 116–124 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Souders, C. L. et al. High-throughput assessment of oxidative respiration in fish embryos: Advancing adverse outcome pathways for mitochondrial dysfunction. Aquat. Toxicol.199, 162–173 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Cai, Z. et al. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohydr. Polym.195, 29–38 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Ali, D., Alarifi, S., Kumar, S., Ahamed, M. & Siddiqui, M. Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquat. Toxicol.124-125, 83–90 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Ji, C. et al. Stage dependent enantioselective metabolism of bifenthrin in embryos of zebrafish (Danio rerio) and Japanese medaka (Oryzias latipes). Environ. Sci. Technol.55, 9087–9096 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Mao, Z. et al. Simultaneous determination of diquat and its two primary metabolites in rat plasma by ultraperformance liquid chromatography-tandem mass spectrometry and its application to the toxicokinetic study. Forensic Toxicol.40, 332–339 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji, C. et al. Evaluation of the toxic effects of fluindapyr, a novel SDHI fungicide, to the earthworms Eisenia fetida. Sci. Total Environ.899, 165697 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Ji, C. et al. AhR agonist activity confirmation of polyhalogenated carbazoles (PHCZs) using an integration of in vitro, in vivo, and in silico models. Environ. Sci. Technol.53, 14716–14723 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Ji, C. et al. Enantioselectivity in the toxicological effects of chiral pesticides: A review. Sci. Total Environ.857, 159656 (2023b). [DOI] [PubMed] [Google Scholar]
- 32.Fenner, K., Canonica, S., Wackett, L. & Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science341, 752–758 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Moreton, M. & Marlatt, V. Toxicity of the aquatic herbicide, reward, to the northwestern salamander. Environ. Sci. Pollut. Res.26, 31077–31085 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Chin, Y. & Sudderuddin, K. Effect of methamidophos on the growth rate and esterase activity of the common carp Cyprinus carpio L. Environ. Pollut.18, 213–220 (1979). [Google Scholar]
- 35.Leung, T., Naqvi, S. & Leblanc, C. Toxicities of two herbicides (Basagran, Diquat) and an algicide (Cutrine-plus) to mosquitofish Gambusia affinis. Environ. Pollut.30, 153–160 (1983). [Google Scholar]
- 36.Jaddi, Y., Safahieh, A. & Salighehzadeh, R. Determination of lethal range and the median lethal concentration (LC50 96 h) of paraquat on benny fish (Mesopotamichthys sharpeyi) (2014).
- 37.Ladipo, M., Doherty, V. & Oyebadejo, S. Acute toxicity, behavioural changes and histopathological effect of paraquat dichloride on tissues of catfish (Clarias gariepinus). Int. J. Biol.3 (2), 67–74 (2011). [Google Scholar]
- 38.Li, H. et al. Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere207, 781–790 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Pourová, J., Kottova, M., Vopršálová, M. & Pour, M. Reactive oxygen and nitrogen species in normal physiological processes. Acta Physiol.198, 1 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Clemente, R. et al. Combination of soil organic and inorganic amendments helps plants overcome trace element induced oxidative stress and allows phytostabilisation. Chemosphere223, 223–231 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Wang, C., Yang, X., Zheng, Q., Moe, B. & Li, X. F. Halobenzoquinone-induced developmental toxicity, oxidative stress, and apoptosis in zebrafish embryos. Environ. Sci. Technol.52 (18), 10590–10598 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Velki, M. & Hackenberger, B. K. Biomarker responses in earthworm Eisenia andrei exposed to pirimiphos-methyl and deltamethrin using different toxicity tests. Chemosphere90 (3), 1216–1226 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Velki, M. et al. Pesticides diazinon and diuron increase glutathione levels and affect multixenobiotic resistance activity and biomarker responses in zebrafish (Danio rerio) embryos and larvae. Environ. Sci. Eur.1, 1 (2019). [Google Scholar]
- 44.Gu, W. et al. Single and joint toxic effects of waterborne exposure to copper and cadmium on Coregonus ussuriensis Berg. Ecotoxicology32, 895–907 (2023). [DOI] [PubMed]
- 45.Ding, N. et al. Polyethylene microplastic exposure and concurrent effect with Aeromonas hydrophila infection on zebrafish. Environ. Sci. Pollut. Res.29, 63964–63972 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Sadi, G. & Güray, T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: Effect of antioxidants. Mol. Cell Biochem.327, 127–134 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Reemtsma, T., Alder, L. & Banasiak, U. Emerging pesticide metabolites in groundwater and surface water as determined by the application of a multimethod for 150 pesticide metabolites. Water Res.47, 5535–5545 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Usenko, C., Harper, S. & Tanguay, R. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol. Appl. Pharmacol.229 (1), 44–55 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boxall, A., Sinclair, C., Fenner, K., Kolpin, D. & Maund, S. When synthetic chemicals degrade in the environment. Environ. Sci. Technol.38, 368–375 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.