Abstract
Wheat stripe rust is a fungal disease caused by Puccinia striiformis f. sp. tritici. The outbreak of wheat stripe rust will have a great impact on wheat production in Xinjiang, China. In order to identify resistance to wheat stripe rust and the distribution of resistance genes in 82 wheat cultivars (41 spring wheat and 41 winter wheat), wheat seedling resistance was evaluated using CYR32, CYR33 and CYR34, and wheat adult plant stage resistance was identified using a combination of 3 races. Six molecular markers were used to identify Yr29, Yr39, Yr46, Yr69 and YrTr1 in 82 wheat cultivars. The results showed that 3 of 82 wheat cultivars (Xinchun No.14, Xinchun No.22, and Xindong No.22) were immune to stripe rust at the adult plant stage. Xinchun No.29, Xinchun No.32, Xindong No.5 and Xindong No.29 were resistant at all stage. The highest detection rates were for Yr69 and YrTr1, at 78.05% and 76.83%. However, the detection rates for Yr39 and Yr46 were only 0 and 2.44%, respectively. The Xindong No.22 were detected with the most resistance genes, which included 4 Yr genes. Furthermore, Xindong No.22 were immune to the disease at adult plant stage. The results confirmed the resistance gene distribution of the wheat cultivars in Xinjiang were heterogeneously, and the number of Yr genes was significantly and positively correlated with wheat cultivars resistant to stripe rust.
Keywords: Xinjiang spring wheat, Stripe rust of wheat, Evaluation of resistance to stripe rust, Detection of resistance genes
Subject terms: Biotechnology, Molecular biology, Physiology, Plant sciences
Introduction
Wheat is one of the four staple crops in China, and its yield has a significant impact on the nation’s economic development. Wheat stripe rust caused by Puccinia striiformis f. sp. tritici (Pst) is one of the most dangerous crop diseases endangering wheat production, causing major reductions in yield and quality1. Historically, Pst has caused catastrophic epidemics and major wheat yield loss in China in 1950, 1964, 1990, 2002, and 2017, resulting in yield losses of over 6.0, 3.2, 1.8, 1.3, and 1.5 million tons, respectively2.
The wheat stripe rust epidemic in China can be divided into several epidemiological regions3. Xinjiang has a large wheat planting area, and wheat is distributed in all crop-growing areas except the Turpan region. Yili Kazak Autonomous Prefecture and Kashgar Prefecture have the largest wheat planting areas4. Because of its unique geographical and meteorological, Xinjiang was designated as a relatively independent epidemiological zone for wheat stripe rust in China5. Wheat stripe rust is a prevalent wheat disease in Xinjiang and has become a pivotal constraint in local wheat production6.
There are many methods for controlling wheat stripe rust, but breeding and promoting cultivars with resistance genes is currently the most cost-effective and environmentally benign option7. Due to their high disease resistance and stable inheritance, resistance genes have been extensively introduced into wheat cultivar breeding. According to the references, more than 100 stripe rust-resistance genes have been detected in wheat and 86 already identified and cataloged, although only a few have been thoroughly researched8–10. For example, resistance genes such as Yr9, Yr29, Yr36, and YrTr1 have been extensively integrated into wheat cultivar breeding11. Some resistance genes were resistant not only to stripe rust, but also to other diseases, such as Yr29/Lr46 and Yr46/Lr67 that were resistant to stripe rust and leaf rust. Wheat cultivars Abbondanza and Fengchan 3 were widely cultivated in South and central Shaanxi Province to control wheat stripe rust for 9 years in 196512,13. Therefore, identifying Yr gene distribution and Yr gene combinations could aid in development of novel wheat cultivars for long-term stripe rust management.
Adult plant resistance (APR) and all stage resistance (ASR) are two major types of resistance to stripe rust14. APR is only resistant to Pst in the adult stage of wheat, and resistance increases as the plant matures. ASR refers to Pst resistance in wheat seedlings and adult stage. ASR is typically race-specific and qualitatively inherited, whereas APR is non-race specific, durable, and frequently quantitatively inherited15. In recent years, researchers discovered resistance to Pst in some wheat cultivars16. These resistant cultivars have better resistance performance in the field. The wide planting of these resistant cultivars in the fields greatly reduces the probability of stripe rust outbreaks, thus ensuring the production of wheat in China. Han et al. screened out 50 resistant cultivarsfrom 1980 cultivars17. Sun et al. screened out 8 resistant cultivars from 100 cultivar18.
Since 1957, Chinese yellow (CY) series have been assigned to Chinese Pst races, and Chinese yellow rust (CYR) series designation for these rust races is still used today. So far, 34 races have been officially named CYR1 - CYR34. In nature, pathogens can rapidly evolve. Wheat rust pathogens can evolve into new virulent races with a high frequency in the field19–21. The frequencies of CYR32 and CYR33 were the highest among all detected races from 1997 to 2009. In 2009, a new Pst race virulent on wheat line G22 was detected in Sichuan Province, China22. Because of its high infection fitness and aggressiveness on wheat cultivars, G22-virulent races have expanded fast throughout China. Among G22 races, G22-9 (previously V26) emerged as a new predominant race and was officially titled as CYR3423,24. So far, CYR32, CYR33, and CYR34 were the predominant races in China.
The longer the planting years, the worse the disease resistance. Meanwhile, high genetic variation in the pathogen population and the rapid rate of selection for new virulent species lead to some cultivars losing their resistance to Pst. In order to evaluate the resistance of spring and winter wheat cultivars to Pst, identify the distribution of the resistance genes, and screen high-resistance cultivars in Xinjiang, China, 82 wheat cultivars were identified using CYR32, CYR33 and CYR34 to study at the seedling stage. At the adult plant stage of wheat, the mixture of races were artificially inoculated to evaluate the resistance of 82 wheat cultivars in field and greenhouses. Furthermore, the resistance genes Yr29, Yr39, Yr46, Yr69 and YrTr1 were identified in 82 wheat cultivars using molecular markers. The resistant cultivars were screened out from spring and winter wheat and provided a scientific foundation for wheat disease resistance breeding and the rational distribution of wheat cultivars in Xinjiang, China.
Results
Wheat seedling stage resistance
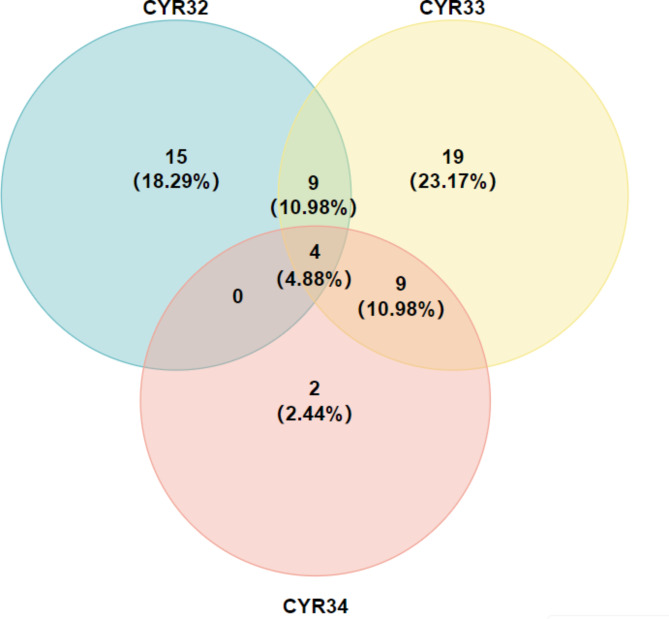
The evaluation results of 82 wheat cultivars for seedling stage resistance to stripe rust in phytotron showed that 28, 41 and 15 wheat cultivars were resistant to races CYR32, CYR33 and CYR34 (IT: 0 ~ 4), accounting for 34.15%, 50% and 18.29% of the total wheat cultivars, respectively (Fig. 1). In recent years, CYR34, a new race has proven to be more virulent than CYR32 and CYR33, only Xinchun No.29 was immune to CYR34 among the 82 cultivars. The proportion of wheat cultivars with resistance to 0 ~ 3 races was 29.27%, 43.90%, 21.95% and 4.88%, respectively. Among them, the cultivars resistant to three Pst races were Xinchun No.29, Xinchun No.32, Xindong No.5 and Xindong No.29.
Fig. 1.
Distribution of resistant wheat cultivars to the three pathogen races CYR32, CYR33 and CYR34. Venn diagram show the ratio of resistant cultivars among 82 wheat cultivars at the seedling stage.
Wheat adult plant stage resistance
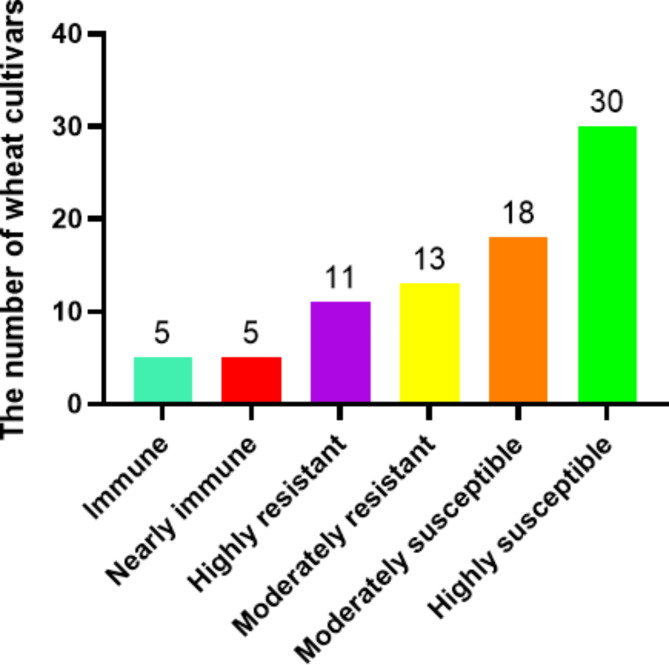
Eighty-two wheat cultivars were evaluated in the field and greenhouse for resistance to stripe rust at the adult plant stage. To ensure the accuracy of the field experiment, the 82 wheat cultivars’ resistance was evaluated in a greenhouse as well. The final evaluate results based on the highest reaction type and disease severity. The results showed that 34 of 82 wheat cultivars were resistant to stripe rust, accounting for 41.46% of the total wheat cultivars (Fig. 2). Among them, 5 cultivars were immune to stripe rust, include Xinchun No.14, Xinchun No.22, Xinchun No.29, Xinchun No.32 and Xindong No.22, accounting for 6.10%. Five cultivars, Xinchun No.9, Xinchun No.16, Xinchun No.19, Xinchun No.21 and Xindong No.38, were nearly immune to stripe rust accounting for 6.10%. In spring wheat cultivars, the proportion of resistant cultivars was 63.41%, whereas in winter wheat cultivars it was only 19.51%. Therefore, spring wheat cultivars were more resistant to stripe rust than winter wheat cultivars. The highest disease severity grade was 80%, and the highest disease severity grade of 80% was displayed by14.63% of all wheat cultivars. According to the research results, there was a poor level of disease resistance and a higher disease severity on wheat cultivars in Xinjiang. However, some cultivars exhibit outstanding resistance in adult plant stage. Therefore, the resistance cultivars may be prioritized for breeding procedures in Xinjiang.
Fig. 2.
The number of wheat cultivars classified .according to resistance reaction in the adult plant stage.
Wheat all stage resistance
The resistance to wheat stripe rust at all stage was comprehensive evaluation which Xinchun No.29, Xinchun No.32, Xindong No.5 and Xindong No.29 were classified as resistant to wheat stripe rust in the seedling stage, with Xinchun No.29 and Xinchun No.32 indicating immune responses in the adult plant stage. Therefore, from the screening of 82 wheat cultivars, 5 were categorized as immune cultivars in the adult plant stage (Xinchun No.14, Xinchun No.22, Xinchun No.29, Xinchun No.32 and Xindong No.22) and 4 as resistant cultivars in all stage (Xinchun No.29, Xinchun 32, Xindong No.5 and Xindong No. 29). According to the pedigree information of these 82 cultivars, most were bred in Xinjiang. But wheat cultivar 85 − 56 and 25 − 3 were the parents of Xinchun No.29, wheat cultivar 97 − 18 and Yongliang 11 were the parents of Xinchun No.32, wheat cultivar Bakhfuk and Beijing No.7 were the parents of Xinchun No.5, and wheat cultivar PH82-2-2 and Luzhi 79 − 1 were the parents of Xindong No.29. Therefore, at least one parent of the 4 all stage resistant cultivars were introduced from other regions, supporting the theory that resistance was inherited from these parents.
The detection of disease-resistance genes
The resistance genes Yr29, Yr39, Yr46, Yr69 and YrTr1 were detected using the molecular markers in 82 wheat cultivars. The results showed that the number of wheat cultivars carrying Yr29, Yr39, Yr46, Yr69 and YrTr1 were 24, 0, 2, 64 and 63, respectively. The detection rates of these 5 resistance genes were 29.27%, 0, 2.44%, 78.05%, and 76.83%, respectively. The number of resistance genes detected also varied among wheat cultivars. The wheat cultivars containing 0 ~ 5 genes were 3, 23, 35, 20, 1 and 0, accounting for 3.66%, 28.05%, 42.68%, 24.39%, 1.22% and 0, respectively. No resistance genes were detected in Xindong No.6, Xindong No.19 and Xindong No.52 and therefore the three cultivars were susceptible in the field evaluation (Table 1). Xindong No.22, which contains 4 resistance genes (Yr29, Yr46, Yr69 and YrTr1), was an adult stage immune cultivar. In this study, wheat cultivars with more resistance genes had higher resistance against stripe rust.
Table 1.
Stripe rust reactions at seedling and adult plant stage, and amplifications by Yr gene specific molecular markers.
| Line | Cultivar | Seedling Stage | Adult Plant Stage | Molecular marker test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CYR32 | CYR33 | CYR34 | Yr29 | Yr39 | Yr46 | Yr69 | YrTr1 | |||
| 1 | Xinchun No.2 | HS | MS | HS | 20MS | − | − | − | − | + |
| 2 | Xinchun No.3 | HS | HS | HS | 60MS | + | − | − | + | + |
| 3 | Xinchun No.5 | HS | MS | HS | 40HR | − | − | − | + | + |
| 4 | Xinchun No.6 | HS | MS | HS | 20MS | − | − | − | + | + |
| 5 | Xinchun No.7 | MS | MS | MS | 20MR | − | − | − | − | + |
| 6 | Xinchun No.8 | MS | MR | HS | 80HS | − | − | − | + | + |
| 7 | Xinchun No.9 | MS | HS | MR | 5NI | + | − | − | + | − |
| 8 | Xinchun No.10 | HS | MS | HS | 60HS | − | − | − | + | + |
| 9 | Xinchun No.11 | HS | HS | HS | 30HR | + | − | − | + | + |
| 10 | Xinchun No.12 | MS | MR | MR | 40MR | − | − | − | + | + |
| 11 | Xinchun No.13 | HS | MS | HS | 40HR | − | − | − | + | + |
| 12 | Xinchun No.14 | MS | MR | HR | 0I | + | − | − | − | − |
| 13 | Xinchun No.15 | HS | MR | MS | 20MR | − | − | − | + | + |
| 14 | Xinchun No.16 | HS | HR | MR | 10NI | + | − | − | + | + |
| 15 | Xinchun No.17 | MS | MS | HS | 40MR | + | − | − | + | + |
| 16 | Xinchun No.18 | MS | MS | HS | 80HS | − | − | − | − | + |
| 17 | Xinchun No.19 | HS | MR | HR | 5NI | − | − | − | + | + |
| 18 | Xinchun No.20 | HS | HS | HS | 40MR | − | − | − | + | + |
| 19 | Xinchun No.21 | MS | MR | MR | 5NI | − | − | − | + | − |
| 20 | Xinchun No.22 | HS | MR | MS | 0I | + | − | − | + | − |
| 21 | Xinchun No.23 | HS | HS | HS | 20HR | + | − | − | + | + |
| 22 | Xinchun No.25 | HS | MR | MS | 80HS | − | − | − | + | + |
| 23 | Xinchun No.26 | MS | HS | HS | 20MR | + | − | − | + | + |
| 24 | Xinchun No.27 | HS | MR | MS | 40MS | + | − | − | + | − |
| 25 | Xinchun No.28 | MR | HS | MS | 20HR | + | − | − | + | + |
| 26 | Xinchun No.29 | I | HR | I | 0I | − | − | − | − | + |
| 27 | Xinchun No.30 | MR | HS | HS | 20HR | + | − | − | + | + |
| 28 | Xinchun No.31 | HS | MR | HR | 20HR | + | − | − | − | + |
| 29 | Xinchun No.32 | MR | MR | HR | 0I | − | − | − | + | − |
| 30 | Xinchun No.33 | MR | HS | MS | 20MR | − | − | − | + | + |
| 31 | Xinchun No.34 | MR | MR | MS | 80HS | − | − | − | + | + |
| 32 | Xinchun No.35 | MR | MS | MS | 60MS | + | − | − | + | − |
| 33 | Xinchun No.36 | HS | MS | MS | 40MR | + | − | − | + | + |
| 34 | Xinchun No.37 | HR | MS | HS | 60MS | + | − | − | + | + |
| 35 | Xinchun No.38 | MS | MR | HS | 10NI | + | − | − | + | + |
| 36 | Xinchun No.39 | I | MR | MS | 80HS | − | − | − | + | + |
| 37 | Xinchun No.40 | MS | MR | MR | 40MR | − | − | − | + | + |
| 38 | Xinchun No.41 | HS | MS | HS | 20MR | − | − | − | + | + |
| 39 | Xinchun No.43 | MS | MR | HR | 40MS | − | − | − | + | + |
| 40 | Xinchun No.44 | HS | MS | MS | 40MS | − | − | − | + | + |
| 41 | Xinchun No.45 | MS | MS | MS | 60HS | − | − | − | + | − |
| 42 | Xindong No.1 | MS | MR | HS | 40MS | − | − | − | − | + |
| 43 | Xindong No.2 | MR | HS | HS | 40MS | − | − | − | − | + |
| 44 | Xindong No.3 | MS | MR | HS | 40MS | − | − | − | + | + |
| 45 | Xindong No.4 | HS | MR | HS | 60HS | − | − | − | − | + |
| 46 | Xindong No.5 | I | HR | HR | 5HR | + | − | − | + | + |
| 47 | Xindong No.6 | MS | MR | MS | 40HS | − | − | − | − | − |
| 48 | Xindong No.7 | MS | MR | HS | 40HS | − | − | − | − | + |
| 49 | Xindong No.9 | MR | MR | HS | 40HS | − | − | − | + | + |
| 50 | Xindong No.10 | MR | HS | HS | 40MS | − | − | − | + | + |
| 51 | Xindong No.11 | MR | MR | HS | 40HS | − | − | − | − | + |
| 52 | Xindong No.12 | MR | HS | HS | 40HS | − | − | − | − | + |
| 53 | Xindong No.13 | MR | MR | HS | 40HS | − | − | − | + | + |
| 54 | Xindong No.14 | HR | MS | MS | 20HR | + | − | − | + | + |
| 55 | Xindong No.15 | MS | MR | HS | 40MS | + | − | − | + | + |
| 56 | Xindong No.17 | MR | MR | HS | 40MS | − | − | − | + | + |
| 57 | Xindong No.18 | HS | MR | MS | 20MS | + | − | − | + | + |
| 58 | Xindong No.19 | MR | MR | HS | 20HS | − | − | − | − | − |
| 59 | Xindong No.20 | HS | MS | HS | 20MR | − | − | − | + | + |
| 60 | Xindong No.22 | MS | MS | MS | 0I | + | − | + | + | + |
| 61 | Xindong No.23 | MR | HS | HS | 40HS | + | − | − | + | + |
| 62 | Xindong No.24 | HS | MR | HS | 80HS | + | − | − | + | − |
| 63 | Xindong No.25 | MR | HS | HS | 60HS | − | − | − | + | + |
| 64 | Xindong No.26 | HS | MR | HS | 80HS | − | − | − | + | + |
| 65 | Xindong No.28 | MR | MS | HS | 80HS | − | − | − | + | − |
| 66 | Xindong No.29 | MR | MR | HR | 5HR | − | − | − | + | + |
| 67 | Xindong No.32 | HS | HS | HS | 60HS | − | − | − | − | + |
| 68 | Xindong No.33 | MS | HS | HS | 40MS | − | − | − | + | − |
| 69 | Xindong No.35 | HS | MS | HS | 60HS | + | − | − | + | + |
| 70 | Xindong No.41 | HS | I | HS | 5HR | − | − | + | + | + |
| 71 | Xindong No.46 | MS | HS | MS | 20MS | − | − | − | + | + |
| 72 | Xindong No.49 | MR | HR | HS | 80HS | + | − | − | + | + |
| 73 | Xindong No.50 | MR | HS | HS | 80HS | − | − | − | + | − |
| 74 | Xindong No.51 | MS | HS | MR | 10MR | − | − | − | + | − |
| 75 | Xindong No.52 | MS | MR | HS | 40MS | − | − | − | − | − |
| 76 | Xindong No.53 | MR | MS | HS | 60HS | + | − | − | + | + |
| 77 | Xindong No.57 | MS | MR | MR | 10MR | − | − | − | + | + |
| 78 | Yili 034 | HS | HS | HS | 80HS | − | − | − | − | + |
| 79 | Yili 053 | MR | MR | HS | 60HS | − | − | − | − | + |
| 80 | Yili 060 | MR | HS | HS | 60HS | − | − | − | + | − |
| 81 | Yili 070 | MS | MR | MS | 60HS | − | − | − | + | − |
| 82 | Yili 086 | MS | MR | MS | 80HS | + | − | − | + | − |
I: immune; NI: nearly immune; HR: highly resistance; MR: moderately resistance; MS: moderately susceptible; HS: highly susceptible; +: the Yr gene was detected; −: the Yr gene was not detected.
Discussion
Xinjiang is located on the northwestern border of China, with a complex and diverse geographical environment and climate. In addition, Xinjiang is an independent endemic area of wheat stripe rust in China. It was reported that the infection regularity and annual change trend of wheat stripe rust in Xinjiang were basically the same as other provinces of China. However, the occurrence time sequence regularity of wheat stripe rust in Xinjiang lags behind other provinces of China25,26. The damage degree of wheat stripe rust in Xinjiang has been relatively large in recent years, exceeding the peak value of wheat stripe rust outbreak in the 1990s27. The disease was most prevalent in Ili Kazak Autonomous Prefecture, Urumqi City, Altai Prefecture, Hami City and Aksu Prefecture4. Planting rust-resistant wheat cultivars has been considered as an effective and green strategy to control wheat stripe rust. In China, comprehensive application of wheat cultivars carrying resistance genes has been successful for long-term disease control28. However, high genetic variation in the pathogen population and the rapid rate of selection for new virulent races lead to some Yr genes have lost their resistance29–31. Therefore, in order to pinpoint the wheat cultivars’ resistance to stripe rust, 4 ASR and 34 APR cultivars were identified from the 82 wheat cultivars of Xinjiang that were screened. According to the results, there are more APR cultivars and fewer ASR cultivars in Xinjiang. Therefore, breeders need to focus on breeding all-stage resistant cultivars in Xinjiang.
At present, 86 wheat stripe rust resistance genes (Yr1-Yr86) have been designated32. Furthermore, there are many unnamed new Yr genes from current wheat cultivars or other Triticum species, such as Yrsp, YrElm4, YrTr1 and YrM85233. A large number of Yr genes have been used in breeding for disease resistance, such as Yr9, Yr29, Yr36, Yr39, Yr46, Yr69 and YrTr134,35,37,39,52. In this study, detection rate of Yr29, locate in the chromosome 1BL region and closely linked to the leaf rust resistance gene Lr46, accounted for 29.27% of all wheat cultivars36. Its derivatives played an important role in wheat disease resistance breeding. Yr39 confers durable high-temperature adult-plant (HTAP) resistance to Pst37. Lin and Chen mapped HTAP resistance gene Yr39 to chromosome 7BL38. Yr46 was found on chromosome arm 4DL and it is closely linked with the leaf rust resistance gene Lr6739. But the detection rate of Yr39 and Yr46 was only 0 and 2.44% of the total wheat cultivars, respectively. Resistance genes in wheat cultivars are frequently defeated by emerging new races, resulting in the wheat cultivars to be vulnerable within a short period after released in the fields. Therefore, breeders can benefit by the combination thereof with resistance genes such as Yr39 and Yr46 in breeding practices in Xinjiang.
Pyramiding rust resistant genes is an important strategy to breed wheat resistance cultivars40. Wheat resistant cultivars with multi-resistance genes exhibit a broader resistance spectrum. Multi-gene pyramiding strategy has been verified for durable control of wheat rusts41,42. By pyramiding Yr15 and Yr64 to the resistance wheat line RIL-Yr64/Yr15, a wider spectrum and durable resistance wheat cultivars were obtained43. Chhetri et al. identified Yr58 by using the method of genotyping, and Yr58 interaction with Yr46 for enhanced wheat resistance to stripe rust44. The deployment of wheat cultivars carrying multiple resistance genes in epidemiological regions can theoretically control disease outbreak. Wheat cultivars with ASR resistance have been grown in epidemic prone regions now and have achieved good results in the control of wheat stripe rust45.
In this study, 4 ASR cultivars were detected and in our previous study, 15 resistance genes were detected in these 82 wheat cultivars of Xinjiang46. Combined the results indicated that Xindong No.5 was detected 13 resistance genes. Xinchun No.29 and Xinchun No.32 were detected 10 and Xindong No.29 oniy 9 resistance genes. There were 4 cultivars that had better resistance performance against wheat stripe rust because of more resistance genes. Only 5 resistant genes were detected in Xinchun No.14, but it displayed immunity to Pst at adult plant stage in the field. This may be due to the presence of other undetected resistance genes or undiscovered resistance genes. Xinchun No.14 was introduced by CIMMYT (The Centro Internacional de Mejoramiento de Maiz y Trigo), so its source of disease resistance is different from other cultivars. It is necessary to make full use of Xinchun No.14 as a before parent to enrich the disease resistance sources of local wheat varieties in Xinjiang in subsequent breeding for disease resistance.
In the future breeding work, high-quality resistance introduction and different Yr gene combinations will be better strategies which will provide more selection for resistance improvement and resistance breeding. Thirty-four wheat cultivars were resistant to stripe rust at adult plant stage. And all of the 34 cultivars were detected multiple resistance genes. Therefore, these cultivars could be preferentially used for spreaded in field. At the same time, in the breeding practices of disease resistance, 34 cultivars could be preferentially selected.Although Xinjiang is assumed to be a relatively independent epidemiological area for wheat stripe rust in China, there is also a perennial risk of wheat stripe rust outbreaks. Once wheat stripe rust spreads, it will have a significant impact on Xinjiang’s agricultural economy47. Therefore to assist with the control of wheat stripe rust in Xinjiang, 82 Xinjiang wheat cultivars were evaluated at seedling and adult plant stages. Disease resistance identification showed that Xinchun No.29, Xinchun No.32 Xindong No.5 and Xindong No.29 were resistant to wheat stripe rust at all stage. Simultaneously, the resistance genes Yr29, Yr39, Yr46, Yr69 and YrTr1 were detected to clarify the resistance genes distribution of 82 wheat cultivars. The results indicated that Yr69 and YrTr1 had higher detection rates, but Yr36 and Yr46 had lower detection rates. The cultivar with gene combinations was comparatively limited. This result is consistent with our earlier findings46. The study provides a theoretical foundation for rational distribution of disease-resistant cultivars and disease-resistant breeding in Xinjiang.
Materials and methods
Materials
A total of 82 wheat cultivars from Xinjiang were used in this study, of which 41 were spring wheat cultivars and 41 were winter wheat cultivars. And the 82 wheat cultivars were mainly cultivated in Xinjiang. Taichun 29 and Mingxian 169 were used as positive controls to evaluate the resistance to stripe rust. AVS near-isogenic lines of wheat with Yr29, Yr39, Yr46, Yr69 and YrTr1 were used as positive controls for resistance genes detection. Three Pst races, including CYR32, CYR33 and CYR34, were used to evaluate resistance at wheat seedling stage. And the mixture of Pst races were employed to evaluate at wheat adult plant stage. All wheat cultivars and Pst were provided by the Laboratory of Plant Disease Epidemiology of Xinjiang Agricultural University.
Evaluation of resistance to stripe rust at seedling stage
Eighty two wheat cultivars, Taichun 29 and Mingxian 169 were planted in 14 cm diameter porcelain pots. Each pot contained 8 wheat seeds and each cultivar was set up with three replicates. Fresh urediniospores and the electron fluoride solution were combined to make a 25 mg/ml spore suspension. When the seedlings reached the Feekes 1 stage, they were inoculated with pipette. After electronic fluorinated liquid evaporated, the seedlings were placed in a sealed barrel for 24 h to maintain humidity and darkness. Then the seedlings were transferred to a phytotron with the temperature in a range of 12 ± 1℃, relative humidity 60 − 80%, light intensity 9000 Lx and Light 14 h/d. Approximately 15 days after inoculation when stripe rust had fully developed on Mingxian 169 and Taichun 29, the infection types (IT) were recorded. Experimental design was arranged in the same way at Xinjiang Agricultural University and Xinjiang Academy of Agricultural Sciences.
Evaluation resistance to stripe rust at adult plant stage in field
Evaluation of resistance of adult wheat to stripe rust in fields in Urumqi City and Changji Hui Autonomous Prefecture of Xinjiang from 2022 to 2023. 41 spring wheat and Taichun 29 were planted in March 2022. 41 winter wheat and Mingxian 169 were planted in September 2022. Each cultivar was planted in 1 m long rows and 0.3 m apart. Mingxian 169 and Taichun 29 were planted at 10-row intervals between the evaluated wheat cultivars to aid in spore dissemination. When the wheat reached the Feekes 3 stage, they were inoculated with 2 mg/mL mixture of fresh urediniospores and water. The inoculation method was sprayed using 1 L watering-can. Cover with black bag for 24 h to keep dark and moist. When Mingxian 169 and Taichun 29 was fully infected, the IT and disease severity were recorded. The experiment was duplicated 3 times and the experimental configuration was the same in two locations.
Evaluation resistance to stripe rust at adult plant stage in greenhouse
Evaluation of wheat resistance to stripe rust at the adult plant stage was carried out in the greenhouse of Xinjiang Agricultural University in 2023. Mingxian 169, Taichun 29 and 82 wheat cultivars were planted in 100 × 30 × 30 cm pots. Thrity wheat seeds were sown in each pot and the experiment was set up with three repetitions. When the wheat grew to Feekes 3 stage, the 30 mg/mL mixture of fresh urediniospores and water was useing watering-can to onto wheat. The inoculation and evaluation methods were consistent with the field experiments.
The detection of resistance genes
The resistance genes Yr29, Yr39, Yr46, Yr69 and YrTr1 of 82 wheat cultivars were detected using the developed molecular markers. DNA from 20 mg leaves of the seedling was extracted using the cetyl trimethyl ammonium bromide (CTAB) method48,49. The DNA concentration was detected using a spectrophotometer; it was diluted to 50 ng/µL by adding 1 × TE. The integrity of the extracted genomic DNA is examined using 1% agarose gel electrophoresis. All of the markers was Simple Sequence Repeat (SSR). The sequence of primers were shown in Table 2. The polymerase chain reactions (PCR) molecular marker primers were synthesized by Beijing Bomade Gene Technology Co., Ltd. (Beijing, China). The PCR were performed in a 30 µL reaction mixture containing 2 µL (50 ng/µL) of template DNA, 15 µL of PCR Mix, 1.5 µL of forward primer, 1.5 µL of reverse primer and 10 µL of ddH2O. Amplification conditions were an initial 5 min of denaturation at 94℃, next by 35 cycles of 1 min of denaturation at 94℃, 1–2 min of annealing at 50–58℃, and 1 min extension at 72℃. Step extension was 10 min at 72℃ and final 10℃ forever. The PCR products were subjected to 1–2% agarose gel electrophoresis (the gel concentration was determined by target fragment size). In order to verify the accuracy of the detection of the stripe rust resistance genes results, the stripe rust resistance genes were sequenced.
Table 2.
Primer sequence of stripe rust resistance gene.
| Genes | Primer name | Primer Sequence | References |
|---|---|---|---|
| Yr29 | WMC44 | F: GGTCTTCTGGGCTTTGATCCTG R: TGTTGCTAGGGACCCGTAGTGG | 50 |
| Yr39 | Xgwm131 | F: AATCCCCACCGATTCTTCTC R: AGTTCGTGGGTCTCTGATGG | 38 |
| Yr46 | Xwe173 | F: CAATAAGTAGGCCGGGACAA R: TGTGCCAGTTGAGTTTGCTC | 51 |
| Yr69 | 2AS111 | F: TCCTGTCCGCTGTATGATCG R: TTGTGGCTCTGGTGTTGTAATC | 52 |
| 2AS171 | F: GACAACAATCACAAGCAGCAA R: CCAACTAACTCTTCTCGGTCTC | ||
| YrTr1 | Xbarc8 | F: GCGGGAATCATGCATAGGAAAACAGAA R: GCGGGGGCGAAACATACACATAAAAACA | 53 |
Identification standards and data analysis
The IT were recorded based on a scale of 0–4 (Table 3). Wheat with IT 0–2 was considered resistant, whereas 3–4 was considered susceptible54. Disease severity was recorded based on a 0–100%, including 0, 5%, 10%, 20%, 40%, 60%, 80% and 100%55. The evaluation results for stripe rust resistance at seedling and adult stage were summarized. GraphPad Prism 9 was used to conduct statistical analysis. The background information of wheat cultivars was obtained from the authoritative website of the Chinese Seed Industry Data Platform “http://202.127.42.47:6010/SDSite/Home/Index”.
Table 3.
Identification standard of wheat stripe rust56.
| Infection Types | Resistant Evaluation | Description of symptoms |
|---|---|---|
| 0 | Immunity (I) | The leaf surface did not have necrosis and chlorosis. |
| 0; | Nearly Immune (NI) | The leaf surface have fewer necrosis but did not have spores. |
| 1 | Highly Resistant (HR) | The leaf surface have fewer sporulation area and necrosis. |
| 2 | Moderately Resistant (MR) | The leaf had significant necrosis and chlorosis, but had less sporulation area. |
| 3 | Moderately Susceptible (MS) | The leaf had significant necrosis and chlorosis, and had larger sporulation area. |
| 4 | Highly Susceptible (HS) | Sporulation areas were large and numerous not the spores |
Acknowledgements
This work was funded by the National Natural Science Foundation of China (#32360659). We would like to thank Laboratory of Plant Disease Epidemiology for their assistance with site and equipment.
Author contributions
Minghao Zhang, Minghao Zeng and Zeyu Ma conceived and designed the experiment. Baishuo Tian and Li Chen performed most of the research. Jing Chen and Haifeng Gao performed some gene expression analysis. Qi Liu and Guangkuo Li analyzed the data, wrote and revised the manuscript. All authors reviewed the manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
Statement
We ensure that we had permission to collect wheat cultivars.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Minghao Zhang and Minghao Zeng have contributed equally to this work.
References
- 1.Dimmock, J. P. R. E. & Gooding, M. J. The influence of foliar diseases, and their control by fungicides, on the protein concentration in wheat grain: A review. J. Agric. Sci.138 (4), 349–366 (2002). [Google Scholar]
- 2.Huang, L. et al. Identification of stripe rust resistance genes in common wheat cultivars from the Huang-Huai-Hai region of China. Plant Dis.104 (6), 1763–1770 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Chen, X. Pathogens which threaten food security: Puccinia Striiformis, the wheat stripe rust pathogen. Food Secur.12 (2), 239–251 (2020). [Google Scholar]
- 4.Liang, S. et al. A GIS based study on wheat stripe rust oversummering zoning in Xinjiang. China Plant. Prot.43(06), 31–37 (2023). [Google Scholar]
- 5.Wan, A., Chen, X. & He, Z. Wheat stripe rust in China. Aust. J. Agric. Res.58(6), 605–619 (2007). [Google Scholar]
- 6.Liu, Q. et al. Detecting the minimum limit on wheat stripe rust in the latent period using proximal remote sensing coupled with duplex real-time PCR and machine learning. Plants12(15), 2814 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, W. et al. Integrated management if wheat stripe rust caused by Puccinia Striiformis f. sp. tritici in China. J. Integr. Agric.46(20), 4254–4262 (2013). [Google Scholar]
- 8.Zhang, C. et al. An ancestral NB-LRR with duplicated 3’UTRs confers stripe rust resistance in wheat and barley. Nat. Commun.10(1), 4023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aktar-Uz-Zaman, M. D., Tuhina-Khatun, M. S. T., Hanafi, M. M. & Sahebi, M. Genetic analysis of rust resistance genes in global wheat cultivars: An overview. Biotechnol. Biotechnol. Equip.31(3), 431–445 (2017). [Google Scholar]
- 10.Dracatos, P. M. et al. Complementary resistance genes in wheat selection ‘Avocet R’ confer resistance to stripe rust. Theor. Appl. Genet.129, 65–76 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Shi, Z. et al. Development of resistance gene analog polymorphism markers for the Yr9 gene resistance to wheat stripe rust. Genome44(4), 509–516 (2001). [PubMed] [Google Scholar]
- 12.Zhao, J. & Kang, Z. Fighting wheat rusts in China: A look back and into the future. Phytopathol. Res.5(1), 6 (2023). [Google Scholar]
- 13.Marais, G. F. et al. Leaf rust and stripe rust resistance genes transferred to common wheat from Triticum dicoccoides. Euphytica143, 115–123 (2005). [Google Scholar]
- 14.Johnson, R. & Taylor, A. J. Isolates of Puccinia Striiformis collected in England from the wheat varieties Maris Beacon and Joss Cambier. Nature238(5359), 105–106 (1972). [Google Scholar]
- 15.Luo, P. et al. Allelic analysis of stripe rust resistance genes on wheat chromosome 2BS. Genome51(11), 922–927 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Zhao, Y. Q. et al. Investigation of urediospore morphology, histopathology and epidemiological components on wheat plants infected with UV-B-induced mutant strains of Puccinia Striiformis f. Sp. Tritici MicrobiologyOpen8, e870 . [DOI] [PMC free article] [PubMed]
- 17.Han, D. et al. Evaluation of resistance of current wheat cultivars to stripe rust in northwest China, north China and the middle and lower reaches of Changjiang. River Epidemic Area. 43(14), 2889–2896 (2010). [Google Scholar]
- 18.Sun, J. et al. Identification of resistance to wheat stripe rust and detection of known resistance genes in 100 wheat cultivars with SSR markers. Plant. Prot.43, 64–72 (2017). [Google Scholar]
- 19.Li, J. et al. Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet.133, 1095–1107 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Kokhmetova, A. Identification of stripe rust resistance genes in common wheat cultivars and breeding lines from Kazakhstan. Plants10 (11), 2303 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmatov, M. et al. Characterization of stem, stripe and leaf rust resistance in Tajik bread wheat accessions. Euphytica215, 1–22 (2019). [Google Scholar]
- 22.Liu, T., Peng, Y., Chen, W. & Zhang, Z. First detection of virulence in Puccinia Striiformis f. sp. tritici in China to resistance genes Yr24 (= Yr26) present in wheat cultivar Chuanmai 42. Plant Dis.94(9), 1163 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Bai, B. et al. High relative parasitic fitness of G22 derivatives is associated with the epidemic potential of wheat stripe rust in China. Plant Dis.102(3), 483–487 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Liu, B. et al. Discovery and pathogenicity of CYR34, a new race of Puccinia Striiformis f. sp. tritici in China. Acta Pharmacol. Sin.47, 682–688 (2017). [Google Scholar]
- 25.Zeng, M. et al. Analysis of population genetic structure of Puccinia Striiformis f. sp. Tritici in Yili prefecture, Xinjiang. J. Triticeae Crops. 44(01), 127–133 (2024). [Google Scholar]
- 26.Zeng, Q. et al. Stripe rust resistance and genes in Chinese wheat cultivars and breeding lines. Euphytica196, 271–284 (2014). [Google Scholar]
- 27.Li, J., Zeng, J., Jiang, Y. & Li, H. Study on the occurrence and epidemic regularity of wheat stripe rust in Xinjiang. China Plant. Prot.30, 16–19 (2010). [Google Scholar]
- 28.Chen, X. Review article: high-temperature adult-plant resistance, key for sustainable control of stripe rust. Am. J. Plant. Sci.4, 608–627 (2013). [Google Scholar]
- 29.Huang, C. et al. Epidemics analysis of wheat stripe rust in China in 2017. Plant. Prot.44(01), 162–166 (2018). [Google Scholar]
- 30.Rosewarne, G. M. et al. Quantitative trait loci of stripe rust resistance in wheat. Theor. Appl. Genet.126, 2427–2449 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponce-Molina, L. J. et al. Characterization of adult plant resistance to leaf rust and stripe rust in Indian wheat cultivar ‘New Pusa 876’. Crop Sci.58(2), 630–638 (2018). [Google Scholar]
- 32.McIntosh, R. A. et al. Catalogue of gene symbols for wheat. In Proceedings of the 12th international wheat genetics symposium, Yokohama, Japan. pp. 8–13 (2013).
- 33.Yang, M. N. et al. Genetic analysis and SSR location of stripe rust resistance of wheat translocation line M8657-1 derived from Triticum aestivum-Leymus mollis (Trin.) Hara. J. Agricultural Biotechnol.18, 861–866 (2010). [Google Scholar]
- 34.Mcintosh, R. A., Wellings, C. R. & Park, R. F. Wheat rusts: an Atlas of Resistance genes. CSIRO Publishing, 9–12 (1995).
- 35.Fang, T. et al. Stripe rust resistance in the wheat cultivar Jagger is due to Yr17 and a novel resistance gene. Crop Sci.51(6), 2455–2465 (2011). [Google Scholar]
- 36.William, M. et al. Molecular marker mapping of leaf rust resistance gene Lr46 and its association with stripe rust resistance gene Yr29 in wheat. Phytopathology93(2), 153–159 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Zheng, X. et al. Transfer of durable stripe rust resistance gene Yr39 into four Chinese Elite wheat cultivars using marker-assisted selection. Agronomy12(8), 1791 (2022). [Google Scholar]
- 38.Lin, F. & Chen, X. Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race-specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theor. Appl. Genet.114(7), 1277–1287 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Herrera-Foessel. Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor. Appl. Genet.127, 781–789 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Dedryver, F. et al. Characterization of genetic components involved in durable resistance to stripe rust in the bread wheat ‘Renan’. Phytopathology99(8), 968–973 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Juliana, P. et al. Genome-wide association map for resistance to leaf rust, stripe rust and tan spot in wheat reveals potential candidate genes. Theor. Appl. Genet.131, 1405–1422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh, R. P. & Rajaram, S. Genetics of adult plant resistance to stripe rust in ten spring bread wheats. Euphytica72, 1–7 (1993). [Google Scholar]
- 43.Qie, Y. et al. Development, validation, and re-selection of wheat lines with pyramided genes Yr64 and Yr15 linked on the short arm of chromosome 1B for resistance to stripe rust. Plant Dis.103 (1), 51–58 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Chhetri, M., Bariana, H., Kandiah, P. & Bansal, U. Yr58: a new stripe rust resistance gene and its interaction with Yr46 for enhanced resistance. Phytopathology106 (12), 1530–1534 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Zhang, G. et al. Evaluation of the potential risk of the emerging Yr5-virulent races of Puccinia Striiformis f. sp. tritici to 165 Chinese wheat cultivars. Plant Dis.106 (7), 1867–1874 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Zhang, M. et al. The detection of yr genes in Xinjiang wheat cultivars using different molecular markers. Int. J. Mol. Sci.24(17), 13372 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia, M. et al. Genome-wide association analysis of stripe rust resistance in modern Chinese wheat. BMC Plant Biol.20, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo, P., Zhang, H., Zhang, H. & Ren, Z. Evidence for the possibility of foreign transposon existence. Mol. Plant. Breed.2, 829–832 (2004). [Google Scholar]
- 49.Riede, C. R. Linkage of RFLP markers to an aluminum tolerance gene in wheat. Crop Sci.36 (4), 905–909 (1996). [Google Scholar]
- 50.Elshafei, A. A. et al. Phenotype and validation of molecular markers associated with rust resistance genes in wheat cultivars in Egypt. Mol. Biol. Rep.29, 1–13 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Herrera-Foessel, S. A. et al. New slow-rusting leaf rust and stripe rust resistance genes Lr67 and Yr46 in wheat are pleiotropic or closely linked. Theor. Appl. Genet.122, 239–249 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Yang, W. et al. Development of linkage markers for stripe rust resistance gene Yr69 in Triticum aestivum L. Plant. Sci. J.40, 197–204 (2022). [Google Scholar]
- 53.Liu, L. et al. Identification of stripe rust resistance loci in US spring wheat cultivars and breeding lines using genome-wide association map and yr gene markers. Plant Dis.104(8), 2181–2192 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Wang, M. et al. Registration of 70 common spring wheat germplasm lines resistant to stripe rust. J. Plant. Registrations. 6(1), 104–110 (2012). [Google Scholar]
- 55.Line, R. F. & Qayoum, A. Virulence, aggressiveness, evolution and distribution of races of Puccinia Striiformis (the cause of stripe of wheat) in North America, 1968-87. Tech. Bull. (USA). 1788, 1–44 (1992). [Google Scholar]
- 56.Roelfs, A. P. Genetic control of phenotypes in wheat stem rust. Annu. Rev. Phytopathol.26, 351–367 .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.