Abstract
We aimed to determine the association between anion gap-to-calcium ratio (ACR) and 30-day mortality in sepsis patients with diabetes mellitus (DM). Data for sepsis patients diagnosed with DM was extracted from Medical Information Mart for Intensive Care Database IV. After screening, 4429 eligible subjects were included in our study finally. The receiver operating characteristic (ROC) curve was used to determine the cut-off value. According to the ROC curve analysis, the ACR demonstrated a higher area under the curve (AUC) of 0.622 compared to AG (0.598). Multivariable logistic regression with inverse probability of treatment weighting (IPTW) based on propensity score were used to detect the association between ACR and 30-day mortality. Our results showed that the patients with the high level of ACR had a higher risk of death within 30 days compared with those with low level of ACR (odds ratio 1.342, 95% confidence interval 1.180–1.526, P < 0.001). In a word, our results suggest that ACR may be an independent prognostic indicator for death with 30 days in critically ill patients with sepsis and DM.
Keywords: Sepsis, Diabetes mellitus (DM), Anion gap, Calcium, Mortality
Subject terms: Infectious diseases, Medical research, Risk factors
Introduction
Diabetes mellitus (DM) is a tremendous health problem worldwide. It is reported that more than 529 million people are living with diabetes worldwide today, and this number is estimated to rise to 1.31 billion by 20501. Because of a compromised immune system, population with DM often have weakened defenses against pathogens2–7. Approximately 2.6 million people with DM develop sepsis each year. Of these, approximately 15% die as the disease progresses8. Therefore, it is necessary to strengthen the supervision and management of sepsis patients with DM, and timely identify the population with a higher risk of death, and ultimately reduce the overall mortality of this group.
Accumulated evidences have revealed that some laboratory indicators are associated with the risk of death in sepsis patients with DM. In 2022, Xin et al. reported that the level of platelet was negatively associated with major adverse kidney events within 30 days in sepsis patients with DM9. In 2023, a large-scale cohort study showed that the high level of red blood cell distribution width was also associated with mortality in sepsis patients with DM10. However, above these indicators are also associated with prognosis for other diseases, with limited specificity in predicting the prognosis of critically ill sepsis patients with DM11–14. Therefore, it is necessary to further screen more reliable indicators to identify these septic patients with a high risk of death at an early stage. Several studies recent reported that the level of anion gap (AG) can be used as a prognostic indicator for sepsis patients15,16, and it was associated with short-term mortality among sepsis patients with DM10. Moreover, hypocalcemia is common in critically ill patients17, and the level of blood calcium is also closely related to insulin secretion18,19. Therefore, it is speculated that a composite of AG and blood calcium level may be an indicators for predicting the prognosis of sepsis patients with DM. However, no published studies have investigated the association between a composite of AG and blood calcium and death in sepsis patients with DM. The aim of this paper was to investigate the association between a composite indicator of AG and calcium and 30-day mortality in critical ill sepsis patients with DM from the intensive care unit (ICU).
Results
Clinical characteristics of included patients
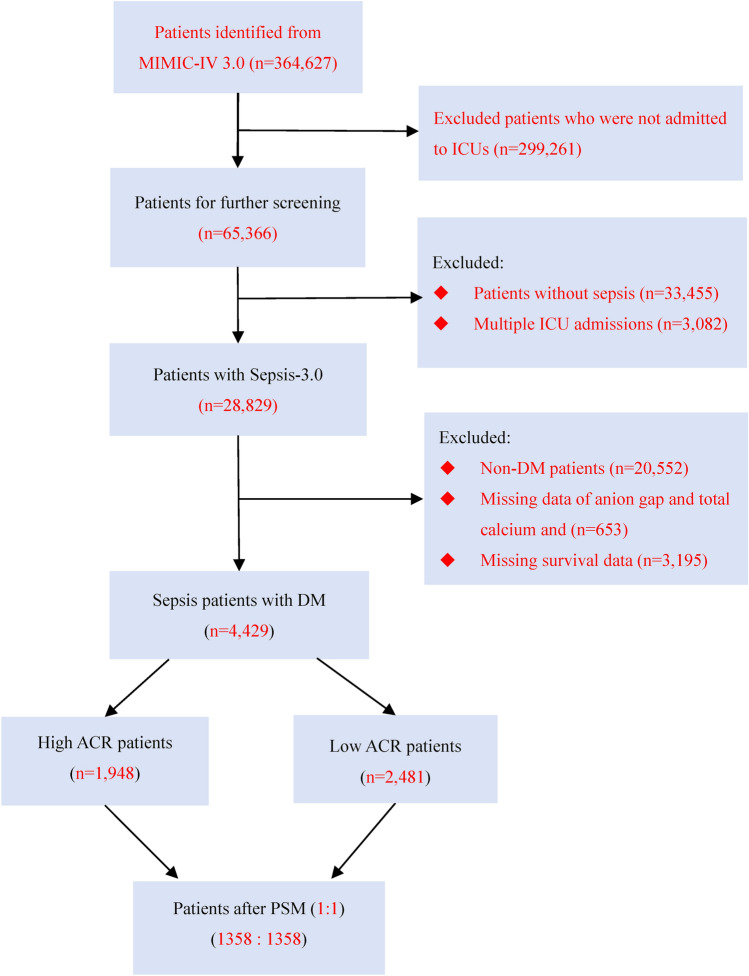
The initial search identified 65,366 ICU admissions from the MIMIC-IV database. After further screening, we identified 4429 patients who met the inclusion criteria to be included in the final analysis. The flow of selection process is shown in Fig. 1.
Fig. 1.
Flowchart of patient selection. Abbreviations: MIMIC-IV 3.0, Medical Information Mart for Intensive Care IV, Version: 3.0; ICU, intensive care unit; DM, diabetes mellitus. ACR, anion gap-to-calcium ratio; PSM, propensity score matching.
The baseline characteristics between survivors and non-survivors were showed in Table 1. Compared with the survivor group, patients in the non-survival group presented higher levels of AG, ACR, Glu, BUN, WBC, RDW, Cr, heart rate, respiratory rate, SAPS II scores and SOFA scores, and lower levels of body mass index and temperature. The patients in non-survival group were older, were more likely to be treated with mechanical ventilation, and had more comorbidities such as coronary artery disease, valvular disease, malignant cancer, and septic shock than those in survival group.
Table 1.
Comparisons of clinical characteristics between survivors and non-survivors.
| Variables | Survivors group (2612) | Non-survivors group (1817) | P-value |
|---|---|---|---|
| Age [mean (SD)], years | 70.2 ± 12.7 | 73.4 ± 12.3 | < 0.001 |
| Gender, male, N (%) | 1535 (58.8) | 1043 (57.4) | 0.365 |
| BMI [mean (SD)], kg/m2 | 30.5 ± 8.0 | 29.3 ± 7.5 | < 0.001 |
| SOFA score [mean (SD)] | 6 ± 3 | 8 ± 4 | < 0.001 |
| SAPS II score [mean (SD)] | 43 ± 12 | 50 ± 14 | < 0.001 |
| Ventilation, N (%) | 1176 (45.0) | 1057 (23.3) | < 0.001 |
| Laboratory tests | |||
| Calcium [mean (SD)], mg/dL | 8.4 ± 0.8 | 8.3 ± 0.8 | 0.2673 |
| Anion gap [mean (SD)], mmol/L | 15 ± 4 | 18 ± 5 | < 0.001 |
| ACR [mean (SD)] | 1.83 ± 0.54 | 2.09 ± 0.69 | < 0.001 |
| Glu [mean (SD)], mg/dL | 175 ± 72 | 185 ± 80 | 0.001 |
| Lac [mean (SD)], mmol/L | 2.2 ± 1.5 | 2.5 ± 1.5 | 0.051 |
| PLT × 109/L [mean (SD)] | 204 ± 110 | 199 ± 111 | 0.143 |
| INR [mean (SD)] | 1.8 ± 1.4 | 1.7 ± 1.1 | 0.254 |
| BUN [mean (SD)], mg/dL | 37 ± 26 | 44 ± 29 | < 0.001 |
| WBC × 109/L [mean (SD)] | 13.2 ± 11.5 | 14.2 ± 9.6 | 0.003 |
| RDW [mean (SD)], % | 16.0 ± 2.4 | 16.5 ± 2.8 | < 0.001 |
| RBC × 1012/L [mean (SD)] | 3.4 ± 0.7 | 3.4 ± 0.8 | 0.795 |
| HCT [mean (SD)], % | 30.9 ± 6.0 | 31.2 ± 6.2 | 0.211 |
| Hb [mean (SD)], g/L | 10.0 ± 2.0 | 10.0 ± 2.1 | 0.711 |
| Cr [mean (SD)], mg/dL | 2.0 ± 1.8 | 2.2 ± 1.8 | < 0.001 |
| Vital signs | |||
| Heart rate [mean (SD)], times/min | 90 ± 20 | 92 ± 21 | 0.004 |
| MAP [mean (SD)], mmHg | 80 ± 18 | 81 ± 19 | 0.993 |
| Resp rate [mean (SD)], times/min | 20 ± 6 | 21 ± 7 | 0.001 |
| Temp [mean (SD)], °C | 36.9 ± 0.7 | 36.8 ± 0.8 | < 0.001 |
| SpO2 [mean (SD)], % | 96 ± 4 | 96 ± 4 | 0.120 |
| Comorbidity, N (%) | |||
| Coronary artery disease | 603 (23.1) | 481 (26.5) | 0.010 |
| Congestive heart failure | 1213 (46.4) | 894 (49.2) | 0.070 |
| Valvular disease | 338 (12.9) | 325 (17.9) | 0.029 |
| Hypertension | 923 (35.3) | 606 (33.4) | 0.171 |
| COPD | 376 (14.4) | 251 (13.8) | 0.585 |
| Malignant cancer | 414 (15.8) | 373 (20.5) | 0.001 |
| Renal failure | 1027 (39.3) | 725 (39.9) | 0.696 |
| Liver disease | 268 (10.3) | 211 (11.6) | 0.836 |
| Rheumatoid arthritis | 56 (2.1) | 30 (1.7) | 0.244 |
| Septic shock | 645 (24.7) | 696 (38.3) | < 0.001 |
N, number; SD, standard deviation; BMI, body mass index; SOFA, Sequential Organ Failure Assessment; SAPS II, Simplified Acute Physiology Score; ACR, anion gap-to-calcium ratio; Glu, glucose; Lac, lactate; PLT, platelets; INR, international normalized ratio; BUN, blood urea nitrogen; WBC, white blood cell; RDW, red blood cell distribution width; RBC, red blood cell; HCT, hematocrit; Hb, hemoglobulin; Cr, creatinine; MAP, mean arterial pressure; Resp rate, respiratory rate; Temp, temperature; SpO2, blood oxygen saturation; Ventilation, the patient needs assisted ventilation on the first day; COPD, chronic obstructive pulmonary disease.
Associations between ACR and 30-day mortality
The significant different variables, including age, BMI, SOFA, SAPS II, ventilation, ACR, BUN, RDW, Cr, WBC, glucose, coronary artery disease, malignant cancer, and septic shock, were used in multivariate logistic regression analyses. The adjusted results showed that ACR (OR 1.342, 95% CI 1.180–1.526, P < 0.001) was independent predictors for death within 30 days from any reason in sepsis patients with DM (Table 2).
Table 2.
Multivariate regression results of 30-day mortality for septic patients with diabetes mellitus.
| Variables | OR | OR (95% CI) | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age, years | 1.019 | 1.013 | 1.026 | < 0.001 |
| BMI, kg/m2 | 0.984 | 0.975 | 0.993 | 0.001 |
| SOFA score | 1.028 | 1.002 | 1.054 | 0.037 |
| SAPS II score | 1.024 | 1.017 | 1.031 | < 0.001 |
| ACR | 1.342 | 1.180 | 1.526 | < 0.001 |
| BUN, mg/dL | 1.001 | 0.998 | 1.003 | 0.665 |
| Cr, mg/dL | 0.916 | 0.872 | 0.962 | 0.010 |
| RDW, % | 1.068 | 1.040 | 1.096 | < 0.001 |
| Glu, mg/dL | 1.001 | 1.000 | 1.002 | 0.004 |
| WBC × 109/L | 0.997 | 0.991 | 1.003 | 0.255 |
| Ventilation | 1.532 | 1.334 | 1.758 | 0.001 |
| Coronary artery disease | 1.125 | 0.969 | 1.306 | 0.122 |
| Malignant cancer | 1.317 | 0.998 | 1.558 | 0.056 |
| Septic shock | 1.372 | 0.925 | 2.034 | 0.116 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; SOFA, Sequential Organ Failure Assessment; SAPS II, Simplified Acute Physiology Score; ACR, anion gap-to-calcium ratio; BUN, blood urea nitrogen; RDW, red blood cell distribution width; Cr, creatinine; Glu, glucose; WBC, white blood cell; Ventilation, the patient needs assisted ventilation on the first day of admission.
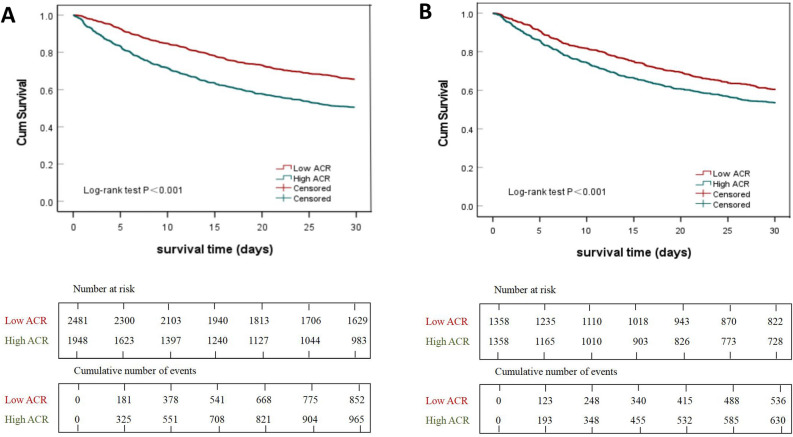
Receiver operating characteristic (ROC) curve was used to evaluate the predictive performance of ACR, SOFA, AG, and Ca for 30-day mortality all-cause mortality of sepsis patients with DM, and ACR has better discrimination and accuracy compared to the other variables (Fig. 2). The areas under ROC of ACR was 0.622 (95% CI: 0.605–0.640), with a sensitivity of 0.501 and a specificity of 0.704. All patients were next divided into two groups according to the cut-off value of ACR: low-ACR group (ACR < 1.89, 2481 patients) and high-ACR group (ACR ≥ 1.89, 1948 patients). Kaplan–Meier analysis was performed between the two groups. As shown in Fig. 3A, the survival curve of the high-ACR group was significantly lower than that of the low-ACR group (log-rank test, P < 0.001).
Fig. 2.
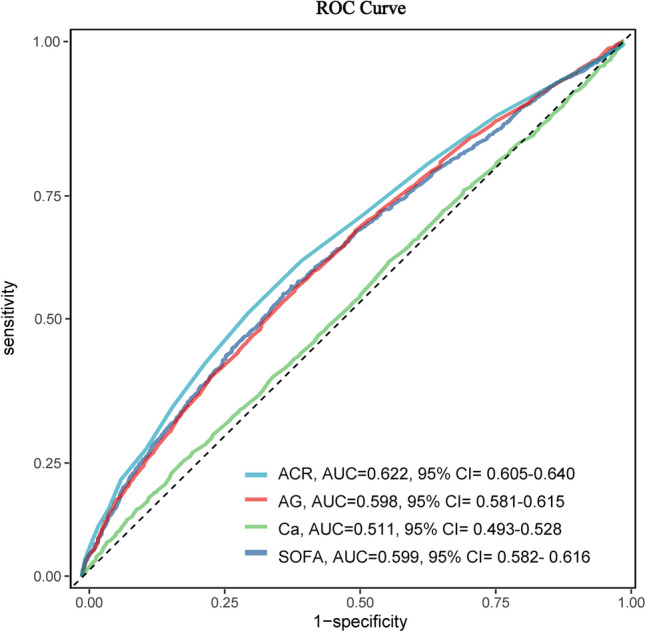
ROC curve of ACR, SOFA, AG and Ca for predicting the all-cause mortality in the overall study population. The optimal cut-off value of ACR for the 30-day all-cause mortality was 1.89, with a sensitivity of 0.501 and a specificity of 0.704. Abbreviations: ROC, receiver operating characteristic; ACR, anion gap-to-calcium ratio; AUC, area under curve; AG, anion gap; Ca, calcium; SOFA, Sequential Organ Failure Assessment.
Fig. 3.
Kaplan–Meier survival curves of septic patients with DM at 30-day, with high (green, ACR ≥ 1.89) and low (red, ACR < 1.89) ACR. (A and B) were reflected the results before and after propensity score matching, respectively. Abbreviations: DM, diabetes mellitus; ACR, anion gap-to-calcium ratio.
Propensity score analysis
To reduce the influence of confounding bias, the PS analyses were next performed in our studies. After PS (1:1), 1358 patients with high-ACR and 1358 patients with low-ACR were included in the final analysis. All covariates were evenly distributed across the two groups after PS analyses (Table 3). Multivariable logistic regression analysis was used to adjust for remaining confounding factors, the results showed that the survival probability 30-day of sepsis patients with DM was significantly higher in the low-ACR than those in high-ACR group (OR 1.622, 95% CI 1.391–1.891, P < 0.001). Multivariable analysis with IPW according to the propensity score also yielded similar results (OR 1.382, 95% CI 1.226–1.558, P < 0.001) (Table 4, Fig. 3B).
Table 3.
Comparison of baseline characteristics between the original cohort and the matched cohort by using propensity score analysis.
| Variables | Original cohort | Matched cohort | ||||
|---|---|---|---|---|---|---|
| Low ACR (2481) | High ACR (1948) | SMD | Low ACR (1358) | High ACR (1358) | SMD | |
| Age [mean (SD)], years | 71.8 ± 12.8 | 71.2 ± 12.8 | − 0.042 | 72.0 ± 12.2 | 72.1 ± 12.6 | 0.010 |
| Gender, male, N(%) | 1441 (58.1) | 1137 (58.4) | 0.006 | 806 (59.4) | 763 (56.2) | 0.016 |
| BMI [mean (SD)], kg/m2 | 30.1 ± 7.8 | 29.9 ± 7.8 | − 0.027 | 29.6 ± 7.5 | 29.7 ± 7.5 | 0.031 |
| SOFA score [mean (SD)] | 6 ± 3 | 8 ± 4 | 0.598 | 7 ± 3 | 7 ± 3 | 0.047 |
| SAPS II score [mean (SD)] | 42 ± 13 | 50 ± 14 | 0.547 | 46 ± 13 | 47 ± 13 | 0.054 |
| Ventilation, N(%) | 1221 (49.2) | 1012 (52.0) | 0.055 | 669 (49.3) | 683 (50.3) | 0.013 |
| Laboratory tests | ||||||
| Cr [mean (SD)], mg/dL | 1.5 ± 1.1 | 2.9 ± 2.2 | 0.617 | 1.9 ± 1.3 | 2.2 ± 1.8 | 0.121 |
| Glu [mean (SD)], mg/dL | 171.7 ± 68.0 | 188.8 ± 83.1 | 0.206 | 184.3 ± 77.1 | 184.0 ± 74.5 | 0.024 |
| WBC × 109/L [mean (SD)] | 12.7 ± 11.0 | 14.8 ± 10.4 | 0.198 | 13.9 ± 13.8 | 14.1 ± 10.0 | 0.002 |
| RDW [mean (SD)], % | 16.0 ± 2.5 | 16.5 ± 2.6 | 0.193 | 16.3 ± 2.6 | 16.3 ± 2.5 | − 0.009 |
| RBC × 1012/L [mean (SD)] | 3.5 ± 0.7 | 3.3 ± 0.7 | − 0.171 | 3.4 ± 0.7 | 3.4 ± 0.7 | 0.005 |
| BUN [mean (SD)], mg/dL | 32.9 ± 21.1 | 49.4 ± 31.1 | 0.531 | 39.7 ± 23.5 | 41.6 ± 26.2 | 0.045 |
| Vital signs | ||||||
| Heart rate [mean (SD)], times/min | 89.7 ± 20.2 | 93.1 ± 21.5 | 0.155 | 92.9 ± 21.5 | 91.9 ± 20.9 | 0.002 |
| Resp rate [mean (SD)], times/min | 20.1 ± 6.4 | 21.1 ± 6.4 | 0.156 | 20.9 ± 6.5 | 20.9 ± 6.3 | 0.029 |
| Temp [mean (SD)], °C | 36.9 ± 0.7 | 36.8 ± 0.8 | − 0.092 | 36.8 ± 0.7 | 36.8 ± 0.8 | − 0.005 |
| Comorbidity, N (%) | ||||||
| Coronary artery disease | 567 (22.9) | 517 (26.5) | 0.098 | 339 (25.0) | 346 (25.5) | 0.006 |
| Congestive heart failure | 1100 (44.3) | 1007 (51.7) | 0.147 | 657 (48.4) | 681 (50.1) | 0.027 |
| COPD | 1100 (44.3) | 1007 (51.7) | − 0.037 | 190 (14.0) | 191 (14.1) | 0.002 |
| Malignant cancer | 456 (18.4) | 331 (17.0) | 0.083 | 248 (18.3) | 243 (17.9) | 0.018 |
| Renal failure | 860 (34.7) | 892 (45.8) | 0.223 | 549 (40.4) | 572 (42.1) | 0.034 |
| Septic shock | 580 (23.4) | 761 (39.1) | 0.321 | 432 (31.8) | 443 (32.6) | 0.032 |
N, number; SD, standard deviation; SMD, standardized mean difference; ACR, anion gap-to-calcium ratio; BMI, body mass index; SOFA, Sequential Organ Failure Assessment; SAPS II, Simplified Acute Physiology Score; Cr, creatinine; Glu, glucose; WBC, white blood cell; RDW, red blood cell distribution width; RBC, red blood cell; BUN, blood urea nitrogen; Resp rate, respiratory rate; Temp, temperature; Ventilation, the patient needs assisted ventilation on the first day; COPD, chronic obstructive pulmonary disease.
Table 4.
Association of ACR and 30-day mortality in Septic patients with DM by using in the crude analysis, multivariable analysis, and propensity-score analyses.
| Analysis | 30-day mortality | P value |
|---|---|---|
| No. of events/no. of patients at risk (%) | < 0.001 | |
| Low ACR | 852/2481 (34.3) | – |
| High ACR | 965/1948 (49.5) | – |
| Crude analysis-OR (95% CI) | 1.877 (1.662, 2.119) | < 0.001 |
| Multivariable analysis-OR (95% CI)* | 1.342 (1.180, 1.526) | < 0.001 |
| Propensity-score analyses-OR (95% CI) | ||
| With matching※ | 1.622 (1.391, 1.891) | < 0.001 |
| With propensity score IPTW† | 1.382 (1.226, 1.558) | < 0.001 |
OR, odds ratio; CI, confidence interval; IPTW, inverse probability-of-treatment weighting; PSM, propensity score matching. *Shown is the odds ratio from the multivariable logistic regression model with additional adjustment for age, body mass index, SOFA score, SAPS II score, anion gap-to-calcium ratio, blood urea nitrogen, red blood cell distribution width, creatinine, glucose, white blood cell, the patient needs assisted ventilation on the first day of admission, coronary artery disease, metastatic cancer and septic shock. The analysis included all 4429 patients. The “event” shown is 30-day mortality; ※Shown is the odds ratio from a multivariable logistic regression model with the same covariates with matching according to the propensity score. The analysis included 2716 patients (1358 in low-ACR group and 1358 in high-ACR group). †Shown is the odds ratio from the multivariable logistic regression model with 30-day mortality as the binary response with the remained unbalanced covariates with inverse probability-of-treatment weighting according to the propensity score. The analysis included all the patients.
The subgroup analyses
Subgroup analysis demonstrated a significant correlation between ACR and a worse prognosis in subgroups of gender, age, comorbidity, and invasive mechanical ventilation. When stratified analysis was performed based on whether septic shock was present, the results showed that patients with high level of ACR have a higher risk of death within 30 days than those with low level of ACR in both patients with or without septic shock (Table S5).
Discussion
Our study observed that the ACR was significantly associated with the risk of death within 30 days in sepsis patients with DM from ICU. Importantly, these results were also robust in a series of sensitivity analyses and showed predictive value of ACR for adverse clinical prognosis. Therefore, our study suggested that ACR may offer clinicians a valuable insights for guiding interventions in this high-risk population.
Sepsis is the life-threatening organ dysfunction caused by the dysfunction of body regulation and infection20. Due to its high fatality rate, sepsis has also been identified by the World Health Organization as a global health priority20–22. Currently, Sequential Organ Failure Assessment (SOFA), Simplified Acute Physiological Score II (SAPS II), and other scoring systems are commonly used to evaluate the severity of the condition and the risk of death for patients with sepsis23,24. However, these methods are not only cumbersome in practice, but also lack specificity for sepsis patients with different characteristics. Therefore, it is necessary to further develop some new simple and effective prediction indicators. Interestingly, in this study, we found for the first time that ACR levels were associated with the risk of death in sepsis patients with DM. Our work extends the understanding established in several previous studies. In 2018, Mohr et al. found that anion gap was associated with the risk of death in patients with sepsis25. In 2022, Li et al. reported that the level of blood calcium were also associated with the risk of death in patients with severe infections26. However, these studies did not further investigate the association between a composite indicator of anion gap and calcium (ACR) and the risk of death in patients with sepsis. Our work suggest that ACR may be a promising indicator in predicting risk of death in sepsis patients with DM.
Septic shock is one of the important causes of mortality27. Our work showed that sepsis patients with high level of ACR have a higher risk of death within 30 days than those with low level of ACR regardless of septic shock, which are in general agreement with several previously published studies. In 2016, Ganesh K et al. reported that high level of AG was associated with an increased risk of death from any cause in patients with septic shock28. In 2017, another cohort study by performed by He et al. also found that sepsis patients with high level of AG had a higher risk of long-term death than those with low level of AG, regardless of whether they had concurrent septic shock29. However, these published studies only looked at the association between AG and the risk of death in sepsis, and our study is the first to report that ACR levels are also associated with the prognosis among sepsis patients with or without septic shock.
Our study has several limitations. First, this is a data study based on a single center, and practice observed in this cohort may not be representative of other settings. Second, the cohort was generated from retrospective data, and therefore confounding factors may contribute to the unreliability of the results, and omission of unmeasured confounders may result in a biased estimation. Indeed, we used PSM to control for measured confounders, but there may be unmeasured or unknown confounders that were not included in the matching process. The results of PSM also depend on the quality of the input data, and errors or biases in the collection of single-center data may affect the calculation and matching process of propensity scores. Third, our analysis was limited to all-cause mortality, mainly because critically ill patients are often complicated by multiple diseases and it is difficult to select a specific cause of death for such patients. However, all-cause mortality is also an objective and useful endpoint that has been widely used in various clinical studies. Fourth, although the AUROC was greater than 0.5, it was less than 0.7 (0.622), indicating scope for improvement. The specificity for ACR suggests that it is effective at identifying the sepsis patients with DM who died in the short term. However, the relatively lower sensitivity also demonstrates the necessity for future studies with larger populations to validate the clinical utility of ACR as a prognostic indicator. Finally, this work also included some variables with missing information. However, we have removed the variables with more than 20% missing data and processed the remaining variables using multiple imputation to minimize bias.
Despite these limitations, our findings also have strengths. First, this study is the first to investigate the association between ACR and death in sepsis patients with DM, and confirmed that the level of ACR was associated with death within 30 days in critically ill sepsis patients with DM. Second, we used several sensitivity analyses to assess the robustness of the findings, which further improve the reliability of our findings. Third, our study provides a new perspective for predicting the prognosis of patients with sepsis and DM. If these results are further validated in future studies, which have a potential to improve strategies for assessing the condition of sepsis patients with DM.
Our results suggested that ACR, as a simple and practical clinical parameter, may be an independent prognostic indicator of death with 30 days in critically ill sepsis patients with DM. But these results still need to be further validated in future studies.
Methods
Study setting
This is a longitudinal, single-center, retrospective cohort study and followed the reporting guidelines of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement30. The publicly available database of Medical Information Mart for Intensive Care Database IV (MIMIC IV, version 3.0) was employed for this work. MIMIC IV was a publicly available real-world clinical database, and maintained by Beth Israel Deaconess Medical Center (BIDMC, Boston, MA, USA) and Massachusetts Institute of Technology (MIT, Cambridge, MA, USA)31. We completed the “Data or Specimens Only Research” course of Collaborative Institutional Training Initiative Program before extracted the MIMIC-IV clinical data. Institutional review board (IRB) approval from Chengdu First People’s Hospital was exempted because this work was an analysis of the publicly available database with pre-existing IRB approval (Certification Number: 62955778).
The dictionary of codes for the International Classification of Diseases and Ninth Revision (ICD-9) codes dictionary were used to screen and extract sepsis, septic shock, and diabetes mellitus from the MIMIC-IV database. Definitions of sepsis was defined by a condition with life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock was defined by the presence of hypotension requiring vasopressor support in patients with sepsis20.
Study population
All patients diagnosed with both sepsis and diabetes mellitus in ICU were included in this study. Detailed inclusion criteria were patients: (1) with sepsis or septic shock; (2) with diabetes mellitus; (3) age ≥ 18 years; (4) with data of anion gap and blood calcium; (5) admitted to the ICU.
Data collection and variable extraction
The categorical and continuous variables were extracted from the first day of ICU admission. Categorical variables included gender, ventilation, comorbidities (coronary artery disease, congestive heart failure, valvular disease, chronic obstructive pulmonary disease, hypertension, malignant cancer, renal failure, liver disease, rheumatoid arthritis and septic shock). Continuous variables included the age at admission, body mass index (BMI), sequential organ failure assessment (SOFA), simplifed acute physiology score II (SAPS II), AG, blood calcium, glucose, lactate, platelets, international normalized ratio (INR), blood urea nitrogen (BUN),white blood cell (WBC), RDW, red blood cell (RBC), hematocrit, hemoglobulin, creatinine (Cr), mean arterial pressure, respiratory rate, temperature, and blood oxygen saturation. Anion gap-to-calcium ratio (ACR) is the ratio of anion gap and calcium. The primary endpoint was the 30-day mortality, which was defined as the status of patient survival within 30 days from admission.
Statistical analysis
The data were analyzed using the statistical software packages R 4.1.2 (http://www.R-project.org, The R Foundation) and Statistical Package for the Social Sciences, version 27 (SPSS 27), which is a comprehensive software program designed for data analysis, reporting, and predictive analytics. We filled in the variables with missing values (< 20%) by the multiple interpolation method. Categorical variables were expressed as the number of the population with the percentage (%). Continuous variables which were non-normally distributed were expressed as median and interquartile range (IQR), and normally distributed variables were expressed as the mean and standard deviation (Mean ± SD). Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the predictive performance of ACR for 30-day mortality. The corresponding sensitivity and specificity were calculated using the cut-off value of ACR, which was determined by maximising the Youden index.
The enrolled patients were next divided into high- and low-ACR groups based on the obtained cut-off values. PS matching (using standard caliper value of 0.2) was used to account for the baseline differences between high and low groups32. Patients with high-ACR were matched to those with low-ACR by nearest neighbor matching. Standardized mean difference (SMD) was calculated before and after matching to examine whether the PSM reduced the differences in pretreatment covariates between low- and high-ACR groups. Multivariable logistic regression model was further used to adjust for residual imbalance by including parameters with P < 0.1 and potential confounders judged by clinical expertise. Inverse probability weighting (IPW) is used to assess the reliability of the results. In the IPW analysis, the predicted probabilities from the propensity-score model were used to calculate the stabilized inverse-probability-weighting weight33. A P value less than 0.05 was considered to be statistically significant.
Several prespecified subgroup analyses were performed by restricting (1) the age of patients at admission (≤ 65 and > 65 years); (2) gender; (3) whether patients received mechanical ventilation (yes or no); (4) the type of comorbidity.
Supplementary Information
Acknowledgements
We acknowledged the contributions of the Medical Information Mart for Intensive Care (MIMIC) Program registries for creating and updating the MIMIC-IV databases.
Author contributions
C.J., P.L., L.K., L.M. and Y.N.B. conceived and designed research; C.J., L.K. and L.M. collected data and conducted research; Y.N.B., G.J and L.J.J. analyzed and interpreted data; C.J. wrote the initial paper; All authors revised the paper; C.J. had primary responsibility for final content. All authors reviewed the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
MIMIC-IV v3.0 (Medical Information Mart for Intensive Care IV, version: 3.0) was accessed on July 23, 2024 from https://physionet.org/content/mimiciv/3.0.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jing Cai and Lin Pu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82700-4.
References
- 1.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet402(10397), 203–234. 10.1016/S0140-6736(23)01301-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schertzer, J. D. & Steinberg, G. R. Immunometabolism: The interface of immune and metabolic responses in disease. Innunol. Cell. Biol.92(4), 303. 10.1038/icb.2014.12 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol.11(2), 98–107. 10.1038/nri2925 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Devaraj, S., Venugopal, S. K., Singh, U. & Jialal, I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta}. Diabetes54(1), 85–91. 10.2337/diabetes.54.1.85 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Berbudi, A., Rahmadika, N., Tjahjadi, A. I. & Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev.16(5), 442–449. 10.2174/1573399815666191024085838 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey, I. M. et al. Risk of infection in type 1 and type 2 diabetes compared with the general population: A matched cohort study. Diabetes Care41(3), 513–521. 10.2337/dc17-2131 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Li, J. et al. Causal relationship between circulating immune cells and the risk of type 2 diabetes: A Mendelian randomization study. Front. Endocrinol.14, 1210415. 10.3389/fendo.2023.1210415 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah, B. R. & Hux, J. E. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care26(2), 510–513. 10.2337/diacare.26.2.510 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Xin, Q. et al. Predictive nomogram model for major adverse kidney events within 30 days in sepsis patients with type 2 diabetes mellitus. Front. Endocrinol.16(13), 1024500. 10.3389/fendo.2022.1024500 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang, C. Y., Jiang, Y., Zhang, C. L., Min, Y. & Huang, X. The predictive values of admission characteristics for 28-day all-cause mortality in septic patients with diabetes mellitus: A study from the MIMIC database. Clin. Diabetes1(14), 1237866. 10.3389/fendo.2023.1237866 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prodan, C. I., Stoner, J. A. & Dale, G. L. Lower coated-platelet levels are associated with increased mortality after spontaneous intracerebral hemorrhage. Stroke46(7), 1819–1825. 10.1161/STROKEAHA.115.009068 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Sarkar, S., Kannan, S., Khanna, P. & Singh, A. K. Role of red blood cell distribution width, as a prognostic indicator in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol.32(2), e2264. 10.1002/rmv.2264 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai, L., Mu, S. & Hu, Y. Prognostic role of RDW in hematological malignancies: A systematic review and meta-analysis. Cancer Cell Int.23(18), 61. 10.1186/s12935-018-0558-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbel, Y. et al. Red blood cell distribution width (RDW) and long-term survival in patients with ST elevation myocardial infarction. Thromb. Res.134(5), 976–979. 10.1016/j.thromres.2014.08.016 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Hu, T. Y., Zhang, Z. W. & Jiang, Y. F. Albumin corrected anion gap for predicting in-hospital mortality among intensive care patients with sepsis: A retrospective propensity score matching analysis. Clin. Chim. Acta.521, 272–277. 10.1016/j.cca.2021.07.021 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Kim, M. J. et al. Serum anion gap at admission as a predictor of mortality in the pediatric intensive care unit. Sci. Rep.7(1), 1456. 10.1038/s41598-017-01681-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steele, T., Kolamunnage-Dona, R., Downey, C., Toh, C. H. & Welters, I. Assessment and clinical course of hypocalcemia in critical illness. Crit. Care17(3), R106. 10.1186/cc12756 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorde, R. et al. Serum calcium and the calcium-sensing receptor polymorphism rs17251221 in relation to coronary heart disease, type 2 diabetes, cancer and mortality: The Tromsø Study. Eur. J. Epidemiol.28(7), 569–578. 10.1007/s10654-013-9822-y (2023). [DOI] [PubMed] [Google Scholar]
- 19.Kim, M. K. et al. Altered calcium homeostasis is correlated with the presence of metabolic syndrome and diabetes in middle-aged and elderly Korean subjects: The Chungju metabolic disease cohort study (CMC study). Atherosclerosis212(2), 674–681. 10.1016/j.atherosclerosis.2010.07.005 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA315(8), 801–810. 10.1001/jama.2016.0287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleischmann-Struzek, C. et al. Incidence and mortality of hospital- and ICU-treated sepsis:results from an updated and expanded systematic review and meta-analysis. Intensive Care Med.46(8), 1552–1562. 10.1007/s00134-020-06151-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito, S., Simone, G. D., Boccia, G. & Caro, F. D. Determinants of the calibration of SAPS II and SAPS 3 mortality scores in intensive care: A European multicenter study Pagliano, P. Sepsis and septic shock—New definitions, new diagnostic and therapeutic approaches. J. Glob. Antimicrob. Resist.10, 204–212. 10.1016/j.jgar.2017.06.013 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Raith, E. P. et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA317(3), 290–300. 10.1001/jama.2016.20328 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Poncet, A., Perneger, T. V., Merlani, P., Capuzzo, M. & Combescure, C. Determinants of the calibration of SAPS II and SAPS 3 mortality scores in intensive care: A European multicenter study. Crit. Care21(1), 85. 10.1186/s13054-017-1673-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohr, N. M. et al. Serum anion gap predicts lactate poorly, but may be used to identify sepsis patients at risk for death: A cohort study. J. Crit. Care44, 223–228. 10.1016/j.jcrc.2017.10.043 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Li, H. et al. Clinical value of serum calcium in elderly patients with sepsis. Am. J. Emerg. Med.52, 208–211. 10.1016/j.ajem.2021.12.019 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Seymour, C. W. & Rosengart, M. R. Septic shock: Advances in diagnosis and treatment. JAMA314(7), 708–717. 10.1001/jama.2015.7885 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesh, K., Sharma, R. N., Varghese, J. & Pillai, M. G. K. A profile of metabolic acidosis in patients with sepsis in an intensive care unit setting. Int. J. Crit. Illn Inj. Sci.6(4), 178–181. 10.4103/2229-5151.195417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He, X. L., Liao, X. L., Xie, Z. C., Jiang, C. & Kang, Y. Albumin corrected anion gap is an independent risk factor for long-term mortality of patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue.29(2), 117–121. 10.3760/cma.j.issn.2095-4352.2017.02.005 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. JAMA326(16), 1614–1621. 10.1001/jama.2021.18236 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Johnson, A. E. W. et al. MIMIC-III, a freely accessible critical care database. Sci. Data24(3), 160035. 10.1038/sdata.2016.35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baek, S., Park, S. H., Won, E., Park, Y. R. & Kim, H. J. Propensity score matching: a conceptual review for radiology researchers. Korean J. Radiol.16(2), 286–296. 10.3348/kjr.2015.16.2.286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robins, J. M., Hernán, M. A. & Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology11(5), 550–560. 10.1097/00001648-200009000-00011 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MIMIC-IV v3.0 (Medical Information Mart for Intensive Care IV, version: 3.0) was accessed on July 23, 2024 from https://physionet.org/content/mimiciv/3.0.