Abstract
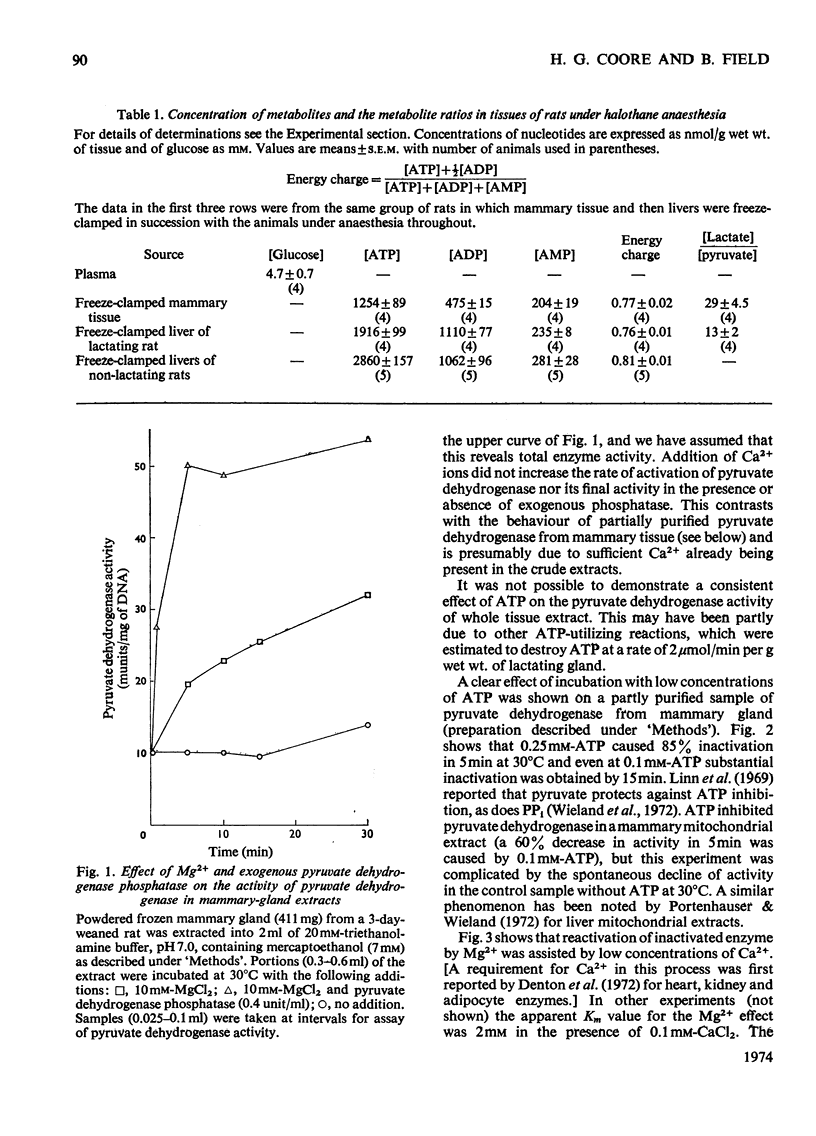
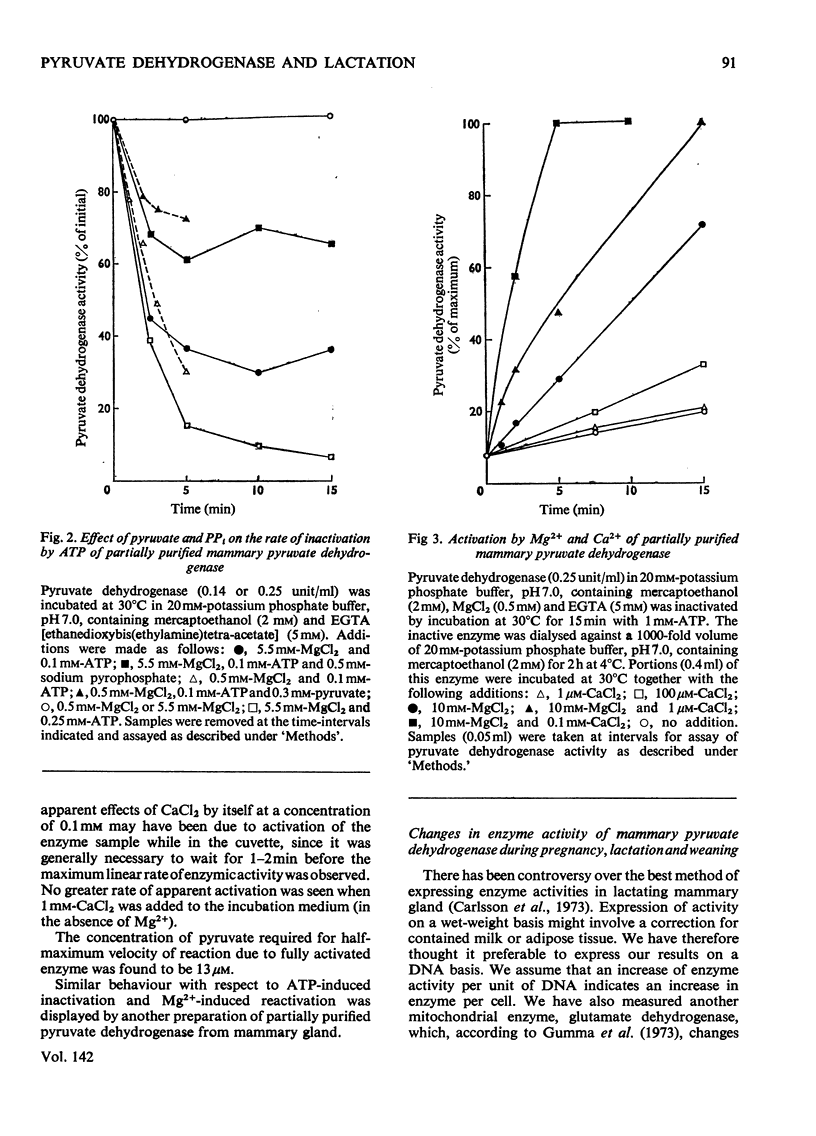
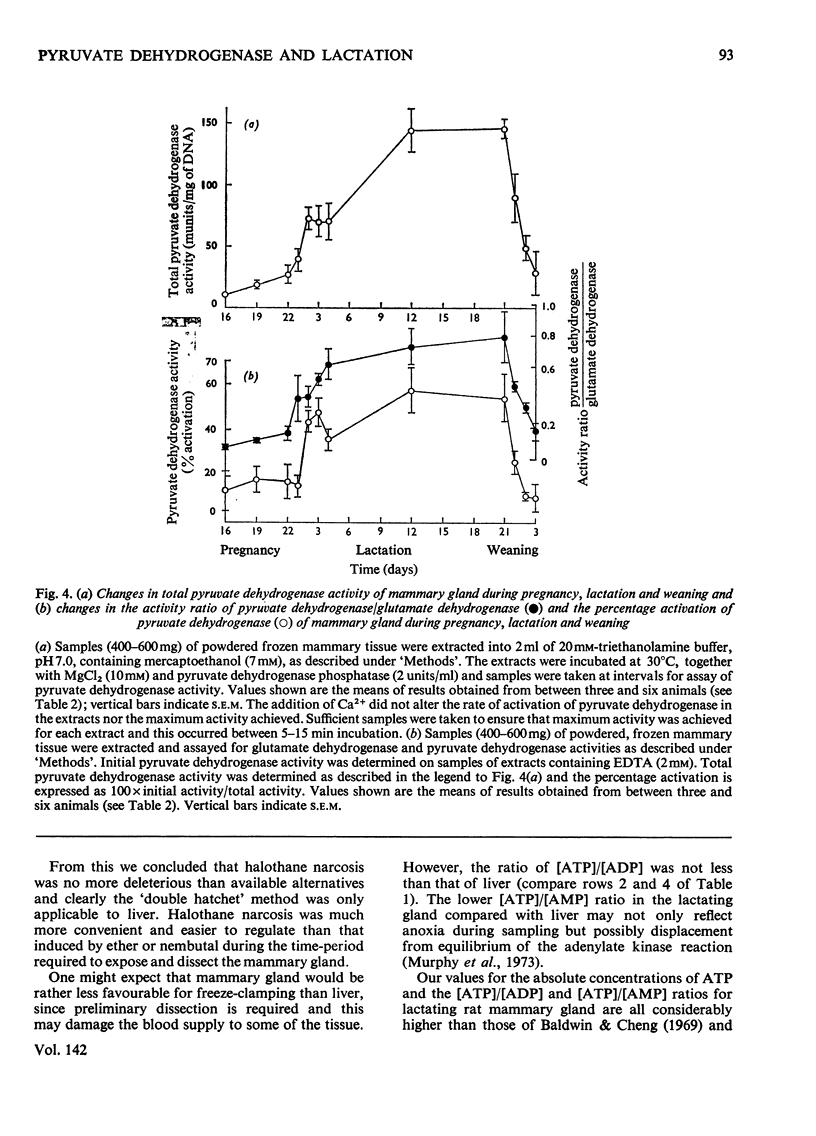
Pyruvate dehydrogenase of rat mammary tissue showed many of the regulatory properties of the analogous enzyme in other mammalian tissues. It was inactivated in the presence of low concentrations of ATP and this rate of inactivation was slowed if pyruvate or PP1 was also present. Reactivation by Mg2+ in the presence of low concentrations of Ca2+ occurred over a similar time-course. The Km value for Mg2+ in this process was about 2mm. The enzyme was assayed in extracts of freeze-clamped mammary glands removed from pregnant, lactating or recently weaned rats under halothane anaesthesia. Both the initial activity and the activity after full activation (`total enzyme activity') were determined. The former parameter, when expressed on a DNA basis, varied within a range of 40 times its lowest value. Maximum total enzyme activity was about 1 unit/g wet wt. The total enzyme activity and the fraction in the active form increased in step from pregnancy to mid-lactation, remained elevated until the end of lactation and then fell steeply within 3 days after weaning. The correlation of these two parameters of enzyme activity may indicate a common regulatory factor or else an interdependence arising from inherent properties of the multi-enzyme complex.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aynsley-Green A., Biebuyck J. F., Alberti K. G. Anaesthesia and insulin secretion: the effects of diethyl ether, halothane, pentobarbitone sodium and ketamine hydrochloride on intravenous glucose tolerance and insulin secretion in the rat. Diabetologia. 1973 Aug;9(4):274–281. doi: 10.1007/BF01221854. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Cheng W. Metabolite changes associated with initiation and maintenance of lactation in rats and cows. J Dairy Sci. 1969 Apr;52(4):523–528. doi: 10.3168/jds.S0022-0302(69)86598-7. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson E. I., Karlsson B. W., Waldemarson K. H. Dehydrogenases and nucleic acids in rat mammary gland during involution initiated at various stages of lactation. Comp Biochem Physiol B. 1973 Jan 15;44(1):93–108. doi: 10.1016/0305-0491(73)90346-5. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaa K. A., Greenbaum A. L., McLean P. Adaptive changes in satellite systems related to lipogenesis in rat and sheep mammary gland and in adipose tissue. Eur J Biochem. 1973 Apr 2;34(1):188–198. doi: 10.1111/j.1432-1033.1973.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Howanitz P. J., Levy H. R. Acetyl-CoA carboxylase and citrate cleavage enzyme in the rat mammary gland. Biochim Biophys Acta. 1965 Oct 4;106(2):430–433. doi: 10.1016/0005-2760(65)90056-1. [DOI] [PubMed] [Google Scholar]
- JACOBSON K. B. Effect of substrate structure on activity of pigeon liver acetyl transferase. J Biol Chem. 1961 Feb;236:343–348. [PubMed] [Google Scholar]
- Jungas R. L. Hormonal regulation of pyruvate dehydrogenase. Metabolism. 1971 Jan;20(1):43–53. doi: 10.1016/0026-0495(71)90058-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ariyanayagam A. D., Kuhn N. J. Progesterone and the metabolic control of the lactose biosynthetic pathway during lactogenesis in the rat. Biochem J. 1973 Dec;136(4):1105–1116. doi: 10.1042/bj1361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C., Löffler G., Wieland O. H. Interconversion of pyruvate dehydrogenase in the isolated perfused rat liver. Eur J Biochem. 1973 Feb 15;33(1):117–122. doi: 10.1111/j.1432-1033.1973.tb02662.x. [DOI] [PubMed] [Google Scholar]
- Portenhauser R., Wieland O. Regulation of pyruvate dehydrogenase in mitochondria of rat liver. Eur J Biochem. 1972 Dec 4;31(2):308–314. doi: 10.1111/j.1432-1033.1972.tb02534.x. [DOI] [PubMed] [Google Scholar]
- Roche T. E., Reed L. J. Function of the nonidentical subunits of mammalian pyruvate dehydrogenase. Biochem Biophys Res Commun. 1972 Aug 21;48(4):840–846. doi: 10.1016/0006-291x(72)90684-5. [DOI] [PubMed] [Google Scholar]
- Seitz H. J., Faupel R. P., Kampf S. C., Tarnowski W. Influence of different types of narcosis and of neck fracture on the concentration of glycolytic intermediates and related compounds in rat liver. Arch Biochem Biophys. 1973 Sep;158(1):12–18. doi: 10.1016/0003-9861(73)90591-2. [DOI] [PubMed] [Google Scholar]
- Sica V., Cuatrecasas P. Effects of insulin, epinephrine, and cyclic adenosine monophosphate on pyruvate dehydrogenase of adipose tissue. Biochemistry. 1973 Jun 5;12(12):2282–2291. doi: 10.1021/bi00736a016. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Purification and characterization of pyruvate-dehydrogenase phosphatase from pig-heart muscle. Eur J Biochem. 1972 Mar 15;26(1):96–105. doi: 10.1111/j.1432-1033.1972.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Siess E., Wittmann J., Wieland O. Interconversion and kinetic properties of pyruvate dehydrogenase from brain. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):447–452. doi: 10.1515/bchm2.1971.352.1.447. [DOI] [PubMed] [Google Scholar]
- Start C., Newsholme E. A. The effects of starvation and alloxan-diabetes on the contents of citrate and other metabolic intermediates in rat liver. Biochem J. 1968 Apr;107(3):411–415. doi: 10.1042/bj1070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR H., MEHLER A. H., STADTMAN E. R. The enzymatic acetylation of amines. J Biol Chem. 1953 Sep;204(1):127–138. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wieland O., Funcke H. v., Löffler G. Interconversion of pyruvate dehydrogenase in rat heart muscle upon perfusion with fatty acids or ketone bodies. FEBS Lett. 1971 Jul 1;15(4):295–298. doi: 10.1016/0014-5793(71)80641-5. [DOI] [PubMed] [Google Scholar]
- Wieland O., Jagow-Westermann B. v. ATP-dependent inactivation of heart muscle pyruvate dehydrogenase and reactivation by Mg(++). FEBS Lett. 1969 Jun;3(4):271–274. doi: 10.1016/0014-5793(69)80156-0. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E. Interconversion of phospho- and dephospho- forms of pig heart pyruvate dehydrogenase. Proc Natl Acad Sci U S A. 1970 Apr;65(4):947–954. doi: 10.1073/pnas.65.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]


