Abstract
Influenza virus infections are a serious danger to people’s health worldwide as they are responsible for seasonal flu outbreaks. There is an urgent need to improve the effectiveness and durability longevity of the immune response to influenza vaccines. We synthesized the CpG HP021 and examined the impact of it on the immune response to an influenza vaccine. In BALB/c mice, hemagglutination inhibition (HI) titers to the vaccine were increased four- to eightfold against H1N1, H3N2, BV, and BY viruses by 3 μg IIV4 + 40 μg CpG HP021 compared with those of the non-adjuvanted IIV4 group, and the CpG HP021 group had a broader HI activity. Additionally, the immune response was directed towards Type 1 T helper (Th1) cells due to the CpG HP021 adjuvant. The CpG HP021-adjuvanted IIV4 induced a higher number of T cells secreting interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), and increased the percentage of effector memory T cells in mice. In SD rats, the immune responses induced by IIV4 with CpG HP021 were similar to those in BALB/c mice. The development of CpG HP021 may expand the options for adjuvants in vaccines against infectious diseases.
Keywords: Quadrivalent influenza vaccine, CpG adjuvant, Hemagglutination inhibition, Antibody, T cell immunity
Subject terms: Influenza virus, Preventive medicine, Influenza virus
Introduction
Vaccination is an effective intervention to prevent influenza, minimize all types of complications, and reducing the healthcare burden. Currently, trivalent and quadrivalent inactivated influenza vaccines (TIV/IIV4) are licensed for use in all age groups. However, the overall efficacy of vaccination is approximately 30 to 40% in individuals over 65 and between 70 and 90% in those under 651,2. Influenza vaccine protection lasts only 6 to 8 months, and vaccine-induced antibodies protect only against influenza strains homologous to the vaccine; therefore, annual seasonal influenza vaccination is required. Consequently, there is a pressing need to enhance the immunization strategy of influenza vaccines to offer comprehensive, efficient, and enduring immune protection across various age groups. Synthetic biology tools show great potential for vaccine and adjuvant development.
Adjuvants play multiple roles in vaccine development, such as increasing the rate of the initial immune response; generating more sustained immune responses; eliciting protective immunity in immunocompromised, aged, and young children3 ,decreasing the dose of antigens and the number of immunizations; and generating immunity against mutated virus strains4–9. Each adjuvant has its own unique immune characteristics, and the choice of an adjuvant needs to be based on the characteristics of the antigen as well as the required immune response for immune protection. There is a long history of using aluminum hydroxide as an adjuvant in human vaccines, but alum does not produce a strong immunological response when combined with influenza antigens10–12. A number of adjuvants, including MF5913,14 and AS0315,16, have also been licensed for use in influenza vaccinations in addition to aluminum adjuvants 17. In 1997, the MF59 adjuvant was approved for use in the influenza vaccine Fluad in Italy 18. Both the H5N1 avian influenza vaccine and the H1N1 pdm09 vaccine contain the AS03 adjuvant 19,20. However, safety issues regarding the H1N1 influenza vaccine with AS03 have been highlighted.
Agonists of Toll-like receptors (TLRs) have been explored as adjuvant candidates in both mice and humans. Synthetic CpG sequences can be employed as TLR9 agonists. It has been demonstrated that every possible combination of sequence variants has unique structural and biological characteristics. Since its approval for use in tumor vaccines in 2002, CpG 7909 has been used in various vaccines, such as malaria and anthrax vaccines 21. CpG 1018 was initially originally licensed for use in HBV vaccines and subsequently added to the COVID-19 vaccines 22–24. CpG HP021 is one of our newly synthesized adjuvants. It has been demonstrated that CpG HP021 stimulates peripheral blood mononuclear cells (PBMCs) to produce cytokines and causes the growth of mouse spleen cells25. We have evaluated the immune effects of CpG HP021 in inactivated COVID-19 vaccines26 and found that the addition of the CpG HP021 adjuvant significantly enhances cross-reactive humoral immunity, providing protection against heterologous challenges, and can induce long-term immunity in K18-hACE2 mice.
In this study, we evaluated the immunogenic efficacy of IIV4, with or without the CpG HP021 adjuvant, at various dose levels. We observed that the inclusion of the adjuvant elicited robust cellular and humoral immune responses. The development of active TLR9 agonists may broaden the options for adjuvants in vaccines against infectious diseases.
Results
CpG HP021-adjuvanted IIV4 induces high levels of HI titers in mice
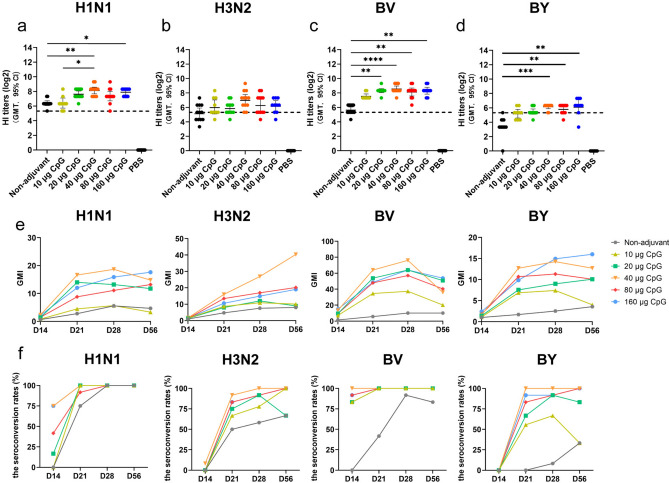
The HI titers against H1N1 and B/Victoria (BV) viruses in the CpG HP021-adjuvanted groups, with CpG doses in the range of 20–160 μg were significantly higher than those in the non-adjuvant group at day 28 (P < 0.05) (Fig. 1a-d). The geometric mean titer (GMT) of the HI antibody responses elicited by 3 μg of IIV4 + 40 μg of CpG HP021 was four to eight-fold greater than the titers induced by the unadjuvanted IIV4 vaccine (P < 0.05). The seroconversion rate (SCR) was 58.3% for the H3N2 type and 8.3% for the BY type in non-adjuvant group at day 28 after the first immunization, at which point the SCRs were more than 90% for all subtypes in the groups adjuvanted with a CpG dose of 20 μg or more (Fig. 1f and Supplementary Table 1). In addition, the geometric mean fold increase (GMI) for the 3 μg of IIV4 + 40 μg of CpG HP021 group was higher than that of the other groups against all four types of viruses (Fig. 1e). The GMI detected by HI against BV was 76.11, which was 7.5 times more than the GMI of the non-adjuvant group.
Fig. 1.
HI titers and GMI at day 28 after first immunization in BALB/c mice. (a-d) The serum HI titers were detected by HI against H1N1, H3N2, BV, and BY at day 28. The data represent geometric mean with 95% CI. (e) Dynamic changes in GMI at various time points. (f) The SCRs for H1N1, H3N2, BY, and BV types. The dotted line indicates HI titers of 1:40 which are suggested to indicate a probability of clinical protection of at least 50%. This titer serves as a threshold for predicting protection. N = 9 to 12. *p < 0.05, **p < 0.01, ***p < 0.001.
The CpG HP021-adjuvanted IIV4 improves the levels of humoral immune antibodies in mice
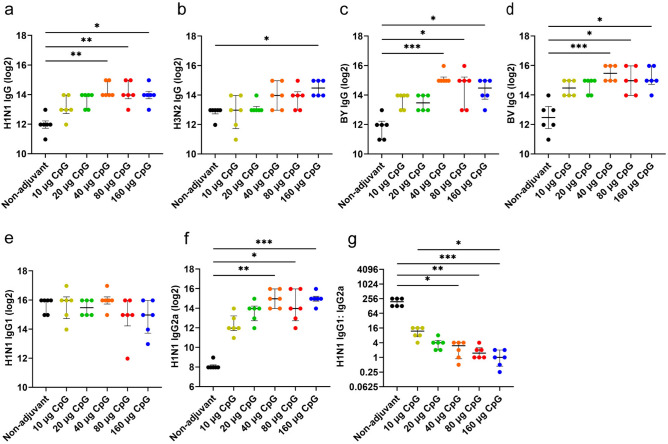
We identified IgG, IgG1, and IgG2a antibodies in serum samples at day 28. The GMT of the binding antibody responses elicited by 3 μg of IIV4 + 40 μg of CpG HP021 at day 28 against H1N1, BV, and BY viruses was significantly higher than those in the non-adjuvant group (P < 0.05). Consistent with the HI antibody responses, 3 μg of IIV4 + 40 μg of CpG HP021 elicited four to eight times higher binding antibody titers than the non-adjuvanted vaccine formulation (Fig. 2a-d). Furthermore, IIV4 formulated with CpG HP021 showed comparable IgG1 titers but higher IgG2a titers in contrast to the non-adjuvant group (P > 0.05) (Fig. 2e-g), indicating that the CpG adjuvant effectively induced a T helper type 1 (Th1) bias in mice. The antigens with 160 μg CpG HP021 group had considerably greater serum levels TNF-α, IL-2, and IFN-γ, compared to the antigen group at day 28 (P < 0.001) (Supplementary Fig. 1). This may suggest an overstimulation of the immune system in the group that received more than 80 μg of CpG HP021.
Fig. 2.
The levels of antibody subtypes after IIV4 immunization in mice measured at day 28. (a-d) Total IgG levels were assessed using ELISA with split H1N1, H3N2, B/Victoria, and B/Yamagata viruses. IgG1 titers (e), IgG2a titers (f), and the ratio of IgG1 to IgG2a titers (g) were assessed using ELISA with split H1N1 viruses. *P < 0.05, **P < 0.01, ***P < 0.001. N = 6.
The CpG HP021-adjuvanted IIV4 induces stronger and broader cross-reactive HI antibody responses in mice
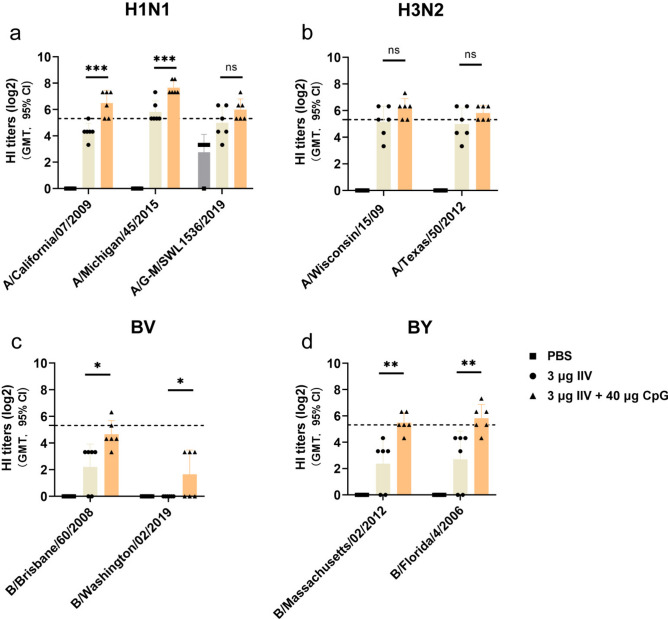
We evaluated the cross-reactive antibody response using an HI assay against influenza viruses from similar and different phylogenetic groups. Serum HI titers of at least 1:40 are suggested to indicate a probability of clinical protection of at least 50%. This titer serves as a threshold for predicting protection 27,28. At day 28 after first immunization, the group receiving 3 μg of IIV4 + 40 μg of CpG HP021 elicited protective HI titres against H1N1, H3N2, and BY viruses (HI titers ≥ 1:40), when non-adjuvant group only induced limited cross-reactivity (HI titer geometric means < 40) (Fig. 3a, b, and d). Neither the non-adjuvant group nor the CpG HP021-adjuvanted IIV4 group did not achieve a post-vaccination titer of ≥ 40 or seroconversion, as measured by HI to heterologous BV virus (Fig. 3c). Overall, our findings indicate that the CpG HP021-adjuvanted IIV4 can effectively neutralize heterologous H1N1, H3N2, and BY influenza viruses.
Fig. 3.
Cross-reactive antibody responses at day 28 after first immunization in BALB/c mice.(a-d) HI antibody responses against the representative heterologous strains. The data represent geometric mean with 95% CI. The dotted line indicates HI titers of 1:40 which are suggested to indicate a probability of clinical protection of at least 50%. This titer serves as a threshold for predicting protection. N = 6. *P < 0.05, **P < 0.01, and ***P < 0.001.
Immunization of mice with IIV4 with CpG HP021 adjuvant induces higher levels of T cell responses and cytokines in mice
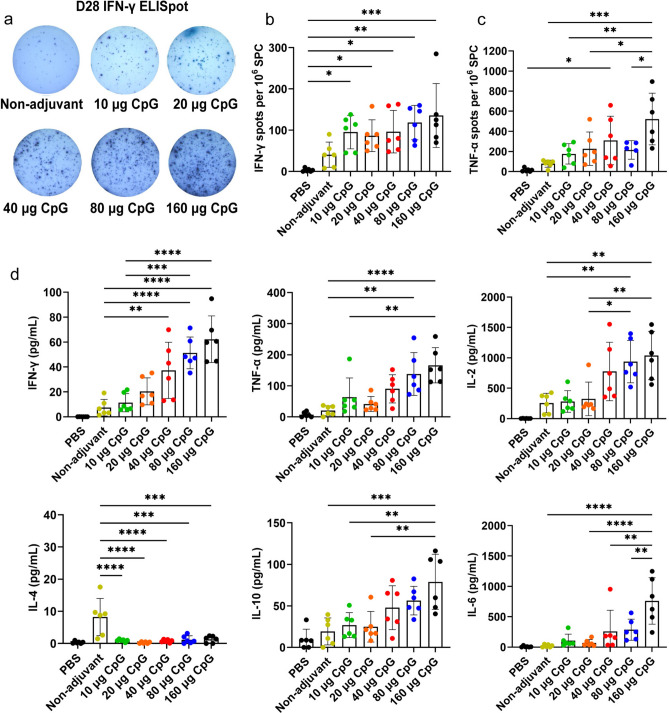
Enzyme-linked immunospot (ELISpot) tests were used at day 28 to detect the T cell responses in the spleens of immunized mice. The mean spot numbers measured by IFN-γ ELISpot in the CpG HP021 groups were 87 to 136 per 106 splenocytes (SPC), which was more than twice those in the non-adjuvant group (mean = 41 per 106 SPC) (Fig. 4a, b). The spot numbers measured by TNF-a ELISpot in the 40 μg or more of CpG HP021 groups were also approximately twice as high as those in the non-adjuvant group (P < 0.05) (Fig. 4c). At day 28, the addition of CpG HP021 significantly increased the levels of IFN-γ, IL-2, TNF-α, IL-6, and IL-10 secreted by splenic lymphocytes (Fig. 4d). These findings imply that adding the appropriate dose of CpG HP021 adjuvant to the vaccine stimulates the production of IFN-γ-secreting T cells, and TNF-α-secreting T cells. It also induces splenic lymphocytes to secrete various cytokines.
Fig. 4.
T cell responses and cytokine levels in the supernatant of splenic lymphocytes at day 28 in BALB/c mice. (a-b) IFN-γ ELISpot test following antigen-specific splenic stimulation in mice. (c) TNF-α ELISpot test following antigen-specific splenic stimulation in mice. (d) Multiplex cytokine analysis of splenic lymphocyte supernatant stimulated by peptides. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. N = 6.
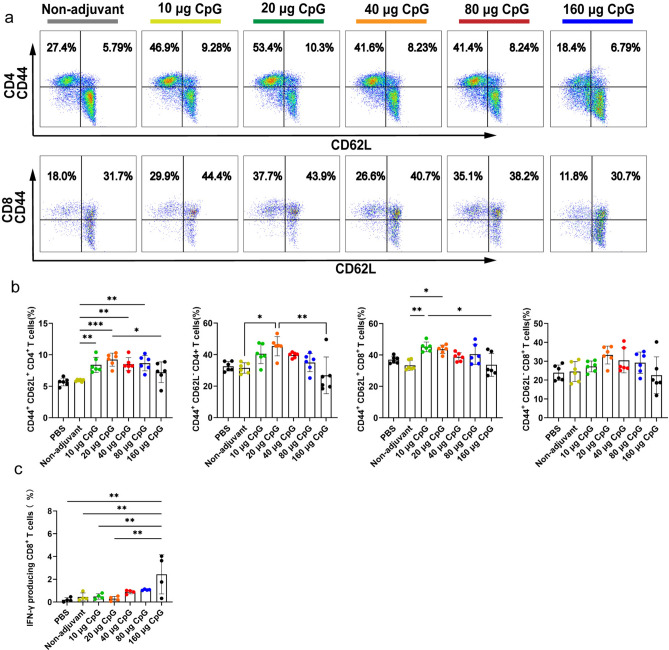
IIV4 with CpG HP021 induces higher levels of memory T cells in mice
Next, we investigated the proportion of memory cells among lymphocytes in mice at day 28. CD62L+ CD44- CD4+ T cells were significantly enriched in the spleens of mice in the CpG HP021 group than non-adjuvant group (P < 0.01) (Fig. 5a, b). Furthermore, the percentage of central memory T cells (CD44+ CD62L+) in the 20 μg CpG HP021 group was considerably greater than that in the non-adjuvant group (Fig. 5a ,b). These findings imply that CpG HP021 may provide sustained immune protection. Consistent with the antigen-specific cytokine production, splenocytes developed the capacity to produce significant levels of the Th1 cytokine IFN-γ when exposed to IIV4-specific stimulation (Fig. 5c).
Fig. 5.
Flow cytometry assay identifying TEM cells and the production of IFN-γ by T cells at day 28 after first immunization in BALB/c mice. (a-b) The proportion of memory cells among lymphocytes was analyzed across the different groups at day 28. (c) Assessments of the IFN-γ producing CD8+ T cells from immunized mice. *P < 0.05, **P < 0.01, ***P < 0.001. N = 5 to 6.
The adjuvant effect of CpG HP021 in SD rats
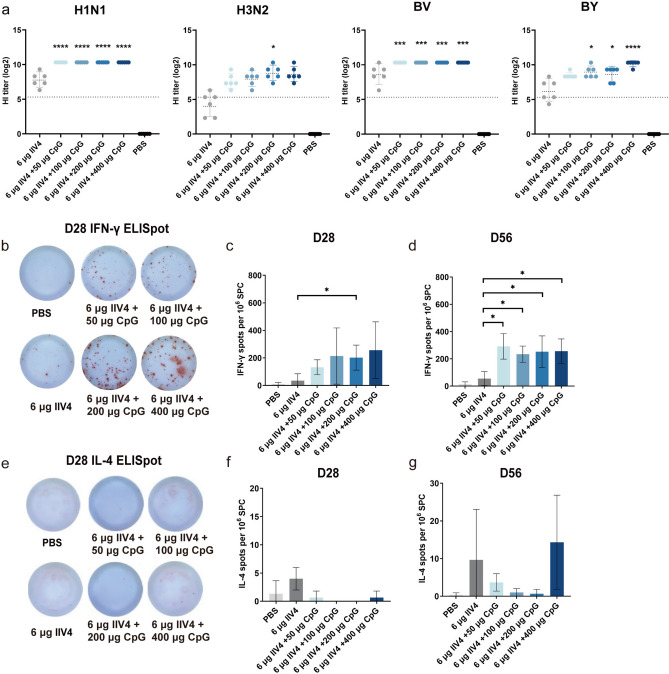
The HI titers against H1N1 and BV viruses in the CpG HP021-adjuvanted groups with CpG HP021 doses ranging from 50 to 400 μg were significantly higher than those in the non-adjuvant group at day 28 after the first immunization (P < 0.001) (Fig. 6a). The CpG HP021-adjuvanted groups also showed an increase in HI titers against H3N2 and BY viruses, especially in the 6 μg of IIV4 + 200 μg of CpG HP021 group and the 6 μg of IIV4 + 400 μg of CpG HP021 group (Fig. 6a). At day 28, the SCRs of H1N1, H3N2, BY, and BV antibodies in the unadjuvanted IIV4 group were 100%, 33%, 100%, and 67%, respectively, while the SCRs in CpG HP021-adjuvanted vaccine groups with CpG HP021 doses ranging from 50 to 400 μg were all 100% (Supplement Table 2). These results imply that the CpG HP021 adjuvant enhanced the humoral responses of IIV4 in SD rats. After antigen stimulation, the 6 μg of IIV4 + 200 μg of CpG HP021 group and the 6 μg of IIV4 + 400 μg of CpG HP021 group both had significantly higher numbers of IFN-γ-secreting cells (mean = 202 per 1 × 106 SPC), more than five times higher than the non-adjuvant group (mean = 35 per 1 × 106 SPC) (Fig. 6b and c). At day 56, the number of IFN-γ-secreting cells was higher in the IIV4 with CpG group compared with that in the non-adjuvant group (P < 0.05) (Fig. 6d). The quantity of cells that secreted IL-4 was similar across the various groups (Fig. 6e-g).
Fig. 6.
HI titers and T cell responses at day 28 after the first immunization in SD rats. (a) The serum HI titers were detected by HI against H1N1, H3N2, BV, and BY at day 28. The data represent geometric mean with 95% CI. (b-d) T cell responses were measured by IFN-γ ELISpot assays for various groups. (e–g) T cell responses were assessed by IL-4 ELISpot. Compared with 6 μg of IIV4, *P < 0.05, ***P < 0.001, ****P < 0.0001. N = 3 to 6.
Discussion
The influenza virus can be effectively prevented through vaccination. Currently, many seasonal influenza vaccines do not contain adjuvants; however, seasonal inactivated influenza vaccinations are less effective, especially against the H3N2 and BY strains, and provide inadequate cross-protection. In order to broaden the range of protection offered by vaccinations, new immunization techniques are being researched 1. Adding the right adjuvant to an influenza vaccination can boost its immunogenicity; however, only a handful of such adjuvanted commercial vaccines are currently available. CpG has the advantages of stability, low cost, easy synthesis, high efficiency, low toxicity, improved antibody and T cell responses, and reduced antigen dosage29,30. Considering the benefits of using a single adjuvant in terms of production efficiency and cost-effectiveness, this study primarily evaluates the immunological effects of an influenza vaccine combined with the novel CpG HP021 adjuvant.
There is an optimal adjuvant for each specific antigen subtype. In this study, the addition of the CpG HP021 adjuvant to IIV4 did not significantly improve HI antibody responses against the H3N2 virus; however, it did enhance HI antibody responses against other subtypes, including H1N1, BV, and BY. Futhermore, the SCRs were more than 90% for all subtypes in the CpG HP021 groups, whereas the SCRs in the unadjuvanted IIV4 group were only 58.3% for H3N2 type and 8.3% for BY types. The CpG HP021-adjuvanted groups exhibited earlier seroconversion and a higher seroconversion rate compared with the non-adjuvant group for all subtypes. The results of HI test and ELISA demonstrated that the CpG HP021 adjuvant boosts antibody responses induced by IIV4 that a 20 to 40 μg dose CpG HP021 is sufficient. The CpG HP021-adjuvanted IIV4 also induces stronger and broader cross-reactive responses in mice. In the SD rat model, the results were consistent with those in BALB/c mice, indicating high serum HI titers in CpG HP021 group.
T cells restrict the duration and severity of influenza virus infection, hence promoting protective immunity against it31,32. They can provide cross-strain protection against various influenza viruses, including emerging strains, because the most virus-specific T lymphocytes are able to identify viral segments that are found in areas that are less mutagenic and more conserved. In our study, the ELISpot and flow cytometry results showed significantly elevated cellular immunity response in the CpG HP021 groups. In comparison to the non-adjuvant group, the CpG HP021 group’s splenic lymphocyte supernatant exhibited notably greater amounts of cytokines. Mechanistically, CpG adjuvant is an artificially synthesized oligonucleotide sequence with unmethylated CpG motifs, possessing immunostimulatory properties similar to bacterial DNA33–35. CpG recognizes and binds to the human or mouse TLR9 and activates dendritic cells (DCs) and B cells expressing TLR936. DCs secrete a variety of pro-inflammatory and antiviral cytokines, which then induce DC migration and aggregation in lymphoid tissues and induce Th1 cell responses26. Other innate immune cells are also activated by DC cells.
The cytokine environment generated by T cells is a primary factor influencing IgG class switching. IgG1, which is the predominant effector antibody for robust humoral immune responses against external infections, is produced in greater quantities by the Th2 cell lineage, primarily through the secretion of IL-4. The Th1 cell lineage primarily produces IL-12 and stimulates the synthesis of IgG2a, which is generally linked to cell-mediated immune responses against intracellular infections. The CpG HP021-adjuvanted vaccine significantly increased the serum IgG2a antibody titer in mice, indicating that the CpG adjuvant effectively induces a Th1 bias in mice. A Th1/IgG2a/b bias with CpG 1018 adjuvant and a Th2/IgG1 bias have been reported previously 26,37,38, which are consistent with this findings 39–43. In addition, B cells activated by TLR9 differentiate into antibody-secreting plasma cells and produce a variety of cytokines (IL-6 and IL-10), which promote the growth, differentiation, and generation of functional antibodies by B cells. These cytokines have an immunomodulatory function that prevents the overactivation of pro-inflammatory factors.
An ideal vaccine adjuvant must have a robust safety profile. The safety of the CpG adjuvant has been demonstrated in preclinical and clinical trials44–46 and the appropriate safe dosage of the CpG HP021 adjuvant can be investigated through animal experiments and future clinical studies. Cytokines such as IFN-γ, TNF-α, IL-2, and IL-6 are associated with systemic reactogenicity47. Early innate immune system activation to fight viral infections and immune system activation regulation to minimize organ damage should be kept in proper balance 48. In this study, we measured these cytokines in mice serum at day 28 after first immunization and found that IIV4 with CpG doses of 80 μg or more resulted in significant systemic reactions. The levels of all cytokines levels comparable to those in the PBS control group, in mice immunized with CpG doses of 80 µg or less, implying the CpG doses of 80 µg or less is safe. Furthermore, CpG may bystander activation leading to activation of autoimmune responses in a susceptible individual49, and the susceptible individuals could choose other appropriate adjuvant based on the specific circumstances.
Our study has several limitations. Our study is main focused on comparing the immune responses between the non-adjuvanted vaccine group and the CpG-adjuvanted vaccine group, as well as determining the optimal dosage of the CpG HP021 adjuvant. However, we have not compared the differences between CpG HP021 and other adjuvants. Furthermore, challenge studies are necessary to further assess the protective efficacy of the CpG HP021-adjuvanted vaccine.
Taken together, we demonstrated that the addition of the CpG HP021 adjuvant can alter the subtype of antibodies and the type of helper T cells, promoting the body’s production of memory immune cells and enhancing the effectiveness of IIV4. This finding encourages us to explore the feasibility of incorporating the CpG adjuvant into other vaccines.
Materials and methods
Animals, vaccines, and viruses
We bought six to eight week-old BALB/c mice and ten-week-old rats from Shanghai Slac Laboratory Animal Co., Ltd (Shanghai, China). The CpG HP021 adjuvant was prepared by Jiangsu Taipurui Biotechnology Co., Ltd (Jiangsu, China). TCGCAACGTTGCCTTCGAAGG-3’ is the sequence of CpG HP021, a newly synthesized adjuvant, whose patent application number is CN 117,568,339. The quadrivalent influenza vaccines of season 2022–2023 (A/Victoria/2570/2019, A/Darwin/9/2021, B/Austria/1,359,417/2021, B/Phuket/3073/2013) and influenza viruses for the evaluation of vaccine cross-reactivity (A/California/07/2009, A/Michigan/45/2015, A/G-M/SWL1536/2019, A/Wisconsin/15/09, A/Texas/50/2012, B/Florida/4/2006, B/Massachusetts/02/2012, B/Brisbane/60/2008, B/Washington/02/2019) were obtained from Zhejiang Tianyuan Bio-Pharmaceutical Co., Ltd (Zhejiang, China).
Immunization
BALB/c mice (n = 12 per group) were injected intramuscularly (IM) in the hind legs, with each leg receiving 50 µL (totaling 100 µL) of two doses (at day 0 and 14) containing IIV4 (3 µg of each HA) with a range of CpG HP021 (0, 10, 20, 40, 80, or 160 μg) (Table 1). A control group was administered phosphate-buffered saline (PBS). SD rats (n = 6 per group) were also immunized via IM injection in the hind legs, with each leg receiving 100 µL (totaling 200 µL) of two doses (at day 0 and 14) containing IIV4 (6 µg of each HA) with a range of doses of CpG HP021 (0, 50, 100, 200, or 400 µg), and the control group was immunized with PBS (Table 2). The carbon dioxide asphyxiation method was used to euthanize the vaccinated mice and SD rats, followed by cervical dislocation. Blood samples were collected before and at day 14, 21, 28, and 56 after the first immunization. The spleens were harvested for cellular response testing when the animals were sacrificed at day 28 and 56 following the initial immunization.
Table 1.
Mice experimental grouping.
| Groups (No.) | IIV4 (μg/each HA) |
CpG (μg) | Volume (μL) | Number of mice |
|---|---|---|---|---|
| PBS | 0 | 0 | 100 | 12 |
| IIV4 | 3 | 0 | 100 | 12 |
| IIV4 + 10 μg CpG | 3 | 10 | 100 | 12 |
| IIV4 + 20 μg CpG | 3 | 20 | 100 | 12 |
| IIV4 + 40 μg CpG | 3 | 40 | 100 | 12 |
| IIV4 + 80 μg CpG | 3 | 80 | 100 | 12 |
| IIV4 + 160 μg CpG | 3 | 160 | 100 | 12 |
Table 2.
SD rats experimental grouping.
| Groups (No.) | IIV4 (μg /each HA) |
CpG (μg) |
Volume (μL) | Number of rats |
|---|---|---|---|---|
| PBS | 0 | 0 | 200 | 6 |
| 6 μg IIV4 | 6 | 0 | 200 | 6 |
| 6 μg IIV4 + 50 μg CpG | 6 | 50 | 200 | 6 |
| 6 μg IIV4 + 100 μg CpG | 6 | 100 | 200 | 6 |
| 6 μg IIV4 + 200 μg CpG | 6 | 200 | 200 | 6 |
| 6 μg IIV4 + 400 μg CpG | 6 | 400 | 200 | 6 |
Ethics statement
All animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals of Zhejiang Province and the study was approved by the Ethics Committee of the Zhejiang Chinese Medical University (Ethical approval No. IACUC-202310–29). All procedures of the study were followed by the ARRIVE guidelines.
HI assay
The HI test was performed, as previously stated50. In brief, 200 µL of receptor destroying enzyme was combined with 50 µL of mouse serum and incubated overnight at 37 °C. After being inactivated for one hour at 56 °C, the mixture was diluted to an initial concentration of 1:20 using PBS, followed by two-fold serial dilutions. 1% fresh chicken red blood cells (RBCs) were used to titrate the viruses. Then, four HA units of virus were added to 96-well U-bottom plate containing the diluted serums. After 30 min of incubation at room temperature (RT), 1% chicken RBCs were added and incubated for 30 min at RT. HI titers were defined as the reciprocal of the highest serum dilution capable of preventing hemagglutination of RBCs. The SCR is defined as the proportion of animals with HI titer < 1:20 before vaccination and ≥ 1:40 at day 28 after first immunization or ≥ 1:20 before vaccination and a ≥ fourfold increase in HI titer at day 28 after first immunization. The GMI is calculated by dividing the GMT after vaccination by the GMT before vaccination.
ELISA
The ELISA was performed, as previously stated 26. Briefly, the influenza antigen (0.5 μg/mL) was added to 96-well plates, which were then stored at 4 °C for all night. A confining solution was used to block the plates for 2 h. From a starting dilution of 1:1000, the serum samples were serially two-fold diluted and then added to the plate. The plate was incubated for 2 h. After that, the plates were incubated with biotin anti-mouse IgG (Cat. No. 1036–08), biotin anti-mouse IgG1 (Cat. No. 1071–08) or biotin anti-mouse IgG2a (Cat. No. 1081–08) (all from Southern Biotech) for one hour at RT. After that, the plates were incubated for 40 min with Streptavidin (HRP). After adding 100 μL of 3, 30, 5, 50-tetramethyl biphenyl anhydride for 5 min, the reaction was stopped with 2 M sulfuric acid. To measure the absorbance at 450 nm, an enzyme-labeling instrument was utilized. Antibody titers are the reciprocal of the maximum dilutions of the antibody measured when the ratio of the optical density (OD) value of the tested antibody to the OD value of the negative control is equal to or greater than 2.1.
ELISPOT
The Mouse IFN-γ ELISpotPLUS kit (Mabtech, Cat. No. 3321-4HPW-10) and the Mouse TNF-α ELISpotPLUS kit (Mabtech, Cat. No. 3511-4HPW-10) were accustomed to assess the T cell responses of mice. The Rat IFN-γ ELISPOT kit (U-CyTech, Cat. No. CT079-PR5) and the Rat IL-4 ELISPOT kit (U-CyTech, Cat. No. CT081-PR2) were accustomed to assess the T cell responses of SD rats. In 96-well plates, 4 × 105 freshly obtained splenocytes were added to each well. The plates were cultured in 1640 medium with or without 5 μg/mL of influenza antigens for 20 h at 37 °C and 5% CO2. Following that, the ELISpot plates were stained according to the manufacturer’s instructions. After that, an ELISPOT® Spot Imaging Analyzer was used to read the plates.
MSD Cytokine analyses
Serum samples from BALB/c mice were collected at 28 day after the initial vaccination. After being freshly isolated, 8 × 105 splenocytes per well of mice and SD rats were stimulated for 28 h with 5 μg/mL of influenza antigens. Subsequently, the supernatants were collected. We analyzed the cytokine levels in the blood samples of mice and the splenic lymphocyte supernatants from both mice and rats using MSD cytokine kits, including the V-PLEX Proinflammatory Panel 1 (mouse) Kit (Meso Scale Diagnostics, Cat. No. K15048D) and the V-PLEX Proinflammatory Panel 2 (rat) Kit (Cat. No. K15059D-1).
Flow cytometry
Mouse spleen cells were stained with Fixable Viability Stain 700. The cells were then stained for 30 min at 4 °C using the FITC anti-mouse CD3, BV605 anti-mouse CD8, Phycoerythrin (PE)-cy7 anti-mouse CD4, BV510 anti-mouse CD62L, and BUV395 anti-mouse CD44 antibodies from BD Pharmingen. The stained cells were then examined on a CytoFLEX flow cytometer. Some mouse spleen cells were harvested and cultured in 1640 medium with or without 5 μg/mL of influenza antigens for 10 h. The cells were then incubated with Fixable Viability Stain 700 and FITC anti-mouse CD3, BV510 anti-mouse CD4 and BV605 anti-mouse CD8 for 30 min at 4 °C. After adding Fixation/Permeabilization Solution and incubating for 30 min at 4 °C, followed by adding Percp-cy5.5 IFN-γ. The samples were then incubated at 4 °C for 40 min. The stained cells were then examined on a CytoFLEX flow cytometer.
Statistical considerations
The data was analyzed using GraphPad Prism 10.2 software. Data analysis was conducted using T-tests, one-way analysis of variance (ANOVA) with Tukey’s test, or Kruskal–Wallis test with a post hoc Dunn’s multiple. P values ≤ 0.05 were considered significant; in the figures, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Supplementary Information
Acknowledgements
We are grateful to every study participant.
Author contributions
Hangping Yao, Jia Ji and Lei Chen designed the study and interpreted the data. Jia Ji, Zhigang Wu, Taoming Tang, Linwei Zhu, Miaojin Zhu and Xiangyun Lu performed the isolation of rat and mouse splenocytes. Jia Ji detects serum antibody levels and T cells response. Lei Chen immunized BALB/c mice and SD rats and measured serum HI titers. Yan Chen provided the CpG HP021. Jia Ji and Hangping Yao wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Zhejiang Plan for the Special Support for Top-notch Talents (2022R52029); the Fundamental Research Funds for the Central Universities (2022ZFJH003); and the National Key Research and Development Program in China (2021YFC2301204).
Data availability
The data in this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
CpG HP021 is patented by Jiangsu Taipurui Biotechnology Co., Ltd. All other authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jia Ji and Lei Chen contributed equally to this work.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83026-x.
References
- 1.Belongia, E. A. et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis.16, 942–951. 10.1016/s1473-3099(16)00129-8 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Ohmit, S. E. et al. Influenza vaccine effectiveness in the community and the household. Clin. Infect. Dis.56, 1363–1369. 10.1093/cid/cit060 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tregoning, J. S., Russell, R. F. & Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccin. Immunother.14, 550–564. 10.1080/21645515.2017.1415684 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma, S. K. et al. New-age vaccine adjuvants, their development, and future perspective. Front. Immunol.14, 1043109. 10.3389/fimmu.2023.1043109 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng, S. et al. Particulate Alum via Pickering Emulsion for an Enhanced COVID-19 Vaccine Adjuvant. Adv. Mater.32, e2004210. 10.1002/adma.202004210 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Pulendran, B., Arunachalam, P. S. & Oagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov.20, 454–475. 10.1038/s41573-021-00163-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med.19, 1597–1608. 10.1038/nm.3409 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Myers, M. L. et al. Impact of adjuvant: Trivalent vaccine with quadrivalent-like protection against heterologous Yamagata-lineage influenza B virus. Front. Immunol.13, 1002286. 10.3389/fimmu.2022.1002286 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesikari, T. et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N. Engl. J. Med.365, 1406–1416. 10.1056/NEJMoa1010331 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Lin, Y. J., Shih, Y. J., Chen, C. H. & Fang, C. T. Aluminum salts as an adjuvant for pre-pandemic influenza vaccines: a meta-analysis. Sci. Rep.8, 11460. 10.1038/s41598-018-29858-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keitel, W. A. et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I-II randomized clinical trial. J. Infect. Dis.198, 1309–1316. 10.1086/592172 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein, D. I. et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J. Infect. Dis.197, 667–675. 10.1086/527489 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Zedda, L. et al. Dissecting the immune response to MF59-adjuvanted and nonadjuvanted seasonal influenza vaccines in children less than three years of age. Pediatr. Infect. Dis. J.34, 73–78. 10.1097/INF.0000000000000465 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Vesikari, T., Groth, N., Karvonen, A., Borkowski, A. & Pellegrini, M. MF59-adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second year seasonal vaccination. Vaccine27, 6291–6295. 10.1016/j.vaccine.2009.02.004 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Folschweiller, N. et al. Reactogenicity, safety, and immunogenicity of chimeric haemagglutinin influenza split-virion vaccines, adjuvanted with AS01 or AS03 or non-adjuvanted: a phase 1–2 randomised controlled trial. Lancet Infect. Dis.22, 1062–1075. 10.1016/s1473-3099(22)00024-x (2022). [DOI] [PubMed] [Google Scholar]
- 16.Winokur, P. L. et al. Safety and Immunogenicity of a monovalent inactivated influenza A/H5N8 virus vaccine given with and without AS03 or MF59 adjuvants in healthy adults. Clin. Infect. Dis.10.1093/cid/ciac983 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Zhu, F. C. et al. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med.361, 2414–2423. 10.1056/NEJMoa0908535 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Block, S. L. et al. Dose-range study of MF59-adjuvanted versus nonadjuvanted monovalent A/H1N1 pandemic influenza vaccine in six- to less than thirty-six-month-old children. Pediatr. Infect. Dis. J.31, e92-98. 10.1097/INF.0b013e318257644f (2012). [DOI] [PubMed] [Google Scholar]
- 19.Carter, N. J. & Plosker, G. L. Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: a review of its use as an active immunization against influenza A subtype H5N1 virus. BioDrugs22, 279–292. 10.2165/00063030-200822050-00001 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Nolan, T. et al. Relative efficacy of AS03-adjuvanted pandemic influenza A(H1N1) vaccine in children: results of a controlled, randomized efficacy trial. J. Infect. Dis.210, 545–557. 10.1093/infdis/jiu173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henning, L. et al. Efficacy of different AV7909 dose regimens in a nonclinical model of pulmonary anthrax. Hum. Vaccin. Immunother.19, 2290345. 10.1080/21645515.2023.2290345 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson, S. et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine36, 668–674. 10.1016/j.vaccine.2017.12.038 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Tzeng, T. T. et al. A TLR9 agonist synergistically enhances protective immunity induced by an Alum-adjuvanted H7N9 inactivated whole-virion vaccine. Emerg. Microbes Infect.12, 2249130. 10.1080/22221751.2023.2249130 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang, X., Li, Y., Zhao, Y. & Chen, X. Overcoming Aging-Associated Poor Influenza Vaccine Responses with CpG 1018 Adjuvant. Vaccines (Basel)10.3390/vaccines10111894 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, L. G. CpG oligonucleotides and their applications. China patent CN 117568339 A (2024).
- 26.Ji, J. et al. Boosting the immune response in COVID-19 vaccines via an Alum:CpG complex adjuvant. Antivir. Res.229, 105954. 10.1016/j.antiviral.2024.105954 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Zakay-Rones, Z. Human influenza vaccines and assessment of immunogenicity. Expert Rev. Vaccines9, 1423–1439. 10.1586/erv.10.144 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Coudeville, L. et al. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med. Res. Methodol.10, 18. 10.1186/1471-2288-10-18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strohmeier, S. et al. A CpG 1018 adjuvanted neuraminidase vaccine provides robust protection from influenza virus challenge in mice. NPJ Vaccines7, 81. 10.1038/s41541-022-00486-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayraklioglu, N., Horuluoglu, B. & Klinman, D. M. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol. Biol.2197, 51–85. 10.1007/978-1-0716-0872-2_4 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Graham, M. B. & Braciale, T. J. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J. Exp. Med.186, 2063–2068. 10.1084/jem.186.12.2063 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinen, P. P., de Boer-Luijtze, E. A. & Bianchi, A. T. J. Respiratory and systemic humoral and cellular immune responses of pigs to a heterosubtypic influenza A virus infection. J. Gen. Virol.82, 2697–2707. 10.1099/0022-1317-82-11-2697 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Shirota, H. & Klinman, D. M. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev. Vaccines13, 299–312. 10.1586/14760584.2014.863715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klinman, D. M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol.4, 249–258. 10.1038/nri1329 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Krieg, A. M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol.20, 709–760. 10.1146/annurev.immunol.20.100301.064842 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Krieg, A. M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug. Discov.5, 471–484. 10.1038/nrd2059 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Lin, H. T. et al. Nanoparticular CpG-adjuvanted SARS-CoV-2 S1 protein elicits broadly neutralizing and Th1-biased immunoreactivity in mice. Int. J. Biol. Macromol.193, 1885–1897. 10.1016/j.ijbiomac.2021.11.020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luan, N., Cao, H., Wang, Y., Lin, K. & Liu, C. LNP-CpG ODN-adjuvanted varicella-zoster virus glycoprotein E induced comparable levels of immunity with Shingrix in VZV-primed mice. Virol. Sin.37, 731–739. 10.1016/j.virs.2022.06.002 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barr, T. A., Brown, S., Ryan, G., Zhao, J. & Gray, D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur. J. Immunol.37, 3040–3053. 10.1002/eji.200636483 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luchner, M., Reinke, S. & Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics10.3390/pharmaceutics13020142 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vollmer, J. & Krieg, A. M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug. Deliv. Rev.61, 195–204. 10.1016/j.addr.2008.12.008 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Nanishi, E. et al. An aluminum hydroxide:CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor binding domain vaccine in aged mice. Sci. Transl. Med.14, eabj5305. 10.1126/scitranslmed.abj5305 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douagi, I. et al. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J. Immunol.182, 1991–2001. 10.4049/jimmunol.0802257 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Li, Q., Ren, J., Liu, W., Jiang, G. & Hu, R. CpG oligodeoxynucleotide developed to activate primate immune responses promotes antitumoral effects in combination with a neoantigen-based mRNA cancer vaccine. Drug. Des. Devel. Ther.15, 3953–3963. 10.2147/dddt.S325790 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girndt, M. et al. Immunogenicity and safety of a booster dose of the hepatitis B vaccine HepB-CpG (HEPLISAV-B®) compared with HepB-Eng (Engerix-B®) and HepB-AS04 (Fendrix®) in adults receiving hemodialysis who previously received hepatitis B vaccination and are not seroprotected: Results of a randomized, multicenter phase 3 study. Hum. Vaccin. Immunother.18, 2136912. 10.1080/21645515.2022.2136912 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruxvoort, K. et al. Association between 2-dose vs 3-dose hepatitis b vaccine and acute myocardial infarction. Jama.327, 1260–1268. 10.1001/jama.2022.2540 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hervé, C., Laupèze, B., Del Giudice, G., Didierlaurent, A. M. & Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines4, 39. 10.1038/s41541-019-0132-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vardhana, S. A. & Wolchok, J. D. The many faces of the anti-COVID immune response. J. Exp. Med.10.1084/jem.20200678 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segal, B. M., Chang, J. T. & Shevach, E. M. CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalitogenic T cells in vivo. J. Immunol.164, 5683–5688. 10.4049/jimmunol.164.11.5683 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Ma, N. et al. Development of an mRNA vaccine against a panel of heterologous H1N1 seasonal influenza viruses using a consensus hemagglutinin sequence. Emerg. Microbes. Infect.12, 2202278. 10.1080/22221751.2023.2202278 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study are available from the corresponding author upon reasonable request.