Abstract
Despite numerous studies investigating the correlation between the serum uric acid and high-density lipoprotein cholesterol ratio (UHR) and fatty liver disease, the evidence for the dose-response relationship between UHR and liver fat content (LFC) remains uncertain. This study employs quantitative computed tomography (CT) to quantify LFC and aims to investigate the correlation and dose-response relationship between UHR levels and LFC in Chinese adults. Based on the health check-up data from 2021 at Henan Provincial People’s Hospital, China, the objective of this cross-sectional study was to investigate the association between UHR levels and LFC among individuals of different genders. The analytical approach encompassed one-way ANOVA, multiple regression analysis, subgroup analysis, smooth curve fitting, and the evaluation of threshold and saturation effects. Upon adjusting for potential influencing factors, the multiple regression analysis indicated a positive correlation between UHR and LFC in both male and female subjects. This positive correlation was more significant in the highest UHR quartile (Male Q4 in model II: β = 2.119, 95% CI: 1.353–2.886, P < 0.05; Female Q4 in model II: β = 1.312, 95% CI: 0.499–2.124, P < 0.05). Subgroup and threshold saturation effect analyses demonstrated a positive correlation between UHR and LFC in the male population, independent of age, although the linear correlation trend was influenced by different body mass index (BMI) groups. In the female population, age also affected the association between UHR and LFC, with a negative association observed when age ≥ 45 years and UHR > 30.63. A positive association exists between UHR levels and LFC in both genders among Chinese adults, albeit exhibiting variations across different age and BMI groups. Consequently, early monitoring of UHR levels may be crucial for the early detection and intervention in high-risk groups exhibiting increased LFC.
Keywords: UHR index, LFC, Physical examination, Cross-sectional study
Subject terms: Endocrinology, Gastroenterology, Health care
Background
With the rise in living standards and shifts in lifestyle habits, such as diet and physical activity, the global incidence of fatty liver disease is on an upward trend. According to a research report from 2020, the occurrence of non-alcoholic fatty liver disease (NAFLD) in China has reached 29.2%1. Fatty liver, the most common intrahepatic metabolic disease, is primarily driven by underlying mechanisms like intrahepatic lipid metabolism disorders and insulin resistance2,3. Recent research indicates that fatty liver is linked to the progression of multiple cancers, both within and outside the liver, and can lead to conditions like cirrhosis and hepatitis4. Therefore, early screening and diagnosis of fatty liver, along with evaluation of treatment effects and follow-up detection, are of paramount importance. Although liver biopsy pathology is widely accepted as the most reliable method for diagnosing fatty liver, its invasive nature and significant cost are notable drawbacks5. This highlights the urgent need to develop non-invasive diagnostic techniques for fatty liver disease. Serum markers, when used in conjunction with other diagnostic criteria, offer numerous benefits such as ease of use, cost-effectiveness, and diagnostic accuracy, and their effectiveness in diagnosing a range of diseases has been substantiated6,7. Considering the interplay between fatty liver and disorders of lipid metabolism, Deprince et al.8have provided evidence that an imbalance in high-density lipoprotein cholesterol (HDL-C) metabolism is implicated in the onset of NAFLD. HDL-C, with its anti-inflammatory and antioxidant capabilities, has been found to be linked with insulin resistance and could be instrumental in the progression of fatty liver disease9. Additionally, serum uric acid (SUA) has been shown to be associated with the development of NAFLD10,11. Sun et al.12reviewed the independent predictive power and internal mechanism of SUA for NAFLD and suggested that reducing SUA concentration may be a potential treatment for fatty liver. Recent studies have widely used the ratio of serum uric acid to high-density lipoprotein cholesterol (UHR), and this combination has been shown to be associated with the development of metabolic syndrome and insulin resistance13,14. Zhao et al.15 investigated the correlation between UHR and NAFLD using ultrasonography and discovered a positive correlation between UHR and the incidence of fatty liver, indicating the potential clinical utility of UHR in diagnosing NAFLD. Notably, Zhu et al.16 conducted a five-year cohort study that the dependent variable included multiple serological indicators, and found that UHR had the highest diagnostic performance among all predictors of NAFLD. This provides a direction for the clinical application and follow-up research of UHR.
Despite the existing research focusing on the link between UHR and the susceptibility to fatty liver disease, there is a noticeable lack of dose-response studies examining the connection between the quantification of liver fat content (LFC) and UHR. This study utilized health check-up data from Henan Provincial People’s Hospital in China, collected in 2021. LFC was quantified using quantitative computed tomography (CT). The objective of this study was to ascertain whether a linear or non-linear relationship exists between UHR levels and LFC across different genders. Additionally, confounding factors, such as biochemical test results associated with LFC, were filtered to confirm the rigor of the study and the confidence of the results.
Materials and methods
Participants and criteria for inclusion
Information for this investigation was obtained from the health records of persons who participated during the year 2021 medical evaluations at the Health Department Center of Henan Provincial People’s Hospital. The criteria for participant selection were as follows: (1) individuals ranging from 20 to 80 years of age, (2) individuals possessing complete demographic and blood biochemistry data, and (3) individuals who have received standard low-dose chest CT scans and evaluations of liver fat. The criteria for exclusion encompassed: (1) existence of various hepatic mass lesions (excluding benign small cysts and minor intrahepatic calcifications), history of hepatic lobectomy, cirrhosis or alcoholic fatty liver disease, (2) history of any form of cancer, (3) endocrine disease, (4) renal disease, and (5) past or present use of lipid metabolism modulators. Skilled personnel gathered fundamental participant data, including gender, age, nationality, medical history, and drug history, through direct investigations.
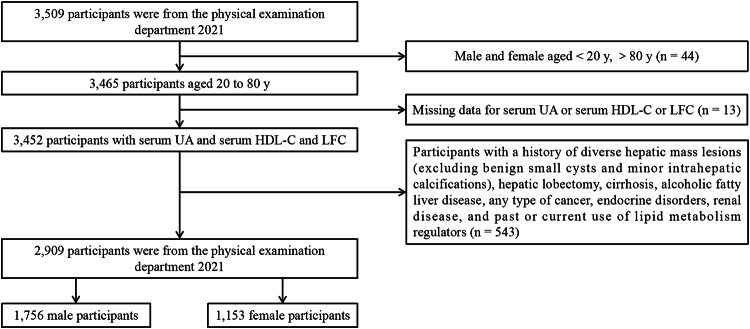
Initially, the study enrolled 3,509 participants. However, 44 participants were omitted as they did not meet the age requirements. Additionally, 13 participants were removed due to incomplete data on SUA, HDL-C, or LFC. Furthermore, 543 participants were disqualified because their medical records were inconsistent with the inclusion criteria. As a result, the final study cohort consisted of 2,909 participants, comprising 1,756 males and 1,153 females. The participant selection process is depicted in Fig. 1.
Fig. 1.
Flowchart of participants selection.
Research methods
In order to uphold the precision and fairness of the data, all investigators participated in a uniform survey training before the initiation of the study. An exhaustive questionnaire was used to collect crucial data about the participants. This survey encompassed information regarding the participants’ medical history, particularly any historical or ongoing instances of diverse liver mass lesions (excluding benign small cysts and minor intrahepatic calcifications), lobectomy, cirrhosis, cancer, endocrine disorders, renal disease, and the usage of lipid metabolism regulators. After the questionnaire was filled out, the data were assembled, synthesized, and validated.
Subsequently, after a 12-hour fasting period, participant measurements were taken in the morning, including height, weight, systolic blood pressure (SBP), and diastolic blood pressure (DBP). To reduce the likelihood of errors, each measurement was performed twice, with the final value being the mean of the two readings. The Body Mass Index (BMI) was computed by dividing the weight by the height2 (kg/m2).
Laboratory tests
Blood specimens were obtained from fasting participants at 8 a.m., and a variety of laboratory parameters were evaluated, including SUA, HDL-C, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), total protein (TP), hemoglobin (Hb), total bilirubin (TB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glutamyl transpeptidase (GGT), fasting blood glucose (FBG), and serum creatinine. An Olympus® AU 5400 automated biochemistry analyzer was utilized for the evaluation of lipid and blood glucose levels. Standard laboratory methods were employed for the assessment of the remaining variables.
LFC measurements
Utilizing a Lightspeed VCT 64-row CT scanner (General Electric), the study conformed to the standard low-dose chest CT scan protocol, setting the tube voltage at 120 kV and the tube current at 100 mA. The scanning field was established at 500 mm × 500 mm, with a slice thickness of 5 mm and a pitch of 0.984. Quantitative CT of LFC was executed using the Measure Liver Fat module scanning analysis software, a supportive tissue measurement application. During this procedure, three circular regions of interest (ROIs) were positioned in the anterior and posterior segments of the left and right lobes, respectively, with a cross-sectional area of 290 ~ 310mm2 for each region. The ROIs were situated in the subcapsular region of the liver, steering clear of the bile ducts and blood vessels. If the left lobe of the liver was too small to be visualized on the section, the slice with the largest area of the left lobe was utilized for measurement. The mean of the three was taken as the final value to determine the liver fat percentage. Quantitative CT software was used by specially trained radiologists to conduct all analyses. Importantly, an earlier research publication confirmed the appropriateness of this technique for individuals of Chinese descent17.
Variables
In this study, UHR was used as the independent variable and LFC as the dependent variable. The confounding variables considered included nationality, marital status, age, BMI, SBP, DBP, TC, TG, LDL-C, TP, Hb, TB, ALT, AST, ALP, GGT, FBG, and serum creatinine. UHR (%) is defined as SUA (µmol/L) divided by HDL-C (mmol/L).
Statistical analysis
EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA) and R software (R, version 4.2.0) were used for statistical analysis. All data underwent normality testing and were expressed as either the mean ± standard deviation for normally distributed continuous variables, the median and interquartile range for non-normally distributed continuous variables, or proportions for categorical variables. Chi-square tests and ANOVAs were used to identify significant differences in the data sets. The study employed univariate analysis to assess the impact of each variable on LFC. In addressing confounding variables, the study examined the relationship between UHR and LFC using a multivariate linear regression model, excluding variables with a variance inflation factor (VIF) > 10 to mitigate multicollinearity. This model was also used to analyze the linear relationship between UHR and LFC in different gender populations, which were further classified by age and BMI. To discern a potential nonlinear relationship between UHR and LFC, a smooth curve fitting technique and a generalized additive model were used. In cases where the relationship was nonlinear, the breakpoint of the correlation between UHR and LFC was determined through calculation. On either side of the inflection point, a two-phase linear regression model was constructed. For all statistical tests, the p-values after Bonferroni correction for multiple comparisons, with two-tailed p-values less than 0.05, are considered statistically significant.
Result
Participant baseline characteristics
A total of 2,909 individuals participated in this study, comprising 1,756 males and 1,153 females. Males (Q1: 8.79 - 23.53, Q2: 23.56 - 28.79, Q3: 28.80 - 35.50, Q4: 35.52 - 59.55) and females (Q1: 5.52- 14.40, Q2: 14.41 - 17.96, Q3: 17.97 - 22.17, Q4: 22.27 - 43.13) were divided into quartile groups according to UHR values. For the male population, except for nationality, marital status, AST, ALP, and FBG, the baseline characteristics of UHR values exhibited significant differences across quartiles (all P < 0.05), and the other quartiles were higher in BMI, SBP, DBP, TG, LDL-C, TP, Hb, ALT, GGT, serum creatinine, and LFC compared with the lowest UHR value quartile, while age, TC, and TB were lower. For females, there were significant differences between the quartiles except for nationality, marital status, TP, TB, and AST in the baseline characteristics of UHR values (all P < 0.05). Compared with the lowest UHR value quartile, the other quartiles had higher age, BMI, SBP, DBP, TG, LDL-C, TP, Hb, ALT, ALP, GGT, FBG, serum creatinine, and LFC, while TC was lower. Table 1.
Univariate analysis
In the cohort of males, LFC showed a positive correlation with BMI, SBP, DBP, TC, TG, LDL-C, TP, Hb, ALT, AST, ALP, GGT, and FBG (all P < 0.05), while a negative correlation was observed with age (P < 0.05). Similarly, in the cohort of females, a positive correlation was found between LFC and BMI, SBP, DBP, TG, TP, ALT, AST, ALP, GGT, and FBG (all P < 0.05). Refer to Table 2 for more details.
Relationship of UHR levels and LFC
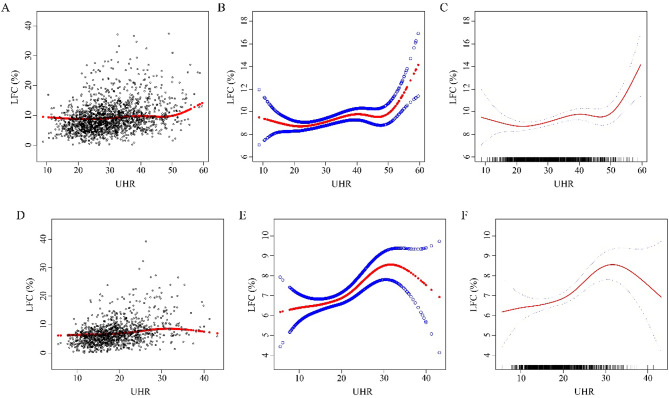
The results of the multivariate regression analysis indicated that for the male population, UHR was positively correlated with LFC in the unadjusted model (β = 0.175, 95% CI: 0.150 - 0.199, P < 0.05). After adjusting for age and nationality in Model I (β = 0.176, 95% CI: 0.151 - 0.202, P < 0.05) and for age, nationality, BMI, SBP, DBP, TC, TG, LDL-C, TP, Hb, ALT, AST, ALP, GGT, FBG, and serum creatinine in Model II (β = 0.090, 95% CI: 0.057 - 0.123, P < 0.05), this correlation remained positive. Additionally, this study used the quartile of UHR levels to convert continuous variables into categorical variables. In all three models, the positive correlation persisted after UHR was converted from a continuous variable to quartiles. For the female population, UHR was also positively correlated with LFC in the model without adjustments (β= 0.270, 95% CI: 0.233 - 0.308, P < 0.05). After adjusting for covariates, the correlation remained positive in Model I (β = 0.269, 95% CI: 0.231 - 0.306, P < 0.05) and Model II (β = 0.093, 95% CI: 0.043 - 0.143, P < 0.05). The curves for UHR and LFC are shown in Figure 2. Except for the subjects in Q2 and Q3 in Model II, the positive correlation between UHR and LFC remained significant in the female population after converting UHR to quartiles in the unadjusted model, Model I, and Model II (Q4 in Model II: β = 1.332, 95% CI: 0.499 - 2.124, P < 0.05). Table 3.
Results of subgroup analysis
The age-based subgroup analysis shows that in the male population aged 45 years and older, there was a significant positive correlation between UHR and LFC (β = 0.050, 95% CI: 0.014 - 0.086, P < 0.05). Similarly, in the female population aged 45 years and older, UHR was positively correlated with LFC (β = 0.110, 95% CI: 0.057 - 0.163, P < 0.05). When BMI was categorized using 24 kg/m² and 28 kg/m² as cut-off points, the BMI subgroup analysis showed that in the male population with a BMI between 24 kg/m² and 28 kg/m², UHR was positively correlated with LFC (β= 0.083, 95% CI: 0.045 - 0.121, P < 0.05). In the female population, when BMI was less than 24 kg/m² (β = 0.075, 95% CI: 0.015 - 0.136, P < 0.05) and between 24 kg/m² and 28 kg/m² (β = 0.163, 95% CI: 0.087 - 0.240, P < 0.05), UHR was positively correlated with LFC. The interaction analysis revealed that the relationship between UHR and LFC in women was influenced by BMI. However, in the male population, no significant interaction was observed. Table 4.
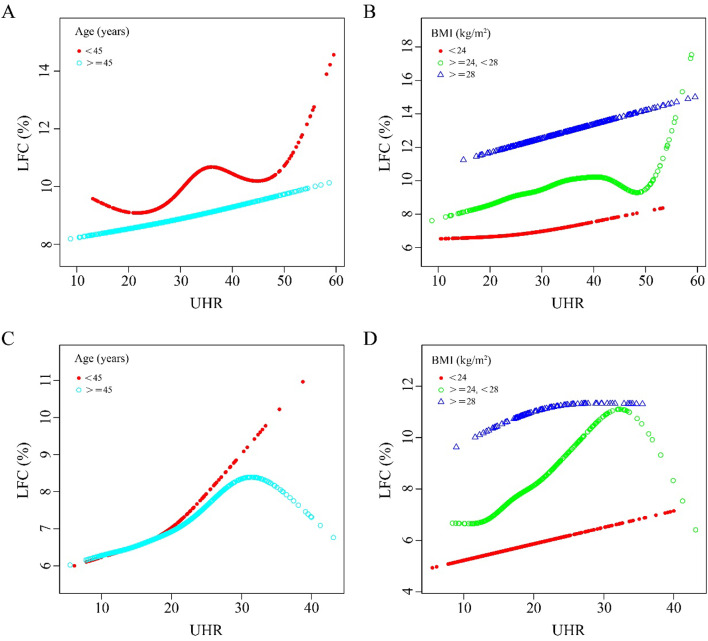
Nonlinear relationship between UHR and LFC
In this study, both piecewise linear regression and smooth curve fitting were applied to scrutinize the relationship between UHR and LFC, taking into account age and BMI subgroups. The results are depicted in Figure 3 and Table 5. For the male population, when the BMI was less than 24 kg/m², there was an inflection point at UHR = 21.23 in the fitting curve (P < 0.05) as shown in Figure 3B. However, when the data were stratified by age, the nonlinear relationship between UHR and LFC was not significant (P > 0.05). In contrast, for the female population, a significant nonlinear relationship between UHR and LFC was observed in both age groups: less than 45 years (with a UHR inflection point at 20.29) and 45 years or older (with a UHR inflection point at 30.63). This relationship was more pronounced on the right side of the curve (β = 0.206, 95% CI: 0.046 - 0.367, P < 0.05 for age < 45 years; β = -0.150, 95% CI: -0.389 - 0.090, P < 0.05 for age ≥ 45 years), as shown in Figure 3C. Moreover, Figure 3D indicates that for women with a BMI between 24 kg/m² and 28 kg/m², there was a significant negative association between UHR and LFC on the right side of the inflection point UHR = 32.02.
Table 1.
Characteristics of the study population.
| Male (n = 1,756) | |||||
|---|---|---|---|---|---|
| UHR (%) | Q1(8.79–23.53) | Q2(23.56–28.79) | Q3(28.80–35.50) | Q4(35.52–59.55) | P value |
| Age (years) | 54.69 ± 11.68 | 51.95 ± 11.81 | 50.32 ± 11.74 | 48.06 ± 12.64 | < 0.001 |
| Nationality (%) | 0.107 | ||||
| non-Han nationality | 2(0.46%) | 1(0.23%) | 5(1.13%) | 7(1.59%) | |
| Han nationality | 434(99.54%) | 439(99.77%) | 436(98.87%) | 432(98.41%) | |
| Marital status (%) | 0.232 | ||||
| Not married | 17(3.90%) | 15(3.41%) | 24(5.44%) | 26(5.92%) | |
| Married | 419(96.10%) | 425(96.59%) | 417(94.56%) | 413(94.08%) | |
| BMI (kg/m2), (%) | < 0.001 | ||||
| < 24 | 233(53.44%) | 166(37.73%) | 111(25.17%) | 68(15.49%) | |
| ≥ 24, < 28 | 180(41.28%) | 212(48.18%) | 258(58.50%) | 245(55.81%) | |
| ≥ 28 | 23(5.28%) | 62(14.09%) | 72(16.33%) | 126(28.70%) | |
| SBP (mmHg) | 130.49 ± 18.44 | 131.31 ± 17.12 | 129.70 ± 18.21 | 133.00 ± 18.61 | 0.046 |
| DBP (mmHg) | 77.33 ± 11.62 | 78.70 ± 10.73 | 78.71 ± 12.04 | 80.71 ± 12.25 | < 0.001 |
| TC (mmol/L) | 4.96 ± 0.94 | 4.94 ± 0.99 | 4.83 ± 0.93 | 4.79 ± 1.00 | 0.019 |
| TG (mmol/L) | 1.22(0.92–1.63) | 1.42(1.04–1.87) | 1.73(1.27–2.29) | 2.22(1.59–3.02) | < 0.001 |
| LDL-C (mmol/L) | 2.87 ± 0.81 | 3.04 ± 0.83 | 3.00 ± 0.75 | 2.99 ± 0.78 | 0.008 |
| TP (g/L) | 70.95 ± 4.00 | 71.29 ± 4.71 | 71.61 ± 3.95 | 71.73 ± 3.98 | 0.028 |
| Hb (g/L) | 150.18 ± 9.68 | 152.23 ± 10.37 | 152.70 ± 9.77 | 152.85 ± 10.59 | < 0.001 |
| TB (µmol/L) | 14.29 ± 5.57 | 13.56 ± 5.30 | 13.50 ± 5.33 | 12.68 ± 5.29 | < 0.001 |
| ALT (U/L) | 17.95(14.10–24.50) | 19.85(14.97–28.65) | 22.60(16.90–30.60) | 25.10(18.10-35.95) | < 0.001 |
| AST (U/L) | 20.00(16.80–24.70) | 20.10(16.80–24.10) | 20.20(17.00-24.10) | 20.70(17.35–25.50) | 0.292 |
| ALP (U/L) | 66.51 ± 14.78 | 67.80 ± 17.43 | 67.80 ± 16.84 | 69.24 ± 17.79 | 0.155 |
| GGT (U/L) | 22.75(17.70-35.05) | 25.90(19.50-36.95) | 30.00(21.20–43.30) | 32.10(22.90-48.45) | < 0.001 |
| FBG (mmol/L) | 5.16 ± 0.59 | 5.16 ± 0.55 | 5.24 ± 0.58 | 5.21 ± 0.59 | 0.088 |
| Serum creatinine (µmol/L) | 72.63 ± 10.00 | 74.96 ± 10.62 | 75.97 ± 12.14 | 77.77 ± 13.65 | < 0.001 |
| LFC (%) | 7.25 ± 3.51 | 8.57 ± 4.44 | 9.47 ± 4.84 | 11.43 ± 5.80 | < 0.001 |
| Female (n= 1153) | |||||
| UHR (%) | Q1(5.52–14.40) | Q2(14.41–17.96) | Q3(17.97–22.17) | Q4(22.27–43.13) | P value |
| Age (years) | 51.07 ± 9.66 | 51.00 ± 10.14 | 52.56 ± 10.72 | 53.34 ± 9.93 | 0.011 |
| Nationality (%) | 0.789 | ||||
| non-Han nationality | 5(1.74%) | 5(1.75%) | 6(2.08%) | 3(1.03%) | |
| Han nationality | 283(98.26%) | 281(98.25%) | 282(97.92%) | 288(98.97%) | |
| Marital status (%) | 0.214 | ||||
| Not married | 10(3.47%) | 13(4.55%) | 7(2.43%) | 5(1.72%) | |
| Married | 278(96.53%) | 273(95.45%) | 281(97.57%) | 286(98.28%) | |
| BMI (kg/m2), (%) | < 0.001 | ||||
| < 24 | 230(79.86%) | 200(69.93%) | 158(54.86%) | 122(41.92%) | |
| ≥ 24, < 28 | 50(17.36%) | 70(24.48%) | 102(35.42%) | 125(42.96%) | |
| ≥ 28 | 8(2.78%) | 16(5.59%) | 28(9.72%) | 44(15.12%) | |
| SBP (mmHg) | 124.64 ± 17.79 | 123.86 ± 19.67 | 126.60 ± 17.73 | 130.06 ± 21.17 | < 0.001 |
| DBP (mmHg) | 73.02 ± 10.47 | 73.71 ± 12.21 | 74.17 ± 10.45 | 76.01 ± 11.72 | 0.011 |
| TC (mmol/L) | 5.25 ± 0.89 | 5.23 ± 0.91 | 5.05 ± 0.94 | 5.02 ± 0.96 | 0.002 |
| TG (mmol/L) | 1.00(0.82–1.28) | 1.17(0.86–1.50) | 1.31(1.02–1.72) | 1.72(1.25–2.25) | < 0.001 |
| LDL-C (mmol/L) | 2.83 ± 0.72 | 3.05 ± 0.78 | 3.03 ± 0.81 | 3.05 ± 0.79 | < 0.001 |
| TP (g/L) | 71.62 ± 4.10 | 71.53 ± 3.57 | 71.73 ± 3.81 | 72.23 ± 3.94 | 0.186 |
| Hb (g/L) | 128.90 ± 11.80 | 131.33 ± 10.62 | 130.64 ± 11.31 | 132.96 ± 10.83 | < 0.001 |
| TB (µmol/L) | 11.13 ± 4.44 | 10.72 ± 4.39 | 10.27 ± 3.58 | 10.68 ± 4.26 | 0.155 |
| ALT (U/L) | 14.00(11.07–18.42) | 15.10(11.20–19.80) | 15.65(12.30-21.32) | 17.20(13.15–23.95) | < 0.001 |
| AST (U/L) | 18.70(15.60-22.42) | 18.55(15.00-22.78) | 18.50(15.90-22.92) | 18.90(16.20-22.75) | 0.408 |
| ALP (U/L) | 62.98 ± 21.51 | 64.60 ± 18.92 | 69.89 ± 20.10 | 73.23 ± 19.70 | < 0.001 |
| GGT (U/L) | 14.50(11.80–17.80) | 15.75(12.43–20.60) | 17.40(13.67–23.82) | 19.30(15.40–26.10) | < 0.001 |
| FBG (mmol/L) | 4.91 ± 0.49 | 4.93 ± 0.45 | 5.03 ± 0.52 | 5.14 ± 0.50 | < 0.001 |
| Serum creatinine (µmol/L) | 55.58 ± 7.76 | 56.86 ± 8.18 | 56.48 ± 8.24 | 59.55 ± 11.98 | < 0.001 |
| LFC (%) | 5.22 ± 2.63 | 6.02 ± 3.29 | 7.13 ± 3.34 | 9.39 ± 5.67 | < 0.001 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; TP, total protein; Alb, albumin; Hb, hemoglobin; TB, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT glutamyl transpeptidase; FBG, fasting blood glucose; LFC, Liver fat content; %, weighted percentage.
Table 2.
The results of univariate analysis.
| Statistics | Effect size (β) | P value | |
|---|---|---|---|
| Male (n = 1,756) | |||
| Age (years) | 51.25 ± 12.20 | -0.02(-0.04, -0.00) | 0.018 |
| Nationality (%) | |||
| non-Han nationality | 15(0.85%) | Reference | |
| Han nationality | 1741(99.15%) | 1.47(-1.05,3.99) | 0.253 |
| Marital status (%) | |||
| Not married | 82(4.67%) | Reference | |
| Married | 1674(95.33%) | 0.75(-0.34,1.85) | 0.179 |
| BMI (kg/m2) | 25.35 ± 2.93 | 0.80(0.73,0.87) | < 0.001 |
| SBP (mmHg) | 131.12 ± 18.13 | 0.04(0.03,0.06) | < 0.001 |
| DBP (mmHg) | 78.86 ± 11.73 | 0.07(0.06,0.09) | < 0.001 |
| TC (mmol/L) | 4.88 ± 0.97 | 0.36(0.12,0.60) | 0.003 |
| TG (mmol/L) | 1.58(1.14–2.22) | 1.13(0.94,1.32) | < 0.001 |
| LDL-C (mmol/L) | 2.98 ± 0.80 | 0.64(0.35,0.93) | < 0.001 |
| TP (g/L) | 71.40 ± 4.18 | 0.13(0.07,0.19) | < 0.001 |
| Hb (g/L) | 152.00 ± 10.16 | 0.05(0.03,0.08) | < 0.001 |
| TB (µmol/L) | 13.51 ± 5.40 | -0.01(-0.06,0.03) | 0.534 |
| ALT (U/L) | 21.25(15.70–30.20) | 0.11(0.10,0.12) | < 0.001 |
| AST (U/L) | 20.20(17.00-24.50) | 0.14(0.11,0.16) | < 0.001 |
| ALP (U/L) | 67.84 ± 16.77 | 0.03(0.02,0.04) | < 0.001 |
| GGT (U/L) | 27.20(20.10-40.85) | 0.03(0.02,0.03) | < 0.001 |
| FBG (mmol/L) | 5.19 ± 0.58 | 1.58(1.18,1.97) | < 0.001 |
| Serum creatinine (µmol/L) | 75.34 ± 11.83 | 0.01(-0.01,0.03) | 0.492 |
| Female (n = 1,153) | |||
| Age (years) | 52.00 ± 10.15 | 0.02(-0.00,0.04) | 0.108 |
| Nationality (%) | |||
| non-Han nationality | 19(1.65%) | Reference | |
| Han nationality | 1134(98.35%) | 1.73(-0.17,3.64) | 0.075 |
| Marital status (%) | |||
| Not married | 35(3.04%) | Reference | |
| Married | 1118(96.96%) | 0.73(-0.69,2.14) | 0.315 |
| BMI (kg/m2) | 23.50 ± 3.02 | 0.62(0.54,0.69) | < 0.001 |
| SBP (mmHg) | 126.30 ± 19.27 | 0.04(0.03,0.06) | < 0.001 |
| DBP (mmHg) | 74.24 ± 11.28 | 0.06(0.04,0.08) | < 0.001 |
| TC (mmol/L) | 5.14 ± 0.93 | -0.16(-0.43,0.10) | 0.221 |
| TG (mmol/L) | 1.25(0.95–1.71) | 1.96(1.62,2.30) | < 0.001 |
| LDL-C (mmol/L) | 2.99 ± 0.78 | 0.22(-0.10,0.53) | 0.176 |
| TP (g/L) | 71.78 ± 3.87 | 0.09(0.02,0.15) | 0.008 |
| Hb (g/L) | 130.96 ± 11.23 | 0.02(-0.00,0.04) | 0.054 |
| TB (µmol/L) | 10.70 ± 4.19 | 0.04(-0.02,0.09) | 0.220 |
| ALT (U/L) | 15.20(11.80–20.80) | 0.12(0.10,0.14) | < 0.001 |
| AST (U/L) | 18.70(15.70–22.60) | 0.11(0.07,0.14) | < 0.001 |
| ALP (U/L) | 67.69 ± 20.47 | 0.03(0.02,0.04) | < 0.001 |
| GGT (U/L) | 16.40(13.00-22.30) | 0.05(0.03,0.06) | < 0.001 |
| FBG (mmol/L) | 5.00 ± 0.50 | 1.74(1.26,2.21) | < 0.001 |
| Serum creatinine (µmol/L) | 57.12 ± 9.31 | -0.01(-0.04,0.01) | 0.377 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; TP, total protein; Hb, hemoglobin; TB, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT glutamyl transpeptidase; FBG, fasting blood glucose; %, weighted percentage.
Table 3.
Relationship between UHR and LFC.
| Crude model | Model I | Model II | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| Male | ||||||
| UHR | 0.175(0.150,0.199) | < 0.001 | 0.176(0.151,0.202) | < 0.001 | 0.090(0.057,0.123) | 0.002 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.325(0.700,1.951) | < 0.001 | 1.324(0.697,1.951) | < 0.001 | 0.627(0.045,1.213) | 0.034 |
| Q3 | 2.217(1.592,2.842) | < 0.001 | 2.240(1.610,2.870) | < 0.001 | 0.971(0.337,1.579) | 0.004 |
| Q4 | 4.179(3.553,4.805) | < 0.001 | 4.216(3.578,4.854) | < 0.001 | 2.119(1.353,2.886) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||
| Female | ||||||
| UHR | 0.270(0.233,0.308) | < 0.001 | 0.269(0.231,0.306) | < 0.001 | 0.093(0.043,0.143) | < 0.001 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.799(0.159,1.440) | 0.015 | 0.800(0.160,1.440) | 0.014 | 0.081(-0.527, 0.689) | 0.794 |
| Q3 | 1.916(1.277,2.555) | < 0.001 | 1.914(1.274,2.554) | < 0.001 | 0.210(-0.468, 0.887) | 0.544 |
| Q4 | 4.174(3.537,4.812) | < 0.001 | 4.153(3.513,4.792) | < 0.001 | 1.312 (0.499, 2.124) | 0.002 |
| P for trend | < 0.001 | < 0.001 | 0.002 |
Crude model: no covariates were adjusted.
Model I: Age and nationality were adjusted.
Model II: Age, nationality, BMI, SBP, DBP, TC, TG, LDL-C, TP, Hb, ALT, AST, ALP, GGT, FBG, and serum creatinine were adjusted.
Table 4.
UHR and LFC were subgroup analyzed and stratified by age and BMI.
| Subgroup analysis | β (95%CI) | P value | P for interaction |
|---|---|---|---|
| Male | |||
| Age (years) | 0.543 | ||
| < 45 | 0.033(-0.016,0.082) | 0.190 | |
| ≥ 45 | 0.050(0.014,0.086) | 0.006 | |
| BMI (kg/m2) | 0.189 | ||
| < 24 | 0.037(-0.019,0.092) | 0.193 | |
| ≥ 24, < 28 | 0.083(0.045,0.121) | < 0.001 | |
| ≥ 28 | 0.033(-0.028,0.095) | 0.289 | |
| Female | |||
| Age (years) | 0.424 | ||
| < 45 | 0.069(-0.027, 0.166) | 0.158 | |
| ≥ 45 | 0.110(0.057, 0.163) | < 0.001 | |
| BMI (kg/m2) | 0.005 | ||
| < 24 | 0.075(0.015, 0.136) | 0.015 | |
| ≥ 24, < 28 | 0.163(0.087, 0.240) | < 0.001 | |
| ≥ 28 | -0.081(-0.219, 0.057) | 0.249 |
Each stratification was adjusted for all factors (Age, nationality, BMI, SBP, DBP, TC, TG, LDL-C, TP, Hb, ALT, AST, ALP, GGT, FBG, and serum creatinine), except for the stratification factor itself.
Table 5.
Multivariate regression analysis of the effect of UHR on LFC in different gender populations.
| Linear regression | Break point (K) | < K | > K | LLR test | |
|---|---|---|---|---|---|
| β (95%CI) | β (95%CI) | β (95%CI) | P | ||
| Male | |||||
| Age (years) | |||||
| < 45 | 0.023(-0.041,0.086) | 49.17 | 0.002(-0.066,0.070) | 0.379(-0.041,0.798) | 0.087 |
| ≥ 45 | 0.060(0.025,0.094) | 20.81 | -0.028(-0.184,0.129) | 0.066(0.029,0.102) | 0.258 |
| BMI (kg/m2) | |||||
| < 24 | 0.089(0.065,0.131) | 21.23 | -0.098(-0.213,0.045) | 0.138(0.094,0.178) | 0.004 |
| ≥ 24, < 28 | 0.123(0.076,0.154) | 31.32 | 0.158(0.083,0.227) | 0.079(0.021,0.140) | 0.220 |
| ≥ 28 | 0.137(0.045,0.236) | 47.41 | 0.126(0.025,0.232) | 0.413(-0.174,0.945) | 0.337 |
| Female | |||||
| Age (years) | |||||
| < 45 | 0.086(-0.025, 0.197) | 20.29 | -0.063(-0.245, 0.119) | 0.206(0.046, 0.367) | 0.037 |
| ≥ 45 | 0.106(0.050, 0.162) | 30.63 | 0.142(0.077, 0.206) | -0.150(-0.389, 0.090) | 0.030 |
| BMI (kg/m2) | |||||
| < 24 | 0.090(0.039, 0.141) | 10.84 | 0.320(-0.132, 0.771) | 0.085(0.033, 0.137) | 0.309 |
| ≥ 24, < 28 | 0.165(0.056, 0.274) | 32.02 | 0.226(0.106, 0.347) | -0.346(-0.800, 0.107) | 0.020 |
| ≥ 28 | 0.176(-0.029,0.384) | 26.27 | 0.357(0.058,0.662) | -0.234(-0.490, 0.022) | 0.142 |
Each stratification was adjusted for all factors (Age, nationality, BMI, SBP, DBP, TC, TG, LDL-C, TP, Hb, ALT, AST, ALP, GGT, FBG, and serum creatinine), except for the stratification factor itself.
Figure 2.
Relationship between UHR and LFC (%). A - C for male, D - F for female. A and D: Each black hollow point exhibits one participant. B, C, E, and F: Solid red line illustrates the fitted smooth curve among variables. Age, nationality, BMI, SBP, DBP, TC, TG, LDL-C, TP, Hb, ALT, AST, ALP, GGT, FBG, and serum creatinine were adjusted.
Figure 3.
Association between UHR and LFC (%) stratified by tertiles of age and BMI. Nationality, SBP, DBP, TC, TG, LDL-C, TP, Hb, ALT, AST, ALP, GGT, FBG, and serum creatinine were adjusted. A-B for male, C-D for female. A and C: The relationship between UHR and LFC (%) stratified by age. B and D: The relationship between UHR and LFC (%) stratified by BMI.
Discussion
Abnormal liver fat accumulation increased the risk of liver cirrhosis and hepatocellular carcinoma. Previous studies also showed that fatty liver was associated with various cardiovascular diseases. Additionally, our previous work confirmed that LFC was closely related to the prevalence of hypertension18. As a non-invasive 3D imaging method, quantitative CT technology could accurately measure LFC without increasing the radiation dose by combining low-dose chest CT with the corresponding module software17. In this study, quantitative CT was used to quantify liver fat, aiming to identify risk factors and new treatment indicators for hepatic steatosis, and to provide a novel research method for the follow-up study of hepatic steatosis.
The objective of this research was to explore the correlation and dose-response relationship between UHR and LFC in both gender groups among Chinese adults, using data from a health management department in 2021 (n = 2,909). After accounting for covariates, the results indicated a positive correlation between UHR and LFC in the male population. This correlation was particularly evident when the BMI was less than 24 kg/m², with a clear curve relationship observed. The point on the right side of the curve indicated a significant positive correlation. In the female population, the relationship between UHR and LFC was more complex. For women under 45 years of age with a UHR greater than 20.29, there was a positive correlation between UHR and LFC. However, for women aged 45 years and older with a UHR greater than 30.63, the correlation between UHR and LFC was negative. Furthermore, for women with a BMI in the range of 24 - 28 kg/m², a significant inflection point was observed at UHR = 32.02, and UHR was negatively correlated with LFC on the right side of the curve.
Earlier research demonstrated a strong connection between SUA, HDL-C, and the onset, progression, and severity of fatty liver disease19–21. Xu22reviewed the predictive ability of SUA on the occurrence of fatty liver disease and the underlying mechanisms, proposing that insulin resistance and oxidative stress enabled SUA to predict the development of NAFLD independently of gender, age, metabolic syndrome, and other clinical variables. Several epidemiological studies confirmed the correlation between SUA and fatty liver disease. In a comprehensive longitudinal study of 3,822 participants in Beijing, China, higher SUA levels identified as a risk factor for NAFLD after accounting for confounding factors such as age, gender, and abdominal obesity23. Wei et al.24investigated the causal relationship between SUA and fatty liver by establishing a follow-up cohort, and also confirmed that elevated SUA could be a standalone predictor of fatty liver. Additionally, it suggested that SUA, as a mediator between obesity and fatty liver disease, should be considered in the intervention of obesity and the treatment of fatty liver disease25.
HDL-C plays many positive roles in human physiology and biochemistry. However, when stimulated by inflammation, oxidative stress, and insulin resistance, HDL-C particles may become dysfunctional molecules and promote atherosclerosis26. Similarly, hepatic steatosis is often accompanied by atherosclerotic dyslipidemia, and the presence of fatty liver is believed to promote alterations in the pro-arteriosclerotic lipoprotein profile27. Zhang et al.28 demonstrating a significant decrease in HDL-C in patients with fatty liver compared to those without NAFLD. This view is also supported by Janac et al.29, who noting that HDL-C concentrations gradually decreased as the fatty liver disease index increased. Crudele et al. and Karami et al. highlighting that HDL-C facilitates the return of dietary cholesterol via the reverse cholesterol transport pathway, while also provides anti-inflammatory and antioxidant benefits30,31. Therefore, the reduction of HDL-C may contribute to the development of NAFLD by reducing cholesterol reflux and antioxidant effects.
Recent studies have identified UHR as a novel metabolic marker linked to various metabolic diseases32,33. Yu et al.14 suggested that UHR could serve as a dependable and valid indicator for detecting metabolic syndrome in non-diabetic males within the Chinese demographic. Additionally, the relationship between UHR and insulin resistance has been noted. Zhou et al.13 conducted a study investigating the relationship between UHR and insulin resistance within the United States population. Their findings reveal a significant association between elevated UHR levels and insulin resistance, suggesting the potential utility of UHR as an indicator for insulin resistance in this population. Furthermore, recent advancements in imaging technologies have yielded novel insights into fat quantification. Wang et al.34 further clarify this correlation by using magnetic resonance imaging to measure abdominal fat accumulation, demonstrating that UHR is closely related to trunk fat accumulation. Xie et al.33find that a positive association between UHR and NAFLD persists in the female population but is not observed in the male population. In this study, we further group participants according to age and BMI and find a positive association between UHR and LFC in the male population, independent of age. However, BMI may mediate the nonlinear relationship between UHR and LFC. In the female population, age also affects the correlation between UHR and LFC, and when the age ≥ 45 years and the UHR > 30.63, the UHR and LFC are negatively correlated. Another study of Chinese adults confirms that subgroup analyses stratified by sex and age find a significant positive association between UHR and NAFLD risk in all groups15. This difference in subgroup analysis of UHR versus liver fat may be due to differences in study populations and confounder selection. Perimenopausal and postmenopausal changes in female sex hormones provide evidence for the negative correlation between UHR and LFC35. Specifically, the alterations in estradiol and sex hormone-binding globulin in postmenopausal women are specifically associated with more severe metabolic abnormalities36. These exacerbated metabolic irregularities may influence the relationship between UHR and hepatic fat accumulation. In this study, we present large data to illustrate the dose-response relationship between UHR and LFC, providing greater confidence in the relationship between UHR and fatty liver development.
The samples in this study were from a uniform geographical region, a factor that significantly enhanced the relevance of the findings for epidemiological investigations within this specific area. This uniformity also bolstered the reliability of our conclusions. Moreover, these samples were sourced from health examination institutions, thereby providing data that was broadly representative of the general population’s health status. More importantly, this study quantified LFC values and executed a stratified analysis based on age and BMI parameters, a facet not explored in prior studies. Despite our best efforts to consider potential confounders inherent to cross-sectional studies, there were certain limitations that warranted acknowledgment. Firstly, this study had a cross-sectional design and could not establish a causal relationship between UHR and LFC. Secondly, covariables such as diet and exercise habits, and other potentially influential conditions, including drinking history, were not included; however, participants with alcoholic fatty liver disease were excluded based on medical history. Lastly, this study originated from a single physical examination department, and the population did not cover the whole of China, so the applicability of the results to the entire Chinese population still needs to be verified by multi-center joint research.
Conclusion
This research demonstrated a positive correlation between UHR and LFC when controlled for covariates. However, the strength of this correlation fluctuated across different age and BMI groups. The correlation between UHR and LFC was found to be more pronounced in the obese population, a trend observable in both male and female cohorts. These findings offered valuable clinical insights for the diagnosis of fatty liver disease among Chinese adults and could assist healthcare providers in the early detection and intervention of high-risk groups with elevated LFC.
Acknowledgements
We express our gratitude to all colleagues for the data collection and quality control.
Author contributions
AL and YL contributed to the central idea. YS, XQ and YZ analyzed most of the data. AL drafted the initial version of the manuscript. JZ assisted in revising the manuscript. ZL, XW, and ZZ contributed to data collection. XL and HL contributed to refining opinions, conducting supplementary analysis, and finalizing the paper. All authors reviewed and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82071884); Central Plains Science and Technology Innovation Leading Talents (244200510016); the National Key R&D Program of China (2022YFC2010000, 2022YFC2010001); Henan Provincial Medical Science and Technology Program Funding (SBGJ202302011); and the Henan Provincial Science and Technology Research Program (242102311121, 242102311018, 242102310299).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethical approval
This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Henan Provincial People’s Hospital (Ethics Committee Number: No. 115, 2022). Informed Consent has been obtained from each participant after a full explanation of the purpose and nature of all procedures used.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou, J. et al. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology71 (5), 1851–1864 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Khan, R. S., Bril, F., Cusi, K. & Newsome, P. N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology70 (2), 711–724 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Kasper, P. et al. NAFLD and cardiovascular diseases: a clinical review. Clin. Res. Cardiol.110 (7), 921–937 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim, G. A. et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. (2017). Nov 2:S0168-8278(17)32294-8. [DOI] [PubMed]
- 5.Castera, L., Friedrich-Rust, M. & Loomba, R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology156 (5), 1264–1281e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gofton, C., Upendran, Y., Zheng, M. H. & George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol.29 (Suppl), S17–S31 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong, V. W., Adams, L. A., de Lédinghen, V., Wong, G. L. & Sookoian, S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat. Rev. Gastroenterol. Hepatol.15 (8), 461–478 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Deprince, A., Haas, J. T. & Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab.42, 101092 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren, X. et al. Association between Triglyceride to HDL-C Ratio (TG/HDL-C) and Insulin Resistance in Chinese Patients with Newly Diagnosed Type 2 Diabetes Mellitus. PLoS One. 11 (4), e0154345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng, X. et al. Serum uric acid and non-alcoholic fatty liver disease in non-obesity Chinese adults. Lipids Health Dis.16 (1), 202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, S. et al. Serum Uric Acid Levels and Nonalcoholic Fatty Liver Disease: A 2-Sample Bidirectional Mendelian Randomization Study. J. Clin. Endocrinol. Metab.107 (8), e3497–e3503 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Sun, D. Q. et al. Serum uric acid: a new therapeutic target for nonalcoholic fatty liver disease. Expert Opin. Ther. Targets. 20 (3), 375–387 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Zhou, X. & Xu, J. Association between serum uric acid-to-high-density lipoprotein cholesterol ratio and insulin resistance in patients with type 2 diabetes mellitus. J. Diabetes Investig. 15 (1), 113–120 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu, X. et al. Serum uric acid to high-density lipoprotein cholesterol ratio is a promising marker for identifying metabolic syndrome in nondiabetic Chinese men. Postgrad. Med.135 (7), 741–749 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Zhao, H., Qiu, X., Li, H. Z., Cui, J. J. & Sun, Y. Y. Association between Serum Uric Acid to HDL-Cholesterol Ratio and Nonalcoholic Fatty Liver Disease Risk among Chinese Adults. Biomed. Environ. Sci.36 (1), 1–9 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Zhu, W. et al. Higher serum uric acid to HDL-cholesterol ratio is associated with onset of non-alcoholic fatty liver disease in a non-obese Chinese population with normal blood lipid levels. BMC Gastroenterol.22 (1), 196 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu, Y. et al. The study protocol for the China Health Big Data (China Biobank) project. Quant. Imaging Med. Surg.9 (6), 1095–1102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun, Y. et al. Association between liver fat level and risk of hypertension: evidence from a Chinese health examination dataset. J. Hypertens. Jun 26. (2024). [DOI] [PMC free article] [PubMed]
- 19.He, J. et al. The Additive Values of the Classification of Higher Serum Uric Acid Levels as a Diagnostic Criteria for Metabolic-Associated Fatty Liver Disease. Nutrients14 (17), 3587 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantinou, C. et al. Advances in high-density lipoprotein physiology: surprises, overturns, and promises. Am. J. Physiol. Endocrinol. Metab.310 (1), E1–E14 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Hoekstra, M. & Van Eck, M. High-density lipoproteins and non-alcoholic fatty liver disease. Atheroscler Plus. 53, 33–41 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu, C. Hyperuricemia and nonalcoholic fatty liver disease: from bedside to bench and back. Hepatol. Int.10 (2), 286–293 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Ma, Z. et al. Changing trajectories of serum uric acid and risk of non-alcoholic fatty liver disease: a prospective cohort study. J. Transl Med.18 (1), 133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei, F. et al. Higher Serum Uric Acid Level Predicts Non-alcoholic Fatty Liver Disease: A 4-Year Prospective Cohort Study. Front. Endocrinol. (Lausanne). 11, 179 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, Q. et al. Serum Uric Acid Is a Mediator of the Association Between Obesity and Incident Nonalcoholic Fatty Liver Disease: A Prospective Cohort Study. Front. Endocrinol. (Lausanne). 12, 657856 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsiki, N., Athyros, V. G., Karagiannis, A. & Mikhailidis, D. P. High-density lipoprotein, vascular risk, cancer and infection: a case of quantity and quality? Curr. Med. Chem.21 (25), 2917–2926 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Almeda-Valdés, P., Cuevas-Ramos, D. & Alberto Aguilar-Salinas, C. Metabolic syndrome and non-alcoholic fatty liver disease[J]. Ann. Hepatol.8, S18–S24 (2009). [PubMed] [Google Scholar]
- 28.Zhang, Y. et al. The liver steatosis severity and lipid characteristics in primary biliary cholangitis. BMC Gastroenterol.21 (1), 395 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janac, J. et al. The association between lecithin-cholesterol acyltransferase activity and fatty liver index. Ann. Clin. Biochem.56 (5), 583–592 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Karami, S. et al. Association of anti-oxidative capacity of HDL with subclinical atherosclerosis in subjects with and without non-alcoholic fatty liver disease. Diabetol. Metab. Syndr.13 (1), 121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crudele, L. et al. Low HDL-cholesterol levels predict hepatocellular carcinoma development in individuals with liver fibrosis. JHEP Rep.5 (1), 100627 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun, H. et al. Serum Uric Acid to High–density Lipoprotein Cholesterol Ratio is Associated with Visceral Fat in Patients with Type 2 Diabetes. Diabetes Metab. Syndr. Obes.16, 959–967 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie, Y. et al. Association of serum uric acid-to-high-density lipoprotein cholesterol ratio with non-alcoholic fatty liver disease in American adults: a population-based analysis. Front. Med. (Lausanne). 10, 1164096 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, Y. et al. Associations of Serum Uric Acid to High-Density Lipoprotein Cholesterol Ratio with Trunk Fat Mass and Visceral Fat Accumulation. Diabetes Metab. Syndr. Obes.17, 121–129 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazo, M. et al. Association Between Endogenous Sex Hormones and Liver Fat in a Multiethnic Study of Atherosclerosis. Clin. Gastroenterol. Hepatol.13 (9), 1686–93e2. 10.1016/j.cgh.2014.12.033 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaidya, D. et al. The association of endogenous sex hormones with lipoprotein subfraction profile in the Multi-Ethnic Study of Atherosclerosis. Metabolism57 (6), 782–790 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.