Abstract
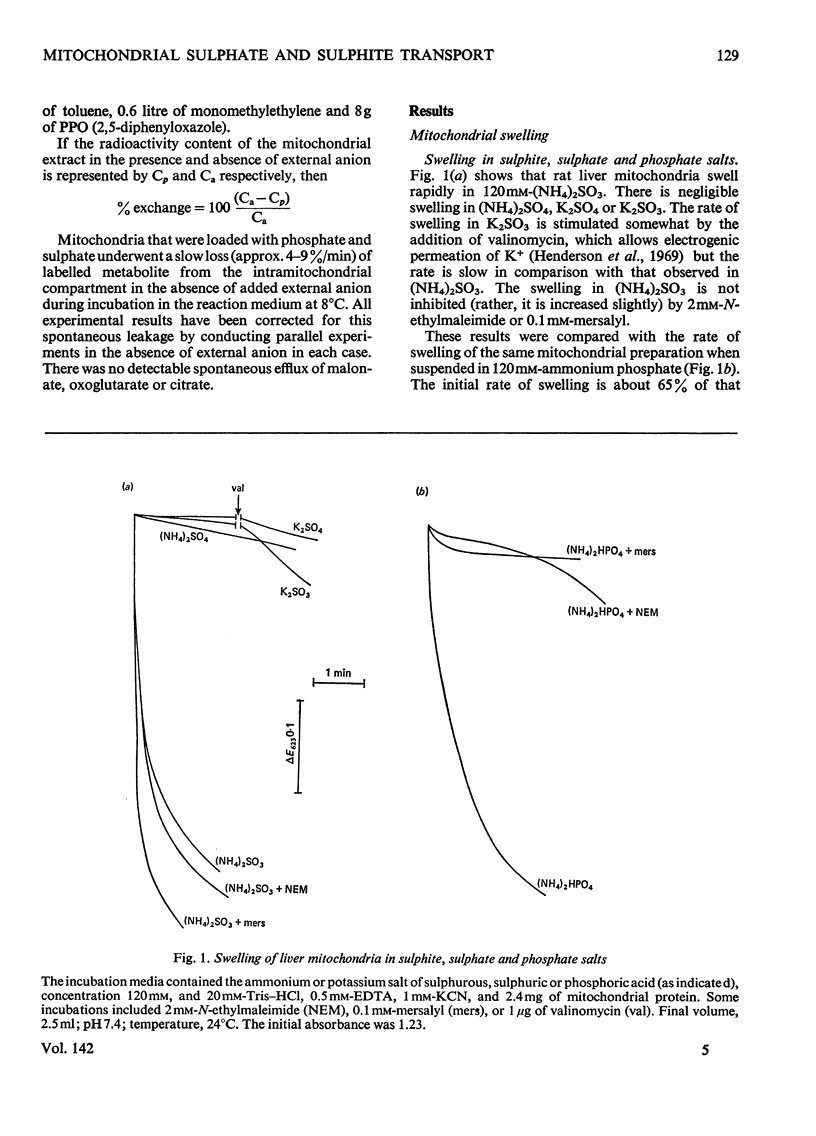
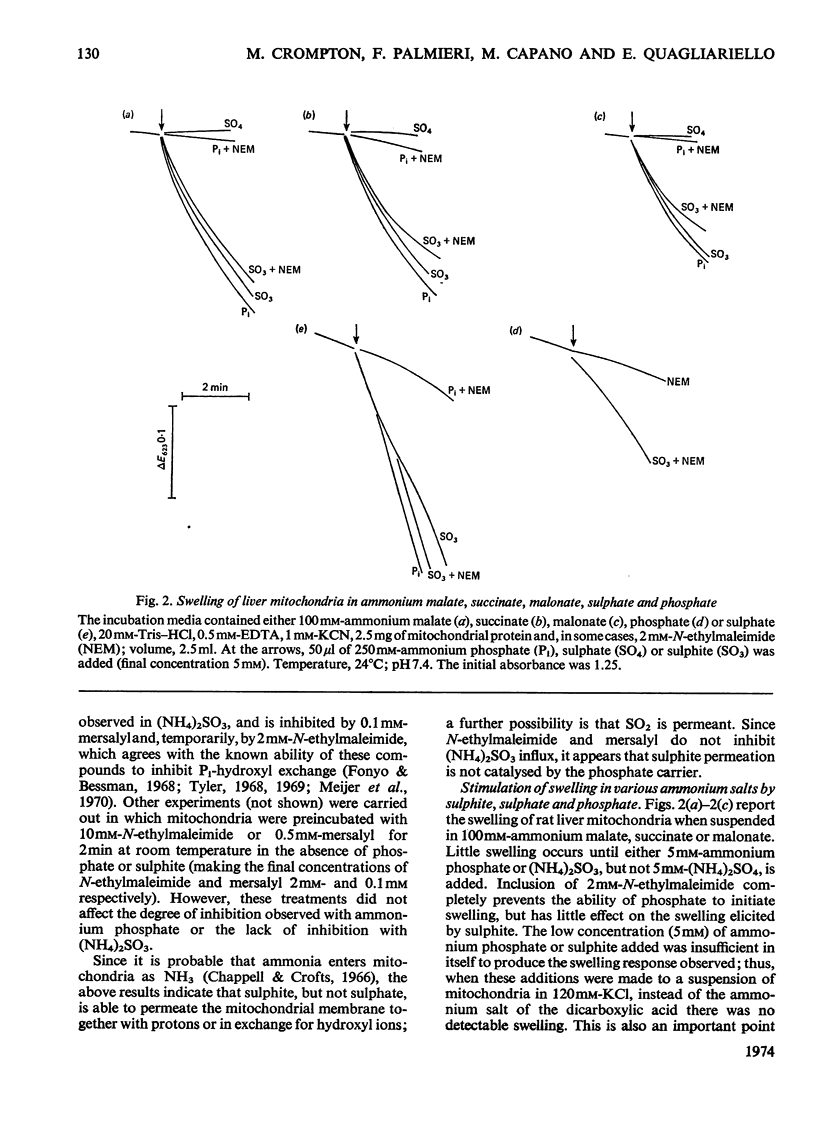
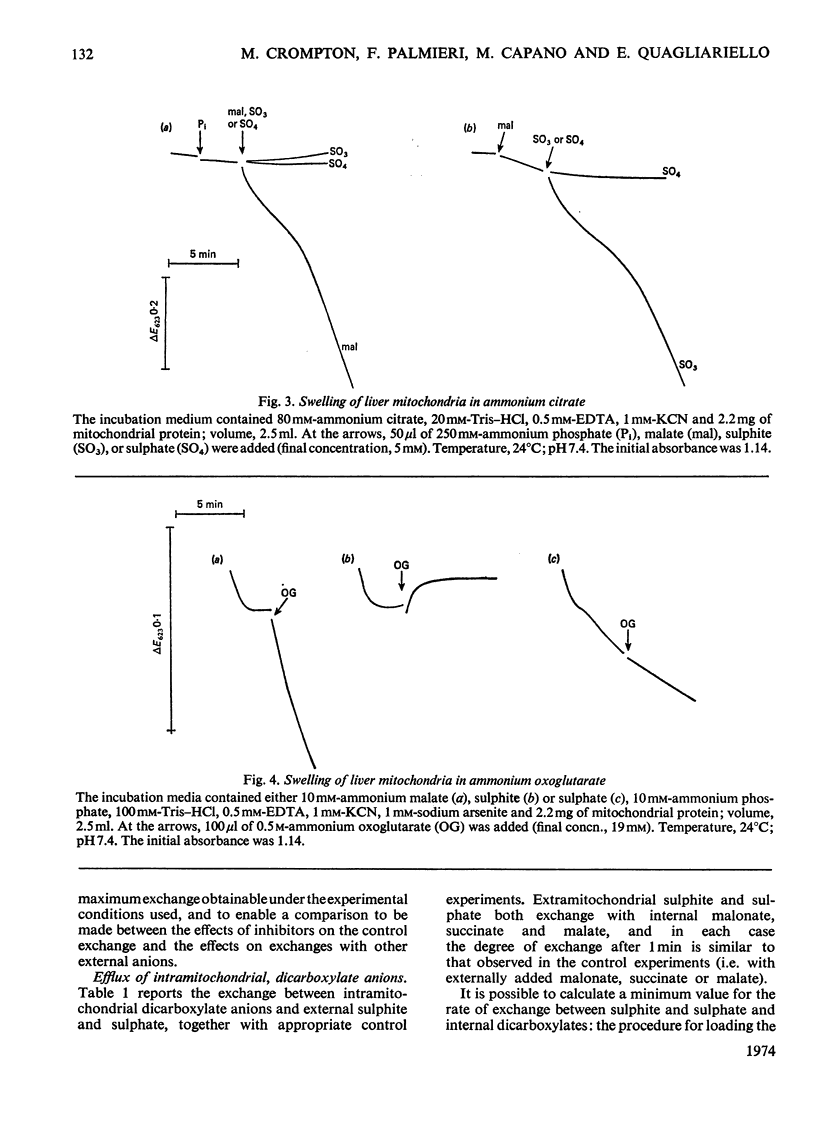
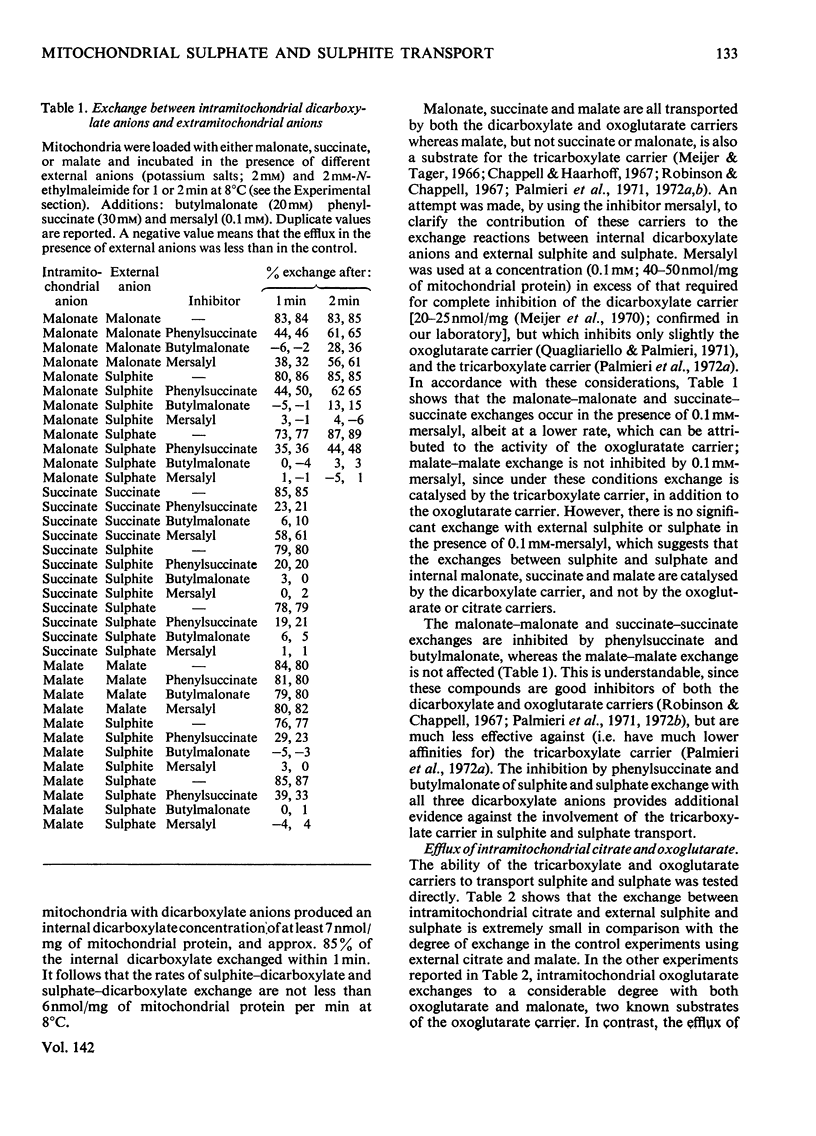
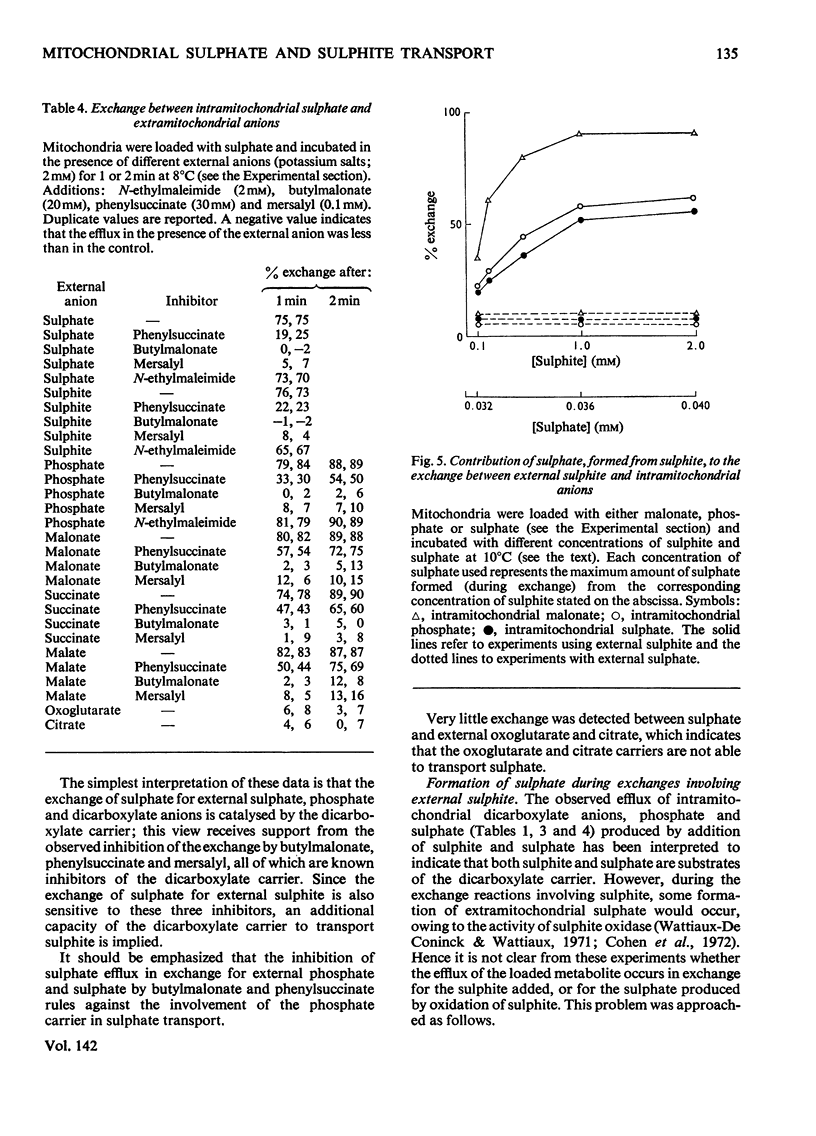
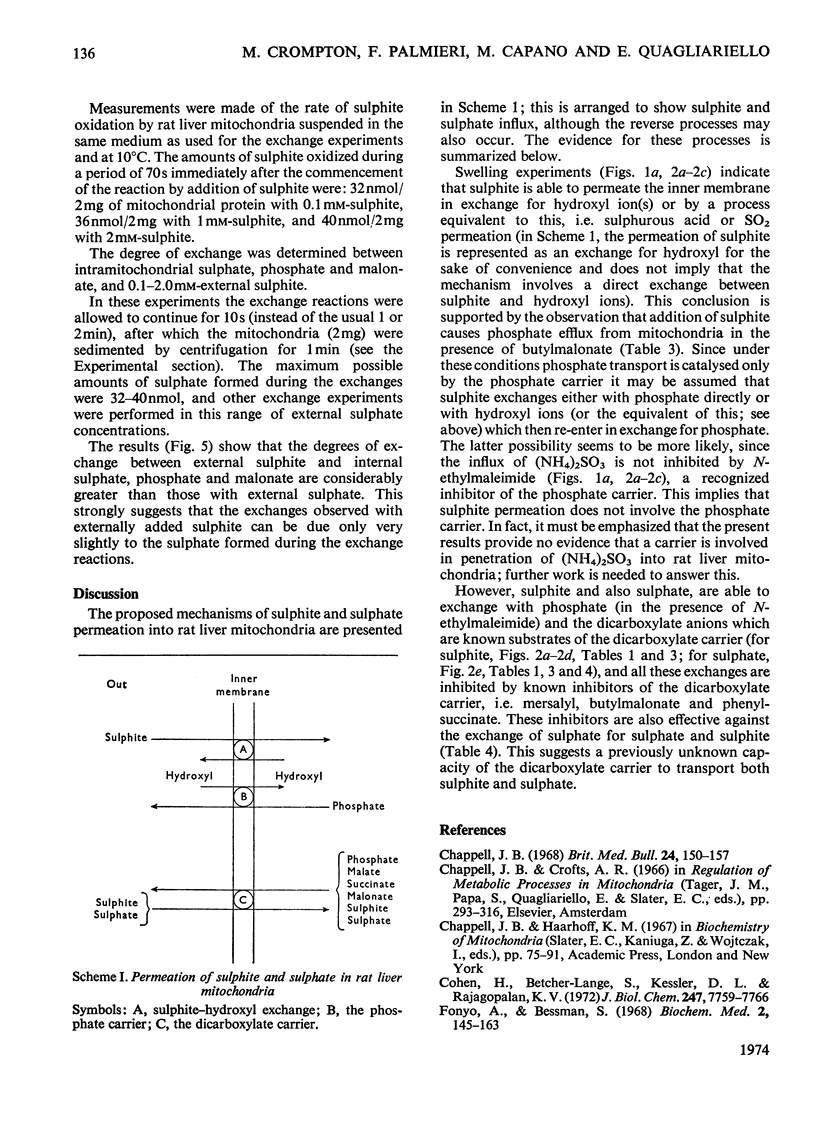
1. The mechanism of sulphite and sulphate permeation into rat liver mitochondria was investigated. 2. Extramitochondrial sulphite and sulphate elicit efflux of intramitochondrial phosphate, malate, succinate and malonate. The sulphate-dependent effluxes and the sulphite-dependent efflux of dicarboxylate anions are inhibited by butylmalonate, phenylsuccinate and mersalyl. Inhibition of the phosphate efflux produced by sulphite is caused by mersalyl alone and by N-ethylmaleimide and butylmalonate when present together. 3. External sulphite and sulphate cause efflux of intramitochondrial sulphate, and this is inhibited by butylmalonate, phenylsuccinate and mersalyl. 4. External sulphite and sulphate do not cause efflux of oxoglutarate or citrate. 5. Mitochondria swell when suspended in an iso-osmotic solution of ammonium sulphite; this is not inhibited by N-ethylmaleimide or mersalyl. 6. Low concentrations of sulphite, but not sulphate, produce mitochondrial swelling in iso-osmotic solutions of ammonium malate, succinate, malonate, sulphate, or phosphate in the presence of N-ethylmaleimide. 7. It is concluded that both sulphite and sulphate may be transported by the dicarboxylate carrier of rat liver mitochondria and also that sulphite may permeate by an additional mechanism; the latter may involve the permeation of sulphurous acid or SO2 or an exchange of the sulphite anion for hydroxyl ion(s).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cañas-Rodriguez A., Smith H. W. The identification of the antimicrobial factors of the stomach contents of sucking rabbits. Biochem J. 1966 Jul;100(1):79–82. doi: 10.1042/bj1000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Betcher-Lange S., Kessler D. L., Rajagopalan K. V. Hepatic sulfite oxidase. Congruency in mitochondria of prosthetic groups and activity. J Biol Chem. 1972 Dec 10;247(23):7759–7766. [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Kinetic study of the tricarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1972 Apr 24;26(4):587–594. doi: 10.1111/j.1432-1033.1972.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Groot G. S.P., Tager J. M. Effect of sulphydryl-blocking reagents on mitochondrial anion-exchange reactions involving phosphate. FEBS Lett. 1970 May 11;8(1):41–44. doi: 10.1016/0014-5793(70)80220-4. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Prezioso G., Quagliariello E., Klingenberg M. Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1971 Sep 13;22(1):66–74. doi: 10.1111/j.1432-1033.1971.tb01515.x. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN H., SALLIS J., FANG M., DELUCA H. F., YOUNG R. PARATHYROID HORMONE AND ANION UPTAKE IN ISOLATED MITOCHONDRIA. Endocrinology. 1964 Mar;74:388–394. doi: 10.1210/endo-74-3-388. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Chappell J. B. The inhibition of malate, tricarboxylate and oxoglutarate entry into mitochondria by 2-n-butylmalonate. Biochem Biophys Res Commun. 1967 Jul 21;28(2):249–255. doi: 10.1016/0006-291x(67)90437-8. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Intermediary metabolism of L-cysteinesulfinic acid in animal tissues. Arch Biochem Biophys. 1956 Apr;61(2):397–409. doi: 10.1016/0003-9861(56)90363-0. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D. Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem J. 1969 Mar;111(5):665–678. doi: 10.1042/bj1110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D. The inhibition of phosphate entry into rat liver mitochondria by organic mercurials and by formaldehyde. Biochem J. 1968 Mar;107(1):121–123. doi: 10.1042/bj1070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTERS R. W., DELLUVA A. M., DEYRUP I. J., DAVIES R. E. Accumulation of sulfate by mitochondria of rat kidney cortex. J Gen Physiol. 1962 Mar;45:757–775. doi: 10.1085/jgp.45.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattiaux-de Coninck S., Wattiaux R. Subcellular distribution of sulfite cytochrome c reductase in rat liver tissue. Eur J Biochem. 1971 Apr 30;19(4):552–556. doi: 10.1111/j.1432-1033.1971.tb01348.x. [DOI] [PubMed] [Google Scholar]


