Abstract
Salmonella enterica serovar 4,[5],12:i:- sequence type 34 (ST34) has recently become a global concern for public and animal health. The acquisition of mobile genetic element ICEmST, which contains two copper tolerance gene clusters, cus and pco, influences the epidemic success of this clone. Copper is used as a feed additive in swine at levels that potentially lead to selection pressure for Enterobacteriaceae; however, it remains unclear whether the copper tolerance system of ICEmST functions in vivo. We performed competition assays with Salmonella 4,[5],12:i:- ST34 wildtype (WT) and deletion mutants of ICEmST (ΔICEmST, Δcus, and Δpco) in groups of mice fed 0, 150, and 500 ppm CuSO4. In the competition of WT against ΔICEmST and Δcus, the competitive index of the 500 ppm-fed group was significantly lower than that of the 0 ppm-fed group. In the swine experiment, all individuals were fed 150 ppm CuSO4. The number of ICEmST-positive strain in the feces was significantly greater than that of ICEmST-negative strain. The serum inflammatory markers were significantly increased in swine infected with the ICEmST-positive strain. These data suggest that ICEmST, especially cus, provides Salmonella with the ability to colonize in the intestine, even at high copper concentrations, leading to swine salmonellosis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83039-6.
Keywords: Copper tolerance; Feed; ICEmST; Salmonella 4,[5],12:i:-; Swine
Subject terms: Bacterial genetics, Bacteriology, Pathogens
Introduction
Salmonella enterica subsp. enterica serovar Typhimurium and its monophasic variant (Salmonella 4,[5],12:i:-) are the most common serovars that cause gastroenteritis in humans and animals worldwide1,2. Previous reports indicated that since 2010, Salmonella Typhimurium and 4,[5],12:i:- were among the top five serovars in cases of human salmonellosis in the United States and European Union (EU)3–8. To date, several globally spread clones of Salmonella Typhimurium and 4,[5],12:i:-, which were determined to be ST19 and ST34 by multilocus sequence typing, have been reported in both humans and animals9,10. Among these clones, ST34 Salmonella 4,[5],12:i:- has recently spread rapidly in Europe, North America, South America, Oceania, Asia, and Africa, and these strains have been isolated from various sources, including humans, swine, cattle, and poultry9,11–17. This clone has been widely disseminated into swine in the EU, the United States, and other regions, including Japan14,18–20. In addition, pork and its products are among the major sources of human salmonellosis caused by ST34 Salmonella 4,[5],12:i:- in the EU13,21. As the selection pressure for this clone in swine is unclear, some background information should be obtained on the mechanism leading to the success of the epidemic.
Most ST34 Salmonella 4,[5],12:i:- strains possess the integrative and conjugative element ICEmST, which was first reported as Salmonella genomic island 3 and subsequently redesigned as an ST34-specific element12,20; this element is found on one of two transfer RNA gene locations on the chromosome, pheR or pheV. ICEmST is approximately 81 kb in size and is composed of genes related to conjugal transfer, DNA partitioning, and heavy-metal tolerance to copper and arsenic compounds. Our previous study demonstrated that ICEmST was excised from the donor chromosome, formed a circular intermediate, transferred by conjugation, and integrated into the pheR or pheV locus on the recipient chromosome22. ICEmST contains the cus and pco gene clusters as copper tolerance systems, and the cus system contributes especially to copper homeostasis in Salmonella spp. under anaerobic conditions in vitro. However, it remains unclear whether these copper tolerance systems in ICEmST are functional in vivo, especially in the swine intestine.
Copper is an essential mineral and is commonly added to commercial feeds to improve growth performance in swine23,24. Under the One Health concept, EU and global institutions have placed restrictions on the use of antimicrobial agents as feed additives to reduce the risk of antimicrobial-resistant bacteria in humans and animals; thus, coppers may be employed as an alternative to antimicrobial agents to prevent postweaning diarrhea in swine25–28. A copper concentration of 100 to 250 ppm in the feed is effective for enhancing the growth performance of piglets28,29, while the minimum copper requirement in the postweaning stage is 5–6 ppm30,31. On the other hand, the high use of heavy metals, such as copper, zinc, and cadmium, in swine feed is a potential risk in terms of poisoning and environmental pollution32. Therefore, in Europe, copper concentrations in feed are limited at each growth stage of swine as follows: 150 ppm for the suckling and weaning stages up to four weeks after weaning and 100 ppm from 5 to 8 weeks after weaning33. In the field, copper sulfate (CuSO4) has been added to feed for piglets at 11.2–248.5 ppm (median 157.7 ppm) in the United States34. Based on previous reports, the concentration of copper as a feed additive increases several times in the starter stage from feed to swine feces35. Some researchers are concerned that high concentrations of copper in the intestine may cause selection pressure for Enterobacteriaceae bacteria, including Salmonella spp.36,37.
In the present study, we performed animal experiments using ST34 Salmonella 4,[5],12:i:- and its mutants to determine the role of ICEmST in Salmonella infection in vivo. We found that ICEmST contributed to the colonization of Salmonella in mouse and swine intestines in the presence of copper, resulting in inflammatory responses.
Results
Contribution of ICEmST to the survival and colonization ofSalmonella in the mouse intestine in the presence of copper
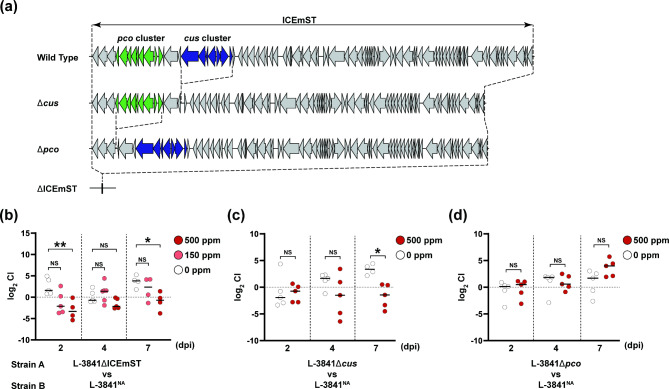
To clarify the role of ICEmST in colonization, we performed a competition assay in mice using deletion mutants of ICEmST, the cus gene cluster, and the pco gene cluster derived from the ST34 Salmonella 4,[5],12:i:- L-3841 strain (Fig. 1). As we previously reported22, the minimum inhibitory concentration (MIC) of CuSO4 under anaerobic conditions for the L-3841ΔICEmST and L-3841Δcus strains was 250 ppm, while that for the L-3841Δpco strain was 1,500 ppm (Table 1). Five C57BL/6 mice in each group were fed copper at 0, 150, or 500 ppm. The levels of copper in the feces of 150 and 500 ppm group were 108.3 ± 44.9 ppm and 505.8 ± 58.8 ppm (mean ± SD), respectively, although the copper concentration in the feces of 0 ppm group was below the detection limit. After pretreatment with streptomycin, C57BL/6 mice were infected with a mixture of equal amounts of the L-3841NA strain and the L-3841ΔICEmST, L-3841Δcus, and L-3841Δpco strains. At 2 and 7 days postinfection with both the L-3841NA and L-3841ΔICEmST strains, the CIs (see “Materials and methods” for the formula) for the 500 ppm CuSO4-fed group were significantly lower than those of the 0 ppm-fed group (P < 0.05) (Fig. 1b). Similarly, the CI obtained for the 500 ppm CuSO4-fed group infected with the combination of the L-3841NA and L-3841Δcus strains was significantly lower than that of the 0 ppm-fed group (P < 0.05) (Fig. 1c). However, after infection with both the L-3841NA and L-3841Δpco strains, there was no significant difference between the 0 ppm and 500 ppm CuSO4 groups at any time (Fig. 1d).
Fig. 1.
Competitive colonization of ICEmST-negative and ICEmST-positive Salmonella 4,[5],12:i:- strains. (a) Schematic view of ICEmST in the wild-type Salmonella 4,[5],12:i:- L-3841 strain, deletion mutants of the cus gene cluster, pco gene cluster, and ICEmST. C57BL/6 mice (n = 4 to 5) were orally infected with a 1:1 mixture (total, 107 CFU/mouse) of Salmonella 4,[5],12:i:- strains A and B, which were ICEmST-negative and ICEmST-positive strains, respectively. The competitive index (CI) was calculated as follows: CI = (CFU strain A recovered/CFU strain B recovered)/(CFU strain A inoculated/CFU strain B inoculated). CIs in mouse feces at days postinfection (dpi) are indicated by white, light red, and red dots indicating the mice that were fed with feeds containing 0, 150, and 500 ppm CuSO4, respectively. Panels (b–d) show comparisons between the L-3841NA strain and the ICEmST deletion mutants, the cus gene cluster, and the pco gene cluster, respectively. Bars indicate medians. NS, not significant. *P < 0.05; **P < 0.01; Dunn–Bonferroni test (b) or Kruskal–Wallis test (c,d).
Table 1.
Bacterial strains used in this study and the susceptibility to copper sulfate
| Strain | Serovar | Description | MIC, mM (ppm) | References | ||
|---|---|---|---|---|---|---|
| L-3841 | 4,[5],12:i:- | Wild type | 6 [1,500] | 18 | ||
| L-3841NA | 4,[5],12:i:- | Nalidixic acid-resistant mutant of L-3841 | 6 [1,500] | This study | ||
| L-3841ΔICEmST | 4,[5],12:i:- | ICEmST deletion mutant derived from L-3841 | 1 [250] | 22 | ||
| L-3841Δcus | 4,[5],12:i:- | cus gene cluster deletion mutant derived from L-3841 | 1 [250] | 22 | ||
| L-3841Δpco | 4,[5],12:i:- | pco gene cluster deletion mutant derived from L-3841 | 6 [1,500] | 22 | ||
| L-3569 | Typhimurium | Wild type | 1 [250] | 45 | ||
| L-3569TC | Typhimurium | L-3569 transconjugant that acquired ICEmST | 6 [1,500] | This study | ||
The copper concentrations in the livers of the 0, 150, and 500 ppm CuSO4-fed groups were 3.5 ± 0.5, 3.4 ± 0.4, and 3.9 ± 0.2 ppm, respectively. On the other hand, copper levels in the spleen were below the detection limit in all mice. In the group infected with the combination of the L-3841NA and L-3841ΔICEmST strains, there were no significant differences among CIs in the liver or spleen (Supplementary Fig. S1). Similarly, no significant differences among CIs were detected in the liver or spleen of mice infected with a combination of the L-3841NA strain and partial ICEmST deletion mutants, namely, L-3841Δcus and L-3841Δpco (Supplementary Fig. S2).
Effect of ICEmST on Salmonella colonization in the presence of copper in swine intestine
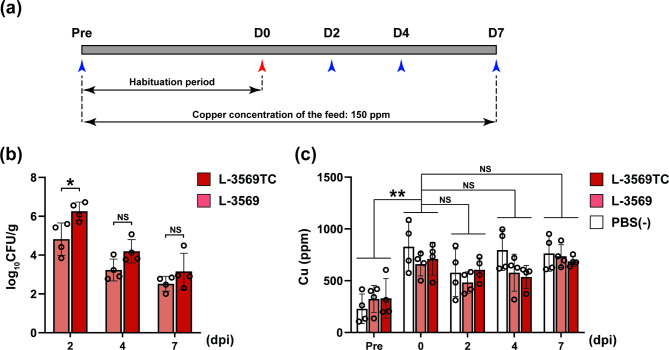
Four 3-week-old Salmonella-free piglets in each group were orally administered Salmonella Typhimurium L-3569 and L-3569TC strains and PBS (−), and all pigs were fed 150 ppm CuSO4 during the experimental period (Fig. 2a). Figure 2b shows the amount of Salmonella Typhimurium shed in the feces in the swine experiments, which were conducted to determine the role of ICEmST on the survival and colonization of Salmonella within swine intestines in the presence of copper. At 2 days postinfection, the number of bacteria of the L-3569TC strain (log10 CFU/g) in the feces was greater than that of the L-3569 strain (log10 CFU/g), and a significant difference at P < 0.05 was detected (Fig. 2b). Subsequently, the mean number of Salmonella shed in feces in the L-3569TC strain-infected group was greater than that in the L-3569 strain-infected group at 4 and 7 days postinfection, but the difference was not statistically significant. Notably, all fecal samples of PBS (−)-administrated pigs were cultured, but no colonies of Salmonella were observed at any time point.
Fig. 2.
Concentrations of copper and shedding of theSalmonella Typhimurium L-3569 and L-3569TC strains in swine feces. (a) Schedule for the swine experiment. All pigs were provided feeds containing 150 ppm CuSO4 during the experiment. The blue arrowheads indicate the day when feces and blood were collected. After 2 weeks of habituation, each of the three groups was orally administered Salmonella Typhimurium L-3569 or L-3569TC strains or PBS (−). The white, light red, and red bars indicate the groups challenged with PBS (−) and the L-3569 and L-3569TC strains, respectively. (b) Salmonella Typhimurium shedding in feces. Each dot represents the amount of Salmonella Typhimurium in the feces of each individual pig as log10 CFU/gram. (c) Copper concentration in feces. The dots indicate copper concentrations in swine feces on the day when pigs were introduced (Pre) and the days postinfection (dpi). Error bars represent standard error. NS, not significant. *P < 0.05; **P < 0.01; one-way ANOVA with unpaired Student’s t test (b) and Tukey’s multiple comparison test (c).
We examined the relationship between swine intestinal copper concentrations and Salmonella colonization in feces and the intestinal contents of the ileum, cecum, proximal colon, and distal colon. As shown in Fig. 2c, the copper concentration in the feces of the pigs on the day of introduction was 294.5 ± 149.5 ppm, reached 733.0 ± 175.7 ppm on the day of infection and remained at similar levels until 7 days postinfection. Among the intestinal contents measured at 7 days postinfection, copper concentrations in the ileum were the lowest, ranging from below the detection limit to 73 ppm; then, the concentrations increased as the contents passed through the intestine and reached 609.3 ± 76.9 ppm in the distal colon (Supplementary Fig. S3).
Fecal characteristics and inflammatory responses of pigs before and after Salmonella was administered
After infection with the L-3569 strain, loose feces were observed in some pigs from 2 to 4 days postinfection (Supplementary Fig. S4). In contrast, diarrhea was observed in pigs infected with the L-3569TC strain at 3 and 4 days postinfection, and loose feces were also observed in some pigs up to 7 days postinfection (Supplementary Fig. S4).
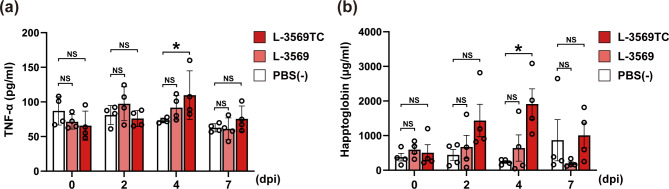
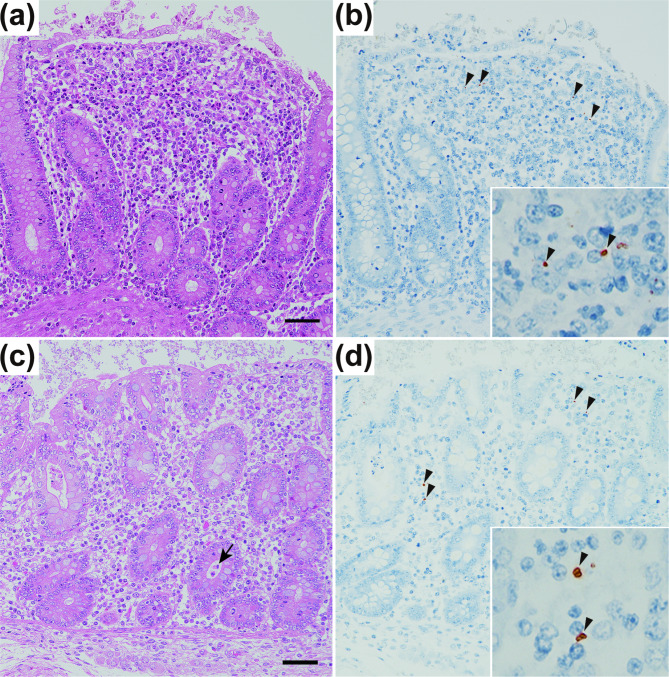
Changes in the concentrations of TNF-α and haptoglobin, two inflammatory markers, in the serum of pigs before and after Salmonella Typhimurium infection were measured by sandwich ELISA and hemoglobin binding assays, respectively. At 4 days postinfection, the concentration of serum TNF-α was significantly greater in the L-3569TC strain-infected group than in the PBS (−)-treated group (P < 0.05), which was designated the negative control group; however, there was no significant difference between the L-3569 strain-infected group and the negative control group (Fig. 3a). The mean concentration of serum haptoglobin in the L-3569TC strain-infected group was greater than that in the L-3569 strain-infected and negative control groups at 2, 4, and 7 days postinfection; there were no statistically significant differences except for a comparison with the negative control group at 4 days postinfection (Fig. 3b). As shown in Fig. 4, histopathological analysis revealed inflammatory responses in the intestine, especially in the cecum, of the pigs infected with the L-3569 and L-3569TC strains at 7 days postinfection. Numerous inflammatory cells, such as macrophages, neutrophils, lymphocytes, and plasma cells, were recruited to the lamina propria of the cecum (Fig. 4a,c). The bacterial cells of Salmonella were occasionally detected in the lamina propria of the cecum, in which inflammatory cells had accumulated (Fig. 4b,d).
Fig. 3.
Changes in serum TNF-α (a) and haptoglobin (b) concentrations before and after Salmonella Typhimurium infection. Each dot represents the concentration of each inflammatory marker in each individual pig at days postinfection (dpi). The white, light red, and red bars indicate the groups challenged with PBS (−) and the L-3569 and L-3569TC strains, respectively. Error bars represent standard error. NS not significant. *P < 0.05; Dunn–Bonferroni test.
Fig. 4.
Histopathology of the cecum of pigs 7 days postadministration of the Salmonella Typhimurium L-3569 (a,b) and L-3569TC (c,d) strains. The lamina propria was expanded and contained many neutrophils, macrophages, lymphocytes, and plasma cells (a,c). Occasionally, small cryptic abscesses were observed (c; arrow). Hematoxylin and eosin stains; bar = 50 μm. Immunohistochemically, Salmonella O4 antigen-positive bacteria (b,d; arrowheads in the insert) were observed in the lamina propria by the antibody-labeled polymer method.
Discussion
Salmonella 4,[5],12:i:- ST34 has been a major clone in humans and food animals within 20 years in many countries, including Europe, the United States, Asia, and Oceania9,14,18,20,38. It was hypothesized that the epidemic of Salmonella 4,[5],12:i:- ST34 has occurred because this clone consists of multidrug-resistant isolates and possesses several genes for heavy metal tolerance encoded by ICEmST39. ST34 Salmonella 4,[5],12:i:- isolates commonly show resistance to ampicillin, streptomycin, sulfonamides, and tetracycline because this clone possesses a composite Tn21-like transposon that contains blaTEM−1B, strA, strB, sul2, and tet(B) instead of the phase 2 flagellin gene fljB. ICEmST contains copper and arsenic homeostasis genes that contribute to tolerance to these chemical compounds. Among the gene clusters in ICEmST that are involved in tolerance to copper, i.e., cus and pco, we found that the Cus system, but not the Pco system, contributes to copper homeostasis under anaerobic conditions in vitro22. However, it remains unclear whether ICEmST contributes to the colonization and inflammatory response caused by Salmonella spp. in vivo, especially in the intestine. In the present study, we performed animal experiments to determine the contribution of ICEmST to Salmonella infection in mice and swine fed CuSO4.
Regarding the dynamics and metabolism of copper in mammals, dietary copper is mainly absorbed in the small intestine as Cu(I), while Cu(II) is not directly taken up by enterocytes and is reduced by six-transmembrane epithelial antigen of prostate (STEAP) family metalloreductases40. Therefore, Cu(I) and Cu(II) are present in the intestine at a certain ratio. The Cus system, CusABC, contains resistance, nodulation, and division (RND) efflux pumps that are expressed at the inner membrane, periplasm, and outer membrane, respectively, in Enterobacteriaceae. CusABC transports Cu(I) and Cu(II) copper ions from the cytoplasm to the extracellular space and leads to copper homeostasis under anaerobic conditions in Escherichia coli41,42. The Pco system may be involved in periplasmic copper detoxification by oxidating Cu(I) to Cu(II)42. In a previous study, we reported that the Pco system in ICEmST does not contribute to copper tolerance in Salmonella spp. in vitro; we found no difference in the MICs of CuSO4 under aerobic and anaerobic conditions between the parental strains and their mutant strains without the pco gene cluster22. In the mouse experiments in the present study, we used the Salmonella 4,[5],12:i:- ST34 L-3841 strain and deletion mutants of ICEmST, the cus gene cluster, and the pco gene cluster to determine whether these elements are functional in the mouse intestine. In experiments with the ICEmST and cus deletion mutants (Fig. 1b, c), the CIs of mice that were fed with feeds containing 500 ppm CuSO4 were significantly lower than the CIs of mice fed 0 ppm CuSO4 at 7 days postinfection (P < 0.05). This result suggested that the Cus system provided favorable results for the survival and colonization of Salmonella spp. within the mouse intestine in the presence of copper. On the other hand, in experiments with the pco deletion mutant (Fig. 1d), no significant difference in CI was observed; therefore, the Cus system may function well without the Pco system as a copper homeostatic system in the intestine. Furthermore, there were no significant differences between the L-3841NA and L-3841ΔICEmST strains in the CIs of the liver and spleen (Supplementary Fig. S1), suggesting that ICEmST does not affect the ability of Salmonella to cause systemic infection.
Heavy metals, such as copper, are essential nutrients for eukaryotes and are vital cofactors for various metalloproteins and enzymes. According to the National Research Council30,31, the daily requirement of copper in swine is relatively low at the postweaning stage (approximately 5 ppm), but these minerals are used at much higher concentrations in the field to improve growth performance and prevent postweaning diarrhea23,24,34,43. On the other hand, the use of copper as a feed additive for swine is regulated in several countries and regions33 due to concerns that high concentrations of copper cause environmental pollution. In this study, we set the copper concentration in the feed at 150 ppm, which is the maximum level permitted by European regulations for the postweaning stage33. The copper concentrations in feces were approximately 2.5 times greater on the day of Salmonella infection than on the day when pigs were introduced. Several previous studies have also reported the concentrations of copper from feed to feces in swine35,37. In terms of anatomy, the copper concentration increased gradually from the small intestine to the large intestine (Supplementary Fig. S3). This increase may result from the presence of surplus copper and the absorption of water. The copper concentration reached a level in the cecum (276 ± 74 ppm) that may cause selection pressure for ICEmST-negative Salmonella. The number of bacteria in the L-3569TC strain tended to be greater than that in the L-3569 strain at the end of the experiment (Fig. 2c), suggesting that ICEmST contributes to survival and colonization within the swine intestine in the presence of copper.
Salmonella Typhimurium is a major gastroenteritis pathogen and causes an inflammatory response in the distal small intestine and colon44. We used the Salmonella Typhimurium L-3569 strain, which has been demonstrated to cause diarrhea and inflammatory responses in piglets in our previous study45, as the parental strain to determine the role of ICEmST in swine salmonellosis. In the present study, the concentration of serum TNF-α, a major proinflammatory cytokine, was significantly greater in the L-3569TC strain-infected group than in the negative control group at 4 days postinfection (P < 0.05) (Fig. 3a). Similarly, the concentration of serum haptoglobin, an acute-phase protein and inflammatory marker, was increased in the group infected with the L-3569TC strain (Fig. 3b). A significant difference was observed in the concentration of haptoglobin between the negative control group and the L-3569TC strain-infected group at 4 days postinfection (P < 0.05), but there was no statistically significant difference between the negative control group and the L-3569 strain-infected group. In addition, the serum haptoglobin concentrations in the L-3569TC strain-infected group tended to be greater than those in the other two groups at 2 and 7 days postinfection, although the differences were not significant (Fig. 3b). These data indicated that the L-3569TC strain successfully colonized in the intestine even in the presence of copper and induced an inflammatory response that peaked at 4 days postinfection. Furthermore, numerous inflammatory cells were observed at the lamina propria in the cecum of L-3569TC strain-infected pigs even 7 days postinfection, after the inflammatory response peaked, and similar histopathological changes were observed in pigs infected with the L-3569 strain (Fig. 4). In addition, bacterial cells of Salmonella were also detected in the lesions, although to a limited extent. Consistent with the above data, L-3569TC strain-infected pigs produced loose feces until the end of the experiment. These data suggest that ICEmST favors the survival and colonization of Salmonella spp. in the early phase of infection in the presence of copper. Based on these results, the number of Salmonella bacteria that harbor ICEmST is sufficient to cause adequate inflammatory responses in swine and the development of salmonellosis, even at high copper concentrations. From another perspective, our data showed that copper as a feed additive helps inhibit Salmonella colonization in the intestine.
Salmonella 4,[5],12:i:- ST34 has been a global concern for both public and animal health9,18,38,46. To date, it has been hypothesized that the epidemic success of this clone results from multidrug resistance and heavy metal tolerance due to Tn21-like composite transposons and ICEmST, respectively39. Bayesian temporal analysis predicted that Salmonella 4,[5],12:i:- ST34 first acquired ICEmST and the composite transposon in 1980 and 1982, respectively39, and the effective population size was estimated to increase in the 2000s9.
In conclusion, we evaluated the role of ICEmST in colonizing and causing salmonellosis within swine in the presence of copper by using a transconjugant of ICEmST and several deletion mutants. We found that the copper tolerance system in ICEmST, especially the cus gene cluster, is functional in vitro and in vivo within both mouse and swine intestines. The present data suggest that ICEmST promotes the survival and colonization of Salmonella spp. in the presence of copper in vivo, further demonstrating the importance of ICEmST as a factor in the epidemic success of Salmonella 4,[5],12:i:- ST34.
Materials and methods
Bacterial strains
The strains used in this study are listed in Table 1. The Salmonella 4,[5],12:i:- ST 34 L-3841 strain and Salmonella Typhimurium L-3569 strain were originally isolated from diseased swine in Japan18,45. The L-3841ΔICEmST, L-3841Δcus and L-3841Δpco strains were the ICEmST, cus gene cluster, and pco gene cluster deletion mutants derived from the L-3841 strain, respectively. Each deletion mutant was produced by the same procedure as previously described22, using the primers listed in Supplementary Table S1 in the supplemental material. Rifampicin-resistant mutants of the L-3569 strain were selected on DHL agar plates (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 100 µg/ml rifampicin (Fujifilm Wako Pure Chemical Corp., Osaka, Japan). Similarly, nalidixic acid-resistant mutants of all strains were selected on DHL agar plates containing 50 µg/ml nalidixic acid (Fujifilm Wako Pure Chemical Corp.).
Conjugation experiment
The L-3569TC strain, a transconjugant of ICEmST into the L-3569 strain, was obtained by a conjugation experiment based on the filter mating method22. Briefly, donor and recipient strains were grown in Luria-Bertani (LB) broth (Becton, Dickinson and Company, Franklin Lakes, NJ) at 37 °C for 18 h with shaking. Each aliquot, 50 µl, was transferred to 5 ml of fresh LB broth and incubated at 37 °C for 4 h with shaking until the exponential phase. The donor and recipient were mixed at a 1:9 ratio and trapped on a sterile mixed cellulose ester filter (0.45 μm pore size, Toyo Roshi Kaisha, Ltd., Tokyo, Japan). Bacterial cells were grown on a filter placed on an LB agar plate at 37 °C for 20 h. Bacterial cells were removed and resuspended from the filter by vortexing with 1 ml of sterile saline. Transconjugants were selected on DHL agar plates supplemented with rifampicin (100 µg/ml) and Na2HAsO4 (8 mM) (Fujifilm Wako Pure Chemical).
CuSO4 susceptibility testing
The MICs of the strains to CuSO4 5H2O (Fujifilm Wako Pure Chemical Corp.) were determined by the agar dilution method using Mueller–Hinton agar plates (Becton, Dickinson and Company). Strains cultured overnight in LB broth were diluted to approximately 106 CFU/ml, and the aliquots were spotted onto Mueller–Hinton agar plates with CuSO4 (0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 12 mM) using the microplanter MIT-27P (Sakuma Co., Ltd., Tokyo, Japan). The spotted plates were incubated under anaerobic conditions (anaerobic jar with AnaeroPack-Anaero; Mitsubishi GAS Chemical Company, Inc., Tokyo, Japan) at 37 °C for 48 h.
Competition assay between copper-resistant and copper-susceptible strains in mice
All procedures involving animals were conducted in accordance with the relevant guidelines and regulations of the National Agriculture and Food Research Organization (Ibaraki, Japan) and the American Veterinary Medical Association47. Six-week-old female C57BL/6NCrSlc mice were purchased from Japan SLC, Inc. (Shizuoka, Japan) and were habituated for one week. Throughout the experiment, each group of mice was fed a low, middle, or high copper feed (CLEA Japan, Inc., Tokyo, Japan) with copper concentrations of 0, 150, or 500 ppm, respectively. After habituation, Salmonella was administered to the mice as described previously with slight modifications48. Briefly, mice were orally pretreated with 20 mg of streptomycin (Fujifilm Wako Pure Chemical Corp.) 24 h prior to infection. The feed was withdrawn from mice 6 h prior to infection. Strains A and B (deletion mutant and parental strains, respectively) were independently grown overnight in LB broth supplemented with 0.3 M NaCl with shaking. The aliquot was diluted 1:20 and subcultured in LB broth for 4 h with shaking. After 5 mg of sodium bicarbonate was administered, the mice were orally infected with a 1:1 ratio of bacterial cells of each strain prepared to 107 CFU/20 µl. Anesthesia of mice was performed by inhaling an excess of isoflurane for veterinary use (Merck & Co. Inc., Rahway, NJ), and mice were humanely euthanized by cervical dislocation under deep anesthesia at 2, 4, and 7 days postinfection. To quantify the number of strains A and B, feces, liver, and spleen were homogenized in sterile dilution A (4.5 g KH2PO4, 6.0 g Na2HPO4,0.5 g L-cysteine HCl H2O, 0.5 g Tween-80, 1.0 g agar per 1.0 L distilled water) and spread on DHL agar plates supplemented with appropriate antimicrobial agents. The competitive index (CI) was calculated as follows: (recovered CFU of strain A/recovered CFU of strain B)/(inoculated CFU of strain A/inoculated CFU of strain B).
Experimental infection of swine
Three-week-old SPF Landrace piglets (CIMCO Corp., Tokyo, Japan) were introduced and reared according to the research animal resource guidelines of the National Institute of Animal Health. To confirm that the piglets were Salmonella-free before the experiment, fecal samples were collected from all the piglets on the day when they arrived, and approximately 1.0 g of each sample was cultured in 10 ml of Hajna tetrathionate broth (Eiken Chemical Co., Ltd., Tokyo, Japan) at 37 °C for 48 h. Subsequently, the culture was spread on DHL agar plate (Eiken Chemical Co., Ltd.) supplemented with 20 µg/ml novobiocin (Merck KGaA, Darmstadt, Germany) and ES Salmonella Agar II (Eiken Chemical Co., Ltd.) and incubated at 37 °C overnight to check for the appearance of Salmonella colonies. To determine the amount of copper used in the postweaning stage, CuSO4 5H2O was added to standard diet SDS No. 1 (Feed One Co., Ltd., Kanagawa, Japan) to adjust the copper concentration to 150 ppm. A total of 12 piglets were equally divided into three different rooms, and all the piglets were provided the above feeds during the experiment. After two weeks of habituation, one of the three groups was provided 1 ml of PBS (−) and served as the uninfected control. The other two groups were orally infected with 1 ml of Salmonella Typhimurium L-3569 and L-3569TC strains (109 CFU/pig) grown in overnight static culture in LB broth. Prior to infection, all piglets were orally administered 10 ml of 0.05 M carbonate-bicarbonate buffer (pH 9.6). Blood was collected from the jugular vein on the day of infection and at 2, 4, and 7 days postinfection, and the serum was separated to determine the concentrations of TNF-α and haptoglobin in the serum by solid phase sandwich ELISA using a Porcine TNF-alpha Quantikine ELISA kit (R&D Systems Inc., Minneapolis, MN) and a hemoglobin-binding assay as described previously45, respectively. Feces were collected from rectal swabs at 2, 4, and 7 days postinfection and scored as previously described45: normal feces = 0, loose feces = 1, diarrhea = 2, and watery diarrhea = 3. Salmonella Typhimurium L-3569 and L-3569TC strains in feces were quantified by spreading serial dilutions on DHL agar plates containing 100 µg/ml rifampicin (Fujifilm Wako Pure Chemical Corp.).
Measurement of copper concentrations
Copper concentrations in feces, intestinal contents, and feed samples were determined by the flame method49 using an AA-7000 atomic absorption spectrophotometer (Shimadzu Co., Kyoto, Japan), which was done by using an air/acetylene flame at wavelengths 324.8 nm. The value of slit width was 0.7 nm, and the value of lamp current was at 8.0 mA. Prior to the measurements, organic materials in feces, intestinal contents, and feed samples were removed by the wet ashing method using the microwave digestion system Multiwave GO (Anton Paar GmBH, Graz, Austria) with nitric acid and hydrochloric acid. The water content of the feed samples was determined by drying at 105 °C for 3 h before wet ashing.
Histopathologic analysis
All pigs were euthanized by exsanguination under deep anesthesia through injection of pentobarbital sodium salt (Kyoritsu Seiyaku Corporation, Tokyo, Japan) (10 mg/0.4 ml/kg body weight), xylazine hydrochloride (Bayer Yakuhin Co., Ltd., Osaka, Japan) (2 mg/0.1 ml/kg body weight), and butorphanol tartrate (Meiji Animal Health Co., Ltd., Kumamoto, Japan) (0.5 mg/0.1 ml/kg body weight). Tissue samples were collected at 7 days postinfection and fixed in 10% neutral-buffered formalin. The samples were embedded in paraffin, sectioned in a conventional manner, and stained with hematoxylin and eosin. For immunohistochemistry analysis, immunostaining was performed using the antibody-labeled polymer method. Antigen retrieval was achieved using 1 mg/ml of actinase E (Kaken Pharmaceutical Co., Tokyo, Japan) in PBS at 37 °C for 10 min. Each section was immunolabeled with Salmonella O4 antigen (rabbit polyclonal, 1:512 dilution, Denka Co. Ltd., Tokyo, Japan). As a secondary antibody, we used Histofine Simple Stain MAX-PO (MULTI) (Nichirei Biosciences Inc., Tokyo, Japan). Immunoreactions were visualized using 3-amino-9-ethylcarbazole (Histofine Simple Stain AEC solution, Nichirei Biosciences Inc.).
Statistical analysis
Statistical significance was tested by R version 4.2.350 using the Mann–Whitney U test, one-way ANOVA with Tukey’s multiple-comparison test, and the Kruskal–Wallis test with Dunn–Bonferroni multiple comparison test, as indicated in the figure legends.
Approval for animal experiments
Mouse and swine experiments were approved by the Animal Ethics Committee of the National Institute of Animal Health, Tsukuba, Ibaraki, Japan, with approval numbers 19-042 and 21-06, respectively. All animal experiments in this study were conducted in accordance with the ARRIVE guidelines51. This paper does not include any studies involving human participants or relevant samples.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by JSPS KAKENHI (Grant Numbers 19J10415 and 21K14980).
Author contributions
Conceptualization: NA, TS, MA, and MK. Investigation: NA, TS, RN, YT, HN, YM, HS, AW, and TI. Data analysis: NA, TS, RN, HN, YM, and MK. Supervision: TS, MA, and MK. Writing-original draft: NA and MK. All authors reviewed the manuscript.
Data availability
All data generated or analyzed in this study are included in this article with supplementary information.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hohmann, E. L. Nontyphoidal salmonellosis. Clin. Infect. Dis.32, 263–269 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Majowicz, S. E. et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis.50, 882–889 (2010). [DOI] [PubMed] [Google Scholar]
- 3.European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2009. EFSA J.9, 2090 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. EFSA J.11, 3129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J.13, 4329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J.16, 5500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Food Safety Authority. The European Union One Health 2020 zoonoses report. EFSA J.19, 6971 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. National Salmonella surveillance annual summaries. https://www.cdc.gov/nationalsurveillance/salmonella-surveillance.html (CDC, 2018).
- 9.Ingle, D. J. et al. Evolutionary dynamics of multidrug resistant Salmonella enterica serovar 4,[5],12:i:- in Australia. Nat. Commun.12, 4786 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mather, A. E. et al. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science341, 1514–1517 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Gallas, N. et al. Quinolone resistance among Salmonella Kentucky and Typhimurium isolates in Tunisia: first report of Salmonella Typhimurium ST34 in Africa and qnrB19 in Tunisia. J. Appl. Microbiol.130, 807–818 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Arai, N. et al. Identification of a recently dominant sublineage in Salmonella 4,[5],12:i:- sequence type 34 isolated from food animals in Japan. Front. Microbiol.12, 690947 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bone, A. et al. Nationwide outbreak of Salmonella enterica serotype 4,12:i:- infections in France, linked to dried pork sausage, March-May 2010. Euro. Surveill. 15, 19592 (2010). [PubMed] [Google Scholar]
- 14.Elnekave, E. et al. Salmonella enterica serotype 4,[5],12:i:- in swine in the United States Midwest: an emerging multidrug-resistant clade. Clin. Infect. Dis.66, 877–885 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Mossong, J. et al. Outbreaks of monophasic Salmonella enterica serovar 4,[5],12:i:- in Luxembourg, 2006. Euro. Surveill. 12, E11–E12 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Tavechio, A. T., Ghilardi, A. C. & Fernandes, S. A. Multiplex PCR identification of the atypical and monophasic Salmonella enterica subsp. enterica serotype 1,4,[5],12:i:- in São Paulo State, Brazil: frequency and antibiotic resistance patterns. Rev. Inst. Med. Trop. Sao Paulo. 46, 115–117 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Trüpschuch, S., Laverde Gomez, J. A., Ediberidze, I., Flieger, A. & Rabsch, W. Characterisation of multidrug-resistant Salmonella Typhimurium 4,[5],12:i:- DT193 strains carrying a novel genomic island adjacent to the thrW tRNA locus. Int. J. Med. Microbiol.300, 279–288 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Arai, N. et al. Phylogenetic characterization of Salmonella enterica serovar Typhimurium and its monophasic variant isolated from food animals in Japan revealed replacement of major epidemic clones in the last 4 decades. J. Clin. Microbiol.56, e01758–e01717 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkwood, M. et al. Ecological niche adaptation of Salmonella Typhimurium U288 is associated with altered pathogenicity and reduced zoonotic potential. Commun. Biol.4, 498 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovska, L. et al. Microevolution of monophasic Salmonella Typhimurium during epidemic, United Kingdom, 2005–2010. Emerg. Infect. Dis.22, 617–624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser, E. et al. Pork contaminated with Salmonella enterica serovar 4,[5],12:i:-, an emerging health risk for humans. Appl. Environ. Microbiol.76, 4601–4610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai, N. et al. Salmonella genomic island 3 is an integrative and conjugative element and contributes to copper and arsenic tolerance of Salmonella enterica. Antimicrob. Agents. Chemother.63, e00429-19 (2019). [DOI] [PMC free article] [PubMed]
- 23.Liao, P. et al. Effects of dietary supplementation with cupreous N-carbamylglutamate (NCG) chelate and copper sulfate on growth performance, serum biochemical profile and immune response, tissue mineral levels and fecal excretion of mineral in weaning piglets. Food Agric. Immunol.28, 1315–1329 (2017). [Google Scholar]
- 24.Liao, P., Shu, X., Tang, M., Tan, B. & Yin, Y. Effect of dietary copper source (inorganic vs. chelated) on immune response, mineral status, and fecal mineral excretion in nursery piglets. Food Agric. Immunol.29, 548–563 (2017). [Google Scholar]
- 25.Heo, J. M. et al. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. (Berl). 97, 207–237 (2013). [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Global Action Plan on Antimicrobial Resistance. https://www.who.int/publications/i/item/9789241509763 (2015). [DOI] [PubMed]
- 27.The European Union. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. Offi. J. Eur. Union (2019).
- 28.Zhao, J. et al. Effects of a chelated copper as growth promoter on performance and carcass traits in pigs. Asian-Australas J. Anim. Sci.27, 965–973 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cromwell, G. L., Stahly, T. S. & Monegue, H. J. Effects of source and level of copper on performance and liver copper stores in weanling pigs. J. Anim. Sci.67, 2996–3002 (1989). [DOI] [PubMed] [Google Scholar]
- 30.National Research Council. Subcommittee on swine nutrition. Nutrient Requirements of Swine. 10th rev. ed. (National Academy Press, 1998).
- 31.National Research Council. Committee on nutrient requirements of swine. Nutrient requirements of swine. 11th rev. ed. (National Academies Press, 2012).
- 32.Shi, J. et al. Potential risks of copper, zinc, and cadmium pollution due to pig manure application in a soil-rice system under intensive farming: a case study of Nanhu, China. J. Environ. Qual.40, 1695–1704 (2011). [DOI] [PubMed] [Google Scholar]
- 33.The European Commission. Commision Implementing Regulation (EU) 2018/1039. Official J. Eur. Union 8–24 (2018).
- 34.Flohr, J. R. et al. A survey of current feeding regimens for vitamins and trace minerals in the US swine industry. J. Swine Health Prod.24, 290–303 (2016). [Google Scholar]
- 35.Li, H. et al. Trace element accumulation from swine feeds to feces in Chinese swine farms: implication for element limits. Integr. Environ. Assess. Manag. 18, 978–987 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Kim, M. et al. Effects of hot-melt extruded nano-copper as an alternative for the pharmacological dose of copper sulfate in weanling pigs. Biol. Trace Elem. Res.199, 2925–2935 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Medardus, J. J. et al. In-feed use of heavy metal micronutrients in U.S. swine production systems and its role in persistence of multidrug-resistant salmonellae. Appl. Environ. Microbiol.80, 2317–2325 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas, S., Li, Y., Elbediwi, M. & Yue, M. Emergence and dissemination of mcr-carrying clinically relevant Salmonella Typhimurium monophasic clone ST34. Microorganisms7, 298 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cadel-Six, S. et al. The spatiotemporal dynamics and microevolution events that favored the success of the highly clonal multidrug-resistant monophasic Salmonella Typhimurium circulating in Europe. Front. Microbiol.12, 651124 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue, Q. et al. Copper metabolism in cell death and autophagy. Autophagy19, 2175–2195 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rademacher, C. & Masepohl, B. Copper-responsive gene regulation in bacteria. Microbiol. (Reading). 158, 2451–2464 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Rensing, C. & Grass, G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev.27, 197–213 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Yazdankhah, S., Rudi, K. & Bernhoft, A. Zinc and copper in animal feed - development of resistance and co-resistance to antimicrobial agents in bacteria of animal origin. Microb. Ecol. Health Dis.25, 25862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bescucci, D. M., Moote, P. E., Ortega, P. R., Uwiera, R. R. E. & Inglis, G. D. Salmonella enterica serovar Typhimurium temporally modulates the enteric microbiota and host responses to overcome colonization resistance in swine. Appl. Environ. Microbiol.86, e01569–e01520 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muneta, Y. et al. In vivo effect of a TLR5 SNP (C1205T) on Salmonella enterica serovar Typhimurium infection in weaned, specific pathogen-free Landrace piglets. Microbiol. Immunol.62, 380–387 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Mather, A. E. et al. New variant of multidrug-resistant Salmonella enterica serovar Typhimurium associated with invasive disease in immunocompromised patients in Vietnam. mBio9, e01056–e01018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals. (2020). Edition https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (2020).
- 48.Barthel, M. et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun.71, 2839–2858 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunderman, F. W. Jr. & Roszel, N. O. Measurements of copper in biologic materials by atomic absorption spectrometry. Am. J. Clin. Pathol.48, 286–294 (1967). [DOI] [PubMed] [Google Scholar]
- 50.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. (2023).
- 51.du Percie, N. et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLOS Biol.18, e3000411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are included in this article with supplementary information.