Abstract
The monocyte-to-Apolipoprotein A1 ratio (MAR) emerges as a potentially valuable inflammatory biomarker indicative of metabolic dysfunction-associated fatty liver disease (MASLD). Accordingly, this investigation primarily aims to assess the correlation between MAR and MASLD risk. A cohort comprising 957 individuals diagnosed with type 2 diabetes mellitus (T2DM) participated in this study. The relationship between MAR and MASLD was analyzed through binomial logistic regression analysis and restricted cubic splines (RCS). Furthermore, a comparative assessment of MAR and monocyte to high-density lipoprotein ratio (MHR) in identifying MASLD efficacy was conducted using receiver operating characteristic curve analysis. Remarkably, even after adjusting for metabolic parameters and hepatic functional markers, MAR stood out as an independent predictor for MASLD (OR 1.58, 95% CI 1.36–1.84; P < 0.001) and displayed a nonlinear positive association with MASLD risk according to RCS analysis (P for nonlinearity and overall < 0.001). Notably, MAR exhibited superior diagnostic accuracy for identifying MASLD compared to MHR (AUC: 0.772 vs 0.722, P < 0.001). In summary, MAR emerges as a promising inflammatory indicator for MASLD, demonstrating potential as a valuable screening tool to bolster the management of MASLD within the T2DM population.
Keywords: Metabolic dysfunction-associated fatty liver disease, Monocyte to Apolipoprotein A ratio, Inflammatory marker, Type 2 diabetes mellitus
Subject terms: Type 2 diabetes, Metabolic syndrome
Introduction
Monocytes, essential components of the innate immune system originating from bone marrow progenitors and disseminated via the circulatory system to extrinsic tissues, play inherent roles in systemic inflammation by instigating and amplifying inflammatory cascades1. Multiple clinical investigations have highlighted an upsurge in peripheral monocyte levels across diverse inflammatory and immune pathologies such as cardiovascular maladies2, inflammatory bowel disease3, metabolic syndrome4, and nonalcoholic fatty liver disease (NAFLD)5. Apolipoprotein A1 (APOA1), a paramount constituent of high-density lipoprotein (HDL-c) assembly, stands as a prognostic indicator for acute cardiac events, acknowledged for its capacity to sequester lipid moieties and facilitate cholesterol efflux. Noteworthy, APOA1 has exhibited immunoregulatory, anti-inflammatory, antioxidant, and anti-thrombotic properties6,7. Recent advancements in lipid metabolism and inflammation research have unveiled the repressive influence of APOA1 on monocyte activation, progenitor cell propagation, and differentiation8. Furthermore, empirical evidence from clinical examinations has affirmed an inverse correlation between ApoA1 serum concentrations and the inflammatory profile of monocytes9. Monocytes exert proinflammatory effects, while APOA1 acts as a mitigating factor during the inflammatory process. The ratio of serum monocytes to APOA1 levels (MAR) has the potential to serve as an indicator for identifying inflammatory and immune disorders.
Metabolic dysfunction-associated fatty liver disease (MASLD), formerly recognized as NAFLD, showcases hepatic triglyceride accumulation alongside assorted metabolic aberrations, embodying a spectrum of liver conditions spanning from steatosis to metabolic dysfunction-associated steatohepatitis. Global epidemiological inquiries into metabolic disorders throughout the period from 2000 to 2019 have unveiled a mounting prevalence of both Type 2 diabetes mellitus (T2DM) and MASLD over the last two decades10. Particularly noteworthy is the escalating incidence of MASLD among individuals afflicted with T2DM. A recent survey delineated a global prevalence rate of 55.2% (95% CI 47.3–63.7) for MASLD in the realm of T2DM11. The liver plays crucial roles in energy metabolism and inflammatory signaling, and the presence of MASLD significantly impacts individuals with T2DM. In addition to its well-recognized association with liver injury, MASLD is considered to synergistically increase the risk of unfavorable extra-hepatic clinical outcomes in T2DM12. Given its pervasive nature and severe implications, MASLD has emerged as a burgeoning quandary necessitating attention to prevention and therapeutic modalities. Unveiling efficacious diagnostic and prognostic markers for MASLD holds the promise of facilitating early interventions to avert these deleterious outcomes in the populace grappling with T2DM.
The intricate pathogenesis of MASLD remains enshrouded in a convoluted tapestry of dysmetabolic processes yet to be entirely unraveled. This hallmark entails perturbations in energy metabolism and activation of inflammatory signaling cascades orchestrated by immune cells. Clinical studies have observed a decrease in serum monocyte levels in patients with MASLD13. Conversely, both T2DM and MASLD exhibit disturbances in lipid metabolism, leading to reduced serum levels of APOA1 and HDL-c. While several studies have investigated the diagnostic value of the monocyte to HDL-c ratio (MHR) in MASLD14, limited research has been conducted on the diagnostic capacity of MAR in this context. This study evaluated the association between MAR and MASLD risk in T2DM. Additionally, this study also sought to compare the diagnostic performance between MAR and MHR in identifying MASLD.
Methods
Study population
This prospective study recruited a total of 1078 consecutive participants with T2DM who were admitted to the inpatient ward at Longyan First Hospital between July 2022 and September 2023. All participants provided informed consent, and the study was approved by the Ethical Committee of Longyan First Hospital (IC-2022-009). All procedures were conducted in compliance with the Declaration of Helsinki. Informed consent was obtained from all participants. The diagnosis of T2DM was based on the criteria set forth by the Chinese Diabetes Society15. To ensure the validity of the results, strict exclusion criteria were applied. Participants with other liver comorbidities that could contribute to fatty liver, such as liver malignancy, were excluded. Individuals with a history of excessive alcohol consumption (≥ 210 g per week for males and ≥ 140 g per week for females) were also excluded. Moreover, participants with underlying conditions that could interfere with circulating monocyte count, such as acute or chronic infections, anemia, hemolytic diseases, and bleeding, were excluded. Additionally, individuals with acute illnesses that may disrupt lipid metabolism and those unable to undergo a complete CT scan, such as pregnant women or those with severe spinal curvature, were excluded. After applying the exclusion criteria, a total of 957 participants with T2DM were included in the final analysis. Of these, 342 participants (35.7%) were newly diagnosed with T2DM. Among the remaining participants, 386 were treated with metformin, 108 with thiazolidinediones (TZDs), 168 with sodium-glucose cotransporter-2 inhibitors (SGLT-2), 98 with glucagon-like peptide-1 receptor agonists (GLP-1RAs), 124 with glucosidase inhibitor, 156 with dipeptidyl peptidase-4 inhibitors, and 98 with insulin. A flowchart illustrating the process of participant selection is provided in Fig. 1.
Fig. 1.
The flowchart illustrates the process of study population enrollment.
Data collection and laboratory assessments
Demographic information, including gender, age, duration of diabetes, medication histories, medical background, and alcohol and tobacco use, were collected during hospitalization through standardized medical history questionnaires administered by trained interviewers. Smoking and drinking habits were determined based on participants’ current smoking or excessive alcohol consumption status. Additionally, anthropometric measurements such as height, weight, waist circumference, and blood pressure (BP) were taken by trained research nurses. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2).
All laboratory assessments were performed at the key laboratory using fasting blood samples. Automated biochemical analysis was conducted to measure various biomarkers, including creatinine, alanine aminotransferase, albumin, uric acid (UA), aspartate aminotransferase (AST), fasting blood glucose (FBG), TG, total cholesterol (TC), HDL-c, low-density lipoprotein cholesterol (LDL-c), and APOA1. Hemoglobin A1c (HbA1c) levels were quantified using the HPLC method. Monocyte and platelet counts were determined using the Coulter LH 780 Analyzer. Insulin resistance (IR) was defined as a homeostasis model assessment-insulin resistance (HOMA-IR) value of ≥ 2.5. HOMA-IR was calculated using the following formula: fasting serum insulin (µU/ml) × FBG (mmol/l)/22.516. MAR and MHR were computed using the formulas: monocytes/APOA1(mmol/l) and HDL-c (mmol/l).
Evaluation of fatty liver
To assess fatty liver, unenhanced abdominal CT scans were conducted on all participants. The image reconstruction was performed using the standard algorithm, resulting in 5 mm thick continuous slices. Two experienced radiologists, who were blinded to clinical data, independently evaluated the presence of fatty liver using CT liver-spleen attenuation measurement (CTL−S). To obtain measurements, the radiologists selected the slice that provided the clearest view of the liver and spleen and then manually drew regions of interest around these structures to calculate average attenuation values. The CTL−S value was determined as the ratio of the average liver attenuation to the average spleen attenuation. Participants with a CTL−S value less than 1.0 were classified as having fatty liver.
Assessment of MASLD and progressive liver fibrosis score
According to the current guidelines for diagnosing MASLD, individuals with T2DM who exhibit hepatic steatosis detected through imaging or liver pathology and no other discernible cause are classified as having MASLD17. In this study, the diagnosis of MASLD was based on the assessment of CTL−S to detect the presence of fatty liver. To evaluate advanced liver fibrosis, the nonalcoholic fatty liver disease fibrosis score (NFS) was calculated. The NFS formula for individuals with T2DM was as follows18: − 0.545 + 0.037 × age (years) + 0.094 × BMI (kg/m2) − 0.013 × PLT (109/L) − 0.66 × albumin (g/dl) + 0.99 × AST/ALT ratio.
Definitions
Metabolic dysfunction was characterized by the presence of specific metabolic disorders, which were defined as follows: (1) Abdominal obesity, indicated by a WC ≥ 90 cm in males and ≥ 80 cm in females. (2) Elevated BP, evidenced by multiple measurements ≥ 130/85 mmHg or the use of prescribed antihypertensive medications. (3) Increased serum TG levels ≥ 1.70 mmol/L or the use of medications targeted at managing elevated TG levels. (4) Low levels of serum HDL-c, specifically < 1.0 mmol/L in males or < 1.3 mmol/L in females, or the use of medications addressing low HDL-c levels. (5) Elevated serum UA levels ≥ 420 µmol/L or the use of medications aimed at controlling UA levels. (6) Presence of insulin resistance (IR), determined by a HOMA-IR value ≥ 2.5. (7) Diagnosis of prediabetes or diabetes.
Statistical analysis
Data analysis was performed using SPSS 26.0 (SPSS Inc., IBM) software. The statistical differences among the MAR quartiles were assessed using either the analysis of variance or the Kruskal–Wallis test. Categorical variables were compared across groups using the chi-squared test. To determine the associations between MAR and CTL−S as well as the NFS, Pearson correlation analysis was conducted. These correlations were further evaluated through multiple regression analysis, with MAR serving as the independent variable, and CTL−S and NFS as the dependent variables. Adjustments were made for potential confounders in the regression models. The impact of MAR on the presence of MASLD was examined by the binomial logistic regression analysis and Restricted cubic spines (RCS) after controlling for confounding variables across different models. Furthermore, a receiver operating characteristic (ROC) curve analysis was performed to compare the diagnostic performance of MAR and MHR in identifying MASLD. Statistical significance was defined as P < 0.05.
Results
Comparison of clinical characteristics between MASLD and non-MASLD groups
A comparison of clinical characteristics between MASLD and non-MASLD groups was conducted, and the findings are presented in Table 1. The overall prevalence of MASLD among participants was 56.4%. Comparing the characteristics based on MASLD status, it was observed that individuals in the MASLD group exhibited higher SBP, DBP, BMI, WC, TG, ALT, UA, AST, monocyte count, and HOMA-IR levels. Conversely, the results also demonstrated lower levels of HDL-c and APOA1 than those in the non-MASLD group. Notably, inflammatory markers such as MHR and MAR were significantly increased in the MASLD group (P < 0.05).
Table 1.
Comparison of clinical characteristics between MASLD and non-MASLD groups.
| Characteristics | Total (n = 957) | MASLD (n = 540) | Non-MASLD (n = 417) | P value |
|---|---|---|---|---|
| Age (year) | 54.0 ± 8.1 | 53.9 ± 8.2 | 54.0 ± 8.1 | 0.972 |
| Male, n (%) | 483 (50.5) | 279 (51.7) | 204 (48.9) | 0.400 |
| Duration (year) | 7.7 ± 3.1 | 7.8 ± 3.2 | 7.7 ± 3.0 | 0.539 |
| BMI (kg/m2) | 24.2 ± 3.0 | 25.3 ± 3.1 | 22.9 ± 2.4 | < 0.001 |
| WC (cm) | 85.3 ± 6.9 | 87.7 ± 7.0 | 82.1 ± 5.5 | < 0.001 |
| SBP (mmHg) | 132.8 ± 18.1 | 139.1 ± 16.9 | 124.7 ± 14.6 | < 0.001 |
| DBP (mmHg) | 81.4 ± 9.3 | 84.5 ± 8.5 | 77.3 ± 8.9 | < 0.001 |
| HbA1c (%) | 8.76 ± 1.05 | 8.76 ± 1.02 | 8.75 ± 1.09 | 0.983 |
| TG (mmol/L) | 2.18 ± 1.36 | 2.58 ± 1.46 | 1.67 ± 1.02 | < 0.001 |
| TC (mmol/L) | 5.30 ± 1.19 | 5.23 ± 1.18 | 5.38 ± 1.25 | 0.068 |
| HDL-c(mmol/L) | 1.09 ± 0.24 | 1.00 ± 0.21 | 1.20 ± 0.23 | < 0.001 |
| LDL-c(mmol/L) | 3.52 ± 0.94 | 3.56 ± 0.93 | 3.47 ± 0.95 | 0.135 |
| APOA1(g/L) | 0.99 ± 0.19 | 0.91 ± 0.17 | 1.09 ± 0.21 | < 0.001 |
| UA (umol/L) | 349.0 ± 85.1 | 369.5 ± 88.2 | 321.4 ± 73.0 | < 0.001 |
| Creatinine (umol/L) | 68.9 ± 13.1 | 68.3 ± 13.2 | 69.7 ± 12.9 | 0.105 |
| ALT (IU/L) | 37.6 ± 8.8 | 39.1 ± 9.3 | 35.7 ± 7.9 | < 0.001 |
| AST (IU/L) | 31.3 ± 6.9 | 32.3 ± 7.5 | 30.1 ± 5.7 | < 0.001 |
|
Albumin (g/L) (g/L) |
40.1 ± 4.9 | 40.0 ± 4.1 | 40.3 ± 4.6 | 0.788 |
| Platelets (109/L) | 187.1 ± 68.2 | 187.1 ± 62.7 | 189.1 ± 47.6 | 0.892 |
| Monocyte (108/L) | 4.75 ± 1.01 | 4.35 ± 1.04 | 3.89 ± 0.95 | < 0.001 |
| HOMA-IR | 3.10 ± 1.74 | 3.59 ± 1.79 | 2.46 ± 1.44 | < 0.001 |
| MAR (108/g) | 4.39 ± 1.43 | 4.93 ± 1.37 | 3.69 ± 1.17 | < 0.001 |
| MHR (108/mmol) | 4.07 ± 1.52 | 4.56 ± 1.53 | 3.43 ± 1.26 | < 0.001 |
| Hypertension, n (%) | 271 (37.2) | 253 (46.9) | 90 (21.6) | < 0.001 |
| Drinking, n (%) | 229 (29.6) | 163 (30.2) | 120 (28.8) | 0.636 |
Metabolic profiles and liver functional indexes according to MAR quartile
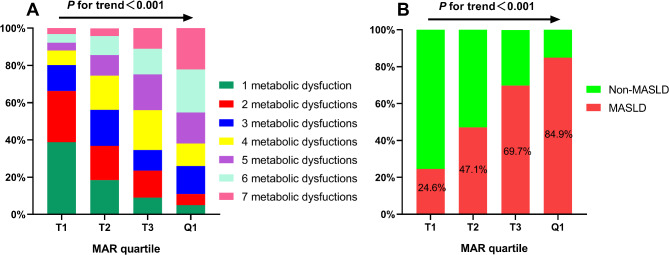
Table 2 displays the metabolic profiles and liver functional indexes classified according to the MAR quartile. The MAR values for the respective quartiles were as follows: 1.26–3.35, 3.36–4.23, 4.24–5.14, and 5.15–10.84. The findings indicated that higher MAR quartile groups exhibited elevated WC, TG, UA, SBP, DBP, and HOMA-IR levels, and a higher proportion of hypertension while demonstrating lower HDL-c and CTL−S levels (P < 0.05). Figure 2A shows that the proportion of participants with 5, 6, and 7 metabolic dysfunctions is higher in the upper MAR quartiles than in the lower MAR quartiles. Additionally, this study also observed a positive correlation between the MAR quartile and MASLD prevalence (P < 0.05), denoted by an increase from 24.6% in the first quartile to 47.1%, 69.7%, and 84.9% in the subsequent higher quartiles (Fig. 2B).
Table 2.
Metabolic profiles and liver functional indexes according to MAR (108/g) quartile.
| Characteristics | MASLD (n = 540) | P value | |||
|---|---|---|---|---|---|
| Q1(1.61–3.35) | Q2(3.36–4.23) | Q3(4.24–5.14) | Q4(5.15–10.84) | ||
| WC (cm) | 82.1 ± 5.1 | 84.5 ± 6.0 | 85.1 ± 5.9 | 89.4 ± 8.2 | < 0.001 |
| SBP (mmHg) | 122.7 ± 15.9 | 130.2 ± 15.1 | 134.6 ± 19.5 | 143.6 ± 15.1 | < 0.001 |
| DBP (mmHg) | 76.5 ± 8.8 | 79.9 ± 6.5 | 83.4 ± 9.2 | 85.8 ± 9.9 | < 0.001 |
| Hypertension, n (%) | 37(15.4) | 74(30.3) | 91(38.9) | 141(59.0) | < 0.001 |
| HbA1c (%) | 8.82 ± 1.00 | 8.67 ± 1.01 | 8.63 ± 0.96 | 8.91 ± 1.21 | 0.010 |
| TG (mmol/L) | 1.50 ± 0.89 | 1.87 ± 0.97 | 2.31 ± 1.24 | 3.06 ± 1.70 | < 0.001 |
| HDL-c (mmol/L) | 1.24 ± 0.23 | 1.13 ± 0.22 | 1.03 ± 0.19 | 0.95 ± 0.21 | < 0.001 |
| HOMA-IR | 2.37 ± 1.47 | 2.81 ± 1.46 | 3.27 ± 1.47 | 3.97 ± 2.08 | < 0.001 |
| UA (umol/L) | 310.2 ± 73.0 | 341.2 ± 72.2 | 360.9 ± 75.2 | 384.2 ± 99.7 | < 0.001 |
| ALT (IU/L) | 37.9 ± 7.5 | 37.7 ± 9.1 | 38.1 ± 9.5 | 38.2 ± 8.8 | 0.336 |
| AST (IU/L) | 31.0 ± 5.9 | 31.4 ± 5.6 | 31.6 ± 6.6 | 31.5 ± 8.6 | 0.382 |
| CTL−S | 1.13 ± 0.24 | 1.01 ± 0.20 | 0.92 ± 0.23 | 0.73 ± 0.24 | < 0.001 |
WC Waist circumference, SBP Systolic blood pressure, DBP Diastolic blood pressure, HbA1c Glycated hemoglobin, TG Triglyceride, HDL-c High-density lipoprotein cholesterol, HOMR-IR Homeostasis model assessment insulin resistance, UA Uric acid, ALT Alanine aminotransferase, AST Aspartate aminotransferase, MAR Monocyte to APOA1 ratio, MASLD Metabolic dysfunction-associated fatty liver disease.
Fig. 2.
MASLD prevalence (A) and metabolic dysfunctions distribution (B) based on MAR quartile. MAR Monocytes to Apolipoprotein A1 ratio, MASLD Metabolic dysfunction-associated fatty liver disease.
Correlation of MAR with CTL−S and NFS
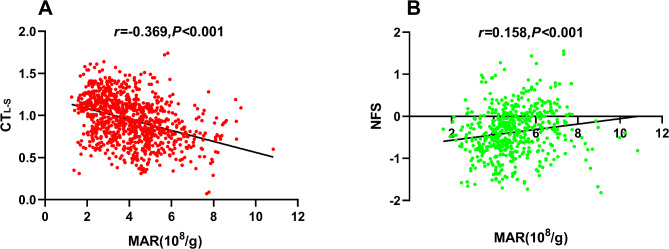
Figure 3 illustrates the correlation between the MAR and CTL−S (Fig. 3A) and the NFS (Fig. 3B) assessed through Pearson correlation analysis. In individuals with MASLD, MAR displayed a positive correlation with NFS (r = 0.158, P < 0.05), while in the entire study population, an inverse association was observed between MAR and CTL−S (r = − 0.369, P < 0.05). Table 3 presents the results of the correlation of MAR with CTL−S and NFS analyzed by the multiple linear regression analysis. Significant correlations of MAR with CTL−S were observed after adjusting potential confounders in Model 1 (including adjustments for gender, age, diabetic duration, and drinking). Subsequently, in Model 2, additional adjustments were made for metabolic profiles such as SBP, DBP, WC, BMI, HbA1c, TG, HDL-c, UA, and HOMA-IR based on Model 1. Moreover, even after further adjusting for liver functional indicators like ALT and AST, as well as the usage of hypoglycemic agents that may influence MASLD like metformin, TZDs, SGLT-2, and GLP-1RAs (Model 3), MAR exhibited significant correlations with both CTL−S (β = − 0.222, P < 0.001) and NFS (β = 0.086, P = 0.008).
Fig. 3.
Correlation of MAR with CTL−S and NFS. (A) the negative association between MAR and CTL−S (r = − 0.369, P < 0.001). (B) the positive association between MAR and NFS (r = 0.158, P < 0.001). NFS Nonalcoholic fatty liver disease fibrosis score, MAR Monocytes to Apolipoprotein A1 ratio.
Table 3.
Multivariate linear regression analysis of the association between MAR and NFS, CTL−S.
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| NFS | 0.142 | < 0.001 | 0.106 | < 0.001 | 0.086 | 0.008 |
| CTL−S | − 0.324 | < 0.001 | − 0.278 | < 0.001 | − 0.222 | < 0.001 |
Model 1: adjustment for age, gender, diabetic duration, and drinking.
Model 2: further adjustment for waist circumference, body mass index, systolic blood pressure, diastolic blood pressure, hemoglobin A1c, triglycerides, high-density lipoprotein, homeostasis model assessment of insulin resistance, and uric acid based on Model 1.
Model 3: additional adjustment for alanine aminotransferase and aspartate aminotransferase, as well as the usage of hypoglycemic agents like metformin, thiazolidinediones, sodium-glucose cotransporter-2 inhibitors, and glucagon-like peptide-1 receptor agonists based on Model 2.
MAR Monocyte to apolipoprotein A1 ratio, NFS Nonalcoholic fatty liver disease fibrosis score.
Correlation of MAR with MASLD risk
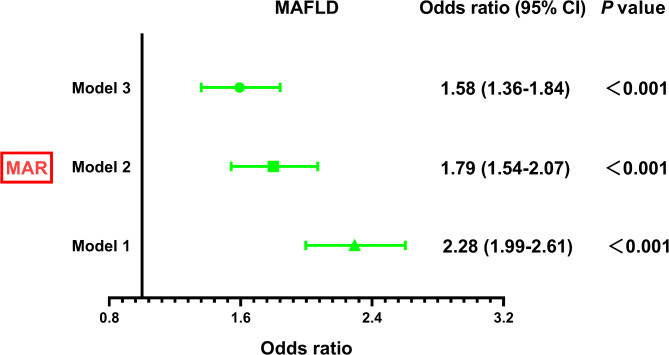
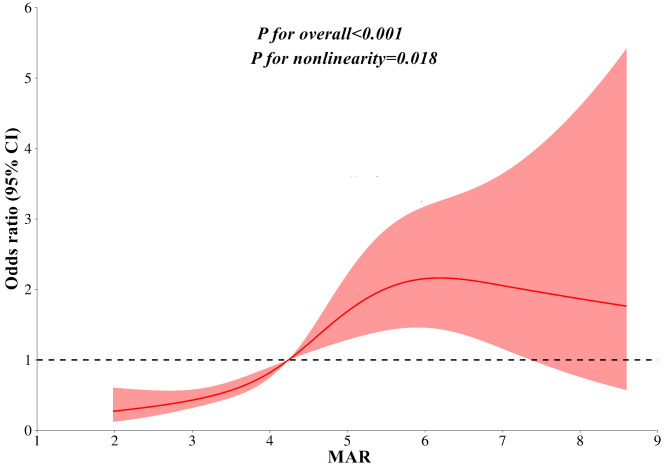
Figure 4 illustrates the correlation between the MAR and the presence of MASLD determined through binomial logistic regression analysis. The findings demonstrate a significant association between higher MAR levels and an increased risk of MASLD in both Model 1 and Model 2. Importantly, even after full adjustments in Model 3, this association remains consistent (OR 1.58, 95% CI 1.36–1.84; P < 0.001). Figure 5 illustrates the relationship between MAR and MASLD analyzed using RCS. The results indicate a nonlinear association between MAR and MASLD risk after accounting for full adjustments in Model 3 (P for nonlinearity = 0.018).
Fig. 4.
The independent correlation of MAR with MASLD in different Models. Model 1: adjustment for age, gender, diabetic duration, and drinking. Model 2: further adjustment for waist circumference, body mass index, systolic blood pressure, diastolic blood pressure, hemoglobin A1c, triglycerides, high-density lipoprotein, homeostasis model assessment of insulin resistance, and uric acid based on Model 1. Model 3: additional adjustment for alanine aminotransferase and aspartate aminotransferase, as well as the usage of hypoglycemic agents like metformin, thiazolidinediones, sodium-glucose cotransporter-2 inhibitors, and glucagon-like peptide-1 receptor agonists based on Model 2. MASLD Metabolic dysfunction-associated fatty liver disease, MAR Monocytes to Apolipoprotein A1 ratio.
Fig. 5.
Restricted cubic spines analysis of the association between MAR and MASLD after accounting for full adjustments in Model 3. MASLD Metabolic dysfunction-associated fatty liver disease, MAR Monocytes to Apolipoprotein A1 ratio.
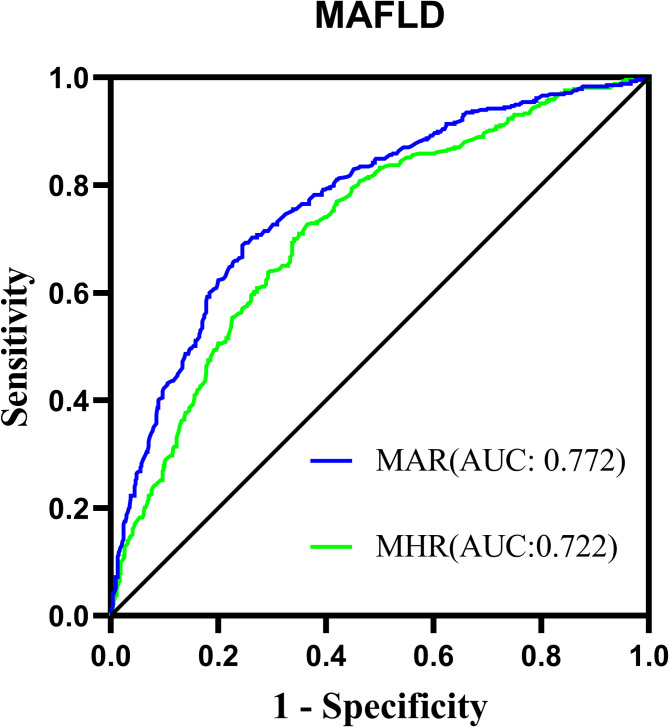
Comparison of MASLD identifying value between MAR and MHR
Figure 6 illustrates the performance comparison between MAR and MHR in identifying MASLD, as determined through ROC curve analysis. The results demonstrated that MAR (AUC:0.772, 95CI 0.742–0.802) exhibits superior performance in identifying MASLD compared to MHR (AUC:0.772 vs 0.722, P < 0.001). The optimal cut-off value of MAR was 4.05 × 108/g (sensitivity: 75.6%, specificity: 68.8%).
Fig. 6.
ROC analysis for comparing MASLD identifying value between MAR and MHR. MASLD Metabolic dysfunction-associated fatty liver disease, MAR Monocytes to Apolipoprotein A1 ratio, MHR Monocytes to high-density lipoprotein, BMI Body mass index, WC Waist circumference, SBP Systolic blood pressure, DBP Diastolic blood pressure, HbA1c Glycated hemoglobin, TG Triglyceride, TC Total cholesterol, HDL-c High-density lipoprotein cholesterol, LDL-c Low-density lipoprotein cholesterol, APOA1 Apolipoprotein A1, UA Uric acid, ALT Alanine aminotransferase, AST Aspartate aminotransferase. HOMR-IR Homeostasis model assessment insulin resistance, MAR Monocyte to APOA1 ratio, MHR Monocyte to HDL-c ratio, MASLD Metabolic dysfunction-associated fatty liver disease.
Sensitivity analysis
Sensitivity analyses were conducted in newly diagnosed T2DM (n = 342) to minimize the impact of hypoglycemic agents on the association between MAR and MASLD. Following full adjustments in Model 3, MAR persisted in significant correlations with CTL−S (β = − 0.269, P < 0.001), NFS (β = 0.088, P = 0.004), MASLD (OR 2.10, 95% CI 1.54–2.87; P < 0.001). Furthermore, MAR demonstrated superior performance in identifying MASLD compared to MHR (AUC:0.817 vs 0.758, P < 0.001).
Discussion
Metabolic dysfunctions, exacerbated by chronic inflammation, constitute a pivotal aspect in the pathogenesis of MASLD. Emerging evidence substantiates the proinflammatory nature of monocytes while underscoring the anti-inflammatory attributes of APOA1 in this context. This study elucidated that MAR emerged as an independent variable for CTL−S, NFS, and MASLD, even after adjustments for metabolic profiles and hepatic functional markers. A notable correlation was also unveiled between elevated MAR levels and an increased risk of MASLD. Noteworthy, MAR displayed superior efficacy in detecting MASLD compared to MHR, suggesting its potential as an innovative inflammatory biomarker for MASLD.
Inflammation and hepatocyte injury serve as the prominent features of MASLD. The activation of innate immune cells is a crucial factor contributing to liver inflammation in cases of MASLD19,20. Monocytes have been recognized as markers of inflammatory status since they facilitate the expression of inflammation. The activation and differentiation of monocytes into monocyte-derived macrophages are key drivers in the pathogenesis of chronic low-grade inflammation diseases21,22. Recent studies utilizing innovative experimental techniques have shed light on the significant involvement of innate monocyte subsets in aggravating liver inflammation and contributing to the progression of MASLD through the recruitment and accumulation of inflammatory cells, including monocyte-derived macrophages, within the hepatic tissue23,24. Consequently, an elevation in circulating monocytes is observed in individuals with MASLD. Perturbations in metabolic homeostasis serve as the main underlying factors for the progression and heightened risk of MASLD. Individuals with MASLD commonly exhibit suboptimal control over their metabolic profiles. Dysregulated lipid metabolism represents a major manifestation of the metabolic dysfunctions associated with MASLD. The activation of innate immunity further contributes to alterations in hepatic lipid metabolism, particularly characterized by elevated TG and decreased levels of APOA1 and HDL-c, which are essential for arterial wall protection and the prevention of atherosclerotic plaque formation25,26. Accordingly, an increase in plasma TG levels accompanies a decrease in APOA1 and HDL-c levels in the MASLD group.
CTL−S has proven to be a valuable tool for categorizing liver fat content and assessing the extent of intrahepatic inflammation27. Intriguingly, this study identifies a noteworthy inverse and independent association between MAR and CTL−S, even after meticulous adjustments for metabolic profiles and liver functional indexes. These findings suggest that MAR could serve as a reliable indicator of both liver fat content and intrahepatic inflammation severity. Liver fibrosis stands as the most robust predictor of long-term clinical outcomes in individuals with MASLD. Current MASLD-related guidelines widely endorse using non-invasive indices, such as the NFS, for risk stratification in patients with liver-related morbidity and mortality, as they demonstrate comparable effectiveness to liver biopsy28. Mounting evidence highlights monocyte-derived macrophages in the progression of liver inflammation and fibrosis. Inhibition of CCR2 + monocyte recruitment through pharmacological means has been shown to efficiently accelerate this progression29,30, further supporting the critical role of monocytes in liver fibrosis. Interestingly, although MAR exhibits a positive correlation with NFS in linear regression analysis, its significance persists even after additional adjustments for metabolic profiles and liver functional indexes. This suggests that an elevated MAR may be associated with an increased risk of progressive liver fibrosis. Currently, it is widely recognized that MASLD arises from a state of chronic inflammation induced by systemic metabolic dysfunction31. In accordance with our previous study32, this study also revealed a consistent pattern whereby individuals with higher MAR exhibited a greater number of metabolic dysfunctions. Among these metabolic dysfunctions, notable contributors to liver inflammation comprise heightened hepatic triglyceride TG accumulation, IR, and adipose tissue expansion33. This study observed that higher MAR quartile groups exhibited elevated TG, HOMA-IR, and WC. The existing data concerning the connection between various monocyte-related inflammatory markers and MASLD demonstrate consistency. Previous studies have independently identified a correlation between MASLD and monocyte-related inflammatory markers such as MHR and lymphocyte-to-monocyte ratio34,35. Corresponding to these monocyte-related inflammatory markers, this study also revealed a significant and sustained correlation between MAR and MASLD, even after accounting for potential confounding factors through binomial logistic regression analysis. Furthermore, results from the RCS analysis demonstrated a nonlinear association between MAR and MASLD risk. This relationship persists even after accounting for potential confounding factors, indicating that the risk of developing MASLD escalates with rising MAR levels. These findings establish MAR as an independent variable for MASLD and suggest its potential utility as an inflammatory marker of MASLD.
MHR has been recognized as an inflammatory predictor for several chronic inflammatory diseases, such as metabolic syndrome36, MASLD14, and cardiovascular disease37. This study conducted a comparison between MAR and MHR in identifying MASLD using Receiver ROC curve analysis. Notably, the results revealed that MAR exhibited superior performance in identifying MASLD compared to MHR. Regarding the underlying mechanisms, the biological features of APOA1 offer a plausible explanation for these findings. Apart from its well-established role in cardioprotection, APOA1 plays essential roles in HDL-c biogenesis and function. Importantly, the beneficial anti-inflammatory properties of APOA1 extend beyond its cardioprotective functions7. It is worth noting that HDL-c functionality depends not only on serum HDL-c levels but also on the apolipoprotein and lipid content functions. Recent studies have demonstrated that APOA1 can modulate cellular inflammation and oxidative stress38,39. For instance, Iqbal A. et al. revealed that apoA1 reduced the recruitment of adoptively transferred monocytes to sites of inflammation in vivo and mice8. Among different monocyte subsets, classical monocytes are believed to possess a greater potential for migration to injured or inflamed tissues compared to intermediate and non-classical subpopulations. Moreover, Patel V et al. revealed that serum APOA1 levels but not HDL-c were inversely correlated with classical monocyte subset recruitment marker profiles40.
To our knowledge, this study is pioneering in examining the link between MAR and MASLD. However, it is crucial to acknowledge several limitations inherent in this investigation. Firstly, this study did not establish a direct causal relationship between MAR and MASLD. Secondly, the study participants were limited to individuals with T2DM, which inherently increases the incidence of MASLD and elevates serum monocyte levels. Therefore, the observed association between MAR and MASLD might not be generalizable to other populations. Thirdly, this study did not delve into further exploration of the underlying mechanisms driving this association.
In conclusion, this study demonstrates that MAR not only serves as an independent variable for CTL−S, NFS, and MASLD. Additionally, MAR exhibits superior performance in identifying MASLD compared to MHR. These findings underscore the paramount clinical significance of MAR as a promising inflammatory marker for MASLD. Consequently, incorporating MAR as an adjunctive screening tool can meaningfully enhance the management of MASLD in T2DM.
Author contributions
W. W. and L. H. took charge of the software and contributed to the original draft. M. T. and X. P. Q. conducted the investigation. X. L. Guo and W. W. contributed to data curation and writing editing.
Funding
This study was sponsored by the Longyan City Science and Technology Plan Project (Grant numbers: 2023LYF17056 and 2022LYF17093). The funder had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors report no conflicts of financial and non-financial interests in this work.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in Figures 1, 2, 3, 4, 5, and 6 where the initial statistical pictures were uploaded instead of the modified Figures.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Wang and Lian Huang contributed equally to this work.
Change history
3/7/2025
A Correction to this paper has been published: 10.1038/s41598-025-90119-8
References
- 1.Shi, C. & Pamer, E. G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol.11(11), 762–774 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratchev, A., Sobenin, I., Orekhov, A. & Kzhyshkowska, J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology.217(5), 476–82 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Ferreiro-Iglesias, R., Barreiro-de Acosta, M., López-Díaz, J., Bastón Rey, I. & Domínguez-Muñoz, J. E. Usefulness of peripheral blood monocyte count to predict relapse in patients with inflammatory bowel disease: A prospective longitudinal cohort study. Rev. Esp. Enferm. Dig.114(1), 10–5 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Jack, et al. Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabet. Metabol. Res. Rev.23(2), 111–118 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Oeztuerk, et al. A nonclassical monocyte phenotype in peripheral blood is associated with nonalcoholic fatty liver disease: a report from an EMIL subcohort. Hormone Metabol. Res.48, 54–61 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Jackson, A., Rahman, G. & Long, S. Apolipoprotein-AI and AIBP synergetic anti-inflammation as vascular diseases therapy: The new perspective. Mol. Cell. Biochem.476, 3065–3078. 10.1007/s11010-020-04037-6 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Montecucco, F. et al. Impact of systemic inflammation and autoimmune diseases on apoA-I and HDL plasma levels and functions. Handb. Exp. Pharmacol.224, 455–482. 10.1007/978-3-319-09665-0_14 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Iqbal, A. J. et al. Acute exposure to apolipoprotein A1 inhibits macrophage chemotaxis in vitro and monocyte recruitment in vivo. eLife.30(5), e15190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel, V., Williams, H., Li, S., Fletcher, J. & Medbury, H. Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis263, 15–23. 10.1016/j.atherosclerosis.2017.05.026 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Chew, N. et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metabol.35, 414-428.e413. 10.1016/j.cmet.2023.02.003 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Younossi, Z. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol.71, 793–801. 10.1016/j.jhep.2019.06.021 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Targher, G., Corey, K., Byrne, C. & Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol.18, 599–612. 10.1038/s41575-021-00448-y (2021). [DOI] [PubMed] [Google Scholar]
- 13.Kim, H. et al. Elevated peripheral blood monocyte fraction in nonalcoholic fatty liver disease. Tohoku J. Exp. Med.223, 227–233. 10.1620/tjem.223.227 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Jia, J. et al. Monocyte to high-density lipoprotein cholesterol ratio at the nexus of Type 2 diabetes mellitus patients with metabolic-associated fatty liver disease. Front. Physiol.12, 762242. 10.3389/fphys.2021.762242 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia, W. et al. Standards of medical care for type 2 diabetes in China (2019). Diabetes Metabol. Res. Rev.35, e3158. 10.1002/dmrr.3158 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Bonora, E. et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care23, 57–63. 10.2337/diacare.23.1.57 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol.79, 1542–1556. 10.1016/j.jhep.2023.06.003 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Bril, F. et al. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with Type 2 diabetes. Diabetes Care43, 290–297. 10.2337/dc19-1071 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Arrese, M., Cabrera, D., Kalergis, A. & Feldstein, A. Innate immunity and inflammation in NAFLD/NASH. Digestive Dis.61, 1294–1303. 10.1007/s10620-016-4049-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huby, T. & Gautier, E. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol.22, 429–443. 10.1038/s41577-021-00639-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapellos, T. S. et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front. Immunol.10, 2035 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ożańska, A., Szymczak, D. & Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol.92, e12883. 10.1111/sji.12883 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Nati, M., Chung, K. J. & Chavakis, T. The role of innate immune cells in nonalcoholic fatty liver disease. J. Innate Immun.14(1), 31–41 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreby, E., Chen, P. & Aouadi, M. Macrophage functional diversity in NAFLD—more than inflammation. Nat. Rev. Endocrinol.18(8), 461–472 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Deprince, A., Haas, J. & Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metabol. Cell.42, 101092. 10.1016/j.molmet.2020.101092 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Z. et al. Impact of NAFLD and its pharmacotherapy on lipid profile and CVD. Atherosclerosis355, 30–44. 10.1016/j.atherosclerosis.2022.07.010 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Kim, H. et al. CT-based Hounsfield unit values reflect the degree of steatohepatitis in patients with low-grade fatty liver disease. BMC Gastroenterology23, 77. 10.1186/s12876-023-02717-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, J. et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int.41, 261–270. 10.1111/liv.14669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, X. et al. STING expression in monocyte-derived macrophages is associated with the progression of liver inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Lab. Invest.100, 542–552. 10.1038/s41374-019-0342-6 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Krenkel, O. et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology67, 1270–1283. 10.1002/hep.29544 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Ampuero, J. et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment. Pharmacol. Ther.48, 1260–1270. 10.1111/apt.15015 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Wang, W., Chen, Z., Guo, X. & Tu, M. Monocyte to high-density lipoprotein and apolipoprotein A1 ratios: Novel indicators for metabolic syndrome in chinese newly diagnosed type 2 diabetes. Front. Endocrinol.13, 935776. 10.3389/fendo.2022.935776 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung, U. & Choi, M. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci.15, 6184–6223. 10.3390/ijms15046184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang, H. et al. Association between monocyte to high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease: A cross-sectional study. Mediators Inflamm.2021, 6642246. 10.1155/2021/6642246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohsari, M. et al. New inflammatory biomarkers (lymphocyte and monocyte percentage to high-density lipoprotein cholesterol ratio and lymphocyte to monocyte percentage ratio) and their association with some cardiometabolic diseases : Results from a large Kurdish cohort study in Iran. Wiener Klinische Wochenschrift134, 626–635. 10.1007/s00508-022-02029-8 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Wang, P. et al. Monocyte-to-high-density lipoprotein ratio and systemic inflammation response index are associated with the risk of metabolic disorders and cardiovascular diseases in general rural population. Front. Endocrinol.13, 944991. 10.3389/fendo.2022.944991 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, N. et al. Relationship between monocyte to HDL cholesterol ratio and concomitant cardiovascular disease in Chinese Han patients with obstructive sleep apnea. Cardiovas. Diagn. Ther.9, 362–370. 10.21037/cdt.2019.08.02 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorci-Thomas, M. & Thomas, M. Why targeting HDL should work as a therapeutic tool, but has not. J. Cardiovas. Pharmacol.62, 239–246. 10.1097/FJC.0b013e31829d48a5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nazir, S. et al. Interaction between high-density lipoproteins and inflammation: Function matters more than concentration!. Adv. Drug Deliv. Rev.159, 94–119. 10.1016/j.addr.2020.10.006 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Patel, V., Williams, H., Li, S., Fletcher, J. & Medbury, H. Monocyte subset recruitment marker profile is inversely associated with blood ApoA1 levels. Front. Immunol.12, 616305. 10.3389/fimmu.2021.616305 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.