Abstract
Long non-coding RNAs (lncRNAs) have emerged as crucial regulators in cancer progression. We found lncRNA DNM1P35 is elevated in ovarian tumors compared to normal tissues, and demonstrated that lncRNA DNM1P35 promoted cancer cell proliferation, migration and invasion in SK-OV-3 and OVCAR-3 cell lines. Furthermore, lncRNA DNM1P35 also facilitated the epithelial-mesenchymal transition (EMT) of ovarian cancer cells. Mechanistic studies identified microRNA-326 (miR-326) as a target of lncRNA DNM1P35. Overexpression of miR-326 diminished the tumor-promoting activity of lncRNA DNM1P35, resulting in reduction of Zinc finger E-box-binding homeobox 1 (ZEB1) expression and EMT features. We further revealed that ZEB1, a master transcription factor for EMT that is negatively regulated by miR-326, was essential for lncRNA DNM1P35-mediated cancer cell progression and EMT. Loss of ZEB1 led to compromised pro-tumoral activity of lncRNA DNM1P35. In vivo studies using a xenograft mouse model of ovarian cancer revealed that tumors with higher levels of lncRNA DNM1P35 led to shorter survival, increased tumor burden, as well as elevated expression of proliferative marker Ki67 and EMT marker ZEB1. Our comprehensive study underscored the significance of lncRNA DNM1P35 in ovarian cancer progression, elucidating the underlying mechanism through miR-326/ZEB1 axis to promote ovarian cancer progression.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83170-4.
Keywords: Ovarian cancer, lncRNA DNM1P35, miR-326, Epithelial-mesenchymal transition, Gene regulation
Subject terms: Gynaecological cancer, Molecular biology
Introduction
Ovarian cancer (OC) is one of the most lethal gynecologic malignancies worldwide1. Ovarian cancer exhibits varying trends in terms of the incidence, mortality, and survival rates. Studies have shown a decline in the overall incidence and mortality rates of ovarian cancer, primarily driven by the epithelial histology2,3. The age-adjusted incidence of epithelial ovarian carcinoma differs among races, with white patients having the highest rates4. Survival rates for ovarian cancer patients in Asia after 1, 3, and 5 years were reported as 73.65%, 61.31%, and 59.60% respectively5. Notably, the incidence-based mortality of specific ovarian epithelial cancer deaths has shown a decreasing trend from 2005 to 20186. Standard treatments involve cytoreductive surgery and platinum-based chemotherapy, with relapse being common in advanced cases7. Recent advancements included maintenance therapies like Poly (ADP-ribose) polymerase (PARP) inhibitors for homologous recombination repair-deficient tumors, improving progression-free survival8. Research highlighted breakthroughs in PARP inhibitors’ impact on overall survival. Given these challenges and advancements, there is an urgent need for a deeper understanding of the molecular mechanisms underlying ovarian cancer and the development of innovative diagnostic and therapeutic approaches.
Long noncoding RNAs (lncRNAs) have emerged as pivotal regulators of the human genome’s epigenetic status9. Transcending the traditional understanding of the genome, these non-protein coding transcripts, longer than 200 nucleotides, have been found to play crucial roles in various cellular processes10. Their interactions with other cellular macromolecules, including DNA, RNA, and proteins, drive many essential cancer phenotypes11. The dysregulation of lncRNAs has been associated with tumorigenesis, metastasis, and therapy resistance. For instance, the lncRNA small nucleolar RNA host gene 6 (SNHG6) has been reported to have an oncogenic role in multiple cancers, including colorectal cancer, by activating the Transforming growth factor beta (TGF-β)/Smad signaling pathway and inducing epithelial-mesenchymal transition via the regulation of ZEB110. Another study on muscle-invasive bladder cancer identified a set of dysregulated lncRNAs that functioned as prognostic biomarkers and potential therapeutic targets12. LncRNA DNM1P35 has recently garnered attention for its potential role in ovarian cancer. While the exact mechanisms of DNM1P35 in tumorigenesis remain to be fully elucidated, preliminary findings suggest its upregulation in ovarian cancer tissues, hinting at its oncogenic role13. The known target miRNAs of lncDNM1P35 are miR-93-5p and miR-17-5p14. These miRNAs are involved in the competing endogenous RNA (ceRNA) network associated with colon cancer progression, where lncDNM1P35 acts as a sponge to regulate their expression and impact the downstream target NHL repeat-containing protein 3 (NHLRC3)15. Additionally, lncDNM1P35 is shown to inhibit the progression of colon cancer through the miR-93-5p/17-5p/NHLRC3 axis16.
The current study aims to delve deeper into the role of the lncRNA DNM1P35 in ovarian cancer. With the backdrop of preliminary findings suggesting its upregulation in OC tissues, the study seeks to elucidate its molecular mechanisms, functional implications, and therapeutic potential. Given the intricate interactions of lncRNAs with other cellular components, including miRNAs and proteins, understanding the role of DNM1P35 could provide valuable insights into the gene-regulation networks in OC.
Materials and methods
Cell culture
Human ovarian cancer cell lines OVCAR-3 (#SCC257, Sigma-Aldrich) and SK-OV-3 (#HTB77, The American Type Culture Collection) were cultured in Dulbecco’s Modified Eagle Medium (DMEM, GIBCO, USA) supplemented with 10% fetal bovine serum (FBS) and antibiotics and maintained in an incubator at 37 °C with a 5% CO2 atmosphere.
Transfection procedure
The cell lines were subjected to transfection using the Lipofectamine 3000 reagent (Cat. #L3000001, Invitrogen). The DNM1P35 gene (NCBI Reference Sequence: NR_024595.3) was cloned into a pLVX-IRES-Puro vector (SANGON Biotechnology). shRNA sequences targeting DNM1P35 or ZEB1 were inserted in pLKO.1 vector (Cat. #8453, Addgene) and validated for knockdown efficiency via Western blot and qPCR. Additionally, synthetic mimics and inhibitors of miR-326 were acquired from Genepharma (Shanghai, China). Sequences targeting ZEB1 and DNM1P35 were listed below:
Scramble: 5’-GCTAAGCGATTGAGAATAGGT-3’.
ZEB1-1: 5’-GATGATGAATGCGAGTCAGAT-3’.
ZEB1-2: 5’-GGTGAATGATAGCACTTGTCT-3’.
DNM1P35-1: 5’-GTTCCCTGACCTGGTAACACT-3’.
DNM1P35-2: 5’-GTCCAGGAGTAGAGGTGAATT-3’.
Quantitative real-time PCR protocol
Total RNA was extracted using TRIzol reagent, followed by the assessment of RNA purity and concentration via a NanoDrop spectrophotometer. One microgram of total RNA was then subjected to reverse transcription (Cat. # 4368814, Applied Biosystems) Subsequent real-time PCR quantification was carried out utilizing an SsoAdvanced Universal SYBR Green Supermix (Cat. #1725270, BIO-RAD). The PCR amplification conditions were set as follows: an initial denaturation at 95 °C for 30 s in one cycle; followed by 50 cycles of denaturation at 95 °C for 5 s and annealing/extension at 60 °C for 34 s; a single cycle of denaturation at 95 °C for 5 s, followed by a temperature ramp from 65 °C for 60 s to 97 °C for 1 s; and a final hold at 42 °C for 30 s. Relative quantification of gene expression was conducted using the 2−ΔΔCT method, with GAPDH serving as the reference gene for normalization of mRNA levels. Primers used for quantification of genes of interest were listed below:
ZEB1-Forward: 5’-GATGATGAATGCGAGTCAGATGC-3’.
ZEB1-Reverse: 5’-ACAGCAGTGTCTTGTTGTTGT-3’.
DNM1P35-Forward: 5’-GAGGAGGAATGCAACGTGTG-3’.
DNM1P35-Reverse: 5’-TGAGTCCCGGAAGTGGTCTT-3’.
CDH1 (E-cadherin)-Forward: 5’-CGAGAGCTACACGTTCACGG-3’.
CDH1-Reverse: 5’-GGGTGTCGAGGGAAAAATAGG-3’.
CDH2 (N-cadherin)-Forward: 5’-TCAGGCGTCTGTAGAGGCTT-3’.
CDH2-Reverse: 5’-ATGCACATCCTTCGATAAGACTG-3’.
ACTA2 (α-SMA)-Forward: 5’-AAAAGACAGCTACGTGGGTGA-3’.
ACTA2-Reverse: 5’-GCCATGTTCTATCGGGTACTTC-3’.
SNAI1-Forward: 5’-TCGGAAGCCTAACTACAGCGA-3’.
SNAI1-Reverse: 5’-AGATGAGCATTGGCAGCGAG-3’.
VIM-Forward: 5’-GACGCCATCAACACCGAGTT-3’.
VIM-Reverse: 5’-CTTTGTCGTTGGTTAGCTGGT-3’.
GAPDH-Forward: 5’-ACAACTTTGGTATCGTGGAAGG-3’.
GAPDH-Reverse: 5’-GCCATCACGCCACAGTTTC-3’.
Cell invasive assay
A 1:1 blend of DMEM and Matrigel, totaling 50 µL, was smoothly coated onto the surface of Transwell inserts (Cat. # CLS3422, Sigma-Aldrich) and then incubated at 37 °C for a duration of 45 min to solidify. Each Transwell upper compartment was seeded with 20,000 cells in serum-free medium, while the lower compartment was filled with 700 µL of medium supplemented with 5% serum to act as a chemoattractant. Following a 12-hour incubation period, non-invading cells were carefully wiped from the upper face of the inserts. Invading cells that reached the bottom surface of the membrane were stained using 0.1% crystal violet (Cat. # C0775, Sigma-Aldrich). Subsequent to staining, cells were visualized and quantified under a microscope.
Cell viability assay
Cancer cells were seeded in 96-well plates at a density of 400 cells per well. To evaluate cellular viability, a CCK-8 assay (Cat. # HY-K0301, MedChemExpress) was conducted at the 24, 48, 72, and 96-hour marks post-seeding. For the assay, 10 µl of the CCK-8 reagent were dispensed into each well. The plates were then placed in a 37 °C incubator for a 2-h incubation period. Post incubation, the absorbance was measured at a wavelength of 450 nm using a microplate reader.
Colony formation evaluation
A colony formation assay was conducted by plating OC cancer cells in 6-well plates at a density of 400 cells per well. The culture medium was refreshed tri-weekly post-seeding. Following a 14-day cultivation period, cells were fixed with 4% paraformaldehyde (Cat. #P0099, Beyotime) and were stained for 5 min using a 0.1% crystal violet staining solution. Subsequently, cells were rinsed twice with PBS to eliminate non-specific staining. Photographic documentation was performed.
Dual-luciferase reporter assay
Cancer cells were seeded in 96-well plates at a seeding density of 1000 cells per well. Twenty-four hours post-seeding, cells were transfected using Lipofectamine 3000 (Cat. #L3000008, Invitrogen) with different combinations of luciferase reporter genes and miRNAs. After a 48-hour incubation at 37 °C, the activity of both firefly and Renilla luciferases was measured utilizing a dual-luciferase reporter assay system (Cat. #E1910, Promega). The activity of firefly luciferase was normalized to that of Renilla.
Western blot
Protein lysates were prepared using RIPA buffer (Cat. #R0278, Sigma-Aldrich,) containing protease (Cat. #04693132001, Roche) and phosphatase inhibitors (Cat. # 4906845001, Roche). Protein concentration was determined by BCA Protein Assay Kit (Cat. #23225, Pierce). Equal amounts of protein (30 µg) were separated on 10% SDS-PAGE gels and transferred to PVDF membranes (Cat. #IPVH00010, Millipore). Membranes were blocked with 5% non-fat dry milk in TBS-T for 1 h at room temperature and incubated overnight at 4 °C with primary antibodies, followed by HRP-conjugated secondary antibodies (Cat. #170–6515 and #170–6516, Bio-Rad, 1:5000). Bands were visualized using Clarity Western ECL Substrate (Cat. # 170–5061, Bio-Rad). Primary antibodies used in this study including β-actin (Cat. #4970, Cell Signaling Technology, 1:10000), E-Cadherin (Cat. #ab40772, Abcam, 1:1000), N-Cadherin (Cat. #ab18203, Abcam, 1:1000), ZEB1 (Cat. #3396, Cell Signaling Technology, 1:1000), α-SMA (Cat. #NB600-536, Novus Biologicals, 1:1000), snail (Cat. #3879, Cell Signaling Technology, 1:1000), Vimentin (Cat. #NB300-223, Novus Biologicals, 1:1000).
Wound healing assay
SK-OV-3 or OVCAR-3 cells were seeded in 12-well plates at a density of 3.0 × 105 cells per well and grown to confluence. A sterile 200 µl pipette tip was used to scratch across the cell monolayer. Cells were then washed three times with PBS to remove detached cells and photographed at 24 h post-scratch using an Olympus IX71 microscope.
Tumor xenograft experimentation
All in vivo experiments were approved by the ethics committee of Wuxi People’s Hospital (KY23115) and conducted in accordance in accordance with ARRIVE guidelines. OC cancer cell was suspended in PBS at a density of 2*106 cells per ml and mixed with Matrigel (Cat. # 356234, Corning) and 1*106 cells were injected into 6-week-old female BALB/C nude mice subcutaneously. The diameter of the tumors (length and width) were measured every 3 days with a caliper and tumor volume was calculated by the formula: V = 1/2*Length*(Width)2. At the endpoint, mice were euthanized with CO2 (5 L per minute), followed by cervical dislocation.
Data analysis and statistics
Results were presented as mean ± standard deviation (SD). Statistical analysis was performed using Student’s t-test and One-way ANOVA test, with Tukey’s honestly significant difference test. Animal survival analysis was conducted using Kaplan-Meier survival analysis and Log-rank (Mantel-Cox) test. All in vitro experiments were independently conducted for at least three times with biological triplicates. A p-value less than 0.05 was defined as the threshold for statistical significance.
Results
DNM1P35 is overexpressed in ovarian cancer tissues
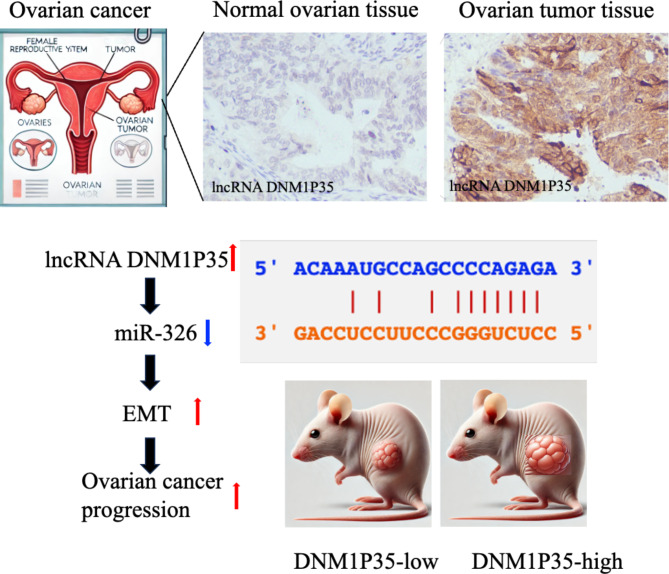
To assess the expression pattern of lncRNA DNM1P35, we analyzed 3 independent datasets (GTEX, TCGA, GSE40595, and GSE18520. The expression of lncRNA DNM1P35 is prominently elevated in ovarian cancer tissues in TCGA dataset compared with healthy controls from GTEX dataset (Fig. 1A). Similar results were observed RNA microarray profiling from GSE40595, which contains 6 normal ovarian epithelial tissues and 32 epithelial ovarian tumor samples, and from GSE18520, which contains 53 advanced stage, high-grade primary tumor specimens and 10 normal ovarian surface epithelium brushings (Fig. 1B and C). RNA in-situ hybridization (ISH) analysis demonstrated that lncRNA DNM1P35 is overexpressed in ovarian cancer tissues compared to normal tissues (Fig. 1D).
Fig. 1.
LncRNA DNM1P35 is elevated in ovarian cancer. (A–C) The expression level of DNM1P35 in the ovarian transcriptome dataset from GTEX and TCGA (A), GSE40595 (B), and GSE18520 (C). (D), RNA-ISH staining of lncRNA DNM1P35 in normal ovarian tissues and ovarian tumor tissues.
DNM1P35 promotes the growth, migration, and invasion of ovarian cancer cells
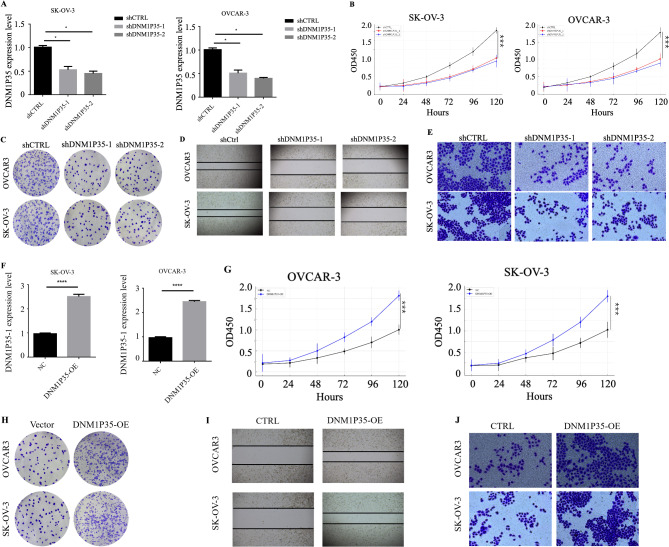
To investigate its role in ovarian cancer progression, lncRNA DNM1P35 was knocked down using two different shRNA sequences (shDNM1P35-1 and shDNM1P35-2) in ovarian cancer cell lines SK-OV-3 and OVCAR-3. The qPCR results showed a marked reduction in DNM1P35 expression in cells expressing shDNM1P35-1 or shDNM1P35-2 (Fig. 2A). SK-OV-3 and OVCAR-3 cells with DNM1P35 knockdown revealed significant attenuated growth rate (Fig. 2B). Crystal violet staining assay similarly revealed noticeable defects in growth (Fig. 2C). The migratory capability evaluated using a wound healing assay demonstrated impaired cell migration (Fig. 2D). Additionally, cell invasion potential of ovarian cancer cells with reduced DNM1P35 expression was disrupted (Fig. 2E).
Fig. 2.
DNM1P35 promotes ovarian cancer cell growth, migration, and invasion. (A–E) Control or DNM1P35 targeting shRNA were expressed in ovarian cancer cells. (A) The expression level of DNM1P35 in SK-OV-3 and OVCAR-3 cells. (B) The growth of ovarian cancer cells was assessed by CCK-8 assay. (C) SK-OV-3 and OVCAR-3 cell colonies were stained by crystal violet. (D) Migration of SK-OV-3 and OVCAR-3 cells was measured by wound healing assay. (E) Invasion of SK-OV-3 and OVCAR-3 cells was measured by trans-well assay. (F–J) DNM1P35 was overexpressed in SK-OV-3 and OVCAR-3 cells. (F) The expression level of DNM1P35 in SK-OV-3 and OVCAR-3 cells. (G) The growth of ovarian cancer cells was assessed by CCK-8 assay. (H) SK-OV-3 and OVCAR-3 cell colonies were stained by crystal violet. (I) Migration of SK-OV-3 and OVCAR-3 cell was measured by wound healing assay. (J) Invasion of SK-OV-3 and OVCAR-3 cell was measured by trans-well assay. *, p < 0.05, ***, p < 0.001. ****, p < 0.0001.
In a parallel set of experiments, the effects of DNM1P35 overexpression on ovarian cancer cells were investigated. QPCR results confirmed the heightened expression of DNM1P35 in SK-OV-3 and OVCAR-3 cells (Fig. 2F). Overexpression of DNM1P35 significantly promoted the growth of SK-OV-3 and OVCAR-3 cells (Fig. 2G and H). In addition, migratory tendencies of cells post DNM1P35 overexpression were also enhanced (Fig. 2I). The invasiveness of DNM1P35 overexpressed SK-OV-3 and OVCAR-3 cells compared with the vector controls were dramatically improved (Fig. 2J).
DNM1P35 promotes epithelial-mesenchymal transition (EMT) in ovarian cancer cells
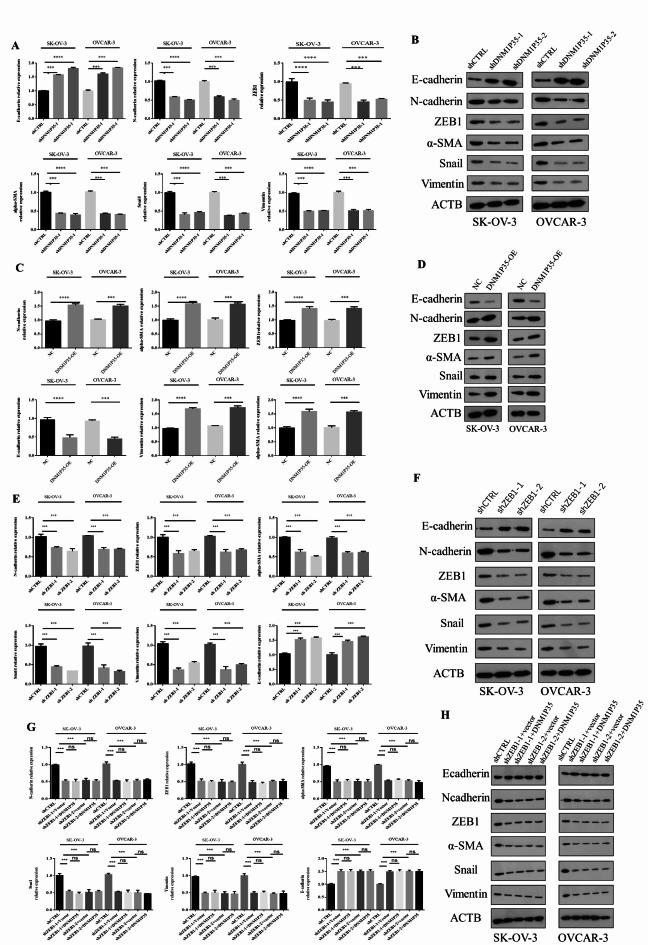
Since DNM1P35 plays an important role in promoting ovarian cancer cell invasion, we aim to investigate its role in the epithelial-mesenchymal transition (EMT) process in ovarian cancer cells. Initially, the expression of EMT-related markers post DNM1P35 knockdown was evaluated. Loss of DNM1P35 reduced the expression of N-cadherin, ZEB1, α-SMA, Snail1, and vimentin, with concomitant elevation of E-cadherin (Fig. 3A and B). Furthermore, to gauge the impact of DNM1P35 overexpression on EMT, an overexpression construct was transfected into both SK-OV-3 and OVCAR-3 cell lines. Significant upregulation of EMT markers and reduction of E-cadherin was observed in both SK-OV-3 and OVCAR-3 cell lines (Fig. 3C and D).
Fig. 3.
DNM1P35 promotes EMT of ovarian cancer cells. (A,B) The expression of EMT markers were assessed by qPCR (A) and Western blotting (B) in SK-OV-3 and OVCAR-3 cells expressing shCTRL and shDNM1P35. (C,D) The expression of EMT markers were assessed by qPCR (C) and Western blotting (D) in SK-OV-3 and OVCAR-3 cells expressing vector and DNM1P35. (E,F) The expression of EMT markers were assessed by qPCR (E) and Western blotting (F) in SK-OV-3 and OVCAR-3 cells expressing shCTRL and shZEB1. (G,H) The expression of EMT markers were assessed by qPCR (G) and Western blotting (H) in SK-OV-3 and OVCAR-3 cells expressing shCTRL and shZEB1 and overexpression of DNM1P35. ***, p < 0.001, ****, p < 0.0001. ns, not significant.
ZEB1 is a well-known regulator of EMT. Knockdown of shZEB1-1 and shZEB1-2 in both cell lines diminished EMT features (Fig. 3E and F). To interrogate the relationship between lncRNA DNM1P35 and ZEB1 in regulating EMT of SK-OV-3 and OVCAR-3 cells, we overexpressed DNM1P35 in both cell lines with shCTRL or shZEB1 to delve deeper into any potential synergistic or antagonistic effects. The analyses using both qPCR and WB methodologies highlighted the interplay between DNM1P35 and ZEB1 in modulating EMT markers in SK-OV-3 and OVCAR-3 cell lines. As loss of ZEB1 disrupted the EMT of both cell lines, further overexpression of lncRNA DNM1P35 failed to rescue these features, indicating that ZEB1 is essential for DNM1P35-mediated EMT in ovarian cancer cells (Fig. 3G and H).
DNM1P35 sponges miR-326 to promote ovarian cancer progression
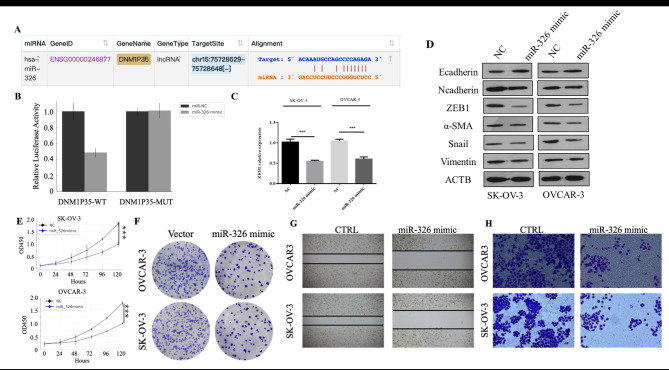
As we previously showed that lncRNA DNM1P35 can regulate the expression of ZEB1, we aim to elucidate the underlying molecular mechanism. One representative function of lncRNA is interacting with miRNAs to prevent them from targeting other RNA molecules including mRNA. Employing the starbase datasets, miR-326 was identified as a potential target of lncRNA DNM1P35 (Fig. 4A). To validate whether miR-326 could specifically target DNM1P35, luciferase reporters of wild-type DNM1P35 (DNM1P35-WT) and mutant DNM1P35 (DNM1P35-MUT) was constructed and co-transfected with miR-326 mimics into the HEK293 cell line to assess luciferase activity. MiR-326 mimics significantly suppressed the luciferase activity of DNM1P35-WT, but didn’t show a noticeable effect on DNM1P35-MUT reporter (Fig. 4B and Supplementary Fig. 1).
Fig. 4.
DNM1P35 targets miR-326 to promote ovarian cancer progression and EMT. (A) The potential target of DNM1P35 was predicted by the STAR (database. (B) Dual-luciferase activity assay in HEK293T cells expressing WT and MUT DNM1P35 luciferase reporter and treated with miR-NC or miR-326. (C) The expression of ZEB1 in SK-OV-3 and OVCAR-3 cells transfected with miR-NC and miR-326 was determined by qPCR. (D) The expression of EMT markers in SK-OV-3 and OVCAR-3 cells transfected with miR-NC and miR-326 was determined by Western blotting. (E) The growth of ovarian cancer cells was assessed by CCK-8 assay. (F) SK-OV-3 and OVCAR-3 cell colonies were stained by crystal violet. (G) Migration of SK-OV-3 and OVCAR-3 cells was measured by wound healing assay. (H) Invasion of SK-OV-3 and OVCAR-3 cells was measured by trans-well assay. ***, p < 0.001.
Prior studies have reported that miR-326 can target ZEB1 directly17. We also observed similar effects of miR-326 in ovarian cancer cells SK-OV-3 and OVCAR-3, transfection of miR-326 mimics resulted in a pronounced reduction of ZEB1 in both cells, along with reduction of N-cadherin, ZEB1, α-SMA, Snail1, and vimentin, and elevation of E-cadherin (Fig. 4C and D).
The overarching influence of miR-326 overexpression on ovarian cancer cells was further explored. Following the transfection of miR-326 mimics into SK-OV-3 and OVCAR-3 cells, several metrics were assessed. Cell proliferation, evaluated by MTT and crystal violet staining assay, was inhibited by miR-326 mimics (Fig. 4E and F). Cell migration and invasion potential were also repressed in the presence of miR-326 mimics (Fig. 4G and H).
Given the function of DNM1P35 and miR-326 in regulating ovarian cancer proliferation and survival, we further investigated if targeting this axis impacted the response of ovarian cancer cells to chemotherapies. Therefore, we knocked down DNM1P35 or transfected miR-326 mimics in SK-OV-3 and OVCAR-3 cells, followed by treatment with cisplatin (10 µM) or paclitaxel (10 µM) for 72 h. Cell viability of ovarian cancer cells with lower levels of DNM1P35 or higher levels of miR-326 were more sensitive to chemo drugs (Supplementary Fig. 2B), insinuating the potential of targeting DNM1P35/miR-326 axis in combination with chemotherapies in enhancing anti-cancer efficacy.
DNM1P35 promotes the progression of ovarian cancer in vivo
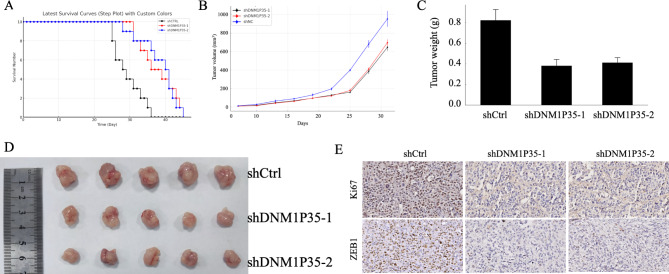
To delve deeper into the in vivo effects of DNM1P35, SK-OV-3 cells expressing shCTRL, shDNM1P35-1 or shDNM1P35-2 were subcutaneously injected into nude mice. The survival time of these animals was closely monitored. Expression of shDNM1P35-1 and shDNM1P35 significantly delayed the growth of SK-OV-3 tumors and prolonged the survival of tumor-bearing mice (Fig. 5A and B).
Fig. 5.
lncRNA DNM1P35 promotes ovarian cancer progression in vivo. (A) Survival curve of nude mice with SK-OV-3 cells expressing shCTRL, shDNM1P35-1, and shDNM1P35-2. N = 10. (B) Tumor volume in nude mice with SK-OV-3 cells expressing shCTRL, shDNM1P35-1, and shDNM1P35-2. N = 5. (C,D) Tumor weight (C) and pictures (D) of SK-OV-3 tumors. N = 5 (E) Ki67 and ZEB1 expression in SK-OV-3 tumors were determined by IHC. **, p < 0.01.
In a parallel series of experiments, after 21 days post tumor implantation, the mice were euthanized. The size and weight of tumors expressing shDNM1P35-1 or shDNM1P35-2 were smaller than the tumors expressing shCTRL (Fig. 5C and D). Immunohistochemistry staining of Ki67 and ZEB1 revealed reduced proliferation and EMT features of tumor DNM1P35 knockdown (Fig. 5E).
Discussion
Ovarian cancer is one of the most lethal gynecological malignancies worldwide. Despite advances in surgical techniques and chemotherapy regimens, the overall survival rate for ovarian cancer patients remains relatively low, primarily due to late-stage diagnosis and recurrence18. LncRNAs have emerged as pivotal regulators oncogenesis19. Dysregulation of lncRNAs has been implicated in numerous cancers, including ovarian cancer20. This study delves into the role of lncRNA DNM1P35 in ovarian cancer progression. Our findings underscore the upregulation of DNM1P35 in ovarian cancer tissues and its subsequent impact on cell proliferation, migration, and invasion. Furthermore, the molecular interactions involving DNM1P35, particularly its sponging effect on hsa-mir-326, provide insights into its mechanism of action and potential therapeutic implications.
In the realm of ovarian cancer research, lncRNAs have been identified as critical regulators of tumorigenesis and metastasis. For instance, the lncRNA–miRNA–mRNA interactome, particularly involving the triplet OIP5-AS1–miR-203a–c-MET, has been highlighted as a marker for the metastatic process in ovarian cancer21. Moreover, the lncRNA HCG11 has been implicated in the repression of ovarian cancer cell growth through the AKT signaling pathway22. Similarly, the lncRNA CRNDE has been found to promote cell proliferation, migration, and invasion via the miR-423-5p/FSCN1 axis23. Another significant finding is the role of lncRNA KCNQ1OT1 in promoting metastasis by altering the methylation status of the EIF2B5 promoter24. While our study sheds light on the role of DNM1P35 in ovarian cancer, other lncRNAs like HOTAIR and MALAT1 have also been implicated in ovarian cancer progression25.
The exact mechanisms through which DNM1P35 exerts its effects remain to be fully elucidated.
One of the intriguing mechanisms through which lncRNAs exert their functions is by acting as molecular sponges for miRNAs26. Bioinformatics tools predicted a potential interaction between DNM1P35 and miR-326. Subsequent experimental validation using luciferase reporters confirmed this interaction. Such lncRNA-miRNA interactions have been previously reported and are known to play crucial roles in various cellular processes, including cell proliferation, migration, and apoptosis27.
While this study delves into the interaction between DNM1P35 and miR-326, it is worth noting that miR-326 has been implicated in other cancers as well. For instance, miR-326 has been reported to act as a tumor suppressor in glioma by targeting the Notch signaling pathway28.
Epithelial-Mesenchymal Transition (EMT) is a fundamental biological process where epithelial cells undergo a phenotypic switch to become mesenchymal stem cells. In the context of cancer, EMT is associated with increased tumor aggressiveness, metastasis, and resistance to therapies29. The process is characterized by the loss of epithelial markers (like E-cadherin) and the gain of mesenchymal markers (like N-cadherin and vimentin). Our study findings suggest that DNM1P35 plays a pivotal role in regulating EMT in ovarian cancer cells. The knockdown of DNM1P35 led to alterations in EMT-related markers. Such regulatory roles of lncRNAs in EMT have been previously documented, where they modulate the expression of key EMT transcription factors or interact with other cellular signaling pathways30. The target of DNM1P35, miR-326 has been implicated in the regulation of EMT in endometrial cancer, lung adenocarcinoma and pulmonary fibrosis31,32. In the current study we found ZEB1, a well-known EMT transcription factor, was a target of miR-326. Its expression was suppressed by miR-326 but was upregulated by DNM1P35. Our study highlights an intriguing interplay between DNM1P35 and ZEB1, suggesting a potential regulatory network that drives EMT in ovarian cancer cells. Previous studies have also emphasized the importance of lncRNA-transcription factor interactions in regulating EMT33. While our study focuses on DNM1P35, other lncRNAs like HOTAIR, MALAT1, and ZEB1-AS1 have been implicated in the EMT process across various cancers. These lncRNAs interact with EMT transcription factors, miRNAs, or other signaling molecules, underscoring the complexity of EMT regulation34. The intricate interactions between DNM1P35, miR-326, and ZEB1 offer a glimpse into the complex molecular networks driving ovarian cancer. Such lncRNA-miRNA-transcription factor interactions have been previously highlighted in various cancers, emphasizing the multifaceted roles of lncRNAs in cancer biology35.
While our current work provides substantial insights into the EMT-related functions of DNM1P35, we recognize the complexity of cancer progression involves multiple interrelated pathways beyond EMT. Such pathways, including cell cycle regulation, apoptosis, and angiogenesis, also play essential roles in tumor biology and could interact with EMT processes or operate independently to affect cancer outcomes. To build on the findings of this study, our future research will explore additional roles of DNM1P35 outside of EMT. We aim to investigate how DNM1P35 may influence other critical pathways in ovarian cancer, such as cell cycle progression, apoptotic signaling, and angiogenic activity. These studies will provide a more comprehensive understanding of DNM1P35’s multifaceted role in cancer biology and may identify novel therapeutic targets that influence multiple aspects of tumor progression.
Our findings indicate that DNM1P35 acts as an oncogenic lncRNA in ovarian cancer by modulating the miR-326/ZEB1 axis and promoting EMT, thereby enhancing tumor progression and metastasis (Fig. 6). The potential for targeting this axis presents a promising avenue for therapeutic development with emerging technology including miRNA-based therapies, antisense oligonucleotides, or small molecule inhibitors36,37.
Fig. 6.
Schematic of DNM1P35-mediated ovarian cancer progression by targeting miR-326 and EMT.
In conclusion, our study on DNM1P35 not only enriches the understanding of ovarian cancer’s molecular landscape but also paves the way for future research and therapeutic strategies targeting lncRNAs.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Author contributions
Study conception and design; JZData collection: MS, YM, JH, and XW; Analysis and interpretation of results: MS, XW, JH and QZ; Draft manuscript preparation: MS and JZ. All authors reviewed the results and approved the final version of the manuscript.
Funding
Wuxi City Health Commission Maternal and Child Health Scientific Research Project Plan (FYKY202301).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mei Shen and Yun Mao contributed equally to this work.
References
- 1.Menon, U. et al. Diagnostic routes and time intervals for ovarian cancer in nine international jurisdictions; Findings from the International Cancer Benchmarking Partnership (ICBP). Br. J. Cancer. 77 (12), 739–740. 10.1097/01.ogx.0000905360.00777.66 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maleki, Z. et al. Survival rate of ovarian cancer in Asian countries: A systematic review and meta-analysis. BMC Cancer. 23(1). 10.1186/s12885-023-11041-8 (2023). [DOI] [PMC free article] [PubMed]
- 3.Somasegar, S., Reddy, R. A. & Karam, A. Trends in ovarian cancer incidence and incidence-based mortality: A 15-year population-based analysis. J. Clin. Oncol.41(16_suppl), 5570. 10.1200/jco.2023.41.16_suppl.5570 (2023). [Google Scholar]
- 4.Mazidimoradi, A. et al. The global, regional and national epidemiology, incidence, mortality, and burden of ovarian cancer. Health Sci. Rep.5(6). 10.1002/hsr2.936 (2022). [DOI] [PMC free article] [PubMed]
- 5.Huang, J. et al. Worldwide burden, risk factors, and temporal trends of ovarian cancer: A global study. Cancers14(9), 2230. 10.3390/cancers14092230 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, A. et al. The effect of advances in epithelial ovarian cancer treatment on population mortality. J. Clin. Oncol.40(16_suppl), 5540. 10.1200/jco.2022.40.16_suppl.5540 (2022). [Google Scholar]
- 7.Skorda, A., Bay, M. L., Hautaniemi, S., Lahtinen, A. & Kallunki, T. Kinase inhibitors in the treatment of ovarian cancer: Current state and future promises. Cancers14(24), 6257. 10.3390/cancers14246257 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asai-Sato, M. Recent advances in ovarian cancer treatment. J. Nihon Univ. Med. Assoc.81(1), 23–28. 10.4264/numa.81.1_23 (2022). [Google Scholar]
- 9.Morlando, M. & Fatica, A. Alteration of epigenetic regulation by long noncoding RNAs in cancer. Int. J. Mol. Sci.19(2), 570. 10.3390/ijms19020570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, X. et al. LncRNA SNHG6 promotes proliferation, invasion and migration in colorectal cancer cells by activating TGF-β/Smad signaling pathway via targeting UPF1 and inducing EMT via regulation of ZEB1. Int. J. Med. Sci.16(1), 51–59. 10.7150/ijms.27359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, Q. et al. LncRNA RSU1P2 contributes to tumorigenesis by acting as a ceRNA against let-7a in cervical cancer cells. Oncotarget8(27), 43768–43781. 10.18632/oncotarget.10844 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, H. et al. Comprehensive analysis of aberrantly expressed profiles of lncRNAs and miRNAs with associated ceRNA network in muscle-invasive bladder cancer. Oncotarget7(52), 86174–86185. 10.18632/oncotarget.13363 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, N. & Zhan, X. Identification of clinical trait–related lncRNA and mRNA biomarkers with weighted gene co-expression network analysis as useful tool for personalized medicine in ovarian cancer. EPMA J.10(3), 273–290. 10.1007/s13167-019-00175-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang, L. et al. DNMBP-AS1 regulates NHLRC3 expression by sponging MIR-93-5p/17-5P to inhibit colon cancer progression. Front. Oncol.12, 765163. 10.3389/fonc.2022.765163 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, P. Y. et al. The long noncoding RNA lncNB1 promotes tumorigenesis by interacting with ribosomal protein RPL35. Nat. Commun.10(1). 10.1038/s41467-019-12971-3 (2019). [DOI] [PMC free article] [PubMed]
- 16.Pan, X., Zheng, G. & Gao, C. LncRNA PVT1: A novel therapeutic target for cancers. Clin Lab.64(05/2018). 10.7754/clin.lab.2018.171216. (2018). [DOI] [PubMed]
- 17. Lee, Y.-H. et al. LINC00084/MIR-204/ZEB1 Axis mediates myofibroblastic differentiation activity in fibrotic buccal mucosa fibroblasts: Therapeutic target for oral submucous fibrosis. J Pers Med.11(8), 707. 10.3390/jpm11080707 (2021). [DOI] [PMC free article] [PubMed]
- 18.Torre, L. A. et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin.68(4), 284–296. 10.3322/caac.21456 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn, J. J. & Chang, H. Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet.17(1), 47–62. 10.1038/nrg.2015.10 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Bhan, A., Soleimani, M. & Mandal, S. S. Long noncoding RNA and Cancer: A new paradigm. Cancer Res.77(15), 3965–3981. 10.1158/0008-5472.can-16-2634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pronina, I. V. et al. Dysregulation of LNCRNA–miRNA–mRNA interactome as a marker of metastatic process in ovarian cancer. Biomedicines10(4), 824. 10.3390/biomedicines10040824 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, X. et al. LncRNA HCG11 represses ovarian cancer cell growth via AKT signaling pathway. J. Obstet. Gynaecol. Res.48(3), 796–805. 10.1111/jog.15083 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Wang, Q. et al. LncRNA CRNDE promotes cell proliferation, migration and invasion of ovarian cancer via miR-423-5p/FSCN1 axis. Mol. Cell. Biochem.477(5), 1477–1488. 10.1007/s11010-022-04382-8 (2022). [DOI] [PubMed] [Google Scholar]
- 24.He, S. L. et al. LncRNA KCNQ1OT1 promotes the metastasis of ovarian cancer by increasing the methylation of EIF2B5 promoter. Mol. Med.28(1). 10.1186/s10020-022-00521-5 (2022). [DOI] [PMC free article] [PubMed]
- 25.Richards, E. J. et al. Long non-coding RNAs (LncRNA) Regulated by Transforming Growth Factor (TGF) β. J. Biol. Chem.290(11), 6857–6867. 10.1074/jbc.m114.610915 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson, D. W. & Dinger, M. E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet.17(5), 272–283. 10.1038/nrg.2016.20 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Cesana, M. et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell147(2), 358–369. 10.1016/j.cell.2011.09.028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi, Z. et al. MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro Oncol.16(10), 1341–1353. 10.1093/neuonc/nou084 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell. Biol.15(3), 178–196. 10.1038/nrm3758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, C. H. & Chen, Y. Targeting long non-coding RNAs in cancers: Progress and prospects. Int. J. Biochem. Cell. Biol.45(8), 1895–1910. 10.1016/j.biocel.2013.05.030 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Cai, M. et al. ADAM17, a target of MIR-326, promotes EMT-Induced cells invasion in lung adenocarcinoma. Cell. Physiol. Biochem.36(3), 1175–1185. 10.1159/000430288 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Das, S. et al. MicroRNA-326 regulates profibrotic functions of transforming growth factor-Β in pulmonary fibrosis. Am. J. Respir Cell. Mol. Biol.50 (5), 882–892. 10.1165/rcmb.2013-0195oc (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez-Tilló, E. et al. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell. Mol. Life Sci.69(20), 3429–3456. 10.1007/s00018-012-1122-2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, H. et al. MIR-7, inhibited indirectly by LinCRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem Cells. 32(11), 2858–2868. 10.1002/stem.1795 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Tay, Y., Rinn, J. & Pandolfi, P. P. The multilayered complexity of ceRNA crosstalk and competition. Nature505(7483), 344–352. 10.1038/nature12986 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen, Y., Gao, D. Y. & Huang, L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv Rev.81, 128–141. 10.1016/j.addr.2014.05.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arun, G., Diermeier, S. D. & Spector, D. L. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol. Med.24(3), 257–277. 10.1016/j.molmed.2018.01.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.