Abstract
Cardiac sex-difference functional studies have centred on measurements of twitch force and Ca2+ dynamics. The energy expenditures from these two cellular processes: activation (Ca2+ handling) and contraction (cross-bridge cycling), have not been assessed, and compared, between sexes. Whole-heart studies measuring oxygen consumption do not directly measure the energy expenditure of these activation-contraction processes. In this study, we directly quantified these energy expenditures in terms of heat production. Left-ventricular trabeculae were dissected from rats aged 9–13 weeks. Mechano-energetics of trabeculae were characterized using our work-loop calorimeter under various conditions including varying muscle lengths, stimulus frequencies, and afterloads. Each trabecula was subjected to protocols that allowed it to contract either isometrically or shorten to perform work-loops. Force production, length change, and heat output were simultaneously measured. We extracted various metrics: twitch kinetics, shortening kinetics, mechanical work, and heat associated with cross-bridge cycling and Ca2+ cycling, and quantified mechanical efficiency. Results show no sex differences in any of the metrics. Peak mechanical efficiency was not affected by sex (10.25 ± 0.57% in female trabeculae; 10.93 ± 0.87% in male trabeculae). We conclude that cardiac mechanics and energetics are not affected by sex at the muscle level, within the rat age range studied.
Keywords: Cardiac efficiency, Force-length, Heat Production, Female, Mechanical work, Cardiac mechanoenergetics
Subject terms: Cardiovascular biology, Bioenergetics
Introduction
Sex differences have increasingly garnered attention in cardiovascular research. While numerous studies have delved into areas in cardiac mechanics1–4 and energetics5–8, whether mechanical efficiency is sex-dependent remains elusive. Understanding the differences in myocardial functional performance between sexes is crucial, as they likely arise from a complex interplay of factors and cellular processes that modulate and affect cardiac mechanical efficiency. Also, such studies can inform the development of tailored therapies for cardiovascular diseases in men and women.
At the whole-heart level, women exhibit distinct cardiac characteristics compared to men. Women generally have smaller hearts3,9–11, faster heart rates8,12,13, higher ejection fraction3,11,12, lower cardiac output1, and lower systolic blood pressure8. These differences are indicative of women’s different cardiac performance profiles from men, with women demonstrating lower stroke volume1 and blood flow kinetic energy but higher vorticity and cardiac strain during the cardiac cycle8,11. Notably, women exhibit reduced exercising oxygen consumption and cardiac index, underscoring the significance of sex as a pivotal variable in cardiac energetics performance8.
Animal studies corroborate findings in humans, revealing lower left-ventricular mass, left-ventricular wall thickness, stroke volume, cardiac output, and end-diastolic volume in female animals compared to males14–17. Experimental interventions simulating exercise conditions further elucidate sex-related differences in cardiac muscle performance, with female hearts exhibiting lower force generation under high stimulation frequencies18,19. A study on isolated rat ventricular trabeculae revealed sex-specific differences in twitch kinetics18. However, studies20–22 investigating sex-related differences noted no variations in the contractile performance of isolated papillary muscles in terms of twitch kinetics between female and male rats. Studies generally report a smaller and slower shortening profile in female cardiomyocytes compared to male23–26. Given the observed differences in cardiac mechanics between female and male animals, it is plausible to infer that there are also likely differences in cardiac energetics between the sexes.
In addition to mechanics, research interests have also centred on intracellular Ca2+ handling. Specifically, lower peak Ca2+ transient24,25,27,28, and lower rate of Ca2+ cycling21,23,27 in female versus male cardiomyocytes have been reported. These observations suggest the possibility that the energy associated with intracellular Ca2+ cycling may be different between sexes, owing to sex-specific Ca2+ transients observed.
Cardiac mechanical efficiency, proposed more than 70 years ago29, has since become a promising alternative approach to evaluate cardiac function comprehensively. Cardiac mechanical efficiency is the ratio of external work to total energy expenditure, representing a crucial parameter for assessing cardiac energy utilisation30,31. While metrics such as stroke volume and ejection fraction provide insights into cardiac performance24,25,32,33, cardiac efficiency has emerged as a more powerful performance index linking mechanics and energetics and recently demonstrated clinical impact34.
Yet conflicting findings on cardiac efficiency have been reported, including higher or lower values in women. In one study5, cardiac efficiency was not affected by sex. However, in a subsequent study by the same investigators35, it was found that women have lower cardiac efficiency than men. Both studies used echocardiography to quantify efficiency. More recent studies have reported that hearts from women have a higher mechanical efficiency, arising from their lower cardiac energy expenditure in terms of oxygen consumption, as compared with men6–8. In rat working-heart experiments, oxygen consumption was found to be independent of sex despite higher stroke work in males, suggesting a higher efficiency in male hearts16,36. Given inconsistent findings in the literature, the question of whether cardiac efficiency is sex-dependent thus remains inconclusive.
This study aims to investigate sex differences in cardiac energetics and efficiency by directly measuring the heat production of contracting muscle. Measurement of heat quantifies the energy expenditure of the actomyosin ATPase and of the ATPases responsible for and involved in intracellular Ca2+ cycling, including the sarcoplasmic reticular Ca2+ ATPase and the Na+-K+ ATPase. Unlike the measurement of oxygen consumption, the measurement of heat provides a direct assessment of cross-bridge and Ca2+ cycling energetics37,38. We designed this study to assess activation heat (thermal output accompanying Ca2+ cycling), cross-bridge heat, and mechanical efficiency over wide ranges of afterload to provide a comprehensive evaluation of sex differences in cardiac energetics.
Methods
Trabeculae preparation
Female and male Wistar rats (9–11 weeks old) were purchased from the University’s animal facility. Protocols for animal handling and euthanasia were approved by the Animal Ethics Committee of The University of Auckland (Ethics No. R1403). This study is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines. On the day of the experiment, a rat was anesthetized by inhalation of isoflurane (< 5% in O2) and received an injection of heparin (1,000 IU kg−1) prior to measurement of body mass and euthanasia via cervical dislocation. The heart was excised and plunged into cold Tyrode solution. Within 30 s, the heart was Langendorff perfused at room temperature with oxygenated Tyrode solution that contained (in mmol L−1) 130 NaCl, 6 KCl, 1 MgCl2, 0.5 NaH2PO4, 0.3 CaCl2, 10 HEPES, 10 glucose, 20 2,3-butanedione monoxime (for halting muscle contraction) and Tris (for achieving a pH of 7.4).
Under a dissecting microscope, trabeculae were excised from the left ventricle. A trabecula was transferred and mounted in a work-loop calorimeter39 between platinum hooks. One end of the muscle was attached to a linear length motor to monitor and control muscle length, while the other end was attached to the force transducer to measure muscle force. In the calorimeter, the trabecula was superfused with oxygenated experimental Tyrode solution, the composition of which was the same as the dissection Tyrode solution but with 1.5 mmol L−1 CaCl2 and no 2,3-butanedione monoxime. Experimental Tyrode solution (superfusate) was gravity-fed and controlled at 0.6 µL s−1 into the measurement chamber of the calorimeter, and down the length of the muscle from upstream to downstream; this flow rate provides optimal sensitivity40 for measuring heat production while maintaining muscle viability41. The temperatures of the superfusate upstream and downstream of the trabecula were measured by thermopile arrays positioned beneath the measurement chamber in the calorimeter. The rate of heat production of the trabecula was inferred from the difference in voltage signals of the downstream and upstream thermopiles.
Once mounted in the calorimeter, each trabecula was given an acclimatisation period of about 1 h. During this period, the trabecula was electrically stimulated at 2 Hz, which was delivered via a pair of platinum electrodes in the calorimeter. The entire calorimeter device was closed in a light-proof and thermally insulated lid, with the interior temperature controlled at 32 °C. Experiments commenced when the mechanical performance of the trabecula and thermal equilibration of the apparatus were established. The electrical stimulation frequency was then increased to 3 Hz. The contracting trabecula was gradually lengthened via the linear length-motor until active twitch force was maximal, to determine the optimal muscle length (Lo). Three protocols were then performed: length-change, work-loop, and frequency protocols. In all protocols, muscle force, muscle length, and the rate of muscle heat production were measured simultaneously.
Length-change protocol: calculation of activation heat
The trabecula was subjected to a series of isometric contractions at six different muscle lengths ranging between Lo (optimal length) and near 0.75 Lo (where muscle force is minimal). At each muscle length, muscle force and rate of heat were simultaneously measured. Electrical stimulation was halted between length steps to allow measurement of baseline heat. Upon stimulation, both force and heat production reached steady states within 2–3 min. Post-experiment data analysis involved plotting muscle heat against force during steady state. A linear regression was applied to the dataset, with the y-intercept representing activation heat and the inverse of slope as muscle economy.
Work-loop protocol: calculation of efficiency
The trabecula contracting at 3 Hz was subjected to work-loop contractions at six different afterloads, which were selected, nominally at around 0.8, 0.7, 0.6, 0.5, 0.3, and 0.1 of the isometric stress. The lowest afterload was in the vicinity of muscle passive force. The mode of contraction was switched to isometric contractions for the measurements of isometric force and rate of heat prior to subjecting the trabecula to each of the six afterloaded work-loop contractions. Steady states of force, length and rate of heat output were reached within 2 min. The area of a work-loop quantified the external work output of the trabecula. Mechanical efficiency of the trabecula was quantified as the ratio of mechanical work output to the change of enthalpy (ΔH; the sum of work and active heat).
Frequency protocol
In this protocol, each trabecula was subjected to isometric contractions at Lo at four different frequencies (3 Hz, 4 Hz, 5 Hz, and 6 Hz) presented in random order. Steady states of force and rate of heat output were measured after 2 min. Electrical stimulation was halted prior to proceeding to the next stimulus frequency.
Post-experiment measurement and quantification
Following the experiment, the lower left limb of the rat was dissected to measure the tibial length. In the dissection bath, and following isolation of trabeculae, the wall thicknesses of both ventricles were measured under a microscope. Both ventricles were then weighed to determine their wet and dry masses (after 12–18 h of oven drying). Force was converted to stress (kPa) by normalizing to muscle cross-sectional area. Afterload was defined as the user-selected stress at which the muscle transitioned from isometric contraction to isotonic shortening during a work-loop. Relative afterload is the ratio of the afterload to the peak isometric total (active plus passive) stress. Extent of shortening was estimated as the difference between the end-systolic length and end-diastolic length in a work-loop. Velocity of muscle shortening was estimated from the length–time trace during the initial 10–20 ms isotonic shortening phase of the work-loop. During this period, the velocity of shortening was maximal and approximately constant. Its value was normalized to the appropriate muscle length and was thus expressed in units of s−1. The product of velocity of shortening and afterload defines power of shortening. Twitch heat (kJ m−3) was calculated by dividing the steady-state rate of heat production by the stimulus frequency and normalizing it to muscle volume. Work output (kJ m−3) was calculated by integrating stress as a function of relative muscle length over the period of the twitch. The duration of twitch stress was quantified between 5% (t95) and 50% (t50) of peak stress.
Measurement of muscle dimensions
Each trabecula was assumed to resemble an ellipse in cross-section and, thus, muscle force was converted to stress from the measurement of muscle diameters in two orthogonal views (top and side). In total, 17 female and 15 male trabeculae completed the study. The trabeculae were isolated from 9 female rats and 12 male rats. There were no statistically significant differences, using Student’s t-test, in trabecula dimension between female and male trabeculae in either cross-sectional area (means ± standard deviation: 0.077 mm2 ± 0.011 mm2 and 0.089 mm2 ± 0.011 mm2, respectively) or muscle volume (means ± standard deviation: 0.242 mm3 ± 0.035 mm3 and 0.279 mm3 ± 0.038 mm3, respectively).
Statistical analyses
Student’s t-test was used to evaluate the differences between the means of the two sex groups. Polynomial regression lines (up to the 3rd order) were used to fit to data. Regression lines (each obtained from a single trabecula) were averaged within groups using the ‘random coefficient’ model within Proc Mixed model of the SAS software package (SAS Institute Inc., Cary, USA). Values are means ± standard errors unless stated otherwise. Statistical significance was declared when p < 0.05.
Results
Morphometrics of rats
Morphometric characteristics of the rats that yielded trabeculae of dimensions suitable for our experiments are presented in Table 1. At around 9–13 weeks of age, female rats were smaller than male rats, as indicated by their lower average body mass, average shorter tibial length, and average body mass relative to tibial length. The heart and left ventricle of female rats were, on average, smaller than those of male rats. However, LV wall thickness normalized to heart wet mass and LV wet mass normalized to tibial length were higher in female than male rats.
Table 1.
Morphometric characteristics of female and male rats.
| Female rats (n = 9) | Male rats (n = 6) | |
|---|---|---|
| Age (day) | 77 ± 3 | 78 ± 3 |
| Body mass (g) | 242.7 ± 8.8 | 378.5 ± 29.0* |
| Tibial length (mm) | 37.0 ± 0.9 | 41.0 ± 1.2* |
| Body mass/tibial length (%) | 6.58 ± 0.24 | 9.20 ± 0.57* |
| Heart | ||
| Wet mass (g) | 0.89 ± 0.03 | 1.27 ± 0.09* |
| Dry mass (g) | 0.173 ± 0.007 | 0.342 ± 0.056* |
| Wet mass/tibial length (g/mm) | 0.024 ± 0.001 | 0.031 ± 0.002* |
| Wet mass/body mass (%) | 0.369 ± 0.016 | 0.338 ± 0.007 |
| Left ventricle | ||
| Wall thickness (mm) | 3.04 ± 0.10 | 3.49 ± 0.08* |
| Wet mass (g) | 0.67 ± 0.03 | 0.96 ± 0.06* |
| Dry mass (g) | 0.127 ± 0.005 | 0.271 ± 0.044* |
| Wet mass/body mass (%) | 0.280 ± 0.013 | 0.256 ± 0.010 |
| Wall thickness/heart wet mass (mm/g) | 3.4 ± 0.1 | 2.8 ± 0.2* |
| Wet mass/tibial length (g/mm) | 0.023 ± 0.001 | 0.018 ± 0.001* |
| Wall thickness/tibial length (%) | 8.54 ± 0.10 | 8.23 ± 0.23 |
Values are means ± standard errors. * P < 0.05.
Results from the Length-change protocol
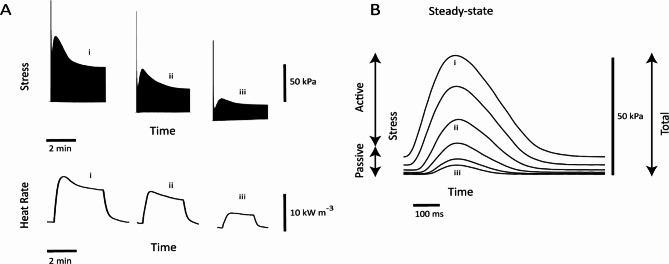
Figure 1 shows representative isometric stress twitches and the associated heat production at a range of muscle lengths. Figure 1A shows simultaneous records of twitches and rate of heat output of a trabecula subjected to the isometric length-change protocol. Both twitch force and heat production decreased progressively with decreasing muscle length. Figure 1B shows steady-state twitch profiles at various muscle lengths.
Fig. 1.
Representative records of a length-change protocol imposed on an isolated trabecula. (A) Records of twitches and the rate of heat production from a representative trabecula subjected to the isometric length-change at progressively diminishing muscle lengths (“i”–“iii”). Records show data of the trabecula upon electrical stimulation until steady states of force and rate of heat. (B) Twitch profiles at steady state and at various muscle lengths superimposed. Labels “i” to “iii” correspond to those in A.
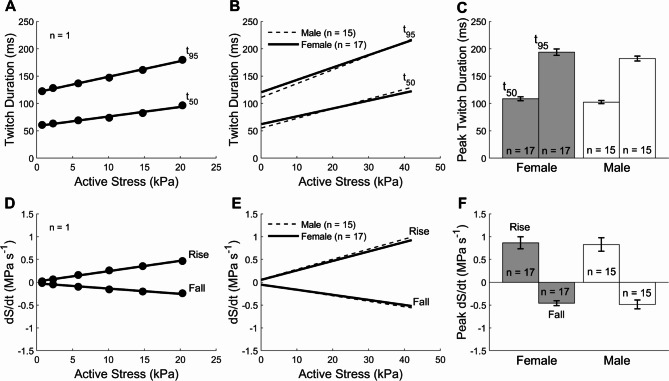
The duration of isometric twitch stress (active stress) was quantified at 5% (t95) and 50% (t50) of peak stress. These were plotted against active stress (Fig. 2 top panels). There were no sex-related differences in the average relation between twitch duration and active stress (Fig. 2B) and the average peak value of twitch duration (as obtained at Lo; Fig. 2C). The rates of rise (+ dS/dt) and fall (− dS/dt) of isometric twitch stress were expressed as functions of active stress (Fig. 2 lower panels). The average relations and peak values were not statistically different between muscles from female and male rats.
Fig. 2.
Steady-state isometric twitch kinetics measured under the length-change protocol. (A) Twitch duration at 5% (t95) and 50% (t50) of peak stress as functions of active stress of a single trabecula. Data fitted using linear regression. (B) Average relations of twitch duration at 5% (t95) and 50% (t50) of peak stress as functions of active stress of 17 trabeculae from female rats and those of 15 trabeculae from male rats. (C) Peak twitch duration at 5% (t95) and 50% (t50) of peak stress, obtained at Lo, for the female and male groups. (D) Rate of twitch stress development (rise; + dS/dt) and relaxation (fall; − dS/dt) as functions of active stress of a single trabecula. Data fitted using linear regression. (E) Average relations of ± dS/dt of 17 trabeculae from female rats and those of 15 trabeculae from male rats. (F) Peak ± dS/dt of female and male rats. No statistical differences between sex groups.
Results from the frequency protocol
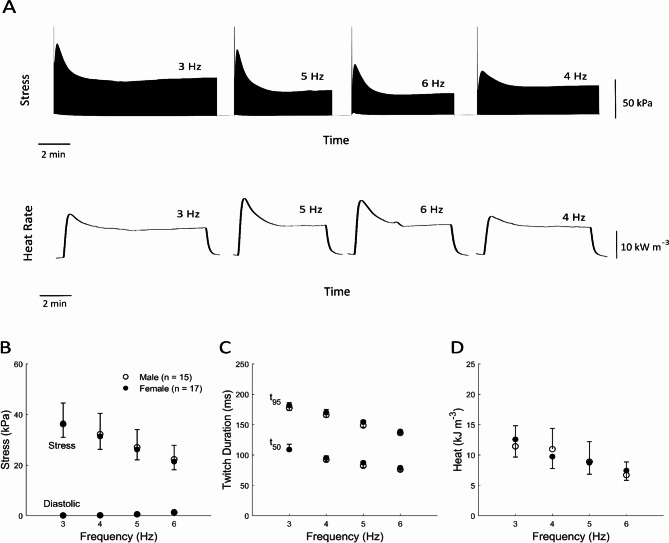
In the Frequency protocol, each trabecula was subjected to isometric contractions at Lo at four different frequencies (3 Hz to 6 Hz; presented in a random order). Figure 3A shows representative experimental records of simultaneous measurements of twitch stress and rate of heat output from a trabecula contracting at the four different frequencies, where stimulation was halted between frequency steps. Steady-state values of stress and heat are plotted in Fig. 3B–D. Average values of active stress, diastolic stress, twitch duration at 5% (t95) and 50% (t50) of peak stress, and heat as functions of stimulus frequency showed no significant sex-difference (Fig. 3B–D). The rates of rise and fall (± dS/dt) as functions of frequencies were also not sex-different (not shown).
Fig. 3.
Sex-difference in mechano-energetics as functions of stimulus frequency. (A) Record of the measured rate of heat production and twitch stress from a trabecula contracting isometrically under the frequency protocol. In this example, the order was as shown: 3 Hz, 5 Hz, 6 Hz, and 4 Hz. Stimulation was halted between frequency steps. (B) Average values of active stress and diastolic stress as functions of stimulus frequency. (C) Average values of twitch duration at 5% (t95) and 50% (t50) of peak stress as functions of stimulus frequency. (D) Average values of heat as functions of frequency. No statistical difference between sex groups can be detected.
Results from the Work-loop protocol
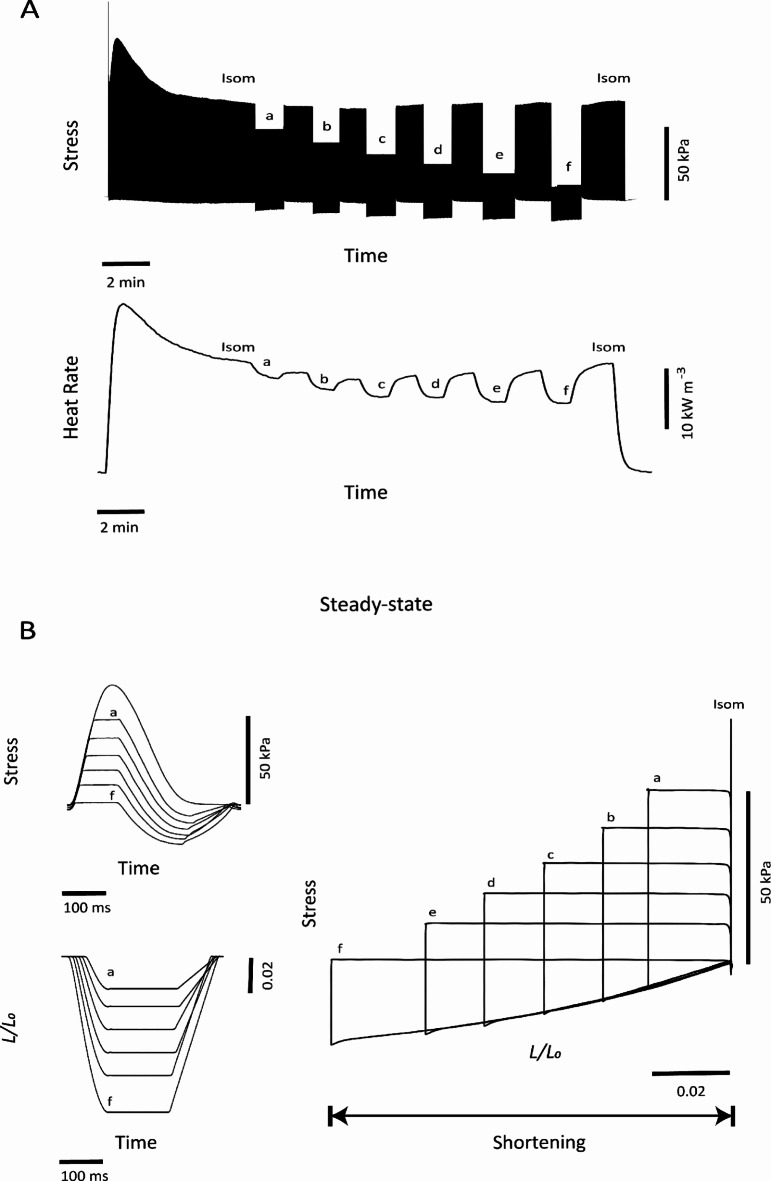
Figure 4 shows representative results from the work-loop protocol imposed on an isolated trabecula. Raw experimental records for stress and rate of heat from a trabecula performing work-loop contractions are depicted in Fig. 4A. Muscle heat production was highest during isometric contractions and decreased progressively with decreasing afterload. Figure 4B presents steady-state force twitches at varying afterloads, and associated length transients throughout each twitch. Parametric plots of force (stress) and length produce stress-length work-loops. The width of each loop quantifies the extent of muscle shortening and increased with decreasing afterloads.
Fig. 4.
Representative example of a work-loop protocol imposed on an isolated trabecula. (A) Upon initiation of stimulation, the trabecula was required to perform isometric contractions (Isom) until a steady state was achieved; it was then required to undergo afterloaded work-loop contractions (a). This protocol was repeated for 6 afterloads (a–f). (B) Superimposed steady-state profiles of relative muscle length versus time (up-left) and overlay of steady-state isotonic twitches of varying afterloads (down-left), data from these two plots are plotted against each other to reveal work-loops in the plot on the right. The width of each loop quantifies the extent of muscle shortening. The area within each stress-length loop represents muscle work output.
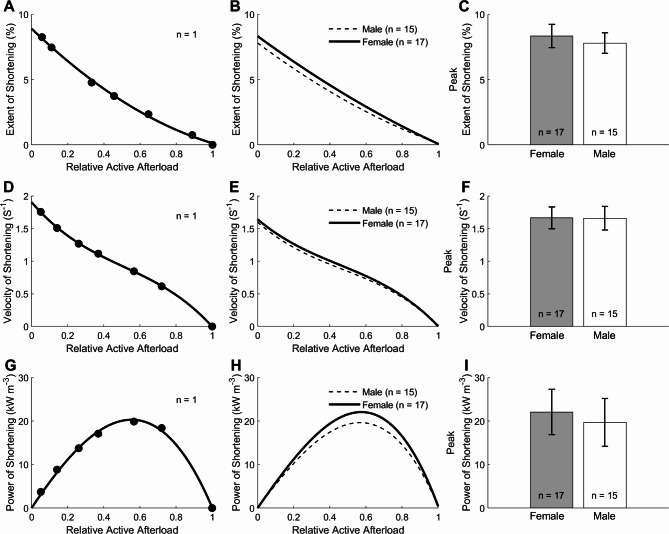
The extent of muscle shortening during work-loop contractions was calculated from the end-systolic length (at which point the muscle transitions from the isotonic shortening phase to the isometric relaxation phase of the work-loop; Fig. 4B length traces) and expressed as a percentage of Lo. Sex had no effects on the average relation between the extent of shortening and relative active afterload (Fig. 5B) and on the peak value of the extent of shortening (as extrapolated to zero relative active afterload), plotted in Fig. 5C. Shortening velocity was calculated as the maximal slope of the length-time trace (Fig. 4B) and normalised to Lo. Sex did not affect the average relation between shortening velocity and relative active afterload (Fig. 5E and on the peak value of shortening velocity; Fig. 5F). Shortening power was computed as the product of shortening velocity and active afterload. Sex did not affect the relation between shortening power and relative active afterload (Fig. 5H) and on the peak value of shortening power (Fig. 5I).
Fig. 5.
Shortening kinetics of trabeculae subjected to the work-loop protocol. (A) The extent of muscle shortening (calculated from the length trace in Fig. 4B) as a function of the relative active afterload of a single trabecula. Data were fitted using a 2nd order polynomial. (B) Average relation of the extent of shortening and relative active afterload of 17 female trabeculae and those of 15 male trabeculae (C) Peak extent of shortening for the muscle groups, calculated from the y-intercepts of the relations in panel (B). (D) Velocity of shortening as a function of relative afterload (calculated from the length trace as in Fig. 4B) of a single trabecula. Data were fitted using a 3rd order polynomial. (E) Average relation of velocity of shortening and relative active afterload of 17 female trabeculae and those of 15 male trabeculae from male rats. (F) Peak velocity of shortening for the muscle groups, calculated from the y-intercepts of the relations depicted in panel (E). (G) Power of shortening as a function of relative afterload of a single trabecula and (H) from the averages of 17 trabeculae from female rats and 15 trabeculae from male rats. Curves were fitted using a 3rd-order polynomials constrained to intercept the x-axis at relative afterloads of 0 and 1. (I) Peak power of shortening for the groups, calculated from the peaks of the curves in H. No differences among groups.
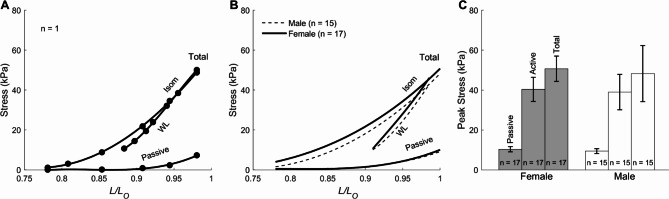
Figure 6A and B respectively show data for an individual muscle, and the average of the isometric twitch stress (derived from Fig. 1A), the work-loop twitch stress (derived from Fig. 4A), and the passive stress (derived from Fig. 1) of muscles at steady state plotted against relative muscle length (L/Lo). Sex had no effect on these relations (Fig. 6B). Peak value of stresses obtained at L = Lo (i.e., isometric contraction) did not differ between muscle groups (Fig. 6C).
Fig. 6.
Steady-state stresses as functions of relative length. (A) Isometric (Isom) stress (derived from Fig. 1A), work-loop (WL) stress (derived from Fig. 4A), and passive stress (derived from Fig. 1) data from a single trabecula. Data were fitted using a 3rd order polynomial. (B) Average relations of 17 female trabeculae were not statistically different to those of 15 male trabeculae (C) Peak values of total, active, and passive stresses were not statistically difference between sex groups.
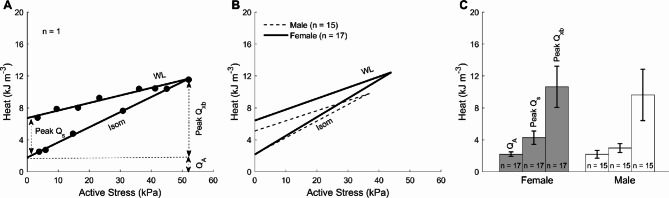
The steady-state rate of heat production (Figs. 1A and 4A) was converted to heat per twitch by dividing by stimulus frequency (3 Hz) and plotted as functions of steady-state values of active stress (Fig. 7A,B) for both the length-change and work-loop protocols. Sex had no effects on the average relations between sex groups (Fig. 7B). Sex also did not affect the average cross-bridge heat (Female: 10.64 kJ m−3 ± 2.58 kJ m−3; Male: 9.62 kJ m−3 ± 3.21 kJ m−3) and the average activation heat (QA) (Female: 2.19 kJ m−3 ± 0.28 kJ m−3; Male: 2.18 kJ m−3 ± 0.48 kJ m−3) as shown in Fig. 7C. Peak shortening heat (Qs), obtained from the difference of y-intercept between the work-loop and isometric heat stress-relations (Fig. 7C), was also not sex-different (Female: 4.26 kJ m−3 ± 0.82 kJ m−3; Male: 2.96 kJ m−3 ± 0.55 kJ m−3).
Fig. 7.
Twitch heat as a function of active stress. (A) Isometric (Isom) heat (derived from Fig. 1A) and work-loop (WL) heat (derived from Fig. 4A) data from a single trabecula. Data were fitted using linear regression. Estimations of activation heat (QA) and peak cross-bridge heat (Peak QXb) were depicted. Cross-bridge heat (QXb) was quantified by subtracting QA from the heat measured under work-loop contractions. Peak shortening heat (Qs) was quantified by subtracting QA from the y-intercept of the work-loop heat stress relation. (B) The average relations of heat and active stress of 17 female trabeculae were not different from those of 15 male trabeculae. (C) Activation heat (QA), peak cross-bridge heat (Peak QXb), and peak shortening heat (Qs) were not different between both sex groups.
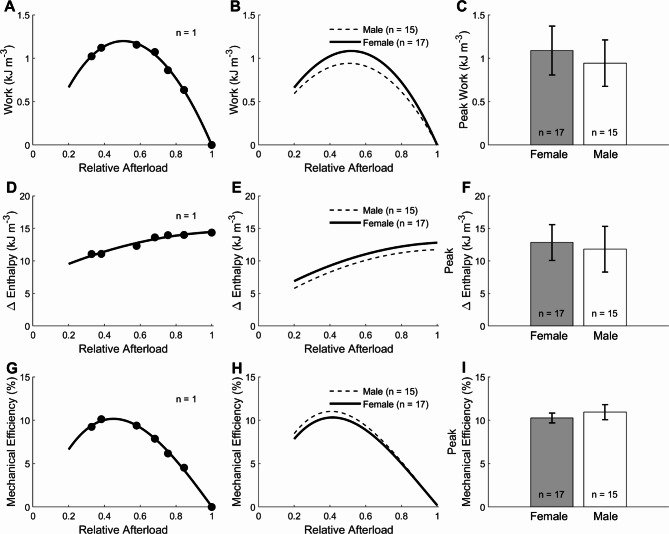
Figure 8 plots the external work output, change of enthalpy (work plus heat), and mechanical efficiency (the ratio of work to change of enthalpy) as functions of relative afterload. In Fig. 8B,E, and H, the relations were not sex-different. Peak values were calculated from these relations. In Fig. 8C,F, and I, peak work (Female: 1.08 kJ m−3± 0.28 kJ m−3; Male: 0.94 kJ m−3± 0.26 kJ m−3), peak change of enthalpy (Female: 12.84 kJ m−3 ± 2.74 kJ m−3, Male: 11.80 kJ m−3 ± 3.51 kJ m−3) and peak mechanical efficiency (Female: 10.25% ± 0.57%; Male: 10.93% ± 0.87%) were not different between the female and male trabeculae.
Fig. 8.
Mechanoenergetics of steady-state work-loop contractions. (A) Mechanical work (the area of each afterloaded work-loop) as a function of relative afterload from a single trabecula. Data were fitted using 3rd-order polynomial, and the peak value of work was quantified for each trabecula. (B) Average relation of work and relative afterload of 17 female trabeculae were not different from those of 15 male trabeculae. (C) Peak work was not different between sex groups. (D) Values of change of enthalpy as a function of relative afterload from a single trabecula and (E) from the averages of 17 trabeculae from female rats and 15 trabeculae from male rats. (F) The peak of change of enthalpy as a function of afterload between groups. (G) Values mechanical efficiency as a function of relative afterload from a single trabecula and (H) from the averages of 17 trabeculae from female rats and 15 trabeculae from male rats. (I) The peak of mechanical efficiency as a function of afterload between groups. Mechanical efficiency is given by the ratio of work to change of enthalpy. Data were fitted using third-order polynomials. In all panels, there were no differences among the groups.
Discussion
This study is the first to assess the effect of sex on cardiac energetics and efficiency by measuring heat output from left-ventricular trabeculae. Mechano-energetics were characterized over a wide range of conditions under varying muscle lengths (Fig. 1), stimulus frequencies (Fig. 3), or afterloads (Fig. 4). We assessed mechanoenergetics using protocols that allowed the muscle to contract either isometrically or shorten to perform mechanical work. From these protocols, we evaluated twitch kinetics, muscle shortening, and energy associated with cross-bridge cycling (actomyosin ATPase) and Ca2+ cycling (activation heat), and, therefore, mechanical efficiency, as functions of condition from which we estimated peak values. We found null effects of sex on (1) muscle shortening and twitch force, and, hence, (2) mechanical work; (3) heat production, and, therefore, (4) efficiency, at the trabecula level.
We chose to study rats between 9 and 13 weeks of age for four reasons. First, the rats are at adulthood—their body’s vital systems have matured, and they have already reached sexual maturity42. Second, studies have reported significant sex-differences in the mechanisms of cardiac excitation-contraction coupling, including cellular Ca2+ handling23–25 and myosin ATPase activity26 at this age range. Third, this age range is commonly used in sex-difference studies in mechanical and physiological experiments19–22,26 to which we can compare our findings. Lastly, this age range is commonly used for interventional studies such as dietary manipulation43,44, exercise intervention33 and for inducing diseases such as diabetes45,46 and hypertension47–49.
At this age range, female rats are smaller than male rats, which is evident from their lower body mass, shorter tibial length, and smaller heart and left ventricle dimensions (Table 1). Our findings are consistent with previous studies highlighting morphological differences between sexes in human and animal models3,11,17,20–22. Female rats have greater LV wall thickness when normalized to heart mass and a higher LV mass when normalized to tibial length than male rats (Table 1), consistent with the study 50 revealing thicker LV mass, LV wall, and chamber dimensions normalized to body mass in females.
While phenotypical sex-differences exist both at the body level and at the whole-heart level, we selected trabeculae of comparable dimensions for our study. There were no differences in the averages of trabeculae dimensions between sex groups. Thus, our comparison of sex-difference in functional performance of trabeculae is not subject to differences in muscle dimensions. This eliminates the confounding effect that muscle dimension contributes to functional performance. Muscle size, specifically radius and cross-sectional area, is known to bear an inverse relationship with muscle stress41,51–53 and with energy expenditure51,54. Muscle heat output likewise depends inversely on muscle length55.
We observed no sex-difference in twitch force. This finding is consistent with several studies on isolated papillary muscles20–22, and trabeculae19, which reported no sex difference in the amplitude of twitch force at the optimal muscle length. In our literature search, no effect of sex on cardiac twitch force is a consistent and unequivocal finding. In our study, this finding of null sex-effect on twitch force extends to twitch force over the full spectrum of muscle lengths (Fig. 6), over the two modes of contraction (i.e., isometric and work-loop56) (Fig. 6), and over a range of stimulus frequencies (Fig. 3).
What is inconsistent in the literature on sex-differences in cardiac muscle is the kinetics of mechanical performance. Thus, whether the female cardiac muscle compared with the male has ‘faster’ kinetics remains inconclusive. Using isometric contraction protocols, the rates of rise and fall of isometric twitch force have been reported to be greater in female than male right-ventricular (RV) papillary muscles contracting at 0.5 Hz (24 °C, 1 mM [Ca2+]o)22, as well as in LV papillary muscles contracting at 0.1 Hz (30 °C, 2.4 mM [Ca2+]o)20. Using isotonic contraction protocols, the latter study by Capasso et al.20 also reported a greater velocity of shortening in female than male LV papillary muscles. These results show that force development in female muscle is ‘fast’. Our results from LV trabeculae contracting at 3 Hz (32 °C, 1.5 mM [Ca2+]o) show no sex-differences in these indices of kinetics, over ranges of muscle lengths (Fig. 2) and stimulus frequencies (Fig. 3). These null effects are consistent with previous reports on LV papillary muscles contracting at 0.1 Hz (29 °C, 1.25 mM [Ca2+]o)21 and RV trabeculae contracting over a range of frequencies (4 Hz, 6 Hz, and 8 Hz, 37 °C, 1.5 mM [Ca2+]o)19. On the other hand, studies on ventricular myocytes reported conflicting findings showing a slower rate of unloaded cell shortening in females than males [(2 Hz, 37 °C, 1.8 mM [Ca2+]o)25, (4 Hz, 37 °C, 2 mM [Ca2+]o)57, and (2 Hz and 4 Hz, 37 °C, 1.8 mM [Ca2+]o)]24, and slower rates of contraction in females than males (0.5 Hz, 25 °C, 1.5 mM [Ca2+]o)23. These results show that unloaded shortening velocity in the female myocyte is ‘slow’.
Conflicting findings of literature (discussed above) on cardiac contractile kinetics may be due to the variety of muscle preparation (papillary muscles, trabeculae, and myocytes), experimental conditions ([Ca2+]o, stimulus frequency, and temperature), and experimental methods (mode of contraction, selection of muscle dimension). For example, papillary muscle preparations over 1 mm thick, as used by Capasso et al.20 have been suggested to have developed tissue hypoxia19 and thereby contributing to the faster kinetics in females. However, this putative suggestion is not compelling as tissue hypoxia would have occurred in both sex groups, and given that the muscles were paced at low stimulus frequencies (0.1–0.8 Hz), tissue hypoxia is perhaps unlikely. Myosin heavy chain (MHC) isoform also could not explain the ‘faster’ kinetics in female muscles found by Capasso et al.20 as studies have reported no sex-difference in the ratio of alpha-MHC to beta-MHC in ventricular tissues33,58. Capasso et al.20 used 2.4 mM [Ca2+]o, which is doubled the physiological concentration of 1.2–1.5 mM. Such a high [Ca2+]o amplifies sex-differences in contractile kinetics where female cardiac myocytes and papillary muscles exhibit slower profiles26. Thus the high [Ca2+]o could not explain the faster kinetics of female muscles found by Capasso et al. We note that Capasso et al.20 used female papillary muscles significantly (27%) shorter than males. In our study, trabecula muscle length was not sex different. Selecting from our data set, a short female muscle (2.20 mm) had greater rates of twitch stress development (0.73 MPa s−1 vs. 0.30 MPa s−1) and relaxation (-0.62 MPa s−1 vs. -0.50 MPa s−1) than a long muscle from the male (3.29 mm), respectively. Future investigations of the effects of [Ca2+]o and muscle length on sex-difference in mechanical kinetics are warranted. We recommend future studies focusing on using trabeculae rather than other muscle preparations, conducting the experiment using physiological [Ca2+]o, at a temperature greater than 30 °C, and accordingly, the muscle can be stimulated at a frequency closer to physiological. Lastly, we strongly recommend using muscles of similar dimensions, preferably with no statistical difference between sex groups.
Using work-loop contractions, we found no sex-related differences in the extent of shortening of trabeculae (Fig. 5) over a range of afterloads. Under isotonic contractions, Leblanc et al.21 also revealed no sex difference in the shortening of papillary muscles. Interestingly, despite greater shortening kinetics in female papillary muscles, Capasso et al.20 also did not find evidence of sex-related differences in peak isotonic shortening. Sex indifference in both force and shortening yields no effect of sex on mechanical work (Fig. 8). Our finding on trabeculae contrasts those studying isolated rat working-heart16,36, where stroke work was found higher in males compared to females at a range of varying afterloads. We cannot explain this difference, but our finding supports those in the whole heart in vivo rat by Oláh et al.33, where no sex difference in pressure-volume stroke work was found. To our knowledge, no studies have compared mechanical work between females and males using isolated cardiac muscle preparations.
We measured activation heat and found no sex differences (Fig. 7). We measured neither calcium transient nor protein expression/activity. We note studies reporting inconsistency in the amplitude of Ca2+ transients: no sex-differences21,24,59–62, and smaller in females23,25. Likewise, we also note several inconsistent findings in the rate of decay of the Ca2+ transient: no sex-difference24,25,60,62, slower23,27 and faster61 in female myocytes. Putative explanations of these discrepancies have been attributed to sex-differences in the abundance and activity of key Ca2+ handling proteins22,63. Our finding of no sex-differences in activation heat is supported on several fronts. First, cardiac Ca2+ transient amplitude is not sex-different21,24,59–62. Second, SERCA abundance and protein expression are not sex-different22,64. This is supported by functional studies of Oknińska et al.60,62 showing no effect of sex on the rates of Ca2+ transport by the SERCA and by the Na+-Ca2+ exchanger. Third, sarcoplasmic reticular Ca2+ content has also been shown to be unaffected by sex25,62. Fourth, the female heart is shown to have greater expressions of L-type Ca2+ channel, RyR, Na+-Ca2+ exchanger22,64, as well as greater Na+-K+ ATPase activity and kinetics (16-week-old rats)65. Despite a higher Na+-K+ ATPase activity and kinetics in the female heart, its contribution to activation heat is small. Activation heat represents the energy expenditure arising from cellular Ca2+-cycling events47, primarily from the ATP hydrolysis by SERCA. In the rat heart, 92% of the Ca2+ removal is achieved by SERCA, 7% by the coupling between Na+-K+ ATPase and Na+-Ca2+ exchanger, and the remaining 1% by the sarcolemma Ca2+ ATPase and mitochondrial Ca2+ uniporter66. Since SERCA transports twice as much Ca2+ per ATP hydrolyzed compared to other Ca2+ transporters, it accounts for 85% of ATP hydrolysis, while Na+-K+ ATPase combined with Na+-Ca2+ exchanger accounts for only 13% 47. Thus, no sex-difference in activation heat largely reflects no sex-difference in SERCA expression and activity.
We found no sex-differences in cross-bridge heat (Fig. 7). In our experiments, muscle force was varied by using two different modes of contraction (Isometric, work-loop) and by varying muscle length and afterload. Measured heat is plotted as a function of force (Fig. 7). Cross-bridge heat is the difference between the heat-stress relation and activation heat67. The heat-stress relation comprises both the isometric and the work-loop heat-stress relations68. At a given force, work-loop cross-bridge heat consists of isometric cross-bridge heat and shortening heat69,70. We found no sex difference in the work-loop heat-stress relation, the isometric heat-stress relation, and shortening heat. These results are not surprising as we found no sex-differences in active force (Fig. 6), and the extent of shortening (Fig. 5). Our finding of comparable cross-bridge heat between females and males suggests similar ATP hydrolysis activity by the myosin ATPase. Indeed, actomyosin ATPase, Ca2+-ATPase of myosin, and K+-EDTA ATPase of myosin have been shown to be sex-indifferent5,35.
The absence of an effect of sex on both mechanical work and active heat is reflected in no change of enthalpy; hence, there is no effect of sex on cardiac mechanical efficiency (Fig. 8). In our study, we measured energy expenditure as heat output directly from Ca2+ cycling and cross-bridge cycling. Previous researchers investigating sex-differences in cardiac efficiency have instead quantified myocardial oxygen consumption in rat working-heart experiments16,36 and in humans using either positron emission tomography71 or indirectly calculated from the double product of systolic blood pressure and heart rate5,11,35. These studies measuring oxygen consumption using different methodologies reported conflicting conclusions regarding the effect of sex on cardiac efficiency (see Introduction). We are not equipped to comment on their inconsistent findings and to reconcile those findings with our heat measurements given the lack of literature data on sex-differences in mitochondrial efficiency in producing ATP per oxygen molecule consumed (i.e., mitochondrial P/O ratio), where ATP is, in turn, used in the processes of Ca2+ cycling and cross-bridge cycling. We do show that the change of enthalpy over a range of afterloads is sex-independent. If we assume sex-indifferent mitochondrial P/O2 efficiency, our results suggest that mitochondrial oxygen consumption is sex-independent at the muscle level—the prediction of which requires future investigation.
Many of the conflicting findings from the literature, as we discussed above, could be argued to have arisen, and could perhaps be reconciled by considering the female estrous cycle. The modulation of the estrous cycle on cardiac function is unlikely to occur in female rodents housed in animal facilities unless they are induced to cycle through exposure to male pheromones72. However, our female rats were housed in the same room as male rats, and thus expected to be cycling. We did not measure the estrous stages of our female rats, but we noted that the influence of the four estrous stages on cardiac mechanics has been investigated in rodents. Specifically, in mouse myocytes, both the extent and velocity of shortening, and Ca2+ transient amplitude are greater only at the estrous stage compared with the other stages (proestrus, metestrus, and diestrus)72. However, in isolated perfused rat hearts, cardiac mechanics are not affected by the estrous stage under normal functioning conditions73–75, but only during extreme interventions induced by hypoxia74 or trauma-hemorrhage73. We also noted that a recent study on humans showed no effects of hormonal changes during the menstrual cycle in women on cardiovascular function76.
Conclusions
We compared mechano-energetics of trabeculae isolated from male and female rats, subjected to isometric and work-loop contractions. Experiments were conducted under various conditions encompassing varying afterloads, muscle lengths, and frequencies. None of the indices of functional performance are different between sexes. That is, twitch kinetics under isometric and shortening contractions, heat production, work output, and mechanical efficiency are affected by sex, at the muscle level and within the rat age range studied.
Acknowledgements
We acknowledge the lives of the animals from whom heart tissue was used in this study.
Author contributions
M.R. and J. C.H. conceived and designed the study. M.R. performed experiments, prepared figures, and drafted the manuscript. T.P., D.C., K.T., and A.T. contributed to the conception of the research and interpretation of the data. All authors edited and revised the manuscript. All authors approved the final version of the manuscript.
Funding
This study was funded by the Heart Foundation of New Zealand through Postgraduate Scholarship (1875, awarded to M.R.), Project Grant (1929, awarded to J.-C.H.) Research Fellowship Grant (1896, awarded to T.P.), and Senior Research Fellowship Grant (awarded to D.C), the Health Research Council of New Zealand through Sir Charles Hercus Health Research Fellowship Grants (20/011 and 21/116; awarded to J.-C.H., K.T., respectively), Explorer Grant (21/758, awarded to J.-C.H.) and Emerging Researcher First Grant (21/653, awarded to T.P.), and the Royal Society of New Zealand through Marsden Project Grant (MFP-UOA2206, awarded to J.-C.H.) and James Cook Research Fellowship (awarded to A.T.).
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu, J. & Yin, L. Sex differences in left ventricular stroke work and cardiac power output per unit myocardium relate to blood pressure in apparently healthy adults. PLoS ONE18, e0280143. 10.1371/journal.pone.0280143 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu, J., Dai, F., Li, C. & Zou, Y. Gender differences in cardiac hypertrophy. J. Cardiovasc. Transl. Res.13, 73–84. 10.1007/s12265-019-09907-z (2020). [DOI] [PubMed] [Google Scholar]
- 3.Wooten, S. V. et al. Age- and sex-differences in cardiac characteristics determined by echocardiography in masters athletes. Front. Physiol.11, 630148. 10.3389/fphys.2020.630148 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam, R. A., Khalsa, S. S. S., Vyas, A. K. & Rahimian, R. Sex-specific impacts of exercise on cardiovascular remodeling. J. Clin. Med.10, 3833 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson, L. R. et al. Sex differences in myocardial oxygen and glucose metabolism. J. Nucl. Cardiol.14, 573–581. 10.1016/j.nuclcard.2007.03.001 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Succurro, E. et al. Sex-specific differences in left ventricular mass and myocardial energetic efficiency in non-diabetic, pre-diabetic and newly diagnosed type 2 diabetic subjects. Cardiovasc. Diabetol.20, 60. 10.1186/s12933-021-01248-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara, F. et al. Physiologic range of myocardial mechano-energetic efficiency among healthy subjects: Impact of gender and age. J. Personal. Med.12, 996 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheatley, C. M., Snyder, E. M., Johnson, B. D. & Olson, T. P. Sex differences in cardiovascular function during submaximal exercise in humans. SpringerPlus3, 445. 10.1186/2193-1801-3-445 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devereux, R. B. et al. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J. Am. Coll. Cardiol.4, 1222–1230. 10.1016/s0735-1097(84)80141-2 (1984). [DOI] [PubMed] [Google Scholar]
- 10.de Simone, G., Devereux, R. B., Daniels, S. R. & Meyer, R. A. Gender differences in left ventricular growth. Hypertension26, 979–983. 10.1161/01.hyp.26.6.979 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Rutkowski, D. R., Barton, G. P., François, C. J., Aggarwal, N. & Roldán-Alzate, A. Sex differences in cardiac flow dynamics of healthy volunteers. Radiol. Cardiothorac. Imaging2, e190058. 10.1148/ryct.2020190058 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley, P. C. et al. Gender-related differences in cardiac response to supine exercise assessed by radionuclide angiography. J. Am. Coll. Cardiol.13, 624–629. 10.1016/0735-1097(89)90603-7 (1989). [DOI] [PubMed] [Google Scholar]
- 13.Umetani, K., Singer, D. H., McCraty, R. & Atkinson, M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J. Am. Coll. Cardiol.31, 593–601. 10.1016/s0735-1097(97)00554-8 (1998). [DOI] [PubMed] [Google Scholar]
- 14.O’Connell, T. D. et al. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J. Clin. Investig.111, 1783–1791. 10.1172/JCI16100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch, S. E. et al. Age- and gender-related changes in ventricular performance in wild-type FVB/N mice as evaluated by conventional and vector velocity echocardiography imaging: A retrospective study. Ultrasound Med. Biol.39, 2034–2043. 10.1016/j.ultrasmedbio.2013.04.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaible, T. F. & Scheuer, J. Comparison of heart function in male and female rats. Basic Res. Cardiol.79, 402–412. 10.1007/BF01908140 (1984). [DOI] [PubMed] [Google Scholar]
- 17.Grilo, G. A. et al. Age- and sex-dependent differences in extracellular matrix metabolism associate with cardiac functional and structural changes. J. Mol. Cell. Cardiol.139, 62–74. 10.1016/j.yjmcc.2020.01.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petre, R. E. et al. Sex-based differences in myocardial contractile reserve. Am. J. Physiol. Regul. Integ. Comp. Physiol.292, R810–R818. 10.1152/ajpregu.00377.2006 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Monasky, M. M., Varian, K. D. & Janssen, P. M. Gender comparison of contractile performance and beta-adrenergic response in isolated rat cardiac trabeculae. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol.178, 307–313. 10.1007/s00360-007-0223-y (2008). [DOI] [PubMed] [Google Scholar]
- 20.Capasso, J. M., Remily, R. M., Smith, R. H. & Sonnenblick, E. H. Sex differences in myocardial contractility in the rat. Basic Res. Cardiol.78, 156–171. 10.1007/bf01906669 (1983). [DOI] [PubMed] [Google Scholar]
- 21.Leblanc, N., Chartier, D., Gosselin, H. & Rouleau, J. L. Age and gender differences in excitation–contraction coupling of the rat ventricle. J. Physiol.511, 533–548. 10.1111/j.1469-7793.1998.533bh.x (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu, S. H. et al. Sex differences in expression of calcium-handling proteins and beta-adrenergic receptors in rat heart ventricle. Life Sci.76, 2735–2749. 10.1016/j.lfs.2004.12.013 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Curl, C. L., Wendt, I. R. & Kotsanas, G. Effects of gender on intracellular [Ca2+] in rat cardiac myocytes. Pflugers Arch. Eur. J. Physiol.441, 709–716. 10.1007/s004240000473 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Howlett, S. E. Age-associated changes in excitation–contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. Am. J. Physiol. Heart Circ. Physiol.298, H659–H670. 10.1152/ajpheart.00214.2009 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Farrell, S. R., Ross, J. L. & Howlett, S. E. Sex differences in mechanisms of cardiac excitation–contraction coupling in rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol.299, H36-45. 10.1152/ajpheart.00299.2010 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Schwertz, D. W., Beck, J. M., Kowalski, J. M. & Ross, J. D. Sex differences in the response of rat heart ventricle to calcium. Biol. Res. Nurs.5, 286–298. 10.1177/1099800403262615 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Wasserstrom, J. A. et al. Characteristics of intracellular Ca2+ cycling in intact rat heart: a comparison of sex differences. Am. J. Physiol. Heart Circ. Physiol.295, H1895-1904. 10.1152/ajpheart.00469.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parks, R. J., Ray, G., Bienvenu, L. A., Rose, R. A. & Howlett, S. E. Sex differences in SR Ca(2+) release in murine ventricular myocytes are regulated by the cAMP/PKA pathway. J. Mol. Cell. Cardiol.75, 162–173. 10.1016/j.yjmcc.2014.07.006 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Bing, R. J. et al. The measurement of coronary blood flow, oxygen consumption, and efficiency of the left ventricle in man. Am. Heart J.38, 1–24. 10.1016/0002-8703(49)90788-7 (1949). [DOI] [PubMed] [Google Scholar]
- 30.Schipke, J. D. Cardiac efficiency. Basic Res. Cardiol.89, 207–240. 10.1007/bf00795615 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Kiriazis, H. & Gibbs, C. L. Effects of aging on the work output and efficiency of rat papillary muscle. Cardiovasc. Res.48, 111–119. 10.1016/s0008-6363(00)00144-9 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Dedkov, E. I. et al. Sex-related differences in intrinsic myocardial properties influence cardiac function in middle-aged rats during infarction-induced left ventricular remodeling. Physiol. Rep.4, e12822. 10.14814/phy2.12822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oláh, A. et al. Sex differences in morphological and functional aspects of exercise-induced cardiac hypertrophy in a rat model. Front. Physiol.10, 889. 10.3389/fphys.2019.00889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sörensen, J. et al. Myocardial efficiency: A fundamental physiological concept on the verge of clinical impact. JACC Cardiovasc. Imaging13, 1564–1576. 10.1016/j.jcmg.2019.08.030 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Peterson, L. R. et al. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc. Imaging1, 424–433. 10.1016/j.jcmg.2008.05.004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaible, T. F., Malhotra, A., Ciambrone, G. & Scheuer, J. The effects of gonadectomy on left ventricular function and cardiac contractile proteins in male and female rats. Circ. Res.54, 38–49. 10.1161/01.res.54.1.38 (1984). [DOI] [PubMed] [Google Scholar]
- 37.Gibbs, C. L. & Barclay, C. J. Cardiac efficiency. Cardiovasc. Res.30, 627–634. 10.1016/s0008-6363(95)00161-1 (1995). [PubMed] [Google Scholar]
- 38.Loiselle, D. S., Johnston, C. M., Han, J. C., Nielsen, P. M. & Taberner, A. J. Muscle heat: a window into the thermodynamics of a molecular machine. Am. J. Physiol. Heart Circ. Physiol.310, H311-325. 10.1152/ajpheart.00569.2015 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Taberner, A. J., Han, J. C., Loiselle, D. S. & Nielsen, P. M. An innovative work-loop calorimeter for in vitro measurement of the mechanics and energetics of working cardiac trabeculae. J. Appl. Physiol. (Bethesda, Md. : 1985)111, 1798–1803. 10.1152/japplphysiol.00752.2011 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Johnston, C. M., Han, J. C., Ruddy, B. P., Nielsen, P. M. & Taberner, A. J. A high-resolution thermoelectric module-based calorimeter for measuring the energetics of isolated ventricular trabeculae at body temperature. Am. J. Physiol. Heart Circ. Physiol.309, H318-324. 10.1152/ajpheart.00194.2015 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Han, J.-C. et al. Radius-dependent decline of performance in isolated cardiac muscle does not reflect inadequacy of diffusive oxygen supply. Am. J. Physiol. Heart Circ. Physiol.300, H1222-1236. 10.1152/ajpheart.01157.2010 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Sudakov, S. K., Alekseeva, E. V., Nazarova, G. A. & Bashkatova, V. G. Age-related individual behavioural characteristics of adult wistar rats. Animals11, 2282 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran, K. et al. Effect of a high-salt diet on the mechano-energetics of left ventricular trabeculae isolated from dahl salt-sensitive rats. Biophys. J.106, 776a. 10.1016/j.bpj.2013.11.4255 (2014).24559979 [Google Scholar]
- 44.Goo, S. et al. Dietary pre-exposure of rats to fish oil does not enhance myocardial efficiency of isolated working hearts or their left ventricular trabeculae. J. Physiol.592, 1795–1808. 10.1113/jphysiol.2013.269977 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han, J.-C., Tran, K., Nielsen, P. M. F., Taberner, A. J. & Loiselle, D. S. Streptozotocin-induced diabetes prolongs twitch duration without affecting the energetics of isolated ventricular trabeculae. Cardiovasc. Diabetol.13, 79–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahin, K. et al. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: The fat-fed, streptozotocin-treated rat. Metab. Clin. Exp.56, 1233–1240. 10.1016/j.metabol.2007.04.021 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Han, J.-C. et al. Cardiac mechanical efficiency is preserved in primary cardiac hypertrophy despite impaired mechanical function. J. Gen. Physiol.153, e202012841. 10.1085/jgp.202012841 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinto, Y. M., Paul, M. & Ganten, D. Lessons from rat models of hypertension: From Goldblatt to genetic engineering. Cardiovasc. Res.39, 77–88. 10.1016/s0008-6363(98)00077-7 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Amenta, F. & Tomassoni, D. In Animal Models of Dementia (eds De Deyn, P. P. & Van Dam, D.) 577–611 (Humana Press, 2011). [Google Scholar]
- 50.Forman, D. E., Cittadini, A., Azhar, G., Douglas, P. S. & Wei, J. Y. Cardiac morphology and function in senescent rats: Gender-related differences. J. Am. Coll. Cardiol.30, 1872–1877. 10.1016/s0735-1097(97)00411-7 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Han, J.-C., Tran, K., Taberner, A. J., Chapman, B. & Loiselle, D. S. In Muscle and Exercise Physiology (ed. Zoladz, J. A.) 505–539 (Academic Press, 2019). [Google Scholar]
- 52.Choi, D. H. et al. The inverse relationship between cardiac muscle stress and cross-sectional area is preserved in Ba2+ contracture and in chemically-permeabilised Ca2+ contracture. Exp. Mech.61, 107–117. 10.1007/s11340-020-00652-y (2021). [Google Scholar]
- 53.Delbridge, L. M. D. & Loiselle, D. S. An ultrastructural investigation into the size dependency of contractility of isolated cardiac muscle. Cardiovasc. Res.15, 21–27. 10.1093/cvr/15.1.21 (1981). [DOI] [PubMed] [Google Scholar]
- 54.Garrett, A. S., Loiselle, D. S., Han, J.-C. & Taberner, A. J. Heat production in quiescent cardiac muscle is length, velocity and muscle dependent: Implications for active heat measurement. Exp. Physiol.106, 2445–2456. 10.1113/EP089800 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Han, J. C., Taberner, A. J., Nielsen, P. M. & Loiselle, D. S. Interventricular comparison of the energetics of contraction of trabeculae carneae isolated from the rat heart. J. Physiol.591, 701–717. 10.1113/jphysiol.2012.242719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han, J.-C., Pham, T., Taberner, A. J., Loiselle, D. S. & Tran, K. Solving a century-old conundrum underlying cardiac force-length relations. Am. J. Physiol. Heart Circ. Physiol.316, H781–H793. 10.1152/ajpheart.00763.2018 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Bell, J. R. et al. Male and female hypertrophic rat cardiac myocyte functional responses to ischemic stress and β-adrenergic challenge are different. Biol. Sex Differ.7, 32. 10.1186/s13293-016-0084-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenkranz-Weiss, P., Tomek, R. J., Mathew, J. & Eghbali, M. Gender-specific differences in expression mRNAs for functional and structural proteins in rat ventricular myocardium. J. Mol. Cell. Cardiol.26, 261–270. 10.1006/jmcc.1994.1029 (1994). [DOI] [PubMed] [Google Scholar]
- 59.Grandy, S. A. & Howlett, S. E. Cardiac excitation-contraction coupling is altered in myocytes from aged male mice but not in cells from aged female mice. Am. J. Physiol. Heart Circ. Physiol.291, H2362-2370. 10.1152/ajpheart.00070.2006 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Oknińska, M. et al. Effect of age and sex on the incidence of ventricular arrhythmia in a rat model of acute ischemia. Biomed. Pharmacother.142, 111983. 10.1016/j.biopha.2021.111983 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Clements, R. T. et al. Sexual dimorphism in bidirectional SR-mitochondria crosstalk in ventricular cardiomyocytes. Basic Res. Cardiol.118, 15. 10.1007/s00395-023-00988-1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oknińska, M. et al. Sex- and age-dependent susceptibility to ventricular arrhythmias in the rat heart ex vivo. Scientific reports14, 3460. 10.1038/s41598-024-53803-9 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parks, R. J. & Howlett, S. E. Sex differences in mechanisms of cardiac excitation–contraction coupling. Pflugers Arch. Eur. J. Physiol.465, 747–763. 10.1007/s00424-013-1233-0 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tappia, P. S., Dent, M. R., Aroutiounova, N., Babick, A. P. & Weiler, H. Gender differences in the modulation of cardiac gene expression by dietary conjugated linoleic acid isomers. Can. J. Physiol. Pharmacol.85, 465–475. 10.1139/y06-104 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Vlkovičová, J., Javorková, V., Pecháňová, O. & Vrbjar, N. Gender difference in functional properties of Na, K-ATPase in the heart of spontaneously hypertensive rats. Life Sci.76, 971–982. 10.1016/j.lfs.2004.10.013 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Bers, D. M. Cardiac excitation–contraction coupling. Nature415, 198–205. 10.1038/415198a (2002). [DOI] [PubMed] [Google Scholar]
- 67.Han, J.-C., Pham, T., Taberner, A. J., Loiselle, D. S. & Tran, K. Resolving an inconsistency in the estimation of the energy for excitation of cardiac muscle contraction. Front. Physiol.14, 1269900. 10.3389/fphys.2023.1269900 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran, K., Taberner, A. J., Loiselle, D. S. & Han, J.-C. Energetics equivalent of the cardiac force-length end-systolic zone: Implications for contractility and economy of contraction. Front. Physiol.10, 1633. 10.3389/fphys.2019.01633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran, K., Han, J. C., Crampin, E. J., Taberner, A. J. & Loiselle, D. S. Experimental and modelling evidence of shortening heat in cardiac muscle. J. Physiol.595, 6313–6326. 10.1113/jp274680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pham, T., Han, J.-C., Taberner, A. & Loiselle, D. Do right-ventricular trabeculae gain energetic advantage from having a greater velocity of shortening?. J. Physiol.595, 6477–6488. 10.1113/JP274837 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martins, H. A. et al. Sex differences in maximal oxygen uptake adjusted for skeletal muscle mass in amateur endurance athletes: A cross sectional study. Healthcare (Basel, Switzerland)11, 1502. 10.3390/healthcare11101502 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacDonald, J. K., Pyle, W. G., Reitz, C. J. & Howlett, S. E. Cardiac contraction, calcium transients, and myofilament calcium sensitivity fluctuate with the estrous cycle in young adult female mice. Am. J. Physiol. Heart Circ. Physiol.306, H938-953. 10.1152/ajpheart.00730.2013 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Yang, S. et al. Estrus cycle: influence on cardiac function following trauma-hemorrhage. Am. J. Physiol. Heart Circ. Physiol.291, H2807-2815. 10.1152/ajpheart.00195.2006 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Broderick, T. L. & Wong, P. Influence of the estrous cycle on hypoxic failure in the female rat heart. Gend Med6, 596–603. 10.1016/j.genm.2009.12.005 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Frasier, C. R. et al. Stage of the estrous cycle does not influence myocardial ischemia-reperfusion injury in rats (Rattus norvegicus). Comp. Med.63, 416–421 (2013). [PMC free article] [PubMed] [Google Scholar]
- 76.Kwissa, M. et al. Cardiovascular function in different phases of the menstrual cycle in healthy women of reproductive age. J. Clin. Med.11, 5861. 10.3390/jcm11195861 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author.