Abstract
The aim of this study was to evaluate the effects of two styles of classical music, based on different tempos (BPM), on the physiological and blood parameters of horses during social isolation and restriction of movements. First experiment was carried out using nine horses of no defined breed, distributed in Control, Slow-tempo music and Moderate-tempo music .For social isolation and restriction of movement, the animals were housed daily in individual stalls for two hours and exposed to the stimuli for 60 min, and eye temperature, heart rate, and respiratory rate were assessed. The second experiment was carried out using ten horses of no defined breed, distributed in a randomized design in treatments: Slow-tempo Music and Moderate-tempo Music. Blood samples were taken at the start and end of the experimental period to assess hematological and biochemical parameters and serum serotonin levels. Horses exposed to moderate-tempo music showed an increase in serum calcium levels, mean corpuscular hemoglobin (MCH), and total hemoglobin concentration, as well as a reduction in lymphocytes. Both types of music led to a significant increase in serotonin levels after one week of stimulation. Both musical rhythms are appropriate for promoting the well-being and health of stabled horses.
Keywords: Environmental enrichment, Equine, Eye temperature, Heart rate, Infrared thermography, Well-being
Subject terms: Animal behaviour, Social behaviour
Introduction
Currently, domestic horses spend most of their time stabled in environments where they have little control, limited or no social interaction, and restricted opportunities to exercise or forage1. The restriction of natural behaviors due to domestic routines and artificial environments causes these animals to remain idle for much of the day, leading to a significant reduction in their well-being. This, in turn, results in an increased incidence of abnormal behaviors as horses attempt to cope with stress2.
In this context, environmental enrichment can enhance animal’s quality of life by encouraging behaviors that are closer to natural ones3. However, some enrichment techniques face challenges, such as costs for implementation and maintenance, and greater need for manpower4. Environmental sound enrichment, however, may offer a viable solution to reduce monotony and improve the well-being of stabled animals.
Different musical genres have shown significant influence on the neural patterns of the human brain. It has been shown that music with a faster tempo and higher pitch evokes “happy” emotions, while a slower tempo and lower pitch initiates “sad” emotional responses5,6. Music has also been reported to positively influence the well-being of animals7,8, including horses9–11, however, there is no conclusive evidence as to which genres or predominant characteristics in music are best suited to evoke positive emotions in animals. Comparative psychological approaches indicate that animals perceive the components of music in a similar way to humans12, making it plausible to hypothesize that the emotional effect of music observed in humans can be experienced by animals, especially those with high cognitive development, such as horses.
Emotional stress can be expressed by behavioral and physiological changes. Cardiac variables and eye temperature are sensitive indicators of the horse’s immediate response to external stimuli, as they are closely related to sympathetic activity, and even mild responses to changes in well-being can be detected13,14. Research results have shown a correlation between ocular temperature and salivary and plasma cortisol concentrations in horses, suggesting that changes in ocular temperature may be associated with the activation of hypothalamic-pituitary-adrenal activity15. Delving deeper into eye temperature16,17, found a positive correlation between ocular temperature, rectal temperature and cortisol concentration during the transport of horses, which reflected changes in the homeostasis and allowing to differentiate reactions resulting from stress or routine handling.
Thus, the research was conducted to evaluate the effect of classical music based on different tempos (BPM) on the physiological responses of horses during social isolation and restricted movement.
Materials and methods
Ethical considerations
Experiments were conducted in accordance with ARRIVE guidelines (https://arriveguidelines.org), and all methods were performed following current regulations. The Ethics Committee on Animal Experimentation of the Federal University of Grande Dourados (CEUA— 1,260/2022) approved all procedures.
Study location
Two experiments were carried out on the premises of the Army’s 4th Mechanized Cavalry Brigade, Dourados-MS, Brazil. The municipality is located at latitude 22º 13’ 18” S, longitude 54º 48’ 23” W and altitude 437 m, and the average temperature during the research was 21.1 ± 3.7oC.
Study location
All the animals had daily access to the paddocks in the afternoon, where they remained in social groups and were only stabled at night. The barn had 19 sequential individual stalls, 2.5 m wide x 3.5 m deep, with a front opening through which the animals could see the outside environment and horses in adjacent stalls. The stalls had a concrete drinking fountain and feeding trough, a natural ventilation system, and a floor with wood shavings, and feces and moisture were removed daily.
The animals were fed two kilos of pelleted commercial concentrate at 04:00. At 10:00, they were released into paddocks with native grass and brought back to the stable in the late afternoon. At 19:00 they received two kilos of oats and at 20:00, six kilos of Coast Cross grass. The horses spent the night in stables, then in the morning they were already in the stalls for the sound stimulus, so only after 30 min the end of the stimuli they were released into the paddock. The animals used in the experiment had no previous riding routine or intense physical exercise.
Before the experiments began, the animals underwent a 15-day period of adaptation to the presence of the researchers, as well as to the equipment to be used in the measurements. Sound enrichment was not introduced during this period.
Experiment 1
Nine male and female horses, without a defined breed, aged between seven and thirteen years, routinely used for equine therapy and riding school activities, were used; however, during the experiment, the animals were not used for any activity.
The animals were distributed in a Greco-Latin square design (3 × 3) in the following treatments: Control, Slow-tempo music (63 to 83 BPM), and Moderate-tempo music (75 to 107 BPM). Three animals were evaluated each day, during three cycles of nine days each, totaling 27 experimental days. For the daily assessments of the three animals, the same three stalls were always used, and physically separated (7.5 m) so that the animals undergoing one treatment did not have access to the sound stimuli of the other treatments. The other animals (n = 6) remained sequentially housed in the stalls at the end of the barn. Additionally, they were physically separated with enough space to prevent access to stimuli on days when they were not being evaluated, as shown in the diagram below.
Sound stimuli
Two different playlists were created, one of which was considered moderate-tempo, based on suites, symphonies, and concertos with Andante (75 to 107 BPM) and Andante Moderato (90 to 100 BPM) tempos, and composed for cello, violin, and string instruments (Bach - SuiteNo 1 for Cello in G major, Bach - Violin Concerto in A minor, Mendelssohn - String SymphonyNo 4, Bach - Brandenburg Concerto #4 In G; Bach - Brandenburg ConcertoNo 1 in F; Bach - English Suites; Mozart - SymphonyNo 33 in B-flat major. The second playlist consisted especially of piano repertoire, with a slow tempo (63 to 83 BPM) (Claude Debussy - Clair de Lune; Chopin - Noturno in E flat major, Op. 9 − 2; Erik Satie - Troisieme Gymnopdie #3; Chopin - Prelude in D flat, Op.28; Chopin - NoturnoNo 3; Gerald Finzi - Eclogue for Piano and Strings).
A speaker was installed in each stall to play the music, and the sound pressure was monitored using a digital decibel meter so as not to exceed 60 dB. The animals were exposed to music daily for a period of one hour (8:00 am to 9:00 am).
Heart rate (HR) and heart rate variability (HRV)
Heart rate and heart rate variability parameters were measured thirty minutes prior to the start of the musical stimuli, one hour during the playback of the music, and thirty minutes after the end, by using a Polar H10 heart rate transmitter (Polar Electro Oy, Kempele, Finland). The heart rate transmitter was attached to the elastic belt and positioned in the thoracic region between the 4th and 5th intercostal space on the left side of the chest. The belt was soaked in water and adjusted on the animals’ bodies 10 min before data collection began. In order to improve the transmission of electrical signals from the body to the electrodes, the hair was cleaned with water.
The data was exported using the Polar ProTrainer Equine Edition software, version 1.2.1 (Polar Electro Oy, Kempele, Finland) and analyzed by Kubios HRV standard software, ver-204 are 3.5.0 (Kubios Oy, Kuopio, Finland). All analyses were reviewed for the percentage of corrected artifacts, with only a correction of 15% or less being accepted.
Five ten-minute samples were then selected for analysis of HRV indices and mean HR. For this study, the following HRV indices were selected for the frequency domain: low frequency power (LF), high frequency power (HF) and low frequency to high frequency ratio (LF/HF). The low frequency band (LF) was defined as 0.04–0.15 Hz, while the high frequency band (HF) was defined as 0.15–0.4 215 Hz18.
Respiratory rate (RR)
Respiratory rate was measured by counting the movements of the flank for 15 s and then multiplying the value by four to express respiratory movements per minute (mpm). Evaluations were carried out before the start, 30 min after the start, and 30 min after the end of the musical stimuli. The control animal was measured at the same times as the others19.
Eye temperature by infrared thermography (IRT)
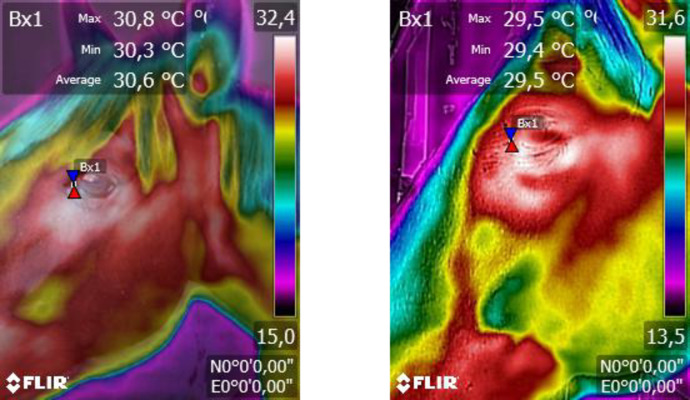
All IRT images of the eye region were taken before the start, 30 min after the start, and 30 min after the end of the musical stimuli, by using a thermographic camera (FLIR Systems Inc, Oregon, USA) positioned at a 90° angle to the sagittal plane and at a distance of approximately 0.5 m from the side of the animal. Several images were taken per animal and per collection period, and the image that provided the most optimal operating conditions for analysis was selected. Using a digital thermo hygrometer (Jiaxi - HTC 2 A) installed inside the stalls, at a height of 1.50 m from the floor, the environment temperature and relative humidity were recorded every time the eye temperature sample was taken in order to calibrate the camera results. Emissivity was set to 0.98 and IRT images were analysed using FLIR tools software. The minimum, average, and maximum eye temperature from the medial posterior palpebral border of the lower eyelid as well as the lacrimal caruncle in a circular area with a 1-cm diameter were recorded (Fig. 1).
Fig. 1.
Infrared thermography images of the eye region. The marker arrows indicate the area of the eye (medial posterior palpebral edge of the lower eyelid and the lacrimal caruncula) where the temperature points were obtained (Flir Thermal studio Suite - FLIR Tools version 6.0).
Experiment 2
The second experiment was conducted thirty days after the conclusion of the previous one. Ten horses (nine from Experiment 1, plus one additional horse) were used, comprising both males and females of no defined breed. The horses were randomly assigned to two treatments: Slow-tempo music and Moderate-tempo music, using the same playlists as in Experiment 1.
The animals were kept stabled in the morning from 06:00 to 08:30 and exposed to musical stimuli for one hour and thirty minutes, for seven consecutive days. The experimental treatments’ stalls were separated by enough physical space so that the sound stimuli of one treatment did not interfere with the other one (22.5 m). The treatments were rotated daily in the stalls to avoid the influence of stall positioning within the shed.
At the start of the experiment, 15 ml blood samples were collected from all the animals via jugular venipuncture for hematological, biochemical, and serotonin level analyses. Hematological analyses were immediately performed using an automated hematology analyzer (Veterinary Hematology Analyzer pocH-100iV Diff, Sysmex).
For biochemical and serotonin level analyses, the samples were centrifuged in an analog centrifuge (Daiki, model 80-2B) at 4,000 RPM for 5 min in a 20 °C air-conditioned room. Biochemical evaluations were conducted immediately after collection using the Cobas C-111 automatic analyzer with appropriate Cobas-Roche exclusive reagents. For serotonin level measurement, the serum was transferred to Eppendorf tubes and frozen at − 20 °C until analysis. The samples were analyzed using a commercial enzyme immunoassay kit (Alpco Diagnostics, Windham, NH, USA), with results expressed in ng/mL.
At the end of the seven-day period, blood samples were collected again to measure the same parameters, following the same collection and analysis procedures.
Statistical analyzes
Experiment 1. The data obtained was submitted to SAS (Version 9.4, SAS Institute, Cary, NC 2015), checking the normality of the residuals and the homogeneity of the variances by using PROC UNIVARIATE. The data was analyzed by using PROC GLM according to the following model:
Where: Yijyklm = dependent variable; µ = general mean; αi(l) = effect of animal nested in square; τj(l) = effect of period nested in square; βk = effect of treatment; δl = effect given square; ζl = effect of stall; eijklm = residual effect.
The data obtained was submitted to analysis of variance by using PROC GLM in SAS, version 9.0 (SAS, 2015), and when significant, the means were compared by using the Tukey test and adopting a significance level of 5%.
Additionally, data were evaluated before and after the musical stimuli, so they were considered as paired data and evaluated by the T-test using the SAS T-Test procedure (Version 9.4, SAS Institute, Cary, NC 2015). The data is presented by comparing the two measurement moments, before and after, for each treatment. The significance level used for all analyses was 5% probability.
Experiment 2. The hematological, biochemical and serotonin concentration data were evaluated before and after the musical stimuli, so they were considered as paired data and evaluated by the T-test using the SAS T-Test procedure (Version 9.4, SAS Institute, Cary, NC 2015). The data is presented by comparing the two measurement moments, before and after, for each treatment. The significance level used for all analyses was 5% probability.
Results
Experiment 1
Physiological parameters (heart rate, respiratory rate, and eye temperature)
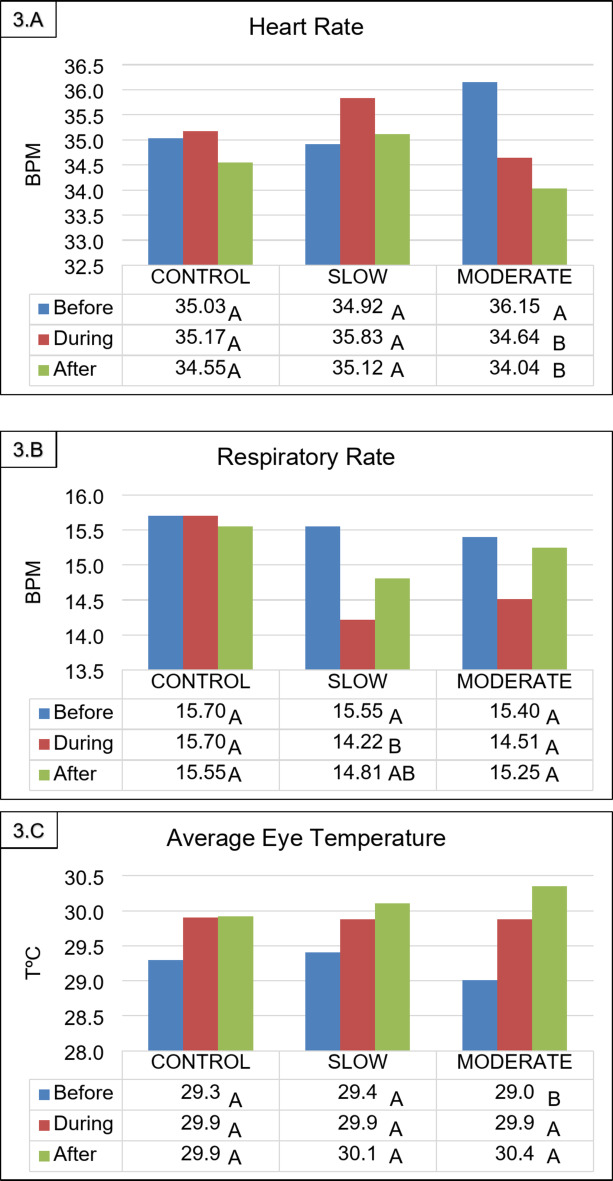
There was no effect between treatments for any of the variables analyzed (p > 0.05); however, when the effects of the treatment were evaluated in the different periods (before, during and after exposure to musical stimuli), different responses were observed depending on the musical rhythm used or the lack of exposure to music. When comparing the physiological parameters measured before and during exposure to musical stimuli, it was observed that horses exposed to moderate-tempo music showed a reduction in heart rate. This reduction coincided with an increase in minimum, average, and maximum eye temperatures compared to the period before the stimuli. Conversely, there was a decrease in respiratory rate when the horses were exposed to slow-tempo music (Table 1).
Table 1.
Heart rate (beats per minute – BPM), respiratory rate (breaths per minute – BPM) and eye temperature (maximum, minimum and average) (°C) of horses before and during exposure to two musical styles.
| Treatment | Exposure to music | P-value | |
|---|---|---|---|
| Before | During | ||
| Heart rate | |||
| Control | 35.03 ± 4.50 | 35.17 ± 6.09 | 0.8338 |
| Slow | 34.92 ± 4.50 | 35.83 ± 7.22 | 0.5535 |
| Moderate | 36.14 ± 3.96 | 34.64 ± 6.80 | 0.0296 |
| Eye temperature (maximum) | |||
| Control | 29.41 ± 1.62 | 30.01 ± 1.47 | 0.0512 |
| Slow | 29.52 ± 1.98 | 29.98 ± 1.74 | 0.2749 |
| Moderate | 28.99 ± 2.21 | 29.95 ± 1.65 | 0.0026 |
| Eye temperature (minimum) | |||
| Control | 29.06 ± 1.86 | 29.81 ± 1.54 | 0.0617 |
| Slow | 28.95 ± 2.59 | 29.82 ± 1.77 | 0.0723 |
| Moderate | 28.94 ± 2.94 | 29.82 ± 1.67 | 0.0112 |
| Eye temperature (average) | |||
| Control | 29.30 ± 1.85 | 29.90 ± 1.51 | 0.0528 |
| Slow | 29.41 ± 2.00 | 29.88 ± 1.74 | 0.2558 |
| Moderate | 29.01 ± 2.15 | 29.88 ± 1.65 | 0.0119 |
| Respiratory rate | |||
| Control | 15.70 ± 2.46 | 15.70 ± 1.53 | 1.0000 |
| Slow | 15.55 ± 3.00 | 14.22 ± 2.56 | 0.0474 |
| Moderate | 15.40 ± 2.64 | 14.51 ± 2.51 | 0.2067 |
It was found that both music tempos had a prolonged effect on the animals, who maintained a lower heart rate and higher eye temperature even 30 min after the end of exposure to the moderate-tempo music, and a lower respiratory rate when subjected to the slower tempo compositions. To reinforce the hypothesis of the effects of exposure to both music tempos, as well as their duration, the same parameters were compared before the start and after the end of the musical stimuli, showing the same differences as previously reported (Fig. 2).
Fig. 2.
(3.A) Heart rate (Beats per minute – BPM), (3.B) respiratory rate (Breaths per minute – BPM), and (3.C) average eye temperature (oC) of horses before, during, and after exposure to different musical tempos. Equal letters in the column demonstrate that the means do not differ from each other at the 5% significance level. Statistical comparisons were made pairwise (before x during, during x after, and before x after).
There was no difference between the treatments in terms of heart rate variability (LF/HF) (p > 0.05), or when compared within each treatment between the periods (during and 30 min after the end of exposure to the musical stimuli) (Table 2).
Table 2.
Heart rate variability during and after exposure to slow and moderate-tempo music.
| Treatment | Exposure to music | P-value | |
|---|---|---|---|
| During | After | ||
| LF/HF | |||
| Control | 3.65 ± 2.04 | 3.031 ± 1.72 | 0.1471 |
| Slow | 3.62 ± 1.87 | 2.75 ± 1.18 | 0.0688 |
| Moderate | 3.06 ± 1.25 | 2.69 ± 1.19 | 0.1605 |
LF low frequency, HF high frequency.
Experiment 2
Serum biochemical parameters
Horses exposed daily to 90 min of moderate-tempo classical music for seven consecutive days showed an increase in serum calcium levels (P < 0.05), but despite the increase, the parameter remained within the reference values (Table 3).
Table 3.
Serum biochemical and hematological parameters of horses before and after exposure to two different musical tempos.
| Parameter | Treatment | Reference values | |||||
|---|---|---|---|---|---|---|---|
| Slow | Moderate | ||||||
| Before | After | P-value | Before | After | P-value | ||
| Leukocytes (10³/mL) | 8.64 ± 0.48 | 8.36 ± 1.11 | 0.5129 | 8.78 ± 1.81 | 8.48 ± 2.47 | 0.7097 | 6–12 |
| Lymphocytes (10³/mL) | 32.30 ± 10.06 | 28.26 ± 7.51 | 0.0969 | 41.28 ± 2.47 | 29.72 ± 2.43 | 0.0027 | 25–60 |
| Neutrophils (10³/mL) | 53.46 ± 9.36 | 54.34 ± 7.63 | 0.7431 | 43.50 ± 3.18 | 49.92 ± 4.16 | 0.0819 | 30–75 |
| Hemoglobin (g/dL) | 12.42 ± 2.06 | 13.04 ± 2.38 | 0.4050 | 11.26 ± 1.59 | 11.60 ± 0.87 | 0.4828 | 10–18 |
| Hematocrit (%) | 32.90 ± 5.53 | 31.32 ± 7.07 | 0.3688 | 30.62 ± 3.59 | 27.5 ± 2.11 | 0.0862 | 32–48 |
| MCV (fL) | 51.04 ± 3.34 | 50.82 ± 3.41 | 0.1894 | 50.16 ± 1.54 | 49.88 ± 1.7 | 0.0800 | 37–51 |
| MCH (pg) | 19.18 ± 1.04 | 21.16 ± 0.61 | 0.0008 | 18.3 ± 0.37 | 20.98 ± 0.83 | 0.0004 | 12–20 |
| MCHC (%) | 37.74 ± 1.96 | 41.92 ± 2.22 | 0.0006 | 36.66 ± 1.84 | 42.24 ± 3.13 | 0.0011 | 31–39 |
| Platelet (mil/mm³) | 67.20 ± 11.99 | 69.00 ± 9.70 | 0.7629 | 80.8 ± 10.94 | 81.6 ± 16.82 | 0.8545 | 100–600 |
| MPV (fL) | 7.96 ± 0.57 | 7.50 ± 0.29 | 0.0234 | 7.56 ± 0.32 | 7.52 ± 0.3 | 0.7292 | 7–12 |
| PDW (fL) | 8.64 ± 2.63 | 8.20 ± 1.10 | 0.6789 | 8.1 ± 1.14 | 8.12 ± 1.16 | 0.9559 | 8–30 |
| PCT (%) | 0.04 ± 0.01 | 1.80 ± 3.96 | 0.3745 | 0.056 ± 0.01 | 0.054 ± 0.02 | 0.6213 | 0.01–9.99 |
| PLCR (%) | 10.82 ± 7.10 | 5.62 ± 5.35 | 0.1649 | 8.06 ± 3.24 | 6.40 ± 6.03 | 0.4454 | 9–55 |
| Calcium (mg/dL) | 11.72 ± 0.41 | 12.22 ± 0.38 | 0.1193 | 11.78 ± 0.58 | 12.30 ± 0.56 | 0.0237 | 11.2–13.6 |
Hematological parameters
Horses exposed to slower tempo music for seven days while stabled showed an increase in mean corpuscular hemoglobin and total hemoglobin concentration, as well as a reduction in mean platelet volume. Similarly, those treated with faster tempo music also exhibited an increase in mean corpuscular hemoglobin and hemoglobin concentration, associated with a reduction in lymphocyte count at the end of the exposure period (Table 3).
Serum serotonin concentration
Horses exposed daily to 90 min of classical music for seven consecutive days showed an increase in serum serotonin levels, regardless of the musical tempo (P < 0.05) (Table 4).
Table 4.
Serum serotonin concentration (ng/ml) before and after a period of seven days of daily exposure to two different musical tempos.
| Treatment | Exposure to music | ||
|---|---|---|---|
| Before | After | P- value | |
| Moderate | 130.52 ± 5.70 | 192.86 ± 18.94 | 0.0012 |
| Slow | 108.94 ± 9.57 | 173.74 ± 32.19 | 0.0051 |
Discussion
There is little information on the effects of music on physiological and hormonal parameters in different animal species, but its effects on autonomic responses, as well as on neurotransmitter levels in humans, are well documented20–22. We believe that part of the results observed in the study may be mediated by changes in hormone and neurotransmitter expression. However, only 5-HT (serotonin) was evaluated in this study, and it was observed that both musical rhythms evaluated promoted a significant increase (62 and 65 ng/ml for Moderate-tempo and Slow-tempo respectively) in serum levels after seven days of exposure to musical stimuli.
Among the effects observed were a reduction in heart rate, eye temperature and an increase in serum calcium levels (moderate-tempo music), a reduction in respiratory rate (slow-tempo music), as well as an increase in the quantity and concentration of mean corpuscular hemoglobin (MCH and MCHC) for both music tempos. These changes observed in horses exposed to both classical music rhythms may be attributed to various physiological mechanisms and their interactions.
Classical music may have a calming and stress-reducing effect, leading to an increase in serotonin levels, neurotransmitter, which is associated with mood regulation and stress reduction. The rhythmic patterns of classical music may stimulate serotonin release in the brain and peripheral tissues, contributing to an overall sense of well-being in the horses. Classical music is associated with reduced occurrence of alertness behavior, increased state of relaxation, lower psychophysiological stress in horses11,23,24 and positive emotional states for race horses25. According to11, music exposure applied in daily sessions of several hours has a positive effect on the relaxation of geriatric horses. Supporting our results22, reported that music intervention on children and adolescents increased 5-HT secretion, and reduced heart rate, blood pressure, and cortisol expression. Other research evaluating humans has shown that music may promote autonomic responses, which automatically causes physiological changes in blood circulation, respiration, skin conductivity, body temperature, heart rate, as well as pain and mood states6,26.
It is believed that both music tempos used were perceived as pleasurable by the animals, with effects prolonged even 30 min after the end of exposure to the treatments. Evaluating the use of classical music during the transportation of horses27, observed that the heart rate of these animals returned to its normal parameters more quickly after transportation when compared to horses transported without music. This way, it is understood that harmonic sounds and their specific combinations of pitch, tempo, intervals, and timbre can provide different responses, with slow-tempo music, low notes, or lower tonality, causing a calming effect21,28.
The physiological changes observed in horses exposed to both classical music rhythms suggest a coordinated response mediated by the autonomic nervous system (ANS), particularly involving the sympathetic (SNS) and parasympathetic (PNS) branches. Despite being subtle, these changes indicate parasympathetic dominance, prompting relaxation responses characterized by bradycardia, bronchoconstriction, and increased peripheral blood flow29.
The reduction in heart rate observed in horses exposed to moderate-tempo music is consistent with a shift toward parasympathetic dominance that acts to decrease heart rate and promote rest and recovery30,31. In turn, the decrease in respiratory frequency in horses during exposure to slow-tempo music can also be attributed to the prevalence of the vagal activity, which influences respiratory functions by decreasing its frequency during periods of rest and relaxation, allowing deeper and more controlled breathing. It should be noted that the average heart rate values obtained in all treatments are close to the reference values (28 to 40 bpm) established for adult horses at rest and in environmental conditions similar to the present study32. This similarity may be attributed to the mild nature of the stressor since the horses were accustomed to spending part of their time in the same stalls, and social isolation was only partial, as they could see other horses in adjacent pens.
Additionally, autonomic innervation of the ocular choroid includes parasympathetic pathways that dilate vessels and increase blood flow and sympathetic pathways that constrict vessels and decrease blood flow33,34. Thus, the induction of a state of relaxation promoted by classical music with a moderate rhythm, with consequent vasodilation of the ocular vessels and increased blood flow, may have contributed to the observed increase in ocular temperature.
Research has revealed a complex relationship between serotonin, parathyroid hormone (PTH), and intestinal calcium absorption. PTH has been shown to stimulate intestinal calcium absorption, leading to a significant increase in serum calcium levels35–39. Consequently, we hypothesize that the increase in central serotonin observed in horses exposed to moderate-tempo classical music may be partly associated with the elevated serum calcium levels found in these animals.
Serotonin also plays a complex role in blood circulation and vascular tone, exhibiting both vasoconstrictor and vasodilator effects. It can promote vasodilation in certain vascular beds, thereby enhancing blood flow and oxygen delivery to tissues40,41. Furthermore, serotonin can attenuate the formation of memories related to fear and stress, consequently lower responses to threatening events through serotonergic projections that depart from the raphe nuclei to the hippocampus42. The higher concentrations of serotonin found after musical stimulation may be related to a greater release of dopamine, a neurotransmitter of the brain reward system. An anticipation of reward system may explain the role of reinforcers in learning process43, associating the musical stimuli with the enjoyment of been released into the paddock.
The alterations seen in serotonin, calcium, MCH, and hemoglobin concentrations in horses exposed to classical music indicate a cohesive physiological response. Music’s ability to induce relaxation and reduce stress may initiate a series of biochemical and hormonal changes, thereby affecting metabolism. These interconnected processes demonstrate the body’s adaptive mechanisms, ensuring homeostasis and optimizing physiological function in reaction to environmental stimuli.
It is important to note that although the effects discussed above were observed, they occurred discreetly and the parameters assessed remained within the values considered normal for the species. It is believed that the restriction of movement for a short period of time and partial social isolation, since, despite being housed individually, the animals still had restricted visual communication between their peers, were probably not enough to induce severe stress in the animals. Future research should be carried out with animals that have higher levels of stress and are housed individually for longer periods without access to paddocks.
In general, the mechanisms of interaction between these parameters highlight the complex interaction between neurotransmitter signaling, mineral metabolism, hematopoiesis and oxygen transport in the body. It is important to note that although these explanations provide a plausible physiological basis for the changes observed, more research is needed to validate these findings and elucidate the specific mechanisms involved in the effects of music, as well as its particularities such as rhythm, on these physiological processes in horses.
Conclusion
Classical music with different characteristics, especially tempo, promoted different physiological responses in stabled horses, however, consistent with a positive emotional state, corroborated by an increase in circulating serotonin levels. In addition, its benefits can extend to better performance during the practice of activities supported by the greater quantity and concentration of hemoglobin and consequently greater oxygen supply.
Author contributions
Conceptualization: F.Y.U. Oliveira; A.M.Odakura; R.G.Garcia and F.R.CaldaraData curation: Not applicable. Formal analysis: F.Y.U. Oliveira; M.F.C.Burbarelli and F.R.Caldara. Funding acquisition: F.Y.U. Oliveira; R.G.Garcia; I.C.L.A.Paz and F.R.Caldara. Investigation: F.Y.U. Oliveira; A.M.Odakura; F.R.Caldara; M.F.C.Burbarelli; I.C.L.A.Paz; J.M. Braz and C.C.Ouros. Methodology: F.Y.U. Oliveira; R.G.Garcia and F.R.Caldara. Project administration: F.Y.U. Oliveira; R.G.Garcia and F.R.Caldara. Supervision: F.R.Caldara. Visualization: F.Y.U. Oliveira; A.M.Odakura; F.R.Caldara; M.F.C.Burbarelli; J.M. Braz I.; C.L.A.Paz and C.C.Ouros. Writing – original draft: F.Y.U. Oliveira; A.M.Odakura; F.R.Caldara; I.C.L.A.Paz and C.C.Ouros. Writing – review & editing: F.Y.U. Oliveira; A.M.Odakura; J.M. Braz; F.R.Caldara and C.C.Ouros.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mcbride, S. & Hemmings, A. A neurological perspective of equine stereotype. Equine Vet. J.29 (1), 10–16. 10.1016/j.jevs.2008.11.008 (2009). [Google Scholar]
- 2.Hemmings, A., Mcbride, S. & Hale, C. Preservative responding of the equine oral stereotypy. Appl. Anim. Behav. Sci.104 (1–2), 143–150. 10.1016/j.applanim.2006.04.031 (2007). [Google Scholar]
- 3.Foppa, L. et al. Environmental enrichment and behaviour of pigs: review. Revista Brasileira De Engenharia De Biossistemas. 8 (1), 1–7. 10.18011/bioeng2014v8n1p1-7 (2014). [Google Scholar]
- 4.Van De Weerd, H. & Ison, S. Providing effective environmental enrichment to pigs: how far have we come? Animals9 (5), 254. 10.3390/ani9050254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peretz, I., Gagnon, L. & Bouchard, B. Music and emotion: perceptual determinants, immediacy, and isolation after brain damage. J. Cogn. 68 (2), 111–141. 10.1016/s0010-0277(98)00043-2 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Khalfa, S., Roy, M., Rainville, P., Simone, S. D. & Peretz, I. Role of tempo entrainment in psychophysiological differentiation of happy and sad music? Int. J. Psychophysiol.68 (1), 17–26. 10.1016/j.ijpsycho.2007.12.001 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Lippi, I. C. C. et al. Effects of music therapy on neuroplasticity, welfare, and performance of piglets exposed to music therapy in the intra- and extra-uterine phases. Anim. (Basel). 12 (17), 2211. 10.3390/ani12172211 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendes, J. P. et al. Performance and welfare of sows exposed to auditory environmental enrichment in mixed or collective housing systems. Animals13, 1226. 10.3390/ani13071226 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houpt, K., Marrow, M. & Seeliger, M. A preliminary study of the effect of music on equine behaviour. Equine Vet. J.20 (11), 691–693. 10.1016/S0737-0806(00)80155-0 (2000). [Google Scholar]
- 10.Wilson, M. et al. Effect of music on the behavioural and physiological responses of stabled weanlings. Equine Vet. J.31 (5), 321–322. 10.1016/j.jevs.2011.03.157 (2011). [Google Scholar]
- 11.Wiśniewska, M., Janczarek, I., Wilk, I. & Wnuk-Pawlak, E. Use of music therapy in aiding the relaxation of geriatric horses. J. Equine Veterinary Sci.78, 89–93. 10.1016/j.jevs.2018.12.011 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Panksepp, J. & Bernatzky, G. Emotional sounds and the brain: the neuro-affective foundations of musical appreciation. Behav. Process.60 (2), 133–155. 10.1016/s0376-6357(02)00080-3 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Stewart, M. et al. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav.92, 520–525. 10.1016/j.physbeh.2007.04.034 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Mcbride, S. D. & Mills, D. S. Psychological factors affecting equine performance. BMC Vet. Res.8, 180. 10.1186/1746-6148-8-180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook, N. et al. Adrenocortical and metabolic responses to ACTH injection in horses: an assessment by salivary cortisol and infrared thermography of the eye. Can. J. Anim. Sci.81 (4), 621 (2001). https://eurekamag.com/research/034/361/034361722.php [Google Scholar]
- 16.Aragona, F. et al. Eye Temperature measured with infrared thermography to assess stress responses to road transport in horses. Animals14, 1877. 10.3390/ani14131877 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aragona, F. et al. Using infrared thermography for the evaluation of road transport thermal homeostasis in athletic horse. J. Equine Vet. Sci. 105102. 10.1016/j.jevs.2024.105102 (2024). [DOI] [PubMed]
- 18.McDuffee, L., Mills, M., McNiven, M. & Montelpare, W. Establishing statistical stability for heart rate variability 775 in HORSES. J. Vet. Behav.32, 30–35. 10.1016/j.jveb.2019.05.003 (2019). [Google Scholar]
- 19.Feitosa, F. F. L. Semiologia veterinária: A Arte do Diagnóstico 4edn, 704 (Roca, 2020).
- 20.Chanda, M. L. & Levitin, D. J. The neurochemistry of music. Trends Cogn. Sci.17 (4), 179–193. 10.1016/j.tics.2013.02.007 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Ooishi, Y., Mukai, H., Watanabe, K., Kawato, S. & Kashino, M. Increase in salivary oxytocin and decrease in salivary cortisol after listening to relaxing slow-tempo and exciting fast-tempo music. PLoS One12 (12). 10.1371/journal.pone.0189075 (2017). [DOI] [PMC free article] [PubMed]
- 22.Park, J. I. et al. Effects of music therapy as an alternative treatment on depression in children and adolescents with ADHD by activating serotonin and improving stress coping ability. BMC Complement. Med. Ther.23, 73. 10.1186/s12906-022-03832-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter, C. & Greening, L. Auditory stimulation of the stabled equine; the effect of different music genres on behaviour. In: Proc. of the 8th International Equitation Science Conference, 167 (Royal (Dick) Veterinary School, 2012).
- 24.Huo, X., Wongkwanklom, M., Phonraksa, T. & Na-Lampang, P. Effects of playing classical music on behavior of stabled horses. Vet. Integr. Sci.19 (2), 259–267. 10.12982/VIS.2021.023 (2021). [Google Scholar]
- 25.Stachurska, A., Janczarek, I., Wilk, I. & Kędzierski, W. Does music influence emotional state in race horses? J. Equine Vet. Sci.35 (8), 650–656. 10.1016/j.jevs.2015.06.008 (2015). [Google Scholar]
- 26.Bernatzky, G., Presch, M., Anderson, M. & Panksepp, J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neurosci. Biobehav. Rev.5 (9), 1989–1999. 10.1016/j.neubiorev.2011.06.005 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Neveux, C. et al. Classical music reduces acute stress of domestic horses. J. Vet. Behav.15, 81. 10.1016/j.jveb.2016.08.019 (2016). 12th International Society of Equitation Science (ISES) Conference Abstract. [Google Scholar]
- 28.Ciborowska, P., Michalczuk, M. & Bień, D. The effect of music on livestock: cattle, poultry and pigs. Animals11 (12), 3572. 10.3390/ani11123572 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travain, T. & Valsecchi, P. Infrared thermography in the study of animals’ emotional responses: a critical review. Animals (Basel)11 (9), 26–2510. 10.3390/ani11092510 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pumprla, J., Howorka, K., Groves, D., Chester, M. & Nolan, J. Functional assessment of heart rate variability: physiological basis and practical applications. Int. J. Cardiol.84, 1–14. 10.1016/s0167-5273(02)00057-8 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Appelhans, B. M. & Luecken, L. J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol.10, 229–240. 10.1037/1089-2680.10.3.229 (2006). [Google Scholar]
- 32.Feitosa, F. L. F. Semiologia Veterinária: A Arte do Diagnóstico (Roca, 2004).
- 33.Reiner, A., Fitzgerald, M. E., Del Mar, N. & Li, C. Neural control of choroidal blood flow. Prog. Retin Eye Res.64, 96–130. 10.1016/j.preteyeres.2017.12.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu, F., Zhao, Y. & Zhang, H. Ocular autonomic nervous system: an update from anatomy to physiological functions. Vision. 10.3390/vision6010006 (2022). [DOI] [PMC free article] [PubMed]
- 35.Fleet, J. C., Bruns, M. E., Hock, J. M. & Wood, R. J. Growth hormone and parathyroid hormone stimulate intestinal calcium absorption in aged female rats. Endocrinology134 (4), 1755–1760. 10.1210/endo.134.4.8137740 (1994). [DOI] [PubMed] [Google Scholar]
- 36.Delling, G., Hehrmann, R. & Montz, R. Effect of dibutyryl cyclic AMP (DBcAMP) and parathyroid hormone (PTH) on intestinal calcium absorption (in vivo studies with 47 ca). Horm. Metab. Res.4 (1), 59–60. 10.1055/s-0028-1097083 (1972). [DOI] [PubMed] [Google Scholar]
- 37.Ducy, P. & Karsenty, G. The two faces of serotonin in bone biology. J. Cell. Biol. 191(1), 7–13. 10.1083/jcb.201006123 (2010). [DOI] [PMC free article] [PubMed]
- 38.Lavoie, B., Lian, J. B. & Mawe, G. M. Regulation of bone metabolism by serotonin. Adv. Exp. Med. Biol.1033, 35–46. 10.1007/978-3-319-66653-2_3 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Franco, R. et al. The effects of serotonin inhibitors on bone metabolism: literature review. J. Stomatol. 73 (3), 136–141. 10.5114/jos.2020.96942 (2020). [Google Scholar]
- 40.Vanhoutte, P.M., Cohen, R.A. & Van Nueten, J.M. Serotonin and arterial vessels. J. Cardiovasc. Pharmacol.6 (Suppl 2), S421–S428 (1984). [DOI] [PubMed]
- 41.Vanhoutte, P. M. Serotonin and the vascular wall. Int. J. Cardiol.14 (2), 189–203. 10.1016/0167-5273(87)90008-8 (1987). [DOI] [PubMed] [Google Scholar]
- 42.Joca, S. R., Padovan, C. R. & Guimarães, F. S. Stress, depression and the hippocampus. Revista Brasileira Psiquiatria2,46 – 1 (2003). [DOI] [PubMed]
- 43.Hausberger, M. et al. Mutual interactions between cognition and welfare: the horse as an animal model. Neurosci. Biobehav. Rev.107, 540–559. 10.1016/j.neubiorev.2019.08.022 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Jain, N. C. Essentials of Veterinary Hematologyv. 1 (Lea & Febiger, 1993).
- 45.Meyer, D. J. & Harvey, J. W. Veterinary Laboratory Medicine: Interpretation and Diagnosis, 3368 (W.B. Saunders Company, 2004).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.