Abstract
The study aimed to determine if virtual reality (VR) games could enhance neuromuscular control and improve anticipatory and compensatory strategies in ball-kicking for soccer players. It was a single-blind randomized clinical trial involving 32 male soccer players with chronic ankle instability. Participants were divided into two groups: VR games and balance training. The primary outcomes measured were the amplitude and onset time of muscle activities in the peroneus longus (PL), tibialis anterior (TA), soleus (SOL), rectus femoris (RF), biceps femoris (BF), and gluteus medius (GM) in three phases: anticipatory (APA), compensatory 1 (CPA1), and compensatory 2 (CPA2). Secondary outcomes included the Y-balance test and perceived ankle instability. Evaluations were done before and after treatment. Both groups underwent 12 sessions, three times a week, each lasting 60 min. In the VR games group, there was a significant decrease in SOL muscle activity amplitude during CPA2 after treatment (P = 0.033), and the BF muscle activated earlier (P = 0.043). The balance training group showed a significant increase in GM muscle activity amplitude during APA (P = 0.037) and earlier activation of the GM muscle post-treatment (P = 0.050). Additionally, this group demonstrated significant decreases in RF activity during CPA1 and PL activity in CPA1 and CPA2 (P = 0.048, P = 0.030, and P = 0.050, respectively). Between-group comparisons indicated a significant increase in GM muscle activity amplitude during APA and a reduction during CPA1 for the balance training group compared to the VR games group (P = 0.050 and P = 0.012, respectively). Both groups showed significant reductions in perceived ankle instability (VR group: P = 0.002, balance training group: P < 0.001) and improvements on the Y-balance test (VR group: P < 0.021, balance training group: P < 0.033), although neither group showed significant improvement in the anterior direction. Overall, both VR games and balance training effectively enhanced perceived ankle stability and dynamic postural control, with neither approach showing clear superiority. Both groups exhibited improvements in muscle activation timing, though neither outperformed the other. While both interventions led to increased muscle activity amplitude during the anticipatory and compensatory phases, the balance training group achieved somewhat greater improvements. These results suggest that both VR games and balance training are effective rehabilitation approaches for chronic ankle instability, providing comparable benefits for enhancing ankle stability and neuromuscular control, without a distinct advantage of one over the other.
RCT Registration: On the Iranian Registry of Clinical Trials (IRCT20230124057197N1). Registration date: 30/06/2023
Keywords: Feedback, Feed forward, Video game, Balance, Ankle instability
Subject terms: Rehabilitation, Health occupations, Orthopaedics
Introduction
The majority of injuries sustained by soccer players occur in the lower extremities, with the ankle accounting for up to a third of all these injuries1. Ankle sprains account for 67% of ankle injuries, and up to 40% of these cases develop into chronic ankle instability (CAI)1. CAI is characterized by ongoing pain, swelling, or the ankle giving way, with recurrent sprains persisting for at least 12 months after the initial injury2. This condition can result in extended absences from sports activities and, over time, can lead to osteoarthritis1.
One feature that contributes to recurrent sprains and a higher risk of re-injury is impaired postural control3. Numerous studies have demonstrated a strong connection between CAI and impaired postural control, which is crucial for athletic performance3,4. A healthy postural control system utilizes two strategies to maintain stability in response to perturbations: anticipatory postural adjustments (APAs) and compensatory postural adjustments (CPAs)5. APAs involve the central nervous system (CNS) controlling the center of gravity (COG) by activating trunk and leg muscles before a perturbation occurs, thus minimizing the risk of losing balance. These adjustments are managed through a feed-forward mechanism. Conversely, CPAs are activated by sensory feedback and help restore the COG after a perturbation5. Studies indicate that stronger anticipatory adjustments (feed-forward) reduce the need for compensatory adjustments (feedback) to maintain stability5–7.
Evidence indicates that individuals with CAI have deficiencies in both feedback and feed-forward postural strategies when dealing with perturbations8. This impairment is partly due to damage to mechanoreceptors from recurrent ankle sprains, which disrupts feedback mechanisms and hinders appropriate responses to perturbations9. Furthermore, the continuous reduction in sensory input from the ankle to the spinal and supra-spinal centers causes changes in the CNS, altering the brain’s motor programs. As a result, feed-forward strategies are compromised, diminishing postural preparation for various movements10.
Postural strategies can be assessed by analyzing muscle activity patterns. Several studies have shown that individuals with CAI exhibit different muscle activity patterns compared to healthy individuals during movements such as jumping, gait initiation, and transitioning from a two-legged to a one-legged stance11–13. They have found that the activation of ankle muscles, particularly the peroneal muscles, is delayed in response to perturbations, and the amplitude of their activity is reduced, increasing the risk of further injury11–13. These alterations in ankle muscle activity during functional activities underscore the differences in neuromuscular control between healthy individuals and those with CAI.
In soccer players, kicking the ball is a commonly repeated movement and is regarded as a perturbation. Rios et al. found that individuals with CAI employ distinct postural strategies for this task; they enhance proximal muscle activity (in the hip and spine) during compensatory phases to offset their ankle deficits and reduce balance sway14. These alterations in postural strategies during kicking are therapeutically significant because this movement is frequently performed in soccer, and such changes may increase the risk of ankle injury and lead to joint deterioration.
Recently, virtual reality (VR)-based rehabilitation programs have gained significant attention from researchers. In VR games, users interact with a virtual world they can observe, assess, and control their movements within. While traditional balance training has proven effective in improving postural control15, it often suffers from being monotonous, failing to engage participants, and sometimes leading individuals to discontinue treatment before achieving the desired results16–18. Consequently, a more engaging and entertaining treatment is needed. VR games present an appealing option. Additionally, traditional balance training may lack immediate feedback, which can reduce motivation. In contrast, VR games provide continuous visual and auditory feedback, enhancing engagement19. Instantaneous feedback allows users to focus more on their movements, with performance-based feedback in gaming environments potentially improving motor task learning20. Moreover, VR-based rehabilitation programs can address various cognitive and motor functions, including managing irrelevant stimuli, decision-making, concentration, strength, and balance21. This cognitive engagement is a notable advantage over conventional balance trainings. Given that cognitive load is a risk factor for recurrent sprains in individuals with CAI, who often have impaired information processing22,23, VR’s dual-task and multi-task training can improve motor skills while reducing reliance on conscious processing24. Researchers suggest that combined cognitive and motor exercises are more effective than motor or cognitive exercises alone, making VR games a promising option for integrated cognitive and motor training25. In VR games, users often need to shift their weight in various directions, speeds, and magnitudes while maintaining their COG within their support base. These controlled movements closely resemble ankle, hip, and trunk strategies, which can be effective in enhancing postural control25.
Despite the potential benefits, studies examining the effects of VR games on musculoskeletal disorders, particularly CAI, are limited. Existing evidence suggests that VR games can positively impact static and dynamic balance26,27, severity of perceived ankle instability28, proprioception28, muscle strength29, information processing speed30, and performance in individuals with CAI30. However, the effects on muscle activity patterns and postural strategies have not been explored. Some research indicates that VR-based exercises have more beneficial effects on anticipatory strategies than routine treatments for individuals with knee osteoarthritis31 and nonspecific low back pain32.
Despite growing interest in VR applications within rehabilitation and the critical role of postural strategies for soccer players with ankle instability, the impact of VR games on anticipatory and compensatory strategies in these individuals—particularly during functional tasks like kicking a ball, a common soccer activity—has yet to be investigated. Therefore, this study aims to address this gap by investigating whether VR games can effectively enhance neuromuscular control and improve these postural strategies during ball-kicking in male soccer players with CAI. Additionally, the findings offer valuable insights for integrating VR into training programs to enhance athletic performance and reduce injury risks among athletes.
As previously mentioned, APAs are used before a perturbation to minimize its impact, while CPAs occur after a perturbation to restore balance. Stronger APAs correlate with a reduced need for CPAs to maintain stability. Thus, an effective treatment would maximize improvements in APAs, thereby minimizing the effects of perturbations beforehand and reducing the need for CPAs. Additionally, a treatment would decrease muscle activation delay.
We hypothesized that, in both groups, the delay time of muscle activity would decrease, the amplitude of muscle activity would increase during anticipatory phase, and muscle activity would decrease during compensatory phase. Moreover, we expected both groups to show improvements in dynamic balance and the severity of perceived ankle instability, as assessed by clinical test and questionnaire, respectively. However, we anticipated that these improvements would be more pronounced with VR games than with traditional balance training.
The hypothesis that VR games offer greater improvements in postural strategies than traditional balance trainings is based on the unique cognitive demands and enhanced feedback mechanisms provided by VR. With VR’s cognitive component and its immersive, interactive design delivering continuous sensory input to the brain, VR games are anticipated to engage brain regions more extensively than balance training, thereby enhancing proactive strategies primarily mediated by the brain. Additionally, VR’s strong focus on real-time, dynamic feedback enables participants to detect and correct movement errors immediately, promoting a more effective learning process. In contrast, traditional balance training provides limited, static feedback, allowing participants to make adjustments only after performance information is provided, which leads to delayed corrections. Therefore, we hypothesize that VR’s enriched cognitive and sensory engagement will results in more substantial improvements in postural strategies.
Method
Study design
This study was a randomized, single-blinded, controlled trial. Participants were randomly assigned to either a group receiving VR games or a group performing balance training. Outcome measures assessed before and after the intervention. Post-tests were performed within 24–48 h after completion of treatment. Primary outcome measures included electromyography-related parameters (normalized integrals of electromyography activity (NIEMG) during the phases of anticipatory and compensatory as well as muscle onset latency). Secondary outcomes included dynamic balance and severity of perceived ankle instability, measured using the Y-balance test (YBT) and the Cumberland ankle instability tool (CAIT), respectively. Both groups underwent individual treatment sessions in a laboratory environment. This study was registered as a clinical trial on the Iranian Registry of Clinical Trials (IRCT20230124057197N1) on 30/06/2023.
Participants
Male soccer players with CAI were recruited from the university community, sports clubs, and federations using posters and social media advertisements (Table 1). A flow diagram based on the CONSORT statement illustrates the participants’ progress from enrollment to analysis (Fig. 1). All participants provided written informed consent. All protocols of this research adhered to the ethical principles outlined in the Declaration of Helsinki and received approval from the Tehran University of Medical Sciences Ethics Committee (IR.TUMS.FNM.REC.1401.149).
Table 1.
The demographical and clinical characteristics of subjects at baseline.
| Characteristics | Intervention (N = 16) | Control (N = 16) | P value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 21.44 | 2.06 | 23.44 | 5.24 | 0.526 |
| Height (cm) | 175.19 | 6.86 | 175.06 | 8.06 | 0.870 |
| Weight (kg) | 72.03 | 8.67 | 73.63 | 8.22 | 0.836 |
| CAIT (0–30) | 17.31 | 2.84 | 15.50 | 4.84 | 0.207 |
| Time since first sprain (months) | 17.63 | 7.08 | 24.38 | 15.56 | 0.315 |
| Time since last sprain (months) | 4.13 | 2.33 | 8.06 | 8.23 | 0.243 |
| Number of giving way episodes | 5.94 | 3.21 | 6.44 | 3.97 | 0.820 |
| Physical activity (hours/week) | 8.94 | 4.70 | 8.59 | 5.73 | 0.894 |
| Affected side (L/R) | 5/11 | 5/11 | 1.000 | ||
| Dominant side (L/R) | 3/13 | 2/14 | 0.626 | ||
CAIT Cumberland Ankle Instability Tool, L Left, R Right.
Fig. 1.
The CONSORT flowchart.
The inclusion criteria were as follow: (1) Male, (2) soccer player, (3) age between 18 and 40 years; (4) self-reported history of the initial acute ankle sprain more than one year ago2; (5) history of at least two episodes of ‘giving way’ and ‘feelings of ankle joint instability’ within the last 6 months2; (6) the most recent ankle sprain occurred > 3 months prior to study enrollment2; (7) CAIT score ≤ 24 (questionnaire scoring ranges from 0–30, with a lower score representing more ankle instability)2; (8) recreationally active (at least three times a week for more than 30 min each session)33; (9) no self-reported history of fracture or surgery in the lower extremity; (10) no psychological or neurological disorders; (11) no prior experience of training with video games. Participants were excluded if they missed three or more non-consecutive sessions or two consecutive sessions, as well as if they were incapable of performing required maneuvers.
Sample size
The sample size was determined using G*Power 3.1.3 software based on a pilot study involving 10 subjects, focusing on the muscle latency and NIEMG of the peroneus longus during the APA phase. The calculation utilized within-between interaction effect sizes of 0.071 and 0.082, derived from preliminary data observed in the pilot study. These effect sizes were selected to reflect the magnitude of the observed effects in our initial findings, providing a foundation for our sample size estimation. A power of 0.8, α = 0.05, non-sphericity correction (ε) = 1, and a correlation among repeated measures of 0.5 were applied. The analysis indicated that at least 28 participants were needed to detect a within-between interaction effect in a test design of 2 groups and 2 measurements with the specified parameters. Consequently, 32 participants were recruited to account for a potential 15% dropout rate.
Randomization and blinding
The participants were randomly assigned to either the intervention group (VR games) or the control group (balance training) with a 1:1 allocation ratio. Block randomization (block size = 4) was performed using a web-based randomization service (http://www.randomization.com). Random allocation was carried out by a third party not involved in the study. Details of the allocated groups were written on cards and concealed using sequentially numbered, opaque, sealed envelopes. In this way, the allocation sequence was concealed from the main researchers.
In this study, the assessor was blinded to group allocation because the assessor and therapist were different individuals; however, the participants were not blinded. Blinding participants was not feasible due to the nature of the two distinct interventions—VR games and balance training—each providing unique experiences. The differences in training methods, including equipment, environment, and physical setup, make it difficult for participants to remain unaware of their group assignment. Both interventions require active participation, which further clarifies to participants which group they belong to, rendering blinding impractical. Moreover, the consent form that participants sign includes the study title and general details about the implementation, informing them of the existence of another group. Therefore, although participant blinding is desirable, it is not feasible in such studies.
Assessments
Assessments were done before and after the intervention. Post-tests were performed within 24 to 48 h after completion of treatment.
Postural strategies during ball-kicking task
The surface EMG of the peroneus longus (PL), tibialis anterior (TA), soleus (SOL), rectus femoris (RF), biceps femoris (BF), and gluteus medius (GM) muscles on the affected limb, as well as adductor longus (ADL) muscle on the unaffected limb, was recorded. This was done using integral dry reusable electrodes (SX230, Biometrics Ltd, Gwent, UK) with a 10 mm diameter, bipolar configuration, and 20 mm inter-electrode distance, connected to an eight-channel EMG system (DataLog P3X8, Biometrics Ltd, Gwent, UK) with a CMRR of > 96 dB at 60 Hz, input impedance > 1012 Ω, gain of 1000, and a band-pass filter from 20 to 450 Hz. After shaving and cleaning the skin with alcohol, the electrodes were placed on the muscle bellies aligned with the muscle fibers. For the PL, the electrode was positioned on the upper quarter of the line connecting the fibula head to the lateral malleolus. For the TA, it was placed on the upper third of the line connecting the fibula head to the medial malleolus. For the SOL, it was placed on the middle two-thirds of the line connecting the femur’s upper condyle to the medial malleolus. For the RF, it was positioned in the middle of the line connecting the anterior superior iliac spine to the upper patella. For the BF, the electrode was placed in the middle of the line connecting the ischial tuberosity to the tibia’s lateral epicondyle. For the GM, it was positioned in the middle of the line connecting the iliac crest to the greater trochanter. For the ADL, it was placed on the upper third of the line connecting the pubic tubercle to the muscle attachment on the femur. These electrode placements were done according to the SENIAM guidelines (http://www.seniam.org). The ground electrode was placed on the wrist.
To complete the ball-kicking task, participants stood on their injured limb in a single-limb stance, with the opposite limb (used for kicking) slightly flexed and externally rotated, positioning the heel of the kicking limb at the height of the medial malleolus of the injured limb. A laboratory-made ball launcher was used to ensure consistency across trials and participants. As shown in Fig. 2, the launcher consisted of a quadripod with a 120 cm long galvanized pipe. The height of the quadripod was 83 cm, and the pipe was angled 30 degrees forward, forming a 60-degree angle with the quadripod. The distance between the participant and the quadripod was 136 cm. In this setup, a 350-g futsal ball was launched at each participant under identical conditions14.
Fig. 2.
Ball-kicking test.
Participants were instructed to kick the ball while maintaining balance on their injured limb. Before launching the ball, participants were asked to indicate when they felt most stable by saying the word “okay.” They were then asked to kick the ball towards a gate in front of them, measuring 40 cm in height and 80 cm in width. Participants had to keep their gaze on the ball, with no requirement for speed or force in the kick. After kicking, they were asked to maintain balance and gently place their foot on the ground. If the ball did not enter the gate, the trial was repeated. Two familiarization trials and five main trials were conducted14.
To determine the anticipatory and compensatory phases, the onset of ADL muscle activity in the kicking leg was identified and marked as time t0. This onset was defined as the point when the EMG amplitude exceeded the mean baseline activity by ± 2 standard deviations for at least 25 ms14. The timing of the four phases (baseline, APA, CAP1, and CPA2) was defined as follows: Baseline phase: 800 to 1000 ms before t0 (− 1000 to − 800 ms); APA phase: 200 ms before t0 to t0 (− 200 to 0 ms); CPA1 phase: t0 to 200 ms after t0 (0 to + 200 ms); CPA2 phase: 200 to 400 ms after t0 (+ 200 to + 400 ms).
Then, the integral EMG activity (IEMG) in each time window was calculated according to the Eq. (1), which was actually the sum of the absolute value of the amplitude of EMG activities34.
| 1 |
Then, IEMG during the APA and CAP time windows were subtracted from the IEMG of the baseline activation (Eq. 2)14.
| 2 |
To compare the amplitude of muscle activity between participants, the data were normalized. For each participant and each muscle, the maximum IEMG across phases and trials was identified, and the IEMG in each phase was divided by this maximum value. This normalization resulted in a range from − 1 to + 1, where positive values indicated muscle activity and negative values indicated muscle inhibition. NIEMGs were then averaged during the APA and CAP time windows across five trials for each participant14,34.
Additionally, the time interval between the onset of muscle activity in the injured limb and the onset of ADL muscle activity in the kicking limb was considered the muscle activity delay time. Earlier onset relative to the ADL muscle was recorded as negative and later onset was recorded as positive14.
Expanding upon Rios’s research on muscle activity during ball-kicking in individuals with CAI, this study selected specific muscles for EMG measurement due to their essential roles in maintaining postural stability. For example, the PL is vital for ankle stability during single-leg stance, preventing excessive foot inversion and aiding in balance throughout the kick. The TA and SOL muscles cover the front and back of the ankle, respectively, and are responsible for stabilizing posture by controlling dorsiflexion and plantarflexion. The RF contributes to hip flexion and knee extension, essential for maintaining stability on these joints. The BF supports knee flexion and helps stabilize the pelvis during dynamic movements, which is crucial for maintaining balance while kicking. Finally, the GM is a key stabilizer of the hip and pelvis, controlling lateral movements and preventing hip drop, essential during the single-leg stance required for the kicking action14.
Y-Balance Test (YBT)
The Y Balance Test (YBT) was employed to evaluate dynamic balance. Participants stood on their injured limb and extended the other limb in three specified directions on the ground: anterior, posteromedial (PM), and posterolateral (PL). The PM and PL directions were set at 90-degree angles to each other, with both forming 135-degree angles with the anterior direction. Participants were instructed to reach the farthest possible point in each direction with their moving foot while maintaining balance. The distance from the center to the reached point was measured. To normalize the results, the distance was divided by the length of the lower limb (measured from the anterior superior iliac spine to the center of the medial malleolus) and then multiplied by 100 to obtain a percentage value relative to the lower limb length. Each participant performed the test three times in each direction, and the average distance from the three trials was used for data analysis. If the participant’s moving foot touched any point other than the intended endpoint, if the supporting foot lifted off the ground, or if the participant lost balance, the trial was deemed invalid and repeated. Previous studies have shown that the YBT is a reliable and valid method for assessing dynamic balance35.
Cumberland Ankle Instability Tool (CAIT)
The CAIT was employed to assess the severity of perceived ankle instability. It consists of 9 questions, with a maximum possible score of 30, where higher scores reflect greater ankle stability. The Persian version of this questionnaire demonstrates good internal consistency (Cronbach’s α of 0.78 for the right ankle and 0.79 for the left ankle) and substantial reliability (ICC(2,1) = 0.88; 95% CI: 0.86–0.90) among athletes36.
Groups
The groups consisted of VR games and balance training. The treatment period for both groups included 12 sessions, occurring three times per week, with each session lasting 60 min. All trainings were done individually and under the supervision of a therapist.
VR games
VR games were conducted using a Wii Balance Board (Nintendo Co. Ltd., Kyoto, Japan), featuring activities such as Single Leg Extension, Torso Twist, Single Leg Twist, Sideways Leg Lift, Rowing Squat, Table Tilt, Penguin Fishing, Soccer Heading, Tightrope Walk, and Snowboard Slalom. The sequence of games in each session was tailored to participants’ preferences, with each game lasting 6 min to include all activities within a session. Typically, each game offered three difficulty levels (beginner, advanced, and expert), with four sub-levels within each level (unstable, amateur, professional, and champion). Participants advanced to higher levels upon successfully completing their current level. Difficulty progression was managed by the Wii Fit game itself, with illuminated stars indicating successful completion and participants earning more stars and points for better balance. Some games required participants to keep their COG within a designated yellow area, while others involved guiding the game character by shifting weight in different directions and speeds. These games imposed cognitive demands, requiring attention and quick responses to visual stimuli. Additionally, games like Table Tilt and Penguin Slide involved planning, while Soccer Heading required decision-making and response inhibition21,37. Thus, the selected games encompassed both cognitive and motor components. Further details about the games are provided in Table 2.
Table 2.
The description of games.
| Games | Explains |
|---|---|
| Single leg extension | The participant balanced on one leg while swinging the opposite leg forward and backward and moving the upper limbs in opposite directions, ensuring that the COG stayed within a designated yellow circle shown on the screen |
| Torso twist | The participant sustained balance while twisting their upper body, ensuring that the COG remained within a specified yellow circle displayed on the screen |
| Single leg twist | The participant sustained balance while lowering one hand down and raising the opposite knee up to touch it with the back of the hand, ensuring that the COG remained within a specified yellow circle shown on the screen |
| Sideways leg lift | The participant maintained balance on one leg while lifting the other leg sideways and raising the opposite arm, ensuring that the COG remained within a designated yellow circle shown on the screen |
| Rowing squat | The participant balanced on their legs, pushing their hips back and bending their knees to about 120 degrees while simultaneously pulling their arms in towards their sides. The goal was to synchronize the squatting motion with the rowing movements. Visual cues displayed on the screen assisted players in maintaining proper form and timing, and they received feedback on their performance. This exercise targets various muscle groups, including the legs, core, and upper body, to improve strength, endurance, and coordination |
| Table tilt | The participant shifted their weight forward and side to side to align their COG with the base of support, guiding balls into corresponding holes |
| Penguin fishing | The participant shifted their weight forward and sideways to adjust the COG over their base of support, allowing them to control the movement of a penguin on ice and catch a fish |
| Soccer heading | The participant adjusted their COG to head the virtual soccer ball while striving to avoid colliding with other objects |
| Tightrope walk | The participant maintained rhythmic weight shifting to walk steadily without stumbling, while also navigating around obstacles |
| Snowboard slalom | The participant shifted their weight from side to side to guide the snowboarder through a series of gates as quickly as possible while maintaining balance and avoiding obstacles |
COG center of gravity.
Overall, the VR games engage participants in dynamic activities that demand constant adjustments to their COG, closely mirroring the ankle, hip, and trunk strategies used in real-world balance and stability tasks. Furthermore, these games deliver real-time feedback that is essential for fine-tuning postural control strategies. This immediate and continuous visual and auditory feedback not only increases awareness of body mechanics but also promotes the development of stronger, more coordinated movements, thereby enhancing APAs. In addition, the cognitive aspects of the games—such as decision-making, reaction time, and response inhibition—are contributor in effective APAs. For instance, in the Soccer Heading activity, players must not only position their bodies accurately to strike the ball but also anticipate its trajectory and speed. This requires rapid cognitive processing and precise motor planning. Such engagement in decision-making tasks activates the brain and fosters the development of neural pathways linked to balance and coordination, thereby enhancing the body’s ability to anticipate and prepare for perturbations.
Balance training
The balance training program encompassed various exercises, including single-limb stance with manipulating visual and proprioceptive inputs, single-limb stance with ball throwing, single-limb stance with ball kicking, single-limb hopping to stabilization, and hopping to stabilization with reaching. Additional details about the training can be found in Table 338–40. Participants progressed to higher levels upon successfully completing their current level, with error-free performance being necessary to advance. The required number of error-free repetitions is outlined in Table 3. During the training, the therapist provided detailed verbal instructions to correct errors and reinforce proper techniques. Over time, these instructions were gradually reduced to encourage participants to perform the exercises successfully and independently.
Table 3.
The description of balance training.
| Difficulty levels | Instructions | Criteria for progression | Errors | |
|---|---|---|---|---|
| Single-limb stance |
(1) Eyes open, hard surface, 30 s, 3 Reps (2) Eyes open, hard surface, 60 s, 3 Reps (3) Eyes open, foam surface, 30 s, 3 Reps (4) Eyes open, foam surface, 60 s, 3 Reps (5) Eyes open, foam surface, 90 s, 3 Reps (6) Eyes open, foam surface, 30 s, ball throwing, 3 Reps (7) Eyes open, foam surface, 60 s, ball throwing, 3 Reps (8) Eyes open, foam surface, 90 s, ball throwing, 3 Reps (9) Eyes closed, hard surface, 30 s, arms out, 3 Reps (10) Eyes closed, hard surface, 30 s, arms across, 3 Reps (11) Eyes closed, foam surface, 30 s, arms out, 3 Reps (12) Eyes closed, foam surface, 30 s, arms across, 3 Reps |
The participant attempted to maintain balance on the affected limb while facing challenges such as altering the base of support, closing eyes, extending the duration, and throwing a ball38,59 | If the participant could complete 3 repetitions without errors at each level of difficulty |
Errors were defined as: A. Touching the ground with the opposite foot B. Excessive trunk motion (more than 30 degrees of lateral flexion) C. Resting the opposite limb against the stance limb D. Lifting the hands from the chest while standing |
| Limb stance with ball kicking |
(1) Double-limb stance, hard surface, ball Kicking, 3 Reps (2) Single- limb stance, hard surface, ball Kicking, 3 Reps (3) Double-limb stance, mini-trampoline, ball Kicking, 3 Reps (4) Single- limb stance, mini-trampoline, ball Kicking, 3 Reps |
The participant was instructed to return the ball to the therapist and maintain balance as much as possible after kicking. During this training, the uninjured limb was used for kicking, while the affected limb served as the supporting limb60 | If the participant successfully completed 3 repetitions without any errors at each difficulty level | Errors included A, B, C, and D mentioned earlier |
| Single-limb hop to stabilization |
(1) Target at 18 inch, using arms to aid in stabilizing, 10 Reps (2) Target at 18 inch, hands on hips while stabilizing, 10 Reps (3) Target at 27 inch, using arms to aid in stabilizing, 10 Reps (4) Target at 27 inch, hands on hips while stabilizing, 10 Reps (5) Target at 36 inch, using arms to aid in stabilizing, 10 Reps (6) Target at 36 inch, hands on hips while stabilizing, 10 Reps |
The participant was instructed to perform 10 hops in four directions: 1. Anterior–posterior 2. Medial–lateral 3. Anteromedial-posterolateral 4. Anterolateral-posteromedial Three target distances (18, 27, or 36 inches) from the starting point were set. The participant hopped to these targets, stabilized their balance on one limb, then hopped back in the opposite direction to the starting position and stabilized again on one limb59 |
If the participant successfully completed 10 repetitions without any errors at each level of difficulty | Errors included A, B, C, and D mentioned earlier |
| Hop to stabilization and reach |
(1) Target at 18 inch, using arms to aid in stabilizing, 5 Reps (2) Target at 18 inch, hands on hips while stabilizing, 5 Reps (3) Target at 27 inch, using arms to aid in stabilizing, 5 Reps (4) Target at 27 inch, hands on hips while stabilizing, 5 Reps (5) Target at 36 inch, using arms to aid in stabilizing, 5 Reps (6) Target at 36 inch, hands on hips while stabilizing, 5 Reps |
The participant hopped, stabilized, and reached back to the starting position. Then, they returned to the starting position and reached to the target position59 | If the participant successfully completed 5 repetitions without errors at each difficulty level | Errors included A, B, C, and D mentioned earlier |
Reps Repetitions.
Overall, balance training exercises like single-limb stance with ball throwing and kicking incorporate perturbation-based elements that simulate on-field sports challenges. This type of training enhances APAs by training the body to respond effectively to anticipated perturbations, thereby improving control and timing of muscle activation. Furthermore, the training includes sensory-motor tasks that manipulate visual and proprioceptive inputs, specifically targeting both spinal and supra-spinal pathways. This comprehensive approach enhances participants’ ability to stabilize themselves in dynamic situations, enhancing both anticipatory and compensatory responses.
Statistical analysis
The Shapiro–Wilk test was used to evaluate the normality of the data distribution. It was determined that the data were normally distributed except for age, physical activity level, number of giving way episodes, and time since the first and last ankle sprain. An independent t-test was applied to compare the two groups at baseline for normally distributed variables. For variables that did not follow a normal distribution, the Mann–Whitney U test was used. In addition, the Chi-square test was used to compare the frequency of affected limb, and dominant limb between the groups. The comparison of baseline values between the two groups revealed significant differences in the NIEMG of the soleus. Consequently, analysis of covariance (ANCOVA) was used to assess between-group differences, with baseline values as covariates. Additionally, a repeated measures ANOVA was conducted for each group to determine within-group (pre-post) effects. Moreover, partial eta squared (η2) was reported as a measure of effect size (small (0.01–0.06), moderate (0.06–0.14), and large (≥ 0.14)). In addition, the percentage difference was calculated according to the following formula:
It should be noted that the Intention to Treat (ITT) approach was used in the analysis. As depicted in the CONSORT flowchart, two participants from the intervention group and two from the control group did not participate in the post-treatment evaluation. Missing data were addressed using the Expectation Maximization (EM) algorithm, which replaces missing values through the Maximum Likelihood (ML) method. Consequently, the analysis was conducted as if there were no dropouts, including data from all 32 participants.
Results
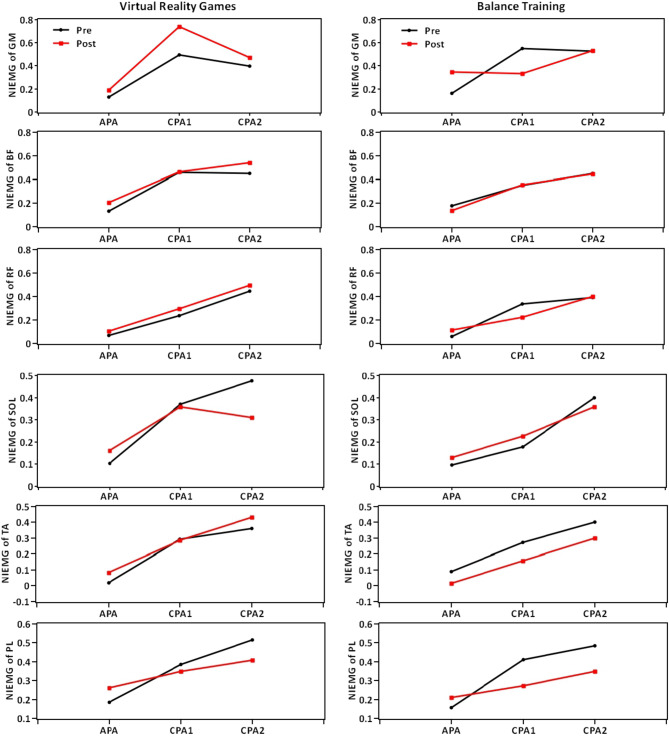
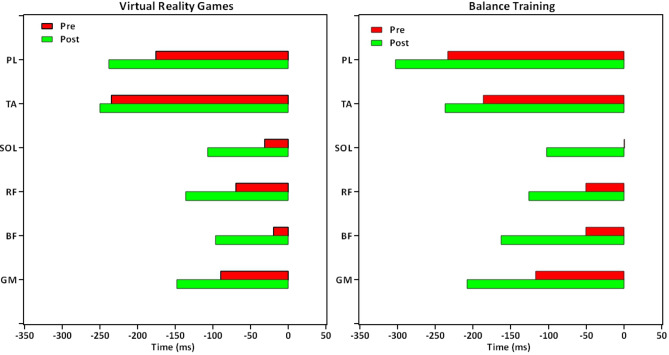
The descriptive data (mean ± standard deviation) and the results for between- and within-group comparisons are presented in Tables 4, 5, 6, 7. Moreover, Figs. 3 and 4 illustrate overall trends in muscle activity during the APA, CPA1, and CPA2 phases, as well as in muscle activation timing across both groups.
Table 4.
The descriptive data (mean and standard deviation) and ANCOVA results for between-group comparisons of the primary outcomes.
| Outcomes | Phase | Virtual reality games (N = 16) | Balance training (N = 16) | Adjusted mean difference | 95% Confidence interval | F | P | η2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Lower bound | Upper bound | ||||||
| NIEMG of GM | APA | 0.131 | 0.124 | 0.189 | 0.212 | 0.161 | 0.147 | 0.347 | 0.254 | − 0.168 | − 0.338 | 0.002 | 4.091 | 0.050* | 0.124 |
| CPA1 | 0.494 | 0.363 | 0.742 | 0.533 | 0.549 | 0.313 | 0.333 | 0.272 | 0.406 | 0.095 | 0.718 | 7.108 | 0.012* | 0.197 | |
| CPA2 | 0.399 | 0.283 | 0.470 | 0.544 | 0.526 | 0.319 | 0.534 | 0.398 | − 0.078 | − 0.436 | 0.280 | 0.200 | 0.658 | 0.007 | |
| NIEMG of BF | APA | 0.131 | 0.150 | 0.204 | 0.142 | 0.177 | 0.129 | 0.138 | 0.072 | 0.070 | − 0.013 | 0.153 | 2.940 | 0.097 | 0.092 |
| CPA1 | 0.458 | 0.211 | 0.465 | 0.192 | 0.349 | 0.160 | 0.355 | 0.173 | 0.144 | 0.011 | 0.277 | 3.363 | 0.077 | 0.104 | |
| CPA2 | 0.451 | 0.237 | 0.542 | 0.180 | 0.452 | 0.207 | 0.447 | 0.216 | 0.095 | − 0.048 | 0.237 | 1.848 | 0.184 | 0.060 | |
| NIEMG of RF | APA | 0.070 | 0.100 | 0.104 | 0.126 | 0.060 | 0.065 | 0.114 | 0.135 | − 0.009 | − 0.105 | 0.087 | 0.039 | 0.845 | 0.001 |
| CPA1 | 0.238 | 0.208 | 0.297 | 0.215 | 0.338 | 0.146 | 0.224 | 0.165 | 0.072 | − 0.074 | 0.219 | 1.016 | 0.322 | 0.034 | |
| CPA2 | 0.446 | 0.189 | 0.497 | 0.193 | 0.393 | 0.161 | 0.398 | 0.197 | 0.089 | − 0.054 | 0.232 | 1.624 | 0.213 | 0.053 | |
| NIEMG of SOL | APA | 0.103 | 0.113 | 0.161 | 0.151 | 0.096 | 0.111 | 0.131 | 0.192 | 0.028 | − 0.096 | 0.153 | 0.220 | 0.643 | 0.008 |
| CPA1 | 0.371 | 0.212 | 0.359 | 0.123 | 0.178 | 0.170 | 0.227 | 0.184 | 0.086 | − 0.038 | 0.211 | 2.021 | 0.166 | 0.065 | |
| CPA2 | 0.475 | 0.234 | 0.310 | 0.175 | 0.400 | 0.211 | 0.359 | 0.157 | − 0.064 | − 0.184 | 0.056 | 1.194 | 0.283 | 0.040 | |
| NIEMG of TA | APA | 0.018 | 0.278 | 0.082 | 0.175 | 0.090 | 0.166 | 0.016 | 0.150 | 0.059 | − 0.062 | 0.179 | 0.998 | 0.326 | 0.033 |
| CPA1 | 0.293 | 0.232 | 0.288 | 0.185 | 0.272 | 0.157 | 0.158 | 0.196 | 0.130 | − 0.010 | 0.271 | 3.600 | 0.068 | 0.110 | |
| CPA2 | 0.361 | 0.276 | 0.431 | 0.158 | 0.402 | 0.221 | 0.300 | 0.231 | 0.134 | − 0.011 | 0.279 | 3.573 | 0.069 | 0.110 | |
| NIEMG of PL | APA | 0.184 | 0.110 | 0.260 | 0.235 | 0.157 | 0.139 | 0.209 | 0.125 | 0.044 | − 0.093 | 0.182 | 0.435 | 0.515 | 0.015 |
| CPA1 | 0.386 | 0.202 | 0.350 | 0.138 | 0.410 | 0.172 | 0.273 | 0.165 | 0.081 | − 0.030 | 0.191 | 2.234 | 0.146 | 0.072 | |
| CPA2 | 0.514 | 0.195 | 0.408 | 0.188 | 0.485 | 0.137 | 0.349 | 0.214 | 0.065 | − 0.082 | 0.211 | 0.814 | 0.374 | 0.027 | |
| Onset latency of GM (ms) | − 89.88 | 94.88 | − 147.98 | 109.75 | − 117.33 | 206.14 | − 207.94 | 108.07 | 52.232 | − 20.942 | 125.405 | 2.131 | 0.155 | 0.068 | |
| Onset latency of BF (ms) | − 19.44 | 110.21 | − 96.72 | 135.01 | − 50.34 | 230.80 | − 162.83 | 138.21 | 63.053 | − 36.948 | 163.054 | 1.663 | 0.207 | 0.054 | |
| Onset latency of RF (ms) | − 69.19 | 124.47 | − 135.93 | 146.50 | − 50.50 | 214.33 | − 125.86 | 101.00 | − 9.351 | − 101.894 | 83.191 | 0.043 | 0.838 | 0.001 | |
| Onset latency of SOL (ms) | − 31.35 | 128.83 | − 106.83 | 109.66 | 1.01 | 189.36 | − 102.30 | 108.58 | − 2.145 | − 82.342 | 78.052 | 0.003 | 0.957 | 0.000 | |
| Onset latency of TA (ms) | − 234.88 | 177.14 | − 250.15 | 184.72 | − 186.35 | 214.18 | − 236.97 | 138.74 | − 8.652 | − 129.003 | 111.699 | 0.022 | 0.884 | 0.001 | |
| Onset latency of PL (ms) | − 176.00 | 126.91 | − 238.00 | 144.91 | − 233.74 | 262.83 | − 302.91 | 159.82 | 60.090 | − 52.541 | 172.721 | 1.191 | 0.284 | 0.039 | |
NIEMG Normalized Integrals of Electromyography Activity, GM Gluteus Medius, BF Biceps Femoris, RF Rectus Femoris, SOL Soleus, TA Tibialis Anterior, PL Peroneus Longus, APA Anticipatory Postural Adjustment, CPA Compensatory Postural Adjustment, SD Standard Deviation.
η2 is effect size (small = 0.01–0.06, medium = 0.06–0.14 and large ≥ 0.14). *Significant differences (P ≤ 0.05).
Table 5.
The results of repeated measures ANOVA for within-group comparisons of the primary outcomes.
| Outcomes | Phase | Within the virtual reality games group | Within the balance training group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% Confidence interval | F | P | η2 | Mean difference | 95% Confidence interval | F | P | η2 | ||||
| Lower bound | Upper bound | Lower bound | Upper bound | ||||||||||
| NIEMG of GM | APA | − 0.058 | − 0.194 | 0.079 | 0.816 | 0.381 | 0.052 | − 0.186 | − 0.359 | − 0.013 | 5.269 | 0.037* | 0.260 |
| CPA1 | − 0.249 | − 0.583 | 0.086 | 2.513 | 0.134 | 0.144 | 0.216 | − 0.035 | 0.467 | 3.373 | 0.086 | 0.184 | |
| CPA2 | − 0.070 | − 0.393 | 0.253 | 0.215 | 0.650 | 0.014 | − 0.009 | − 0.305 | 0.287 | 0.004 | 0.951 | 0.000 | |
| NIEMG of BF | APA | − 0.072 | − 0.170 | 0.025 | 2.505 | 0.134 | 0.143 | 0.039 | − 0.044 | 0.122 | 1.006 | 0.332 | 0.063 |
| CPA1 | − 0.007 | − 0.181 | 0.168 | 0.007 | 0.935 | 0.000 | − 0.006 | − 0.151 | 0.139 | 0.007 | 0.933 | 0.000 | |
| CPA2 | − 0.090 | − 0.258 | 0.077 | 1.317 | 0.269 | 0.081 | 0.005 | − 0.101 | 0.111 | 0.010 | 0.920 | 0.001 | |
| NIEMG of RF | APA | − 0.034 | − 0.118 | 0.050 | 0.728 | 0.407 | 0.046 | − 0.054 | − 0.141 | 0.033 | 1.756 | 0.205 | 0.105 |
| CPA1 | − 0.059 | − 0.222 | 0.105 | 0.584 | 0.457 | 0.037 | 0.114 | 0.001 | 0.226 | 4.654 | 0.048* | 0.237 | |
| CPA2 | − 0.051 | − 0.169 | 0.066 | 0.859 | 0.369 | 0.054 | − 0.006 | − 0.143 | 0.132 | 0.007 | 0.933 | 0.000 | |
| NIEMG of SOL | APA | − 0.059 | − 0.127 | 0.010 | 3.324 | 0.088 | 0.181 | − 0.035 | − 0.155 | 0.086 | 0.373 | 0.550 | 0.024 |
| CPA1 | 0.012 | − 0.083 | 0.107 | 0.077 | 0.785 | 0.005 | − 0.049 | − 0.175 | 0.078 | 0.672 | 0.425 | 0.043 | |
| CPA2 | 0.166 | 0.015 | 0.316 | 5.487 | 0.033* | 0.268 | 0.040 | − 0.062 | 0.143 | 0.705 | 0.414 | 0.045 | |
| NIEMG of TA | APA | − 0.064 | − 0.242 | 0.114 | 0.589 | 0.455 | 0.038 | 0.074 | − 0.064 | 0.213 | 1.303 | 0.272 | 0.080 |
| CPA1 | 0.005 | − 0.156 | 0.166 | 0.004 | 0.949 | 0.000 | 0.113 | − 0.021 | 0.248 | 3.222 | 0.093 | 0.177 | |
| CPA2 | − 0.071 | − 0.239 | 0.098 | 0.799 | 0.386 | 0.051 | 0.102 | − 0.052 | 0.256 | 1.989 | 0.179 | 0.117 | |
| NIEMG of PL | APA | − 0.076 | − 0.197 | 0.046 | 1.760 | 0.204 | 0.105 | − 0.052 | − 0.152 | 0.048 | 1.216 | 0.287 | 0.075 |
| CPA1 | 0.036 | − 0.077 | 0.148 | 0.464 | 0.506 | 0.030 | 0.138 | 0.015 | 0.260 | 5.740 | 0.030* | 0.277 | |
| CPA2 | 0.107 | − 0.056 | 0.270 | 1.946 | 0.183 | 0.115 | 0.135 | − 0.003 | 0.274 | 4.328 | 0.050* | 0.224 | |
| Onset latency of GM (ms) | 58.101 | − 12.187 | 128.389 | 3.104 | 0.098 | 0.171 | 90.608 | 0.132 | 181.085 | 4.556 | 0.050* | 0.233 | |
| Onset latency of BF (ms) | 77.286 | 2.690 | 151.882 | 4.877 | 0.043* | 0.245 | 112.489 | − 28.705 | 253.682 | 2.884 | 0.110 | 0.161 | |
| Onset latency of RF (ms) | 66.744 | − 41.511 | 174.999 | 1.727 | 0.209 | 0.103 | 75.356 | − 40.237 | 190.949 | 1.931 | 0.185 | 0.114 | |
| Onset latency of SOL (ms) | 75.473 | − 15.487 | 166.433 | 3.128 | 0.097 | 0.173 | 103.304 | − 2.465 | 209.072 | 4.334 | 0.055 | 0.224 | |
| Onset latency of TA (ms) | 15.271 | − 93.900 | 124.442 | 0.089 | 0.770 | 0.006 | 50.618 | − 94.565 | 195.802 | 0.552 | 0.469 | 0.036 | |
| Onset latency of PL (ms) | 62.004 | − 53.448 | 177.456 | 1.310 | 0.270 | 0.080 | 69.171 | − 72.385 | 210.726 | 1.085 | 0.314 | 0.067 | |
NIEMG Normalized Integrals of Electromyography Activity, GM Gluteus Medius, BF Biceps Femoris, RF Rectus Femoris, SOL Soleus, TA Tibialis Anterior, PL Peroneus Longus, APA Anticipatory Postural Adjustment, CPA Compensatory Postural Adjustment.
η2 is effect size (small = 0.01–0.06, medium = 0.06–0.14 and large ≥ 0.14). *Significant differences (P ≤ 0.05).
Table 6.
The descriptive data (mean and standard deviation) and ANCOVA results for between-group comparisons of the secondary outcomes.
| Outcomes | Virtual reality games (N = 16) | Balance training (N = 16) | Adjusted mean difference | 95% Confidence interval | F | P | η2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Lower Bound | Upper Bound | |||||
| A YBT (%) | 120.02 | 16.81 | 121.18 | 16.09 | 128.31 | 17.27 | 130.34 | 16.99 | − 3.034 | − 11.179 | 5.111 | 0.580 | 0.452 | 0.020 |
| PM YBT (%) | 100.52 | 14.62 | 111.03 | 11.19 | 108.22 | 15.27 | 117.38 | 7.17 | − 5.300 | − 12.281 | 1.681 | 2.411 | 0.131 | 0.077 |
| PL YBT (%) | 87.74 | 10.14 | 100.95 | 9.78 | 94.75 | 12.12 | 107.71 | 11.29 | − 2.029 | − 7.740 | 3.682 | 0.528 | 0.473 | 0.018 |
| CAIT (0–30) | 17.31 | 2.85 | 22.93 | 4.65 | 15.50 | 4.84 | 22.05 | 3.11 | 0.737 | − 2.244 | 3.717 | 0.255 | 0.617 | 0.009 |
YBT Y Balance Test, A Anterior, PM Posteromedial, PL Posterolateral, CAIT Cumberland Ankle Instability Tool, SD Standard Deviation.
η2 is effect size (small = 0.01–0.06, medium = 0.06–0.14 and large ≥ 0.14). *Significant differences (P ≤ 0.05).
Table 7.
The results of repeated measures ANOVA for within-group comparisons of the secondary outcomes.
| Outcomes | Within the virtual reality games group | Within the balance training group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% Confidence interval | F | P | η2 | Mean difference | 95% Confidence interval | F | P | η2 | |||
| Lower bound | Upper bound | Lower Bound | Upper Bound | |||||||||
| A YBT (%) | − 1.166 | − 7.028 | 4.695 | 0.180 | 0.677 | 0.012 | − 2.035 | − 8.546 | 4.476 | 0.444 | 0.515 | 0.029 |
| PM YBT (%) | − 10.518 | − 19.108 | − 1.928 | 6.811 | 0.020* | 0.312 | − 9.160 | − 17.446 | − 0.874 | 5.551 | 0.032* | 0.270 |
| PL YBT (%) | − 13.207 | − 18.509 | − 7.905 | 28.189 | < 0.001* | 0.653 | − 12.960 | − 16.176 | − 9.743 | 73.766 | < 0.001* | 0.831 |
| CAIT (0–30) | − 5.617 | − 8.801 | − 2.433 | 14.136 | 0.002* | 0.485 | − 6.549 | − 9.057 | − 4.041 | 30.978 | < 0.001* | 0.674 |
YBT Y Balance Test, A Anterior, PM Posteromedial, PL Posterolateral, CAIT Cumberland Ankle Instability Tool.
η2 is effect size (small = 0.01–0.06, medium = 0.06–0.14 and large ≥ 0.14). *Significant differences (P ≤ 0.05).
Fig. 3.
Trends in muscle activity during the APA, CPA1, and CPA2 phases across both groups. NIEMG Normalized Integrals of Electromyography Activity, GM Gluteus Medius, BF Biceps Femoris, RF Rectus Femoris, SOL Soleus, TA Tibialis Anterior, PL Peroneus Longus, APA Anticipatory Postural Adjustment, CPA Compensatory Postural Adjustment.
Fig. 4.
Trends in muscle activation timing across both groups. GM Gluteus Medius, BF Biceps Femoris, RF Rectus Femoris, SOL Soleus, TA Tibialis Anterior, PL Peroneus Longus.
NIEMG of muscles during APA, CPA1 and CPA2
Between-group comparisons
The results indicate that the amplitude of GM muscle activity during the APA phase was significantly higher in the balance training group compared to the VR games group, with a medium effect size (F(1,30) = 4.091, P = 0.050, η2 = 0.124).
Conversely, during the compensatory postural adjustment (CPA1) phase, the amplitude of GM muscle activity was significantly lower in the balance training group than in the VR games group, with a large effect size (F(1,30) = 7.108, P = 0.012, η2 = 0.197) (Table 4).
There were no significant differences in other variables between the groups (P > 0.05).
Within-group comparisons
In the VR games group, the amplitude of the SOL muscle activity in the CPA2 significantly decreased by 25% after treatment compared to before, with a large effect size (F(1,15) = 5.487, P = 0.033, η2 = 0.268).
In the balance training group, the amplitude of GM muscle activity in the APA phase significantly increased by 115% after treatment compared to before (F(1,15) = 5.269, P = 0.037, η2 = 0.260).
Moreover, in the balance training group, the amplitude of RF muscle activity in CPA1 significantly decreased by 33% after treatment compared to before, with a large effect size (F(1,15) = 4.654, P = 0.048, η2 = 0.237).
In the balance training group, the amplitude of PL muscle activity in CPA1 significantly decreased by 50% after treatment, with a large effect size (F(1,15) = 5.740, P = 0.030, η2 = 0.277). Additionally, the amplitude of PL muscle activity in CPA2 decreased by 28% after treatment, with a large effect size (F(1,15) = 4.328, P = 0.050, η2 = 0.224) (Table 5 and Fig. 3).
Onset latency of muscles
Between-group comparisons
There was no significant difference between the groups (P > 0.05) (Table 4).
Within-group comparisons
In the VR games group, the BF muscle activated significantly earlier by approximately 77 s after treatment compared to before (F(1,15) = 4.877, P = 0.043, η2 = 0.245) .
In the balance training group, the GM muscle activated significantly earlier by approximately 90 s after treatment compared to before (F(1,15) = 4.556, P = 0.050, η2 = 0.233) (Table 5 and Fig. 4).
Y Balance Test
Between-group comparisons
There were no significant between-group effects in this parameter (P > 0.05) (Table 6).
Within-group comparisons
In the VR games group, the Y-balance test showed significant improvement in all directions except the anterior direction after treatment. In the PM direction, there was a 10% increase with a large effect size (F(1,15) = 6.811, P = 0.020, η2 = 0.312). In the PL direction, there was a 15% increase (F(1,15) = 28.189, P < 0.001, η2 = 0.653).
In the balance training group, the Y-balance test showed significant improvement in all directions except the anterior direction after treatment. In the PM direction, there was an 8% increase with a large effect size (F(1,15) = 5.551, P = 0.032, η2 = 0.270). In the PL direction, there was a 14% increase (F(1,15) = 73.766, P < 0.001, η2 = 0.831) (Table 7).
CAIT
Between-group comparisons
There was no significant difference between the groups (P > 0.05) (Table 6).
Within-group comparisons
There was a significant reduction of 32% in ankle instability perception in the VR games group after treatment (F(1,15) = 14.136, P = 0.002, η2 = 0.485).
In the balance training group, there was a significant reduction of 42% in ankle instability perception after treatment (F(1,15) = 30.978, P < 0.001, η2 = 0.674) (Table 7).
Discussion
The results of this study indicated that both the VR games and balance training groups effectively improved perceived ankle instability and enhanced dynamic postural stability, with no group showing superiority over the other. Concerning muscle activity delay time, both groups also exhibited relative improvement, yet neither outperformed the other. Regarding the amplitude of muscle activity during the anticipatory and compensatory phases, both groups showed improvements, but the balance training group demonstrated more significant performance.
As previously mentioned, damage to peripheral mechanoreceptors that provide proprioceptive input is believed to lead to changes in neuromuscular control. In other words, when mechanoreceptors are damaged, feedback-based strategies are disrupted, preventing individuals from adequately responding to perturbations9. The ongoing reduction in sensory input from the ankle to the spinal and brain leads to changes in the CNS and alters the motor programs stored in the brain. As a result, feed-forward strategies are also affected, decreasing the body’s preparation for various movements10. Consequently, both anticipatory and compensatory strategies, which depend on the brain and sensory feedback respectively, are impaired in individuals with ankle instability.
Therefore, the improved postural strategies observed in the balance training group may be attributed to enhanced proprioceptive inputs, which likely facilitate both spinal and supra-spinal adaptations. Indeed, proprioceptive enhancements allow for more effective neuromuscular control by influencing both the spinal cord (spinal adaptations) and higher brain centers (supra-spinal adaptations). Spinal adaptations refer to alterations in reflex pathways that result in quicker, more automatic responses to perturbations, along with enhanced feedback-based compensatory adjustments. However, supra-spinal adaptations involve the brain’s reorganization and greater engagement of cortical areas, which facilitate improved voluntary control and feed-forward-based anticipatory adjustments41.
The balance training regimen in this study included various sensory-motor components, such as visual stimuli (eyes open and closed) and proprioceptive stimuli (firm and foam surfaces, small and large support bases), which together helped stimulate both spinal and supra-spinal pathways. Additionally, the exercises involved perturbations, requiring individuals to throw or kick a ball in both stable and unstable positions, which further strengthened the sensory-motor system by encouraging precise control and adaptation. Since motor outputs are largely dependent on sensory inputs, these exercises likely improved proprioceptive feedback, which plays a crucial role in selecting appropriate motor strategies and enhancing postural stability42.
By targeting both sensory and motor functions, the exercises stimulate proprioceptive organs, increase their sensitivity, and improve responses to sensory stimuli, thereby boosting coordination between different muscle groups42. This enhanced proprioception leads to better motor responses in dynamic and challenging environments, manifesting as improved anticipatory and compensatory strategies when kicking a ball. A meta-analysis suggests that balance training can enhance proprioception in individuals with CAI43. Moreover, some studies indicate that balance training can improve muscle activation patterns and reduce activation time in individuals with ankle instability44,45. Therefore, we believe that balance training, probably through the improvement of proprioception, played an effective role in restoring postural strategies.
A 2017 study investigated the effects of balance training using a wobble board on individuals with ankle instability. After four weeks, the intervention group exhibited changes in muscle activation patterns during a single-limb landing, engaging muscles that provided optimal joint stability post-landing. Specifically, the TA and PL muscles were less active when the gastrocnemius was more engaged, and vice versa. This selective activation suggested adaptations in postural control, likely involving spinal and supra-spinal changes46.
Additionally, a 2008 review indicated that a four-week balance training program can modify spinal reflexes, induce brain plasticity, and enhance cortical excitability post-training41. Therefore, based on the results of these studies, it can be inferred that the balance training likely lead to selective and more effective muscle activity and create spinal and supra-spinal adaptations, which can make the role of feed-forward strategies more prominent and, thereby minimizing the need for compensatory strategies.
As mentioned, the training also included perturbation-based exercises that required individuals to throw or kick a ball on both stable and unstable surfaces. These tasks consistently applied perturbations to the body, stimulating postural strategies. It seems that repeated practice over 12 sessions have facilitated motor learning and skill acquisition in strategy application. Moreover, the test condition (kicking the ball) was more similar to the balance training conditions than to those in the VR games group. This similarity might have contributed to better performance of balance training in the ball-kicking test, as both groups showed equal improvement in other clinical tests, such as the balance Y-test and perceived instability, with neither group demonstrating superiority.
In this context, research has indicated that perturbation-based exercises can enhance anticipatory muscle activity. For instance, a study involving healthy participants demonstrated that engaging in a functional task, such as throwing a ball at various angles, which introduces predictable perturbations to the whole body, could boost anticipatory muscle activity in several lower limb muscles. This single-session study revealed significant post-treatment increases in the activity of the SOL, BF, semitendinosus, RF, and both vastus lateralis and medialis muscles47. Additionally, a similar study with elderly participants found that four weeks of ball-catching exercises enhanced anticipatory muscle activity in response to both external perturbations and internal perturbations (such as raising both arms)48.
One of the most comparable studies examining the impact of perturbation-based exercises on anticipatory and compensatory strategies is by Conceição et al. In this study, individuals with ankle instability were divided into two groups: one received balance exercises involving ball kicking, while the control group did not receive any treatment. Each session lasted 30 min and consisted of a single session. The test involved ball kicking and the study investigated anticipatory and compensatory postural strategies through an IEMG. The study found changes in postural strategies, primarily during CPA1, evidenced by reduced activity in both anterior and posterior muscles of lower extremity after treatment. The researchers suggested that CAI might lead individuals to overreact and increase muscle activity and stiffness to reduce COG displacement. However, following balance exercises, participants may experience improved stability and better management of perturbations, as indicated by reduced muscle activity in the compensatory phase39.
In contrast to the current study, Conceição et al. observed increased activity in the PL and TA muscles during the CPA2 (following ball impact) when these muscles were examined individually. The authors attributed this increase to the opposing rotational forces generated by these muscles (inversion by TA and eversion by PL), suggesting that simultaneous increased activity was necessary to maintain a neutral ankle joint position after the kick39. This discrepancy may be due to differences in the number of treatment sessions and the types of exercises used. Conceição’s study involved only one session, while the current study included 12 sessions. Additionally, the current study featured a greater variety of exercises compared to Conceição’s study. These factors could contribute to broader changes in the sensory-motor system, leading to more substantial adaptations in both the central and peripheral nervous systems, and enhancing muscle efficiency in maintaining joint stability without necessitating increased activity.
In the balance training group, the results showed a significant increase in the amplitude of GM muscle activity during the anticipatory phase following treatment, along with a notable decrease during the compensatory phase. Additionally, the muscle began to activate earlier than before. Previous research has indicated that during ball kicking, proximal muscles like the GM are more active than distal muscles in individuals with CAI. These individuals often increase pelvic muscle activity to compensate for their unstable ankle joint14. Han et al. investigated EMG of GM in both healthy and “copers”—those who, despite having had at least one severe sprain in the past year, returned to their activities without feeling instability or re-injury. Their study found that copers showed greater GM activity compared to healthy subjects while standing on one limb. The GM is crucial for preventing pelvic tilt and maintaining stability in the frontal plane, which helps reduce lateral movement and lower limb injuries49. Thus, the balance exercises used in the present study, which mainly involved single-limb standing, appear to have effectively improved GM function. This is reflected in the earlier onset of muscle activation and decreased need for compensatory activity, suggesting a more efficient use of the muscle.
Another feature of the exercises in this study was the inclusion of hop-to-stabilization exercises. A 2024 study compared the immediate effects of these exercises on muscle preparatory activity during cutting movements in healthy individuals and soccer players with ankle instability to a simple warm-up. The results showed that hop-to-stabilization exercises improved preparatory muscle activity compared to the control group, suggesting their potential to enhance neuromuscular control50. These exercises require precise muscle coordination to maintain balance and are based on plyometric principles, including stretch-shortening cycles and afferent pathway stimulation. This type of stimulation, similar to that found in proprioceptive exercises, challenges balance and joint position sense50. Therefore, it can be inferred that the hop-to-stabilization exercises used in this study, with their rapid muscle length changes through eccentric and concentric phases, might enhance afferent input. This could improve proprioception and result in more precise, coordinated muscle responses, potentially benefiting individuals with CAI by refining their postural strategies.
Another finding of this study was that, in the balance training group, there was a significant improvement in the Y balance test in the PL and PM directions after treatment, but no significant improvement in the anterior direction. This result is consistent with the findings of Terada et al. and Gabriner et al.51,52. These researches suggested that the distance traveled in the anterior direction in this test is more influenced by mechanical limitations, while the distances in the PM and PL directions are more dependent on strength and postural control51,52. Thus, the improvement observed in the PL and PM directions in this study may be attributed to increased strength of the relevant muscles and enhanced postural stability in the internal–external plane.
Similarly, McKeon et al. found that balance exercises led to improvements in the PM and PL directions in this test, but did not show significant changes in the anterior direction40. In contrast, Hale et al. reported that after 4 weeks of a comprehensive exercise program—including balance, strengthening exercises with a thera-band, and gastrosoleus stretching—improvements were observed in all movement directions for individuals with CAI53. It appears that a greater focus on range of motion exercises and thereby reducing mechanical limitations could have a more pronounced effect on improving the distance traveled in the anterior direction.
The current study demonstrated that balance training reduces perceived ankle instability. This increased sense of stability is likely due to the correction of behavioral-motor disorders in these patients, including improved muscle activity timing, enhanced neuromuscular control, and increased muscle strength54. Additionally, balance training has been shown to enhance joint proprioception, which is the ability to recognize the position of a joint in space55. Even minor errors in joint position sense can lead to inappropriate foot contact during sports and daily activities. Therefore, improving joint position awareness can lead to a greater sense of joint stability. A 2024 meta-analysis found that traditional balance exercises, compared to a control group without treatment, as well as better than strengthening exercises, can reduce perceived ankle instability15.
This study is the first to investigate the effect of VR games on postural strategies in individuals with CAI. The results indicated a significant reduction in the amplitude of SOL muscle activity during CPA2 post-treatment. Additionally, the BF muscle activated significantly earlier after the treatment compared to before. Investigating the activity of the SOL muscle in the anticipatory phase revealed an increase after treatment compared to before, though this change was not statistically significant, despite having a large effect size. Effect size is independent of sample size, whereas statistical significance is influenced by both sample size and effect size. Consequently, P values can be biased due to their reliance on sample size; a statistically significant result might simply indicate a large sample size. Therefore, relying solely on P values is insufficient56. With this understanding, the observed reduction in SOL muscle activity during the compensatory phase may be due to the increased activity in the anticipatory phase, which better equips the muscle to handle the perturbation (ball impact) and reduces the necessity for compensatory responses.
It appears that VR games, which provide a multi-component environment incorporating sensory, motor, and cognitive factors, have effectively restored appropriate muscle activity patterns, likely by improving proprioception and increasing muscle strength. In most Nintendo Wii Fit exercises, maintaining the COG within a specified area is necessary, which likely heightens patients’ awareness of their ankle condition, reduces errors, and enhances proprioception. Furthermore, VR games offer continuous audio and visual feedback, and as previously mentioned, sensory inputs are crucial for neuromuscular and postural control. Therefore, VR-based exercises, being feedback-oriented treatments, can leverage these benefits.
Supporting this, a 2015 study by Kim et al. demonstrated that 12 sessions of balance games using the Nintendo Wii Fit Plus, with each session lasting 20 min, significantly improved proprioception in both the transverse and sagittal planes in individuals with CAI28. Additionally, a 2018 study by Kim and colleagues showed that VR games using the Nintendo Wii Fit Plus could enhance the strength of the plantar flexor muscles in individuals with CAI. Traditional exercises, which included strengthening and balance training, also increased strength in all ankle muscles (plantar flexors, dorsiflexors, everters, and inverters). Both groups trained three times a week for four weeks, with each session lasting 20 min. Therefore, the researchers suggested that VR games could be added as an optional component to routine training programs29.
This study demonstrated that in the VR games group, all muscles exhibited faster activity after the treatment, with the change in the onset of activity of the BF muscle being statistically significant. This finding aligns with the results of Li et al., who found that individuals with non-specific chronic low back pain experienced an earlier onset of transversus abdominis muscle activity after VR treatment compared to a control group receiving only conventional thermal magnetic therapy and another group receiving motor control exercises. Additionally, the IEMGs of the transversus abdominis and tibialis anterior muscles during CPA1 significantly decreased only in the VR group after the intervention32. One possible reason for improved anticipatory strategies in VR is visual feedback, which increases the activity of frontoparietal and sensory-motor networks57.
In the current study, games such as Soccer Heading, Snowboard Slalom, and Tightrope Walk were used, which can stimulate the user’s predictability, as the user must anticipate when to avoid obstacles or hit the ball with their head. Therefore, it seems that the information obtained from the virtual environment increases the activity of the frontal lobe, which participates in the predictive process. However, this study did not directly investigate brain activity using imaging techniques to confirm this. Nonetheless, some studies using electroencephalography have shown that the prefrontal and motor cortex of older adults can be reorganized after playing video games17,58.
In Oliveira’s study, the effects of VR exercises combined with routine physical therapy were compared to routine physical therapy alone on pain, physical capacity, balance, and APAs in patients with knee osteoarthritis. Both groups showed improvements, but only the VR group demonstrated enhancements in APA parameters. The researchers attributed this improvement to the diverse sensory inputs provided by VR, which enhance sensory-motor interaction, integrate proprioception and vision, and activate various brain regions. Consequently, VR offers a broad range of sensory benefits, including improved concentration, coordination, balance, and strength, all within an engaging and enjoyable format31.
Comparing these findings suggests that VR not only benefits individuals with knee osteoarthritis and chronic low back pain but also serves as an effective intervention for soccer players with CAI. Although all three groups encountered challenges with postural control, the findings indicated that they experienced improved neuromuscular control and enhanced balance strategies, likely due to the multifaceted sensory stimulation offered by VR. This suggests that the mechanisms by which VR enhances APAs could be applicable across different populations with balance deficits, reinforcing its versatility and effectiveness as a therapeutic tool in rehabilitation. Indeed, our study adds to the growing body of evidence supporting VR’s effectiveness in enhancing postural strategies. This comparative analysis not only contextualizes our results within the existing literature but also highlights the potential of VR as a versatile intervention across diverse rehabilitation settings.
The improvement in postural strategies may stem from the fact that VR requires the user to continuously shift their weight in various directions, speeds, and magnitudes, closely resembling ankle, hip, and trunk strategies25. Over 12 sessions, these strategies were practiced repeatedly, possibly leading to motor learning and modification of previously stored movement programs in the brain. This can manifest in earlier muscle activity, enhanced anticipatory strategies, and a reduced need for compensatory strategies.
Additionally, in this study, some games involved standing on one limb while moving the upper limb or opposite limb in different directions, all while maintaining the COG within a designated area. These exercises have a perturbation-oriented nature, during which an internal perturbation was created and the person tried to maintain the balance. As mentioned in the balance training, these types of exercises play an effective role in improving neuromuscular control.
In this study, significant improvement in dynamic postural stability was observed in the VR games group, with increased distance traveled in the PL and PM directions on the Y balance test, showing a large effect size. This improvement can be attributed to the emphasis on lateral weight shifting in many VR games such as Single Leg Twist, Sideways Leg Lift, Table Tilt, Penguin Fishing, Soccer Heading, Tightrope Walk, and Snowboard Slalom. High repetition of weight shifts in the internal–external direction has likely led to enhanced dynamic postural stability in the PL and PM directions.
Supporting these findings, Kim et al. reported that video game training was more effective than traditional balance training for improving dynamic balance in the ML direction on the Biodex screen for individuals with CAI. Although the balance assessment tools differed between the studies (Biodex vs. Y balance test) and Kim’s study had shorter intervention duration (12 sessions of 20 min each vs. 60 min each), both studies indicated that VR is effective in improving dynamic balance. The similarity in game types used in both studies likely contributed to the comparable results.
In this study, the VR games group experienced a significant reduction in perceived ankle instability after treatment. This improvement in joint stability may be attributed to the correction of behavioral-motor disorders and enhanced proprioception. Consistent with this finding, a study by Kim et al. demonstrated that 12 sessions of 20 min each of balance-oriented video games using the Nintendo Wii Fit Plus significantly reduced perceived ankle instability28.
As previously noted, the mechanisms of APAs and CPAs are not mutually exclusive in response to perturbations. Instead, they interact dynamically to maintain stability; thus, an increase in APAs can effectively reduce reliance on CPAs. Moreover, we believe that while the underlying mechanisms driving the improvement of these strategies are somewhat similar across both groups, the contributions and weights of specific beneficial features differ. For instance, previous studies have demonstrated that traditional balance training enhances both spinal and supra-spinal adaptations. However, due to the cognitive component inherent in VR games, it was anticipated that VR would engage the brain more extensively than traditional balance trainings, thereby playing a more significant role in enhancing feed-forward strategies mediated by the brain. Moreover, VR games, which emphasize feedback, include more advanced processes for error detection and correction than traditional balance training. As a result, the enhanced feedback provided in VR was expected to improve postural strategies by helping individuals more effectively identify and rectify their error compared to traditional exercises. However, our findings ultimately indicated that both training methods are effective, each contributing to improvements in postural control strategies.
Strengths and limitations
Although VR has been increasingly applied in general rehabilitation, this research was among the first to assess its specific effects on postural control strategies, focusing on anticipatory and compensatory responses in male soccer players with CAI. By utilizing VR games as a form of cognition-sensory-motor training, this study provides new insights into how semi-immersive, feedback-oriented virtual environments can enhance neuromuscular control, dynamic balance, and perceived ankle stability. These findings provide a foundation for incorporating VR into sports rehabilitation protocols, potentially expanding treatment options for CAI and establishing VR as a valuable approach in modern rehabilitation programs.
A notable strength of this study lies in its direct comparison of VR-based training and traditional balance training, yielding valuable insights into the effectiveness of each method for rehabilitating CAI. This comparison enables practitioners to understand how both approaches can improve neuromuscular control, dynamic balance, and perceived ankle stability, thereby guiding them in selecting the most suitable interventions tailored to specific rehabilitation needs. Ultimately, this study equips sports rehabilitation professionals with evidence-based recommendations to more effectively customize their programs.
One limitation of this study is the absence of kinematic and kinetic assessments alongside EMG recordings, which could have been helpful in interpreting the results. Additionally, the absence of follow-up assessments limits our ability to evaluate the long-term sustainability of the treatment effects. In clinical practice, understanding whether the benefits of such interventions are maintained over time is crucial for determining their overall effectiveness. Without follow-up data, we cannot ascertain whether the improvements in ankle stability and neuromuscular control are sustained or diminish after the intervention concludes.
Furthermore, the sample size is small. Moreover, the generalizability of the findings may be limited when applied to other populations, such as athletes from other sports, females, or individuals with different levels of CAI severity. The specific characteristics of our sample may not adequately represent the broader athlete population, which includes a range of age groups, skill levels, and training backgrounds. Furthermore, differences in injury susceptibility and rehabilitation responses between genders could further limit the applicability of the findings. Consequently, caution should be taken when generalizing these results to other populations.
Another notable limitation of this study is the reliance on the Wii Balance Board as the training tool. While this equipment is useful, it may not fully replicate the dynamic and complex movements encountered in real-life sports situations. The controlled environment of exergaming may restrict the training effects, as athletes typically engage in complex, unpredictable movements that are challenging to simulate within a laboratory setting.
Conclusion
The study found that both the VR games and balance training groups significantly improved perceived ankle instability and dynamic postural stability, with no group showing superiority over the other. Both groups also exhibited relative improvement in muscle activity delay time without one outperforming the other. While improvements were seen in the amplitude of muscle activity during the anticipatory and compensatory phases for both groups, the balance training group demonstrated more significant performance.
Clinical implications
Practitioners can effectively incorporate both VR and traditional balance training into rehabilitation programs, leveraging the distinct benefits of each approach to improve dynamic stability, neuromuscular control, and perceived ankle stability in athletes with CAI. This study underscores the contributions of both approaches in enhancing these key factors, which are fundamental for minimizing re-injury risk and ensuring a safe return to play for athletes recovering from CAI. By optimizing muscle activity amplitude and timing while improving postural stability, these interventions empower athletes to regain control over their movements, thereby reducing the likelihood of re-injury. Consequently, incorporating VR and balance training into rehabilitation protocols can facilitate a more efficient and effective recovery, enabling athletes to return to their sports with greater confidence and a diminished risk of recurrent ankle instability. Additionally, this study shows that VR games, designed as cognition-sensory-motor training in semi-immersive, feedback-oriented environments, have a positive impact on CAI rehabilitation. These positive findings provide a foundation for incorporating VR into sports rehabilitation protocols, potentially expanding treatment options for CAI and establishing it as a modern, effective approach in rehabilitation programs.
Future research
Future research should prioritize longitudinal studies to investigate the long-term effects of VR and balance training on injury prevention and postural control in soccer players and other athletic populations. Understanding how these interventions influence athletes over extended periods will provide valuable insights into their effectiveness and sustainability. Additionally, exploring the combination of VR and traditional balance training may optimize rehabilitation outcomes. Integrating VR can help alleviate the monotony often associated with conventional exercises while enhancing engagement and motivation among participants. Research in this area could reveal innovative approaches to rehabilitation that leverage the strengths of both methodologies.
Author contributions
R.KH. contributed to conceptualization, resources, methodology, supervision, and writing—review and editing. R.F. contributed to data curation, formal analysis, and writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Data availability
Data are available upon reasonable request to the corresponding author via Email address.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study involves human participants. All protocols of this research adhered to the ethical principles outlined in the Declaration of Helsinki and received approval from the Tehran University of Medical Sciences Ethics Committee (IR.TUMS.FNM.REC.1401.149). Participants gave informed consent to participate in the study before taking part.
Patient consent for publication
The two individuals depicted in Fig. 2 have provided written informed consent for the publication of their images in this online open-access publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Walls, R. J. et al. Football injuries of the ankle: A review of injury mechanisms, diagnosis and management. World J. Orthop.7, 8–19. 10.5312/wjo.v7.i1.8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gribble, P. A. et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. Br. J. Sports Med.48, 1014–1018. 10.1136/bjsports-2013-093175 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Xue, X. et al. Postural control deficits during static single-leg stance in chronic ankle instability: a systematic review and meta-analysis. Sports Health16, 29–37. 10.1177/19417381231152490 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeon, P. O. & Hertel, J. Systematic review of postural control and lateral ankle instability, part I: can deficits be detected with instrumented testing. J. Athl. Train.43, 293–304. 10.4085/1062-6050-43.3.293 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hejazi, H. S., Khanmohammadi, R., Olyaei, G. & Qanbari, S. The effects of combining sensorimotor training with transcranial direct current stimulation on the anticipatory and compensatory postural adjustments in patients with chronic low back pain. Disabil. Rehab.10.1080/09638288.2024.2375756 (2024). [DOI] [PubMed] [Google Scholar]
- 6.Aruin, A. S. Enhancing anticipatory postural adjustments: a novel approach to balance rehabilitation. J. Nov. Physiother.10.4172/2165-7025.1000e144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos, M. J., Kanekar, N. & Aruin, A. S. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J. Electromyogr. Kinesiol.20, 398–405. 10.1016/j.jelekin.2010.01.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez, G. M., Kaminski, T. W. & Douex, A. T. Neuromuscular control and ankle instability. PM R1, 359–365. 10.1016/j.pmrj.2009.01.013 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Yen, S. C., Corkery, M. B., Donohoe, A., Grogan, M. & Wu, Y. N. Feedback and feedforward control during walking in individuals with chronic ankle instability. J. Orthop. Sports Phys. Ther.46, 775–783. 10.2519/jospt.2016.6403 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Xue, X. et al. Maladaptive neuroplasticity in corticospinal tract after ankle sprain: causal links established by Mendelian randomization. Med. Sci. Sports Exerc.55, 1114–1120. 10.1249/mss.0000000000003134 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Jeon, H. G., Lee, S. Y., Park, S. E. & Ha, S. Ankle instability patients exhibit altered muscle activation of lower extremity and ground reaction force during landing: a systematic review and meta-analysis. J. Sports Sci. Med.20, 373–390. 10.52082/jssm.2021.373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Deun, S. et al. Relationship of chronic ankle instability to muscle activation patterns during the transition from double-leg to single-leg stance. Am. J. Sports Med.35, 274–281. 10.1177/0363546506294470 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Labanca, L. et al. Muscle activations during functional tasks in individuals with chronic ankle instability: a systematic review of electromyographical studies. Gait Posture90, 340–373. 10.1016/j.gaitpost.2021.09.182 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Rios, J. L., Gorges, A. L. & dos Santos, M. J. Individuals with chronic ankle instability compensate for their ankle deficits using proximal musculature to maintain reduced postural sway while kicking a ball. Hum. Mov. Sci.43, 33–44. 10.1016/j.humov.2015.07.001 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Guo, Y. et al. A systematic review and meta-analysis of balance training in patients with chronic ankle instability. Syst. Rev.13, 64. 10.1186/s13643-024-02455-x (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry, G., van Schaik, P., MacSween, A., Dixon, J. & Martin, D. Exergaming (XBOX Kinect™) versus traditional gym-based exercise for postural control, flow and technology acceptance in healthy adults: a randomised controlled trial. BMC Sports Sci. Med. Rehab.8, 25. 10.1186/s13102-016-0050-0 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olyaei, G. et al. The effect of exergaming on cognition and brain activity in older adults: A motor- related cortical potential study. Physiol. Behav.255, 113941. 10.1016/j.physbeh.2022.113941 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Elaraby, A. E. R., Shahien, M., Jahan, A. M., Etoom, M. & Bekhet, A. H. The efficacy of virtual reality training in the rehabilitation of orthopedic ankle injuries: a systematic review and meta-analysis. Adv. Rehab. Sci. Pract.12, 11795727231151636. 10.1177/11795727231151636 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, H., Han, K. & Ruan, B. Effect of virtual reality on functional ankle instability rehabilitation: a systematic review. J. Healthc. Eng.2021, 7363403. 10.1155/2021/7363403 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Gioftsidou, A. et al. Typical balance exercises or exergames for balance improvement?. J. Back Musculoskelet. Rehab.26, 299–305 (2013). [DOI] [PubMed] [Google Scholar]
- 21.dos Santos Mendes, F. A. et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease—effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy98, 217–223. 10.1016/j.physio.2012.06.001 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Burcal, C. J., Needle, A. R., Custer, L. & Rosen, A. B. The effects of cognitive loading on motor behavior in injured individuals: a systematic review. Sports Med.49, 1233–1253. 10.1007/s40279-019-01116-7 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Rosen, A. B., McGrath, M. L. & Maerlender, A. L. Males with chronic ankle instability demonstrate deficits in neurocognitive function compared to control and copers. Res. Sports Med. (Print)29, 116–128. 10.1080/15438627.2020.1723099 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagheri, H. et al. Video game and motor-cognitive dual-task training could be suitable treatments to improve dual-task interference in older adults. Neurosci. Lett.760, 136099. 10.1016/j.neulet.2021.136099 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Khanmohammadi, R. et al. The effect of video game-based training on postural control during gait initiation in community-dwelling older adults: a randomized controlled trial. Disabil. Rehab.44, 5109–5116. 10.1080/09638288.2021.1925360 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Kim, K. J. & Heo, M. Comparison of virtual reality exercise versus conventional exercise on balance in patients with functional ankle instability: A randomized controlled trial. J. Back Musculoskelet. Rehab.32, 905–911. 10.3233/bmr-181376 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Shousha, T. M., Abo-Zaid, N. A., Hamada, H. A., Abdelsamee, M. Y. A. & Behiry, M. A. Virtual reality versus Biodex training in adolescents with chronic ankle instability: a randomized controlled trial. Arch. Med. Sci.19, 1059–1068. 10.5114/aoms/134635 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, K. J. Effects of virtual reality programs on proprioception and instability of functional ankle instability. J. Int. Acad. Phys. Ther. Res.6, 891–895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, K., Choi, B. & Lim, W. The efficacy of virtual reality assisted versus traditional rehabilitation intervention on individuals with functional ankle instability: a pilot randomized controlled trial. Disabil. Rehab. Assist. Technol.14, 276–280. 10.1080/17483107.2018.1429501 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi, N., Hadian, M. R. & Olyaei, G. R. Comparison of the effects of Wii and conventional training on functional abilities and neurocognitive function in basketball-players with functional ankle instability: Matched randomized clinical trial. Clin. Rehab.35, 1454–1464. 10.1177/02692155211010249 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Oliveira, L. K. R. et al. Virtual reality in improving anticipatory postural adjustments to step initiation in individuals with knee osteoarthritis: a randomized controlled trial. Games Health J.13, 100–108. 10.1089/g4h.2023.0154 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Li, Z. et al. The effect of virtual reality training on anticipatory postural adjustments in patients with chronic nonspecific low back pain: a preliminary study. Neural Plast.2021, 9975862. 10.1155/2021/9975862 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynall, R. C., Campbell, K. R., Mauntel, T. C., Blackburn, J. T. & Mihalik, J. P. Single-legged hop and single-legged squat balance performance in recreational athletes with a history of concussion. J. Athl. Train.55, 488–493. 10.4085/1062-6050-185-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Azevedo, A. K., Claudino, R., Conceição, J. S., Swarowsky, A. & Dos Santos, M. J. Anticipatory and compensatory postural adjustments in response to external lateral shoulder perturbations in subjects with Parkinson’s disease. PLoS One11, e0155012. 10.1371/journal.pone.0155012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plisky, P., Schwartkopf-Phifer, K., Huebner, B., Garner, M. B. & Bullock, G. Systematic review and meta-analysis of the Y-Balance Test lower quarter: reliability, discriminant validity, and predictive validity. Int. J. Sports Phys. Ther.16, 1190–1209. 10.26603/001c.27634 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirshahi, M., Halabchi, F., Golbakhsh, M. & Saadat, S. Reliability and recalibration of the Persian version of Cumberland ankle instability tool cut-off score in athletes with functional ankle instability. Adv. J. Emerg. Med.3, e26. 10.22114/ajem.v0i0.180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pompeu, J. E. et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy98, 196–204. 10.1016/j.physio.2012.06.004 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Anguish, B. & Sandrey, M. A. Two 4-week balance-training programs for chronic ankle instability. J. Athl. Train.53, 662–671 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conceição, J. S., Schaefer de Araújo, F. G., Santos, G. M., Keighley, J. & Dos Santos, M. J. Changes in postural control after a ball-kicking balance exercise in individuals with chronic ankle instability. J. Athl. Train.51, 480–490. 10.4085/1062-6050-51.8.02 (2016). [DOI] [PMC free article] [PubMed]
- 40.McKeon, P. O. et al. Balance training improves function and postural control in those with chronic ankle instability. Med. Sci. Sports Exerc.40, 1810–1819. 10.1249/MSS.0b013e31817e0f92 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Taube, W., Gruber, M. & Gollhofer, A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol. (Oxford, England)193, 101–116. 10.1111/j.1748-1716.2008.01850.x (2008). [DOI] [PubMed] [Google Scholar]
- 42.Page, P. Sensorimotor training: A “global” approach for balance training. J. Bodyw. Mov. Ther.10, 77–84 (2006). [Google Scholar]
- 43.de Vasconcelos, G. S., Cini, A., Sbruzzi, G. & Lima, C. S. Effects of proprioceptive training on the incidence of ankle sprain in athletes: systematic review and meta-analysis. Clin. Rehab.32, 1581–1590. 10.1177/0269215518788683 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Yoshida, N. et al. Changes in the muscle reaction time of ankle periarticular muscles by balance training. J. Phys. Fit. Sports Med.2, 493–500 (2013). [Google Scholar]
- 45.Plangtaisong, P., Shen, W., Wheeler, P. C. & Fong, D. T. Effect of exercise interventions and prophylactic devices on reducing peroneal muscle reaction time by sudden ankle perturbation: A systematic review and meta-analysis. Med. Nov. Technol. Devices11, 100082 (2021). [Google Scholar]
- 46.Silva, P. B., Oliveira, A. S., Mrachacz-Kersting, N. & Kersting, U. G. Effects of wobble board training on single-leg landing neuromechanics. Scand. J. Med. Sci. Sports28, 972–982. 10.1111/sms.13027 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Kanekar, N. & Aruin, A. S. Improvement of anticipatory postural adjustments for balance control: effect of a single training session. J. Electromyogr. Kinesiol.25, 400–405. 10.1016/j.jelekin.2014.11.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jagdhane, S., Kanekar, N. & Aruin, A. S. The effect of a four-week balance training program on anticipatory postural adjustments in older adults: a pilot feasibility study. Curr. Aging Sci.9, 295–300. 10.2174/1874609809666160413113443 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Han, S., Oh, M., Lee, H. & Hopkins, J. T. EMG analysis during static balance in chronic ankle instability, coper and controls. Int. J. Sports Med.45, 48–54. 10.1055/a-2156-2644 (2024). [DOI] [PubMed] [Google Scholar]
- 50.Laddawong, T., Saito, H., Soga, T. & Hirose, N. Acute effects of a hop-stabilization warm-up program on dynamic balance, ground reaction force, and muscle activity during cutting movements in collegiate athletes with chronic ankle instability. Int. J. Exerc. Sci.17, 343–358 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terada, M., Harkey, M. S., Wells, A. M., Pietrosimone, B. G. & Gribble, P. A. The influence of ankle dorsiflexion and self-reported patient outcomes on dynamic postural control in participants with chronic ankle instability. Gait Posture40, 193–197. 10.1016/j.gaitpost.2014.03.186 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Gabriner, M. L., Houston, M. N., Kirby, J. L. & Hoch, M. C. Contributing factors to star excursion balance test performance in individuals with chronic ankle instability. Gait Posture41, 912–916. 10.1016/j.gaitpost.2015.03.013 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Hale, S. A., Hertel, J. & Olmsted-Kramer, L. C. The effect of a 4-week comprehensive rehabilitation program on postural control and lower extremity function in individuals with chronic ankle instability. J. Orthop. Sports Phys. Ther.37, 303–311. 10.2519/jospt.2007.2322 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Hertel, J. & Corbett, R. O. An updated model of chronic ankle instability. J. Athl. Train.54, 572–588. 10.4085/1062-6050-344-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sefton, J. M., Yarar, C., Hicks-Little, C. A., Berry, J. W. & Cordova, M. L. Six weeks of balance training improves sensorimotor function in individuals with chronic ankle instability. J. Orthop. Sports Phys. Ther.41, 81–89. 10.2519/jospt.2011.3365 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Sullivan, G. M. & Feinn, R. Using effect size-or why the p value is not enough. J. Grad. Med. Educ.4, 279–282. 10.4300/jgme-d-12-00156.1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bohil, C. J., Alicea, B. & Biocca, F. A. Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci.12, 752–762. 10.1038/nrn3122 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Schättin, A., Arner, R., Gennaro, F. & de Bruin, E. D. Adaptations of prefrontal brain activity, executive functions, and gait in healthy elderly following exergame and balance training: a randomized-controlled study. Front. Aging Neurosci.8, 278. 10.3389/fnagi.2016.00278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McKeon, P. O. et al. Balance training improves function and postural control in those with chronic ankle instability. Med. Sci. Sports Exerc.40, 1810–1819 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Conceicao, J. S., Schaefer de Araújo, F. G., Santos, G. M., Keighley, J. & Dos Santos, M. J. Changes in postural control after a ball-kicking balance exercise in individuals with chronic ankle instability. J. Athl. Train.51, 480–490 (2016). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the corresponding author via Email address.