Abstract
Nanomaterial-biomembrane interactions constitute a critical biological process in assessing the toxicity of such materials in theoretical studies. However, many investigations simplify these interactions by using membrane models containing only one or a few lipid types, deviating significantly from the complexity of real membrane compositions. In particular, cholesterol, a ubiquitous lipid essential for regulating membrane fluidity and closely linked to various diseases, is often overlooked. Consequently, the role of cholesterol in nanomaterial-biomembrane interactions remains poorly understood. In this study, we employ molecular dynamics (MD) simulations to explore the effect of graphene quantum dots (GQDs) on a realistic placental lipid membrane model, aiming to elucidate the role of cholesterol in these interactions. Our MD results reveal that both GQD monomers and clusters can spontaneously insert into the placental lipid membrane model, driven by strong van der Waals interaction energy. Further analyses indicate that cholesterol and POPC lipids primarily contribute to interfacial interactions. Notably, cholesterol can be squeezed into the bilayer interface, forming a unique structure where it is sandwiched between the GQD cluster and the membrane’s bottom leaflet. More significantly, cholesterol, together with the GQD cluster, exhibits free lateral movement, suggesting a strong affinity of cholesterol for GQD clusters. These findings highlight the critical role of cholesterol in mediating GQD insertion into the biomembrane. Structural analyses of the membrane further demonstrate deformation of the placental lipid membrane model during GQD penetration. Finally, free energy calculations confirm that the insertion of both GQD monomers and clusters into the placental lipid membrane model is energetically favorable. Overall, this study not only sheds new light on the potential harmful effects of GQDs on realistic placental membranes but also provides the first theoretical evidence of the pivotal role of cholesterol in nanomaterial-biomembrane interactions, contributing to a deeper understanding of nanomaterial-cell membrane interactions.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82805-w.
Keywords: Realistic placental lipid membrane model, Graphene quantum dot, Interaction, Cholesterol, Molecular dynamics simulation
Subject terms: Computational biophysics, Biological physics
Introduction
Since the pathbreaking discoveries of graphene1, carbon nanotube2 and fullerene3, carbon nanomaterials have gathered great research interests from many areas, including energy conversion and storage4–7, nanomedicine,8,9 biological medicine10–16, optics17, and electronics18,19, due to their unique and outstanding properties8,20–25. Thus, carbon nanomaterials have presented great promise in near future. However, the extensive application will probably elicit the wide exposure of carbon nanomaterials into the environment, which may be thereby uptake by animals, plants and even human bodies. Various studies have demonstrated that such direct exposure of human bodies to carbon nanomaterial can cause potential harmful influence, i.e., toxicity26–31. In particular, at 2015, French scientists, Moussa and co-workers, have directly observed the existence of anthropogenic carbon nanotubes in the airways of Parisian children, which may lead to detrimental effect, e.g., asthmatic32. This also indicates that humans are often surrounded by nanomaterials. Therefore, investigating the effect of nanomaterials to human, particularly the corresponding molecular mechanism, is of great significance to understand how the nanomaterials generate toxicity.
Nanomaterial-cellular membrane interaction is closely related to the nanotoxicity of such nanomaterial, because nanomaterial often suffers from the biological membrane once entering into human body. Numerous investigations have exposed the potential toxicity of nanomaterials via perturbing the membrane. For example, experimental and theoretical evidences have both demonstrated that graphene nanosheet can spontaneously insert into cellular membrane and even extract the lipids from the membrane, yielding the robust toxicity33–36. In addition, carbon nanotubes were also confirmed having the ability to enter into membrane bilayer, which affects the normal function of the membrane37–40. Similarly, graphene quantum dots (GQDs) also present comparable capacity of spontaneous entry into cellular membrane41–44. However, due to the much smaller size of GQDs compared with graphene nanosheets, GQDs were often less toxic45,46 The theoretical investigations can clearly show the molecular insights of the potential toxicity of nanomaterials to cellular membrane. However, most existing theoretical simulations utilized the cellular membrane model with only one or few types of lipids to mimic the realistic cellular membrane35,39,47,48 In particular, these studies commonly overlooked the existence of cholesterol in membrane model. Cholesterol is ubiquitous in higher-vertebrate plasma membranes (with mole fraction accounting for up to 45% of the total lipids in biomembranes49), which shows a very important role as the membrane composition. Importantly, previous studies have confirmed that cholesterol can robustly regulate the fluidity of cellular membrane, wherein increasing the concentration of cholesterol can decrease the membrane fluidity50–53. Various experimental evidences also suggested that the high concentration of cholesterol can arouse breast cancer, hepatocellular carcinoma, etc., due to the declined membrane fluidity54,55 Experimental evidences have confirmed that the lipid rafts, comprising abundant cholesterol, often mediate the entry of nanomaterials (including GQDs) into cellular membrane43,56,57 Therefore, in this study, we specifically investigate the interaction between graphene quantum dots (GQDs) and the realistic placental cell membrane, taking into consideration of the plentiful presence of cholesterol in the membrane model. Some previous studies have demonstrated that nanosized materials have the potential to transport across the placental membrane, but the underlying molecular mechanism is still elusive58,59 Therefore, it is meaningful to explore the molecular insights into the effect of nanosized GQDs on the realistic placental lipid membrane model. Our MD simulation results demonstrated that GQDs can interfere the placental lipid membrane model via inserting into the membrane interior. The cholesterol shows an important role during the GQDs-membrane interaction. On the one hand, the cholesterol tightly surrounds the inserted GQDs; on the other hand, the cholesterol molecule can be squeezed into the interface of two membrane leaflets. Meanwhile, the squeezed cholesterol molecule closely touches the GQD cluster, and freely moves along with the intimately contacted GQD cluster in the membrane. In addition, various analyzes confirm that the placental lipid membrane model is distinctly affected by the inserted GQDs, indicating the corresponding adverse influence to the biomembrane.
Methods
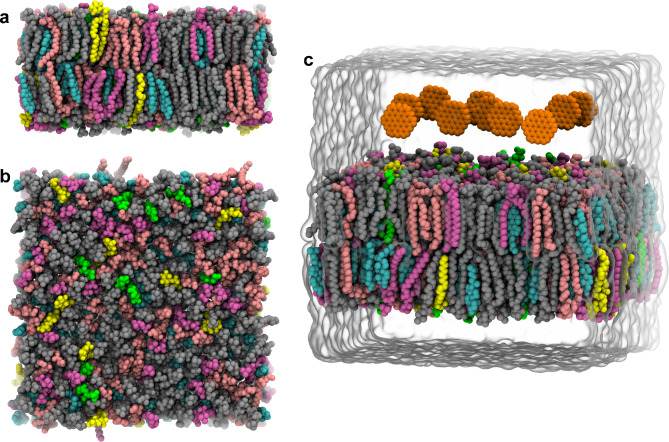
The realistic placental lipid membrane model was built using the CHARMM-GUI server,60,61 which was according to an experimental test62. In detail, this membrane model comprised 28.06% cholesterol, 10.79% PSM (palmitoyl sphingomyelin), 33.09% POPC (phosphatidylcholine), 3.60% POPS (phosphatidylserine), 3.60% POPI (phosphatidylinositol), and 20.86% POPE (phosphatidylethanolamine) in each leaflet. The membrane model had a surface dimension of 12.44 × 12.44 nm2, which can be found in Fig. 1a,b. Following, this membrane model was placed into a water box with the size of 12.44 × 12.44 × 10.11 nm3, which was also added 0.15 M NaCl. This box was then performed a 500-ns simulation, as a control simulation. The final membrane structure was extracted and ten GQDs were randomly put on the membrane with their initial minimum distance less than 1.5 nm (as shown in Fig. 1c). The GQD had a circle shape with a dimeter of 1.2 nm. The obtained complex was dissolved into the solution with 0.15 M NaCl, wherein the box size was 11.22 × 11.22 × 11.48 nm3. This box was finally performed three parallel simulations with each of duration of 200 ns without any position restraint. We have examined the box size along x/y directions as shown in Figure S1, which demonstrate the membrane dimensions during simulations. Clearly, the membrane dimensions just show limited fluctuations at the end of simulations, indicating that these simulations have converged.
Fig. 1.
The placental lipid membrane model from side view (a) and top view (b). The placental membrane comprises six main lipid compositions, including cholesterol (cyan), PSM (mauve), POPE (pink), POPC (silver), POPS (green) and POPI (yellow). (c) Simulation setup. The GQDs are shown in orange spheres. The box boundary is also illustrated.
All molecular dynamics (MD) simulations were performed using the GROMACS software package (version 2018)63, and the trajectories and snapshots were analyzed using the VMD software64. The CHARMM 36 force field65 and the TIP3P water model66 were employed for the lipid and water molecules, respectively. The force field parameters of GQD was derived from a previous work67. The temperature was maintained at 310 K using the v-rescale thermostat68 and the pressure was restrained at 1 atm with Parrinello-Rahman barostat method69 (semi-isotropic pressure of x + y and z). Periodic boundary conditions were used in all directions (x, y and z). Long-range electrostatic interactions were treated using the particle mesh Ewald (PME) method,70,71 and the van der Waals (vdW) interactions were calculated within a cutoff distance of 1.2 nm. All bonds involving hydrogen atoms were constrained to their equilibrium values using the LINCS algorithm72, while the SETTLE algorithm73 was employed to constrain the geometry of the water molecules. A time step of 2.0 fs was applied, and the coordinates of all atoms were saved every 10 ps.
In order to calculate the potential of mean force (PMF) profiles for the insertion capacity of the GQDs into the placental lipid membrane model, umbrella sampling simulations were performed by pulling the GQD monomer and cluster from the insertion position to solution74–76. During the pulling process, the vertical distances (d) between the center of masses (CoMs) of GQD and the membrane were restrained at a reference distance (d0) using a harmonic force of F = k × (d − d0), where k was the force constant (2000 kJ mol-1 nm-2). Sampling windows were spaced at 0.1 nm intervals, and each d0 distance was equilibrated for 2.0 ns before a 10 ns productive run. Free energy profiles were obtained using the g_wham tool77, which implements the weighted histogram analysis method.
Results
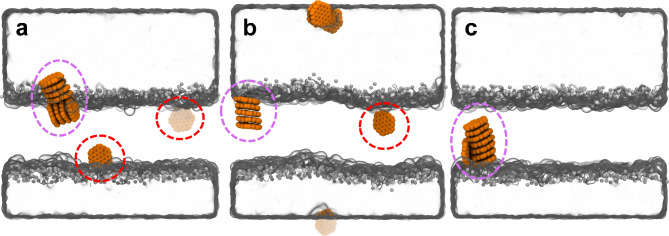
Figure 2 illustrates the final binding sites of GQDs in the final simulation boxes of three parallel simulations. Notably, the GQDs can enter into the placental lipid membrane model interior in all three simulations. Interestingly, the GQDs permeate into membrane either with monomer (denoted by red dashed circle) or formed as a cluster (purple dashed circle), indicating that both GQD monomer and cluster has the capacity to penetrate into placental lipid membrane model. In addition, the GQD clusters also feature two major forms, one with multiple layers aligned face-to-face (Fig. 2b), and another where clusters or monomers contacted the side surfaces of other GQD cluster (Fig. 2a,c). Overall, the GQDs have a strong capacity to enter into placental lipid membrane model, because the hydrophobic GQDs are more likely to enter into the hydrophobic core of the membrane rather than stay in the water phase or lipid-water interface.
Fig. 2.
(a–c) The final binding sites of GQDs in three parallel simulations, run1 (a), run2 (b) and run3 (c). The water boundary is shown with gray surfaces. The membrane is canceled, which locates at the position between two water boundaries in each figure. The red and purple dashed circles denote the insertion of GQD cluster and monomer into membrane, respectively.
The dynamics of GQDs inserting into the placental lipid membrane model is monitored by analyzing the contact atom number and interaction energy between membrane and GQDs, along with showing the binding conformations at four key simulation times (Fig. 3). The interaction between GQDs and membrane is quickly started, featuring the fast improvement of atom contact number and interaction energy. At 6.0 ns, one GQD monomer rapidly penetrates into the membrane interior with a vertical orientation, which is accompanied by the increase of atom contact number (to 539) and vdW interaction energy (to − 505 kJ/mol). Then, after 7.5 ns, the inserted GQD monomer slightly moves and the GQD cluster attaches the membrane surface as well. Meanwhile, the contact atom number and interaction energy have limited fluctuations. At 36.0 ns, two GQD clusters are formed and moves laterally on the membrane surface. At 51.5 ns, one GQD cluster enters into the membrane interior, with the contact atom number and vdW interaction energy sharply approaching 1206 and − 1092 kJ/mol, respectively. And the other GQD cluster maintains its contact on the membrane surface. This conformation persists till the end of simulation, featuring minimal fluctuations. This dynamic process suggests that both GQD monomer and cluster can spontaneously insert into the placental lipid membrane model.
Fig. 3.
Dynamic analysis of a typical trajectory. (a) Time dependent atom contact number of membrane to GQDs. (b) Time dependent interaction between GQDs and membrane. (c) Four binding conformations of GQDs to membrane at four key time points.
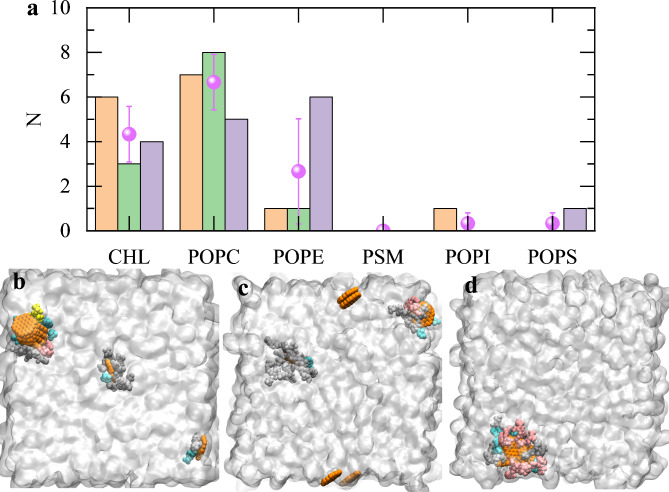
We also show the numbers of each lipid binding to GQDs at the final conformations of three parallel simulations (Fig. 4). The cholesterol and POPC lipids are primarily involved in the interfacial binding of GQDs and the membrane across all three simulations, with POPC having the highest contact number, followed by cholesterol. The high contact number of cholesterol and POPC is attributed to their significant proportions in the placental lipid membrane model (i.e., 28.06% and 33.09%). By normalizing the lipid numbers versus the total number of each lipid in the membrane (as shown in Fig. S2), we find that the cholesterol and POPC lipids often present relatively high normalized contact ratios, although other lipids occasionally have the higher ratio. The high inherent fraction of cholesterol in the placental lipid membrane model makes the cholesterol possessing a significant role in interacting with nanomaterials. From the binding conformations, we can observe that the inserted GQD monomer and cluster are fully surrounded by the lipids, indicating their high binding affinity. The previous experimental study43 have demonstrated that cholesterols can pack GQDs forming lipid rafts, which effectively helps the entry of GQD into membranes. Our simulations also support the abundant adsorption of cholesterol molecules to GQDs (i.e., Fig. 4). Apart from the robust adsorption, GQD cluster can also squeeze the cholesterol molecule into the membrane bilayer interface, damaging the normal conformation and function of cholesterol in the membrane. For example, the lateral fluidity of the membrane is highly restrained upon the GQDs’ insertion, which is a significant factor of cancer54,55.
Fig. 4.
(a) Number of each lipid binding to GQDs at the final conformations. Three colors indicate three parallel simulations. The data are obtained from the final binding conformations of each simulation. The mean number of binding lipids and corresponding error bars are also illustrated. (b–d) Conformations of different lipids binding to GQDs, run1 (b), run2 (c) and run3 (d). The gray surface denotes the membrane boundary. The lipid colors are same as Fig. 1.
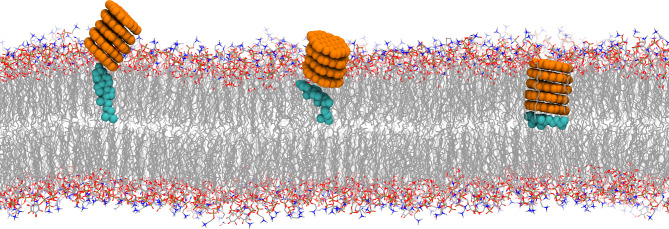
Surprisingly, we observed that one cholesterol molecule is squeezed into the membrane bilayer interface by the inserted GQD cluster, becoming finally sandwiched by the bottom membrane leaflet and the GQD cluster as shown in Fig. 5. During this process, the cholesterol undergoes a sharp orientation rotation from upright to flat. More importantly, such binding pattern (the cholesterol sandwiched by the bottom membrane leaflet and the GQD cluster) maintains till the end of simulation. That is to say, the squeezed cholesterol molecule tightly attaches to the GQD cluster basal surface, and freely move laterally along with the inserted GQD cluster. Specifically, when the GQD cluster inserts into the membrane, GQD cluster has possibility to touch the cholesterol molecule (e.g., Fig. 5). Due to the smaller size of cholesterol compared with other lipids, the GQD cluster pushes the adsorbed cholesterol into the membrane bilayer interface. This finding clearly demonstrates that the GQDs inserting into the placental lipid membrane model can robustly damage the normal conformation of cholesterol in the membrane, indicating the potential adverse influence.
Fig. 5.
Robust perturbation of GQD cluster to cholesterol molecule. During the insertion of GQD cluster into membrane, one cholesterol molecule is extruded into the interface of membrane bilayer. The cholesterol is displayed by cyan spheres.
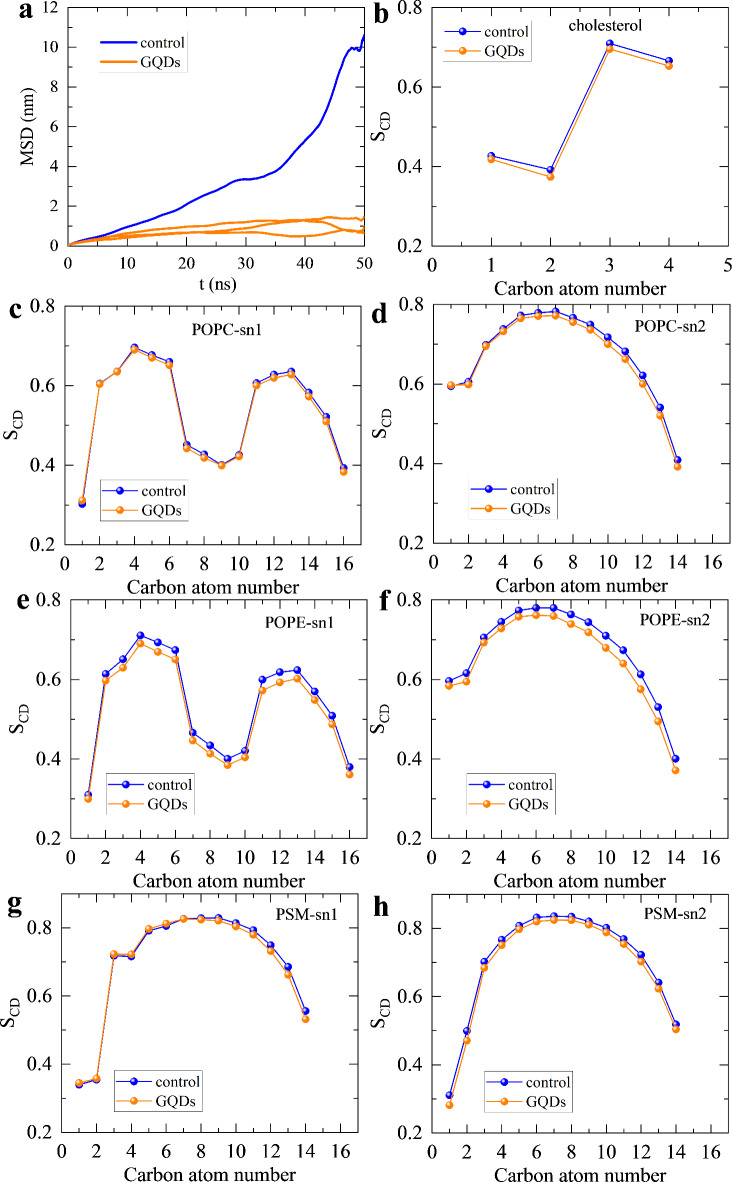
We then assessed the critical structural parameters of the placental lipid membrane model upon the insertion of GQDs. Figure 6a shows the mean square displacement (MSD) of membrane lipids along lateral direction during the final 50 nanoseconds of the control and three parallel simulations. The control simulation denotes the 500-ns simulation in absence of GQDs (more see “Methods” section). The MSD profiles distinctly show that the placental lipid membrane model treated with GQDs in three parallel simulations presents much lower MSD values compared with that in control simulation. This suggests that the insertion of GQDs is capable of strongly reducing the lateral fluidity of the placental lipid membrane model, which represents a detrimental indicator to the cellular membrane54,55 Moreover, the deuterium order parameters (SCD) of both tail chains in lipids are also examined as shown in Fig. 6b,h, Figs. and S4. The lipids with treatment of GQDs show slightly changed SCD values relative to those of control simulation in most cases. In addition, the order parameters of lipids in the extended 100 ns of GQDs treated membrane also have small difference compared with the control simulation (Fig. S5). Intuitively, increased lipid diffusion was expected due to the incremental area per lipid with a slightly lower order parameters of lipid tails. But, the GQDs inserting into the membrane robustly adsorb the lipids around them, forming “artificial” lipid rafts. Such large “artificial” lipid rafts in the membrane can hinder the free lateral movement of other lipids, leading to the declined lipid fluidity.
Fig. 6.
(a) Mean square displacement (MSD) of membrane lipids in control (blue) and three parallel (orange) simulations along the lateral direction. (b–h) Order parameters (SCD) of membrane lipids’ carbon tails of cholesterol (b), POPC (c,d), POPE (e,f) and PSM (g,h). The sn1 and sn2 denote two carbon tails of lipids.
To further investigate the impact of the GQDs insertion on the placental lipid membrane model, the membrane thickness and the area per lipid were calculated using the GridMAT-MD software package78. The control simulation features a smooth membrane thickness (Fig. 7a), whereas the membranes after GQDs insertion (Fig. 7b,d) have many distinct “hump” and “valley”, indicating that the insertion of GQDs is able to deform the lipids membrane. The area per lipid calculations (Table 1) show the increased value of membrane lipids after GQDs insertion. These results collectively support that the insertion of the GQDs into the placental lipid membrane model can cause damage to the membrane structural integrity leading to changes in lipid dynamics and membrane morphology.
Fig. 7.
2D thickness distribution of membrane in control and three parallel simulations (GQDs-run1, GQDs-run2 and GQDs-run3). The unit of color bar is nanometer.
Table 1.
Area per lipid in each simulation (unit in Å2).
| Control | GQDs-run1 | GQDs-run2 | GQDs-run3 | |
|---|---|---|---|---|
| Top leaflet | 43.86 | 44.79 | 43.95 | 44.70 |
| Bottom leaflet | 43.86 | 44.79 | 43.95 | 44.70 |
Finally, we also performed the potential of mean force (PMF) calculations based on umbrella sampling method to evaluate the free energy of the GQD monomer and cluster inserting into the placental lipid membrane model. We have calculated the count distribution of PMF calculation along the pulling direction of two systems as shown in Fig. S6, wherein the entire PMFs are fully sampled. As shown in Fig. 8, the GQD monomer and cluster are pulled away from the inserted conformation, and the corresponding PMF profiles are achieved. The GQD monomer and cluster have the lowest potential wells at the inserted conformations, with their PMF values of -268 and − 471 kJ/mol, respectively. These negative values suggest that GQD monomer and cluster are energetically favorable to insert into the placental lipid membrane model, which also supports the above three parallel simulation results. In addition, the lower potential well of the GQD cluster relative to GQD monomer also implies that GQD cluster are more likely to persist within the membrane interior.
Fig. 8.
PMF profiles of one GQD inserting into membrane (a) and GQD cluster inserting into membrane (b).
Conclusion
In summary, utilizing MD simulations, we explore the potential impact of GQDs on a realistic placental lipid membrane model, with a focus on revealing the crucial role of cholesterol in this interaction. Our simulations show that both GQD monomers and clusters can spontaneously insert into the placental lipid membrane model, eventually stabilizing within the membrane interior. Dynamic analyses indicate that the insertion process is driven by vdW interaction energy. Cholesterol and POPC lipids play key roles associating to GQD insertion into the membrane. Specifically, cholesterol is robustly affected, being squeezed into the interface between the two membrane leaflets, where it is sandwiched between the bottom leaflet and the GQD cluster. This finding further underscores the critical role of cholesterol in the GQD insertion process. Additional membrane structure analyses reveal that the lateral fluidity of the placental lipid membrane model is affected following GQD penetration. Membrane thickness, and area per lipid further confirm that the placental lipid membrane model is strongly impacted by the presence of GQDs. The PMF profiles show the lower free energy of GQD monomers and clusters in the membrane model, indicating that both GQD monomers and clusters are energetically favorable for insertion into the placental lipid membrane model. Therefore, GQDs are favorable in terms of promoting toxic interactions. These findings not only demonstrate the harmful effects of GQDs on realistic placental lipid membrane models, but also emphasize the significant role of cholesterol in nanomaterial-biomembrane interactions, offering valuable insights into the interaction between nanomaterials and cell membranes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Zonglin Gu acknowledges the support of National Natural Science Foundation of China (No. 12104394) and Natural Science Research of Jiangsu Higher Education Institutions of China (No. 21KJB140024).
Author contributions
J.C. conceived the concept and designed the study. J.C. and Z.G. conducted the simulations and analyses J.C. and Z.G. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science306 (5696), 666–669 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Iijima, S. Helical microtubes of graphitic carbon. Nature354 (6348), 56–58 (1991). [Google Scholar]
- 3.Kroto, H. W., Heath, J. R., Obrien, S. C., Curl, R. F. & Smalley, R. E. C60: Buckminsterfullerene. Nature 318 (6042), 162–163 (1985).
- 4.Dai, L. M. Functionalization of graphene for efficient energy conversion and storage. Acc. Chem. Res.46 (1), 31–42 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Sun, Y. Q., Wu, Q. O. & Shi, G. Q. Graphene based new energy materials. Energy Environ. Sci.4 (4), 1113–1132 (2011). [Google Scholar]
- 6.Pumera, M. Graphene-based nanomaterials for energy storage. Energy Environ. Sci.4 (3), 668–674 (2011). [Google Scholar]
- 7.Raccichini, R., Varzi, A., Passerini, S. & Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater.14 (3), 271–279 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Feng, L. & Liu, Z. Graphene in biomedicine: opportunities and challenges. Nanomedicine6 (2), 317–324 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Mao, H. Y. et al. Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev.113 (5), 3407–3424 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Cha, C., Shin, S. R., Annabi, N., Dokmeci, M. R. & Khademhosseini, A. Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano. 7 (4), 2891–2897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao, H. et al. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small7 (11), 1569–1578 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Li, B. et al. Direct optical imaging of graphene in vitro by nonlinear femtosecond laser spectral reshaping. Nano Lett.12 (11), 5936–5940 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao, Y. et al. Graphene based electrochemical sensors and biosensors: a review. Electroanal22 (10), 1027–1036 (2010). [Google Scholar]
- 14.Lee, J. S., Joung, H. A., Kim, M. G. & Park, C. B. Graphene-based chemiluminescence resonance energy transfer for homogeneous immunoassay. ACS Nano. 6 (4), 2978–2983 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Yang, K. et al. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials33 (7), 2206–2214 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Yang, Z., Kang, S. & Zhou, R. Nanomedicine: de novo design of nanodrugs. Nanoscale6 (2), 663–677 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Liu, M., Yin, X. & Zhang, X. Double-layer graphene optical modulator. Nano Lett.12 (3), 1482–1485 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Das, A. et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 3 (4), 210–215 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Bolotin, K. I. et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun.146 (9–10), 351–355 (2008). [Google Scholar]
- 20.Sanchez, V. C., Jachak, A., Hurt, R. H. & Kane, A. B. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem. Res. Toxicol.25 (1), 15–34 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, J. Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis17 (1), 7–14 (2005). [Google Scholar]
- 22.Katsnelson, M. I. Graphene: carbon in two dimensions. Mater. Today. 10 (1–2), 20–27 (2007). [Google Scholar]
- 23.Geim, A. K. Status and prospects. Science324 (5934), 1530–1534 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Wang, C. et al. A novel hydrazine electrochemical sensor based on the high specific surface area graphene. Microchim. Acta. 169 (1–2), 1–6 (2010). [Google Scholar]
- 25.Coleman, J. N., Khan, U., Blau, W. J. & Gun’ko, Y. K. Small but strong: a review of the mechanical properties of carbon nanotube-polymer composites. Carbon44 (9), 1624–1652 (2006). [Google Scholar]
- 26.Pietroiusti, A., Stockmann-Juvala, H., Lucaroni, F. & Savolainen, K. Nanomaterial exposure, toxicity, and impact on Human Health. Wires Nanomed. Nanobi10 (5), e1513. (2018). [DOI] [PubMed]
- 27.Monteiro-Riviere, N. A. & Inman, A. O. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon44 (6), 1070–1078 (2006). [Google Scholar]
- 28.Eckelman, M. J., Mauter, M. S., Isaacs, J. A. & Elimelech, M. New perspectives on nanomaterial aquatic ecotoxicity: production impacts exceed direct exposure impacts for carbon nanotoubes. Environ. Sci. Technol.46 (5), 2902–2910 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Lam, C. W., James, J. T., McCluskey, R., Arepalli, S. & Hunter, R. L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol.36 (3), 189–217 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Du, J., Wang, S. T., You, H. & Zhao, X. S. Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: a review. Environ. Toxicol. Pharmacol.36 (2), 451–462 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Fadeel, B. et al. Safety assessment of graphene-based materials: focus on human health and the environment. ACS Nano. 12 (11), 10582–10620 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Kolosnjaj-Tabi, J. et al. Anthropogenic carbon nanotubes found in the airways of parisian children. Ebiomedicine2 (11), 1697–1704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, Y. et al. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. PNAS110 (30), 12295–12300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhavan, O. & Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 4 (10), 5731–5736 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Mao, J., Guo, R. & Yan, L. T. Simulation and analysis of cellular internalization pathways and membrane perturbation for graphene nanosheets. Biomaterials35 (23), 6069–6077 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Tu, Y. et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 8 (8), 594–601 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Corredor, C. et al. Disruption of model cell membranes by carbon nanotubes. Carbon60, 67–75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Höfinger, S. et al. A computational analysis of the insertion of carbon nanotubes into cellular membranes. Biomaterials32 (29), 7079–7085 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Shi, X. H., von dem Bussche, A., Hurt, R. H., Kane, A. B. & Gao, H. J. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat. Nanotechnol. 6 (11), 714–719 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu, W. P. et al. Nanomechanical mechanism for lipid bilayer damage Induced by carbon nanotubes confined in intracellular vesicles. PNAS113 (44), 12374–12379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong, Z. et al. Effect of shape on the entering of graphene quantum dots into a membrane: a molecular dynamics simulation. Acs Omega. 6 (16), 10936–10943 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, P. Z. et al. Molecular dynamics simulation of transport mechanism of graphene quantum dots through different cell membranes. Membranes12 (8), 753 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, X. Y. et al. The permeability and transport mechanism of graphene quantum dots (Gqds) across the biological barrier. Nanoscale7 (5), 2034–2041 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Liang, L. J. et al. Theoretical evaluation on potential cytotoxicity of graphene quantum dots. ACS Biomater. Sci. Eng.2 (11), 1983–1991 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Chong, Y. et al. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials35 (19), 5041–5048 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Zhang, D. et al. Systematic evaluation of graphene quantum dot toxicity to male mouse sexual behaviors, reproductive and offspring health. Biomaterials194, 215–232 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Wong-Ekkabut, J. et al. Computer simulation study of fullerene translocation through lipid membranes. Nat. Nanotechnol. 3 (6), 363–368 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Qiao, R., Roberts, A. P., Mount, A. S., Klaine, S. J. & Ke, P. C. Translocation of C-60 and its derivatives across a lipid bilayer. Nano Lett.7 (3), 614–619 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Liu, S. L. et al. Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nat. Chem. Biol.13 (3), 268–274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, Y., Li, Q. C., Dong, M. D. & Han, X. J. Effect of cholesterol on the fluidity of supported lipid bilayers. Colloid Surf. B. 196, 111353 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Pöhnl, M., Trollmann, M. F. W. & Böckmann, R. A. Nonuniversal impact of cholesterol on membranes mobility, curvature sensing and elasticity. Nat. Commun.14, 8038 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wennberg, C. L., van der Spoel, D. & Hub, J. S. Large influence of cholesterol on solute partitioning into lipid membranes. J. Am. Chem. Soc.134 (11), 5351–5361 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Shepherd, J. C. & Büldt, G. The influence of cholesterol on Head Group mobility in Phospholipid Membranes. Biochim. et Biophys. Acta (BBA)-Biomembranes. 558 (1), 41–47 (1979). [DOI] [PubMed] [Google Scholar]
- 54.Zhang, J. R. et al. Cholesterol content in cell membrane maintains surface levels of Erbb2 and confers a therapeutic vulnerability in Erbb2-Positive breast Cancer. Cell. Commun. Signal.17, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribas, V., García-Ruiz, C. & Fernández-Checa, J. C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Translational Med.5, 1–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parton, R. G. & Richards, A. A. Lipid rafts and Caveolae as portals for endocytosis: New insights and Common mechanisms. Traffic4 (11), 724–738 (2003). [DOI] [PubMed] [Google Scholar]
- 57.He, B. et al. The transport pathways of polymer nanoparticles in Mdck epithelial cells. Biomaterials34 (17), 4309–4326 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Wick, P. et al. Barrier capacity of human placenta for Nanosized materials. Environ. Health Perspect.118 (3), 432–436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kucki, M. et al. Impact of graphene oxide on human placental trophoblast viability, functionality and Barrier Integrity. 2d Mater.5 (3), 035014 (2018). [Google Scholar]
- 60.Jo, S., Kim, T., Iyer, V. G. & Im, W. Charmm-Gui: a web-based graphical user interface for Charmm. J. Comput. Chem.29 (11), 1859–1865 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Jo, S., Lim, J. B., Klauda, J. B. & Im, W. Charmm-Gui membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J.97 (1), 50–58 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang, X. et al. Increased placental phospholipid levels in pre-eclamptic pregnancies. Int. J. Mol. Sci.14 (2), 3487–3499 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abraham, M. J. et al. Gromacs: high performance Molecular simulations through Multi-level parallelism from laptops to supercomputers. SoftwareX1, 19–25 (2015). [Google Scholar]
- 64.Humphrey, W., Dalke, A. & Schulten, K. Vmd: visual molecular dynamics. J. Mol. Graph Model.14 (1), 33–38 (1996). [DOI] [PubMed] [Google Scholar]
- 65.Klauda, J. B. et al. Update of the Charmm All-Atom Additive Force Field for lipids: validation on six lipid types. J. Phys. Chem. B. 114 (23), 7830–7843 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating Liquid Water. J. Chem. Phys.79 (2), 926–935 (1983). [Google Scholar]
- 67.Gu, Z. L. et al. Multifaceted regulation of Potassium-Ion channels by Graphene Quantum dots. Acs Appl. Mater. Inter. 13 (24), 27784–27795 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys.126 (1), 014101 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Parrinello, M. & Rahman, A. Polymorphic transitions in single-crystals—a new molecular-dynamics method. J. Appl. Phys.52 (12), 7182–7190 (1981). [Google Scholar]
- 70.Darden, T., York, D. & Pedersen, L. Particle Mesh Ewald—an N.Log(N) method for Ewald Sums in large systems. J. Chem. Phys.98 (12), 10089–10092 (1993). [Google Scholar]
- 71.Essmann, U. et al. A smooth particle Mesh Ewald Method. J. Chem. Phys.103 (19), 8577–8593 (1995). [Google Scholar]
- 72.Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. Lincs: a linear constraint solver for molecular simulations. J. Comput. Chem.18 (12), 1463–1472 (1997). [Google Scholar]
- 73.Miyamoto, S. & Kollman, P. A. Settle—an analytical version of the shake and rattle algorithm for rigid water models. J. Comput. Chem.13 (8), 952–962 (1992). [Google Scholar]
- 74.Roux, B. The calculation of the potential of mean force using computer-simulations. Comput. Phys. Commun.91 (1–3), 275–282 (1995). [Google Scholar]
- 75.Torrie, G. M. & Valleau, J. P. Non-physical Sampling distributions in Monte-Carlo Free-Energy estimation - umbrella sampling. J. Comput. Phys.23 (2), 187–199 (1977). [Google Scholar]
- 76.Kumar, S., Rosenberg, J. M., Bouzida, D., Swendsen, R. H. & Kollman, P. A. Multidimensional free-energy calculations using the weighted histogram analysis method. J. Comput. Chem.16 (11), 1339–1350 (1995). [Google Scholar]
- 77.Hub, J. S., de Groot, B. L. & van der Spoel, D. G_Wham-a free weighted histogram analysis implementation including robust error and Autocorrelation estimates. J. Chem. Theory Comput.6 (12), 3713–3720 (2010). [Google Scholar]
- 78.Allen, W. J., Lemkul, J. A. & Bevan, D. R. Gridmat-Md: a Grid‐based membrane analysis tool for use with molecular dynamics. J. Comput. Chem.30 (12), 1952–1958 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.