Abstract
Corneal endothelial cells, situated on the innermost layer of the cornea, are vital for maintaining its clarity and thickness by regulating fluid. In this study, we investigated the differences in the transcriptome between young and old corneal endothelial cells using next-generation sequencing (NGS). Cultured endothelial cells from both young and elderly donors were subjected to NGS to unravel the transcriptomic landscape. Subsequent analyses, facilitated by Metascape, allowed for the dissection of gene expression variances, unearthing pivotal biological pathways. A total of 568 genes showed differences, and were related to Endomembrane system organization, nuclear receptors meta pathway, efferocytosis, etc. Notably, a reduction in the expression of 260 genes was observed in the aged cells form old donors, and in the related analysis, eukaryotic translation initiation, integrator complex, and Hippo YAP signaling were significant. Conversely, 308 genes exhibited elevated expression levels in the elderly, correlating with processes including transition metal ion transport and glycoprotein biosynthesis. In conclusion, our investigation has revealed critical genes involved in the aging process of corneal endothelial cells and elucidated their underlying biological pathways. These insights are instrumental in selecting targets for therapeutic intervention, thereby facilitating the advancement of novel therapeutic approaches for the restoration and preservation of corneal endothelial cell function.
Subject terms: Eye diseases, Corneal diseases
Introduction
Corneal endothelial cells, residing in the innermost layer of the cornea, are vital for maintaining its clarity and thickness through fluid regulation1. Severe damage to these cells leads to corneal blindness or bullous keratopathy requiring corneal transplantation, because corneal endothelial cells have very limited regenerative abilities in vivo2. The mechanisms by which corneal endothelial cells fail to regenerate has been reported to include cell cycle arrest, abundant negative cytokine in anterior chamber, and senescence3. Senescence is a hallmark of aging process, playing a crucial role in both the biological aging of organisms and the development of age-related diseases4, and is similar to in vivo wound healing of corneal endothelial cells in that cells do not proliferate and are enlarged5. Thus, understanding the differences between the corneal endothelial cells of the young and the old is important for pioneering future therapeutic strategies for corneal endothelial regeneration. Differences in corneal endothelial cells between old and young donors have been reported, including proliferative capacity, cell cycle dynamics and protein expression6–9. This study employed next-generation sequencing (NGS) to analyze the transcriptome differences between young and old corneas. NGS represents an array of advanced sequencing technologies designed for fast, high-throughput analysis of DNA and RNA sequences10. Gene expression analysis involves quantifying the levels of mRNA produced from genes in a cell, providing insights into the functional state of those cells10. This comparison could reveal significant insights into gene expression changes, regulatory mechanisms, and pathways that are influenced by aging11. In this study, we investigated the differences in the transcriptome of corneal endothelial cells between young and old corneas using NGS, thereby elucidating the regulatory mechanisms and pathways influenced by aging.
Methods
This study was performed in accordance with the tenets of the Declaration of Helsinki and was reviewed and approved by the institutional review board/ethics committee (IRB) of the Hallym University Medical Center. Cells were cultured according to previously published methods12. Corneas were purchased from Eversight (Ann Arbor, MI), which had obtained informed consents for donated corneas. Because it was practically impossible to obtain consent from research subjects or human material donors in the case of human material research during the research process, the consent form was waived by the institutional review board/ethics committee of the Hallym University Medical Center. Corneas from three donors in each group were used. Human corneal endothelial cells-Descemet’s membrane complex was incubated for 10 min in 0.25% trypsin/0.02% ethylenediaminetetraacetic acid (EDTA) solution. Cells were then plated in 6-well plates coated with a fibronectin–collagen combination (FNC) coating mix (Athena Environmental Sciences, Inc., Baltimore, MD, USA). Cells were cultured to confluence for 10–14 days and then passaged at a ratio of 1:3 using 0.25% trypsin/0.02% EDTA solution. Donor ages were 26.6 ± 6.2y in young cornea (n = 5) and 69.3 ± 9.0y in old corneas (n = 4).
Cell shape evaluation and immunofluorescence staining
Cell shape was evaluated and microscopic images were obtained using an inverted fluorescence microscope (DMi8, Leica, Wetzlar, Germany). Immunofluorescence of ZO-1 was performed. Samples were initially rinsed with phosphate-buffered saline (PBS) and subsequently fixed in a 4% paraformaldehyde solution for 20 min. Permeabilization was performed with a 0.5% Triton X-100 solution for 10 min, followed by a blocking step with 1% bovine serum albumin (BSA) at 25 °C for one hour. Overnight incubation at 4 °C was performed with one of several antibodies: rat anti-ZO-1 (sc-33725, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing with PBS, samples were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG (1:100) for 2 h at 25 °C in darkness, followed by counterstaining with Hoechst 33,342 nuclear staining dye (1:2000; Molecular Probes, Eugene, OR, USA). Observations were made using a fluorescence microscope (DMi8), and images were documented.
Transcriptome analysis and Analysis of differentially expressed genes (DEGs) and functional analyses of DEGs
RNA extraction was meticulously conducted using the ReliaPrep™ RNA Miniprep Systems (Promega, Madison, WI, USA), ensuring the retrieval of high-quality RNA for further analysis. The sequencing of the extracted RNA was performed at MacroGen Inc. (www.macrogen.com), utilizing the advanced Illumina HiSeq 2000 platform13. This high-throughput sequencing technology facilitated a comprehensive examination of the transcriptome, enabling precise quantification and identification of gene expression differences across samples. For the analysis of differentially expressed genes (DEGs), the edgeR package and R 3.6.3 program (R Foundation, Vienna, Austria) were employed, a robust statistical tool designed for examining RNA sequencing data14. DEGs were identified based on stringent criteria: a log2(fold change (FC)) ≥ 1 combined with a false discovery rate (FDR) of < 0.05, ensuring that only statistically significant alterations in gene expression were considered. StringTie version 1.3.4d and DESeq2 software were used to calculate transcript abundances and confirm DEGs between young and old corneal endothelial cells15,16. The calculation of transcript abundances was performed using the Fragments Per Kilobase of transcript per Million mapped reads (FPKM) metric, providing a normalized measure of gene expression levels. To address the multiple comparison problem and reduce the likelihood of type I errors, FDR control was meticulously applied using the Benjamini–Hochberg algorithm, adjusting p-values to more accurately reflect the discovery of true positives.
Functional annotation and network analysis were performed using a Kyoto Encyclopedia of Genes and Genomes (www.kegg.jp/kegg/kegg1.html) or Metascape (https://metascape.org/gp/index.html#/main/step1), which was employed for the identification of metabolic pathways or signal transduction pathways that were significantly enriched in DEGs17. In addition, STRING database (https://string-db.org/) and ShinyGO0.80 were used for network analysis and functional annotation. GO terms and pathways with an adjusted p-value < 0.05 were considered significantly enriched.
Function and pathway enrichment analysis
Metascape (http://metascape.org/gp/index.html#/main/step1)18 serves as a sophisticated tool for gene function annotation, leveraging advanced bioinformatics methodologies for the batch analysis of genes and proteins to elucidate their biological functions. It offers researchers the capability to annotate an extensive array of genes or proteins comprehensively, facilitating the exploration of their roles within biological contexts. Furthermore, Metascape enables the performance of enrichment analysis, a crucial step in interpreting large-scale genomics and proteomics data by identifying over-represented functional categories that may shed light on the underlying biological processes. Additionally, the construction of protein–protein interaction (PPI) networks through Metascape provides invaluable insights into the molecular interactions and signaling pathways, allowing for a deeper understanding of cellular mechanisms. This multifaceted approach not only streamlines the functional analysis of gene sets but also significantly enhances the ability to uncover novel insights into the complex dynamics of biological systems19.
Results
Cell shape and DEGs
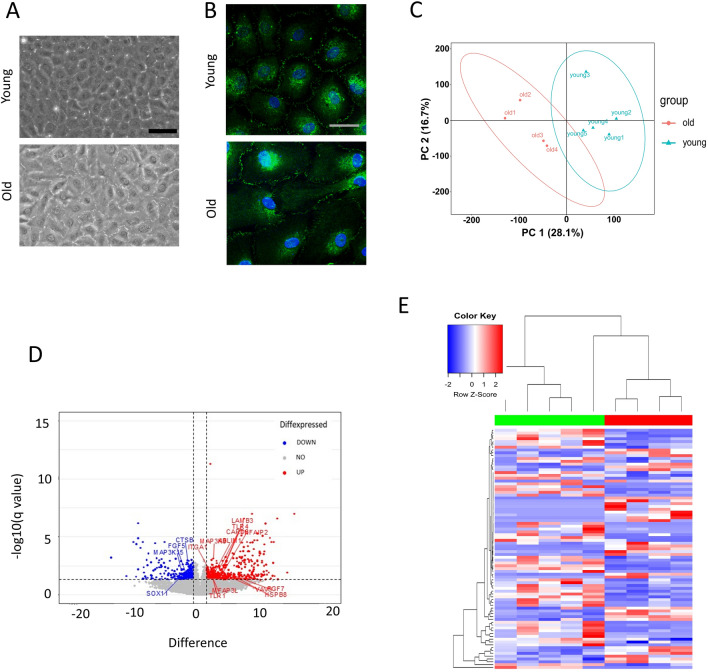
The morphology of corneal endothelial cells from young and old donors was evaluated to gain the insight into the health of cells (Fig. 1A). Compared to young cells, old corneal endothelial cells were larger. Immunofluorescence staining of ZO-1 showed the distribution of the ZO-1 protein within cells (Fig. 1B). ZO-1 is a key protein found in tight junctions, which are structures that tightly seal cells together in corneal endothelial cells, creating a barrier and controlling the passage of molecules. ZO-1 appeared as continuous lines at the cell borders, outlining where cells meet and form junctions. We selected all significantly up-regulated and down-regulated mRNAs in corneal endothelial old donor to plot their expression on principal component analysis (PCA) plot, heat-maps and volcano plots of differentially expressed mRNAs (Fig. 1C-1E). The significantly up-regulated and down-regulated DEGs are shown in Tables 1. The NGS analysis resulted in the identification of 568 DEGs. Of this total, 308 were characterized by upregulation and 260 by downregulation in corneal endothelial cells from older donors. These DEGs underwent further examination through the ShinyGO 0.80 (http://bioinformatics.sdstate.edu/go/) and the Metascape tool (http://metascape.org/gp/index.html#/main/step1).
Fig. 1.
(A) Morphology of corneal endothelial cells from young and old donor. Scale bar = 100 μm. (B) Immunofluorescence staining of ZO-1 was performed. Scale bar = 25 μm. (C) Principal component analysis (PCA) plot, (D) Volcano plot, (E) Heatmap of differentially expressed mRNAs from corneal endothelial cells from young and old donor.
Table 1.
The differential expressed genes.
| Gene | P value | q value |
|---|---|---|
| Up-regulated | ||
| SGSM3 | 0.000808 | 0.045453 |
| SUPT7L | 0.000805 | 0.045448 |
| DGCR6L | 0.000343 | 0.035461 |
| TIMM29 | 0.000567 | 0.040427 |
| KDELR1 | 0.000036 | 0.01458 |
| SHOC2 | < 0.000001 | 0.002559 |
| IPO13 | 0.000054 | 0.016326 |
| ZSCAN18 | 0.000226 | 0.03223 |
| BET1L | 0.000404 | 0.036897 |
| EXOC6B | 0.000108 | 0.0238 |
| FAM222B | 0.000284 | 0.033477 |
| STAT2 | 0.00019 | 0.03021 |
| SLC39A9 | 0.000049 | 0.015769 |
| ZBTB47 | 0.000497 | 0.039341 |
| S100A11 | 0.00054 | 0.039993 |
| ATP6AP2 | 0.000442 | 0.037836 |
| TMEM219 | 0.000342 | 0.035461 |
| SEC16A | 0.001104 | 0.049351 |
| ITGAE | 0.000666 | 0.041674 |
| TESK1 | 0.001066 | 0.048858 |
| SELENOW | 0.000914 | 0.046274 |
| TAX1BP1 | 0.00107 | 0.048874 |
| B4GALT3 | 0.000595 | 0.040784 |
| ROCK1P1 | 0.000895 | 0.045822 |
| ZDHHC9 | 0.000457 | 0.038152 |
| TOR1AIP2 | 0.000286 | 0.033477 |
| TMED7 | 0.000803 | 0.045448 |
| AP1B1 | 0.000046 | 0.015333 |
| SLC39A7 | 0.000982 | 0.047339 |
| SCYL1 | 0.00058 | 0.040704 |
| ZNF275 | 0.000182 | 0.029422 |
| POMT1 | 0.000836 | 0.045574 |
| FBXL7 | 0.00078 | 0.044762 |
| SLC4A2 | 0.000044 | 0.015295 |
| PRDX2 | 0.000029 | 0.012822 |
| NBPF3 | 0.000089 | 0.021595 |
| PPP1R12A-AS1 | 0.000245 | 0.032923 |
| MINPP1 | 0.000688 | 0.042365 |
| ATXN10 | 0.00054 | 0.039993 |
| CPD | 0.001026 | 0.047944 |
| PKP4 | 0.000364 | 0.035865 |
| SLC30A7 | 0.001095 | 0.049199 |
| PIP5K1C | 0.000075 | 0.019397 |
| MICOS10 | 0.000227 | 0.03223 |
| EMC10 | 0.000156 | 0.028623 |
| YIPF2 | 0.000448 | 0.038084 |
| TCTN3 | 0.000215 | 0.031763 |
| ABHD15 | 0.000761 | 0.044109 |
| PXN | 0.000108 | 0.0238 |
| BDH2 | 0.000013 | 0.008443 |
| KIAA2013 | 0.00093 | 0.046401 |
| SDF4 | 0.000173 | 0.029412 |
| TBC1D12 | 0.000543 | 0.039993 |
| THEM4 | 0.001016 | 0.047924 |
| ZSCAN16-AS1 | 0.000008 | 0.007177 |
| MYDGF | 0.000631 | 0.041521 |
| SLC35B3 | 0.000351 | 0.035587 |
| DEXI | 0.000626 | 0.041267 |
| C12orf73 | 0.000546 | 0.039993 |
| SLC35E1 | 0.00029 | 0.033477 |
| RNF121 | 0.001018 | 0.047924 |
| SLC39A11 | 0.00036 | 0.035721 |
| ATP6AP1 | 0.000358 | 0.035721 |
| MGAT2 | 0.000588 | 0.040704 |
| ATP1B3 | 0.00065 | 0.041674 |
| DNAJC15 | 0.000526 | 0.039977 |
| DNASE2 | 0.000472 | 0.038402 |
| ZC3H12B | 0.000608 | 0.040872 |
| PGRMC2 | 0.000077 | 0.019618 |
| EVI5 | 0.000643 | 0.041674 |
| ZFYVE27 | 0.000589 | 0.040704 |
| B3GAT3 | 0.000185 | 0.029654 |
| MFSD12 | 0.001068 | 0.048858 |
| ALG11 | 0.000589 | 0.040704 |
| AFF1 | 0.000195 | 0.030564 |
| PGAP6 | 0.000136 | 0.02705 |
| GLS | 0.000896 | 0.045822 |
| ENG | 0.000324 | 0.035114 |
| RNU6-3P | 0.000337 | 0.035461 |
| P3H4 | 0.00109 | 0.049199 |
| WIPI1 | 0.000495 | 0.039341 |
| BTBD3 | 0.000044 | 0.015295 |
| TPR | 0.000865 | 0.045651 |
| SLC38A10 | 0.000212 | 0.031713 |
| SEZ6L2 | 0.001108 | 0.049366 |
| ABCD3 | 0.001115 | 0.049496 |
| RNASET2 | 0.00066 | 0.041674 |
| PBLD | 0.000067 | 0.017747 |
| TCTA | 0.000446 | 0.037982 |
| BMPR1B | 0.000246 | 0.032923 |
| PICALM | 0.000227 | 0.03223 |
| LOC101927365 | 0.000667 | 0.041674 |
| H2AJ | 0.00005 | 0.015769 |
| DYNLL2 | 0.001052 | 0.048783 |
| ERN1 | 0.001085 | 0.049199 |
| ATP2C1 | 0.000441 | 0.037836 |
| RNU6-1 | 0.000704 | 0.042803 |
| TECTA | 0.000829 | 0.045574 |
| SMPD1 | 0.000179 | 0.029422 |
| NUAK1 | 0.000254 | 0.032923 |
| EMC1-AS1 | 0.000528 | 0.039977 |
| ARHGEF5 | 0.000137 | 0.02705 |
| LONP2 | 0.000122 | 0.02551 |
| SLC41A2 | 0.000196 | 0.030564 |
| IER3-AS1 | 0.000209 | 0.031713 |
| HTRA1 | 0.000997 | 0.047567 |
| CHPF | 0.000312 | 0.034655 |
| CHST3 | 0.000124 | 0.025547 |
| TGFB1 | 0.000517 | 0.039977 |
| GPR157 | 0.000037 | 0.014924 |
| SLC5A10 | 0.001006 | 0.047889 |
| NPIPB15 | 0.000434 | 0.037836 |
| RNU6-36P | 0.000177 | 0.029412 |
| ENTPD7 | 0.000945 | 0.046804 |
| GADD45B | 0.000214 | 0.031713 |
| SNORA14B | 0.000871 | 0.045651 |
| PPP1R13B | 0.001138 | 0.049979 |
| ARHGEF34P | 0.000537 | 0.039993 |
| MAPK13 | 0.000658 | 0.041674 |
| CKAP4 | 0.000439 | 0.037836 |
| SCARB1 | 0.000961 | 0.047339 |
| PLA2G15 | 0.001144 | 0.049979 |
| SLC22A23 | 0.001134 | 0.049958 |
| PAPSS2 | 0.000301 | 0.034253 |
| CFHR1 | 0.000848 | 0.045574 |
| MIR770 | < 0.000001 | 0.000005 |
| GSTT2B | 0.000001 | 0.002777 |
| MFSD6 | 0.000854 | 0.045643 |
| SIRPA | 0.000849 | 0.045574 |
| IRF5 | 0.000107 | 0.0238 |
| ARHGEF35 | 0.000108 | 0.0238 |
| FBN1 | 0.000985 | 0.047339 |
| IER3 | 0.000182 | 0.029422 |
| CSRNP1 | 0.000617 | 0.041144 |
| VEGFC | 0.00006 | 0.017196 |
| CYB561 | 0.000327 | 0.035225 |
| PODXL2 | 0.000335 | 0.035461 |
| MR1 | 0.000834 | 0.045574 |
| WDR66 | 0.000981 | 0.047339 |
| DOK1 | 0.000007 | 0.007148 |
| IGFBP7 | 0.000709 | 0.042809 |
| GRAMD2B | 0.000207 | 0.031713 |
| SLC46A3 | 0.000761 | 0.044109 |
| DOCK9-DT | 0.000659 | 0.041674 |
| SLC16A2 | 0.000526 | 0.039977 |
| SEMA3C | 0.000966 | 0.047339 |
| GSTT2 | < 0.000001 | 0.002036 |
| ANO5 | 0.000985 | 0.047339 |
| BNC2-AS1 | 0.000261 | 0.032923 |
| ABAT | 0.000774 | 0.044592 |
| GATA2-AS1 | 0.001099 | 0.049199 |
| CFH | 0.000608 | 0.040872 |
| GALNT5 | 0.000283 | 0.033477 |
| KBTBD8 | 0.000879 | 0.045667 |
| MAP3K9 | 0.000007 | 0.007177 |
| MID2 | 0.000727 | 0.043521 |
| TMEM255B | 0.000171 | 0.029406 |
| REPS2 | 0.00102 | 0.047924 |
| H1-4 | 0.000026 | 0.012261 |
| H2AC6 | 0.000594 | 0.040784 |
| ABLIM1 | 0.000289 | 0.033477 |
| SGK1 | 0.000623 | 0.041267 |
| EPHX2 | 0.000381 | 0.036676 |
| OXCT2P1 | 0.00062 | 0.041256 |
| ZNF365 | 0.000032 | 0.013726 |
| USP32P2 | 0.000082 | 0.020537 |
| CD55 | 0.000597 | 0.040797 |
| PCDHGA4 | 0.000884 | 0.045777 |
| KCTD16 | 0.000742 | 0.043844 |
| MEGF10 | 0.000993 | 0.047471 |
| KCNK1 | 0.000436 | 0.037836 |
| RNF180 | 0.000554 | 0.040233 |
| PRKG2 | 0.000874 | 0.045651 |
| NFASC | 0.000218 | 0.031852 |
| TLR4 | 0.000403 | 0.036897 |
| PMEPA1 | 0.000692 | 0.042369 |
| SLC4A11 | 0.000538 | 0.039993 |
| PLEKHH1 | 0.00032 | 0.034952 |
| KCNT2 | 0.000887 | 0.045818 |
| SLC22A15 | 0.000101 | 0.023385 |
| PDZD2 | 0.00016 | 0.028639 |
| ACSL5 | 0.000502 | 0.039341 |
| RGS4 | 0.000432 | 0.037836 |
| H4C8 | 0.00105 | 0.048783 |
| LINC01138 | 0.000372 | 0.036369 |
| ANKRD6 | 0.000332 | 0.035296 |
| GPRC5D-AS1 | 0.000008 | 0.007177 |
| ACOT11 | 0.000352 | 0.035587 |
| HSPA12A | 0.000049 | 0.015769 |
| TPD52 | 0.000554 | 0.040233 |
| TMEM233 | 0.000267 | 0.032923 |
| ADAMTS5 | 0.000993 | 0.047471 |
| ZBED2 | 0.000485 | 0.039158 |
| NEDD9 | 0.000043 | 0.015295 |
| CES4A | 0.000063 | 0.017352 |
| GPRC5B | 0.001098 | 0.049199 |
| CNTNAP3 | 0.000352 | 0.035587 |
| HERC2P7 | 0.000039 | 0.015132 |
| TRPM3 | 0.000913 | 0.046274 |
| APOL1 | 0.000467 | 0.038324 |
| WSCD1 | 0.000193 | 0.030515 |
| GALNT18 | 0.000462 | 0.038321 |
| POLRMTP1 | 0.000331 | 0.035296 |
| TMEM229B | 0.000896 | 0.045822 |
| TENM2 | 0.000737 | 0.0437 |
| LARGE1 | 0.000098 | 0.022834 |
| FGF7 | 0.000406 | 0.036897 |
| SLC2A3P2 | 0.000059 | 0.017107 |
| CNTNAP3P2 | 0.00009 | 0.021772 |
| GBP4 | 0.000001 | 0.002571 |
| BEND7 | 0.000848 | 0.045574 |
| CHST15 | 0.000217 | 0.031852 |
| RNASEH1P2 | 0.000415 | 0.037188 |
| PTGFRN | 0.000051 | 0.015769 |
| MX2 | 0.001041 | 0.048539 |
| HTR1D | 0.000287 | 0.033477 |
| MPZL3 | 0.000012 | 0.008443 |
| PAX8-AS1 | 0.000129 | 0.026371 |
| DIO2 | 0.000556 | 0.040233 |
| LINC00639 | 0.00052 | 0.039977 |
| WDR93 | 0.000067 | 0.017747 |
| SMOC1 | 0.000331 | 0.035296 |
| CNTNAP3B | 0.000023 | 0.011409 |
| LOC101929268 | 0.000258 | 0.032923 |
| RPS10P1 | 0.000993 | 0.047471 |
| SAMD9 | 0.00017 | 0.029406 |
| IL21-AS1 | 0.000637 | 0.041674 |
| GAS2L1P2 | 0.00008 | 0.020215 |
| JPH2 | 0.000317 | 0.034952 |
| COL10A1 | 0.000965 | 0.047339 |
| TTBK1 | 0.000698 | 0.042626 |
| LINC01235 | 0.000004 | 0.006366 |
| P4HA3-AS1 | 0.000045 | 0.015295 |
| OR2S2 | 0.000058 | 0.017015 |
| CYP51A1P1 | 0.000115 | 0.024852 |
| MYBPC1 | 0.000012 | 0.008443 |
| OGFR-AS1 | 0.001107 | 0.049366 |
| LINC00511 | 0.000397 | 0.036897 |
| IQCA1 | 0.000868 | 0.045651 |
| LINC02542 | < 0.000001 | 0.001529 |
| ERMN | 0.00002 | 0.010222 |
| ANGPTL7 | 0.000244 | 0.032923 |
| TPTE2 | 0.000118 | 0.024907 |
| PDE6A | 0.000143 | 0.027256 |
| MESTIT1 | 0.000132 | 0.026713 |
| TECTB | 0.000397 | 0.036897 |
| LOC105373553 | 0.000083 | 0.02069 |
| UBE2QL1 | 0.000282 | 0.033477 |
| MYRFL | 0.000176 | 0.029412 |
| LINC00856 | 0.000006 | 0.007148 |
| CCN4 | 0.000013 | 0.008443 |
| GPR68 | 0.000342 | 0.035461 |
| CXADRP3 | 0.000087 | 0.021254 |
| LIMCH1 | 0.000024 | 0.011594 |
| LINC02613 | 0.000024 | 0.011594 |
| GPAT2P1 | 0.000235 | 0.032746 |
| CD1D | 0.000028 | 0.012309 |
| LINC01592 | 0.000044 | 0.015295 |
| C8orf34-AS1 | 0.000377 | 0.036426 |
| VWA2 | 0.00001 | 0.007485 |
| RNU5E-1 | 0.000687 | 0.042365 |
| CDH10 | 0.000309 | 0.034574 |
| RSPO4 | 0.00026 | 0.032923 |
| FOXO6 | 0.000013 | 0.008443 |
| ARMC4 | 0.001097 | 0.049199 |
| H2AC21 | < 0.000001 | 0.001303 |
| TSPEAR-AS1 | 0.000679 | 0.042008 |
| FGF16 | 0.00002 | 0.010222 |
| FAM95C | 0.00022 | 0.031852 |
| LOC100132057 | 0.000018 | 0.009982 |
| ADGRF4 | 0.000964 | 0.047339 |
| MIR412 | 0.000278 | 0.033477 |
| EGLN3 | 0.000571 | 0.040459 |
| SNORD114-13 | 0.000009 | 0.007271 |
| CYP24A1 | 0.000204 | 0.031489 |
| HLA-V | 0.000562 | 0.040404 |
| FGFR2 | 0.000159 | 0.028639 |
| LINC02575 | 0.000005 | 0.00691 |
| CNTNAP3P4 | 0.000002 | 0.00346 |
| FBP1 | 0.000248 | 0.032923 |
| KRTAP5-AS1 | 0.000925 | 0.046274 |
| NYAP2 | 0.000415 | 0.037188 |
| MAPK4 | 0.000865 | 0.045651 |
| LINC01561 | 0.000406 | 0.036897 |
| VIT | 0.000047 | 0.01558 |
| FAM201A | 0.000027 | 0.012267 |
| TRBJ2-1 | 0.000033 | 0.013816 |
| MIR369 | 0.000039 | 0.015132 |
| IDO1 | 0.000454 | 0.038152 |
| GPAT2 | 0.000063 | 0.017352 |
| LINC01239 | 0.00014 | 0.02705 |
| SLC37A1 | 0.000781 | 0.044762 |
| SMCO3 | 0.000662 | 0.041674 |
| KLHL4 | 0.000004 | 0.006425 |
| DHRS2 | 0.000011 | 0.008198 |
| SPINK1 | 0.00004 | 0.015132 |
| ADORA1 | 0.001127 | 0.049814 |
| ENTPD3 | < 0.000001 | 0.002024 |
| DLX5 | 0.000354 | 0.035587 |
| PTPN20 | 0.000163 | 0.028856 |
| CECR7 | 0.001139 | 0.049979 |
| SNORD113-3 | 0.000004 | 0.006366 |
| CGA | 0.000009 | 0.007271 |
| TDRD1 | 0.000016 | 0.009926 |
| PIEZO2 | 0.000919 | 0.046274 |
| BEX1 | 0.000257 | 0.032923 |
| MEG9 | < 0.000001 | 0.001584 |
| PSPHP1 | < 0.000001 | 0.001303 |
| Down-regulated | ||
| SPOCK3 | 0.000064 | 0.017352 |
| LINC00491 | 0.000006 | 0.007148 |
| CDIPTOSP | 0.000009 | 0.007271 |
| LINC01925 | 0.000003 | 0.005406 |
| CDKL4 | < 0.000001 | 0.001584 |
| GABRA4 | 0.000547 | 0.039993 |
| LLPH-DT | 0.000016 | 0.009643 |
| GNG3 | 0.000006 | 0.007148 |
| CDK2AP2P1 | 0.000384 | 0.03677 |
| RPL36AP15 | 0.000097 | 0.022834 |
| HCG22 | 0.000266 | 0.032923 |
| NPFFR2 | 0.000035 | 0.01458 |
| TBX4 | 0.001112 | 0.049443 |
| MLLT10P1 | 0.000156 | 0.028623 |
| SGCZ | 0.00073 | 0.043558 |
| SFRP1 | 0.001143 | 0.049979 |
| FAUP1 | 0.000008 | 0.007177 |
| RPS25P2 | 0.000564 | 0.040404 |
| RSL24D1P11 | 0.000073 | 0.018976 |
| FAM225A | 0.000442 | 0.037836 |
| RPS4XP22 | 0.000164 | 0.028909 |
| GABRB1 | 0.000117 | 0.024907 |
| FAM225B | 0.000387 | 0.03686 |
| RPS7P3 | 0.000404 | 0.036897 |
| TP53TG3B | 0.000263 | 0.032923 |
| TP53TG3C | 0.00032 | 0.034952 |
| EMILIN3 | 0.001012 | 0.047924 |
| USP32P1 | 0.000289 | 0.033477 |
| DBF4P1 | 0.00017 | 0.029406 |
| HTATSF1P2 | 0.000007 | 0.007148 |
| KCNN2 | 0.000376 | 0.036426 |
| TP53TG3 | 0.00037 | 0.036319 |
| BCHE | 0.000667 | 0.041674 |
| RPS2P7 | 0.000286 | 0.033477 |
| RPS2P20 | 0.000648 | 0.041674 |
| LY6K | 0.000658 | 0.041674 |
| CCDC144A | 0.000306 | 0.034464 |
| TPPP3 | 0.00087 | 0.045651 |
| PCDHGA11 | 0.000267 | 0.032923 |
| LOC100288175 | 0.000296 | 0.033862 |
| APLN | 0.000556 | 0.040233 |
| LOC440568 | 0.000239 | 0.032923 |
| LRRCC1 | 0.000817 | 0.045535 |
| MAMDC2 | 0.000309 | 0.034574 |
| APOBEC3D | 0.000514 | 0.039977 |
| SSC5D | 0.000044 | 0.015295 |
| WDR17 | 0.000131 | 0.026551 |
| ZNF560 | 0.000657 | 0.041674 |
| SNORD135 | 0.00042 | 0.037192 |
| USP44 | 0.000736 | 0.0437 |
| LINC01140 | 0.000304 | 0.034464 |
| NR1H3 | 0.000051 | 0.015769 |
| PGGHG | 0.000806 | 0.045448 |
| DDIT4 | 0.000559 | 0.040315 |
| MNS1 | 0.000648 | 0.041674 |
| DPYSL2 | 0.000758 | 0.044109 |
| AOC3 | 0.000812 | 0.045484 |
| CGAS | 0.000027 | 0.012261 |
| SNCA | 0.00098 | 0.047339 |
| RPS2P55 | 0.000601 | 0.040865 |
| MYO15B | 0.000918 | 0.046274 |
| EIF4EBP1 | 0.00023 | 0.03223 |
| CEBPD | 0.000799 | 0.045357 |
| RAPGEF4 | 0.000546 | 0.039993 |
| A2M-AS1 | 0.001065 | 0.048858 |
| RPL23AP87 | 0.000625 | 0.041267 |
| RPL9P8 | 0.000835 | 0.045574 |
| POU2F2 | 0.000242 | 0.032923 |
| FAM161A | 0.000063 | 0.017352 |
| GOLGA8H | 0.000438 | 0.037836 |
| IL7 | 0.000672 | 0.041899 |
| ARL6IP6 | 0.000604 | 0.040872 |
| CDCA4 | 0.000974 | 0.047339 |
| ARNTL2 | 0.000093 | 0.022161 |
| THAP9-AS1 | 0.000641 | 0.041674 |
| RPL23AP4 | 0.000866 | 0.045651 |
| B3GNT5 | 0.000455 | 0.038152 |
| CHRAC1 | 0.00026 | 0.032923 |
| VEGFA | 0.000757 | 0.044109 |
| SIAH1 | 0.000879 | 0.045667 |
| PTPRG-AS1 | 0.001141 | 0.049979 |
| SKAP2 | 0.000174 | 0.029412 |
| PSD3 | 0.000118 | 0.024907 |
| SLCO3A1 | 0.000985 | 0.047339 |
| MST1 | 0.000343 | 0.035461 |
| RPS2P5 | 0.000757 | 0.044109 |
| KIAA1324 | 0.000354 | 0.035587 |
| EIF3E | 0.000608 | 0.040872 |
| LONRF1 | 0.000527 | 0.039977 |
| TCEA1 | 0.000493 | 0.039341 |
| ATP23 | 0.001019 | 0.047924 |
| KIFC2 | 0.000586 | 0.040704 |
| MED30 | 0.000323 | 0.035095 |
| RPL7 | 0.000874 | 0.045651 |
| PPP1R3B | 0.000407 | 0.036897 |
| ZFP69B | 0.000797 | 0.045349 |
| PLAG1 | 0.000285 | 0.033477 |
| RN7SL832P | 0.000212 | 0.031713 |
| EFNA3 | 0.000653 | 0.041674 |
| AMZ2P1 | 0.00011 | 0.023831 |
| CENPP | 0.000936 | 0.046543 |
| NECTIN3 | 0.000657 | 0.041674 |
| FMNL2 | 0.000757 | 0.044109 |
| TBPL1 | 0.000144 | 0.027256 |
| AGER | 0.000103 | 0.023571 |
| BNIP3L | 0.000791 | 0.045171 |
| LOXL2 | 0.000183 | 0.029422 |
| DCLRE1B | 0.000611 | 0.040872 |
| NCOA2 | 0.000455 | 0.038152 |
| RPS2P46 | 0.000834 | 0.045574 |
| WHAMMP1 | 0.00027 | 0.033189 |
| SAV1 | 0.00112 | 0.049594 |
| STK17B | 0.000543 | 0.039993 |
| CUL7 | 0.001026 | 0.047944 |
| NSMCE2 | 0.000211 | 0.031713 |
| TRAF3IP2-AS1 | 0.000856 | 0.045651 |
| STK3 | 0.000019 | 0.010222 |
| RPL23AP79 | 0.001059 | 0.048858 |
| RBIS | 0.000058 | 0.017015 |
| RPL30 | 0.000587 | 0.040704 |
| RPS20 | 0.000376 | 0.036426 |
| RPS27P3 | 0.000145 | 0.027256 |
| INTS8 | 0.0004 | 0.036897 |
| FAM86B3P | 0.000834 | 0.045574 |
| PPM1M | 0.000566 | 0.040426 |
| SNX16 | 0.000013 | 0.008443 |
| PABPC1 | 0.000107 | 0.0238 |
| VPS13B | 0.000293 | 0.033638 |
| SLC66A1L | 0.000496 | 0.039341 |
| SPIDR | 0.00069 | 0.042367 |
| POLG2 | 0.000278 | 0.033477 |
| GASAL1 | 0.000874 | 0.045651 |
| ASH2L | 0.00042 | 0.037192 |
| RPL29P11 | 0.000494 | 0.039341 |
| RPS3AP5 | 0.000253 | 0.032923 |
| TMEM256 | 0.000953 | 0.047083 |
| MRPL13 | 0.000709 | 0.042809 |
| DNALI1 | 0.000025 | 0.012148 |
| DPH6 | 0.001134 | 0.049958 |
| DUS4L | 0.000045 | 0.015295 |
| ENY2 | 0.001066 | 0.048858 |
| AFDN | 0.000141 | 0.02705 |
| LRRC37A2 | 0.000178 | 0.029412 |
| ZNF623 | 0.000839 | 0.045574 |
| DHRS4-AS1 | 0.000409 | 0.036932 |
| ZNF706 | 0.000649 | 0.041674 |
| CAMK2D | 0.00101 | 0.047924 |
| C11orf54 | 0.001061 | 0.048858 |
| SNHG29 | 0.000244 | 0.032923 |
| NEO1 | 0.000775 | 0.044592 |
| ARHGEF10 | 0.000472 | 0.038402 |
| HMGN1P18 | 0.000916 | 0.046274 |
| FAM66B | 0.000265 | 0.032923 |
| PGBD1 | 0.000339 | 0.035461 |
| AP1S2 | 0.000907 | 0.046274 |
| ANP32B | 0.000064 | 0.017352 |
| NLN | 0.000468 | 0.038324 |
| WRN | 0.000149 | 0.02771 |
| ERICH1 | 0.000229 | 0.03223 |
| WASHC5 | 0.0006 | 0.040865 |
| SINHCAF | 0.001017 | 0.047924 |
| ATF1 | 0.001065 | 0.048858 |
| ZFAND1 | 0.000263 | 0.032923 |
| HILPDA | 0.001052 | 0.048783 |
| TPT1 | 0.000006 | 0.007148 |
| UBXN2B | 0.000314 | 0.034792 |
| LRRC37A | 0.000056 | 0.01668 |
| IQCH | 0.000459 | 0.038228 |
| PABPC5 | 0.000676 | 0.041996 |
| PBX2P1 | 0.000727 | 0.043521 |
| NSD3 | 0.00036 | 0.035721 |
| STARD3NL | 0.000468 | 0.038324 |
| SMIM19 | 0.000914 | 0.046274 |
| RPS15A | 0.000761 | 0.044109 |
| TRIQK | 0.000826 | 0.045574 |
| ALPK1 | 0.000506 | 0.03944 |
| CACYBP | 0.000362 | 0.035727 |
| ARHGAP21 | 0.000229 | 0.03223 |
| EMC2 | 0.0004 | 0.036897 |
| WASHC1 | 0.000043 | 0.015295 |
| ZNF251 | 0.000924 | 0.046274 |
| PRSS53 | 0.000569 | 0.040427 |
| SLC2A1 | 0.00054 | 0.039993 |
| EEF1D | 0.000738 | 0.0437 |
| CCDC25 | 0.000002 | 0.003769 |
| INTS10 | 0.001084 | 0.049199 |
| XPO7 | 0.000634 | 0.041617 |
| VDAC3 | 0.000477 | 0.038633 |
| AASDH | 0.000976 | 0.047339 |
| ARHGAP4 | 0.000388 | 0.03686 |
| GARS-DT | 0.000792 | 0.045171 |
| RPL27A | 0.00061 | 0.040872 |
| MAGOHB | 0.000176 | 0.029412 |
| PPP1R12B | 0.001085 | 0.049199 |
| PLEC | 0.00109 | 0.049199 |
| ZFP41 | 0.000853 | 0.045643 |
| ZNF558 | 0.000204 | 0.031489 |
| RESF1 | 0.000647 | 0.041674 |
| PTGES3 | 0.000159 | 0.028639 |
| DDHD2 | 0.000018 | 0.009982 |
| DDAH2 | 0.000722 | 0.043475 |
| MYL5 | 0.000942 | 0.046727 |
| MORC3 | 0.000518 | 0.039977 |
| SPTSSA | 0.000584 | 0.040704 |
| PACRGL | 0.000499 | 0.039341 |
| R3HDM4 | 0.000465 | 0.038324 |
| PEX2 | 0.000529 | 0.039977 |
| SRXN1 | 0.000018 | 0.009982 |
| FAM193B | 0.000018 | 0.009982 |
| ZNF333 | 0.000051 | 0.015769 |
| TRNAU1AP | 0.000139 | 0.02705 |
| ACAP3 | 0.000935 | 0.046543 |
| ATXN2L | 0.00109 | 0.049199 |
| CRYZL1 | 0.000925 | 0.046274 |
| ADAT2 | 0.00081 | 0.045458 |
| TRMT12 | 0.000924 | 0.046274 |
| FXR1 | 0.0005 | 0.039341 |
| C2orf74 | 0.000408 | 0.036897 |
| CAB39 | 0.000821 | 0.045574 |
| LYST | 0.000728 | 0.043521 |
| ZSWIM7 | 0.000503 | 0.039341 |
| AFMID | 0.000436 | 0.037836 |
| ZSCAN26 | 0.000288 | 0.033477 |
| BIN3 | 0.000847 | 0.045574 |
| ZNF397 | 0.000395 | 0.036897 |
| IFT88 | 0.00016 | 0.028639 |
| PUF60 | 0.000832 | 0.045574 |
| SLC25A43 | 0.000842 | 0.045574 |
| YWHAZ | 0.000861 | 0.045651 |
| MOCS2 | 0.000454 | 0.038152 |
| PIP4P2 | 0.000985 | 0.047339 |
| SFXN3 | 0.000749 | 0.044109 |
| ATPSCKMT | 0.001093 | 0.049199 |
| COG4 | 0.000763 | 0.044109 |
| TAF15 | 0.000138 | 0.02705 |
| ERLIN2 | 0.000242 | 0.032923 |
| RAB2A | 0.000894 | 0.045822 |
| PI4KAP1 | 0.00088 | 0.045667 |
| PARP4 | 0.000703 | 0.042803 |
| FNTA | 0.001023 | 0.047944 |
| ABCD4 | 0.000261 | 0.032923 |
| RHBDD1 | 0.000417 | 0.037192 |
| TBXAS1 | 0.000392 | 0.036897 |
| FBXO38 | 0.000345 | 0.035538 |
| SRSF4 | 0.000579 | 0.040704 |
| RNF139 | 0.000421 | 0.037192 |
| RNF214 | 0.000709 | 0.042809 |
| UBA3 | 0.000258 | 0.032923 |
| INTS11 | 0.000825 | 0.045574 |
| SPRED2 | 0.000582 | 0.040704 |
| GNPAT | 0.000592 | 0.040784 |
| JRKL | 0.000502 | 0.039341 |
| RBMS1 | 0.000985 | 0.047339 |
| TBC1D15 | 0.00035 | 0.035587 |
| MORF4L1 | 0.000888 | 0.045818 |
| LAMTOR4 | 0.000546 | 0.039993 |
| PSMG3 | 0.000677 | 0.041996 |
| FCHSD1 | 0.000523 | 0.039977 |
| SCAMP1 | 0.000847 | 0.045574 |
| MIPEP | 0.000817 | 0.045535 |
Enrichment analysis of total differentially expressed genes
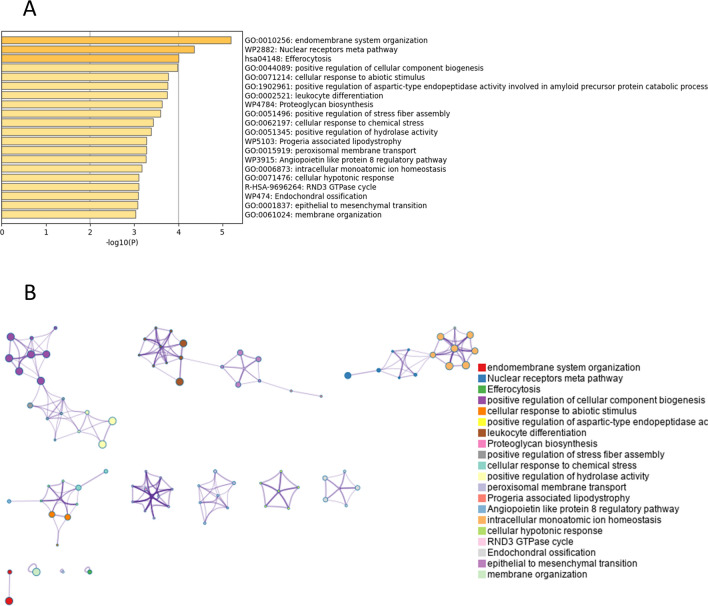
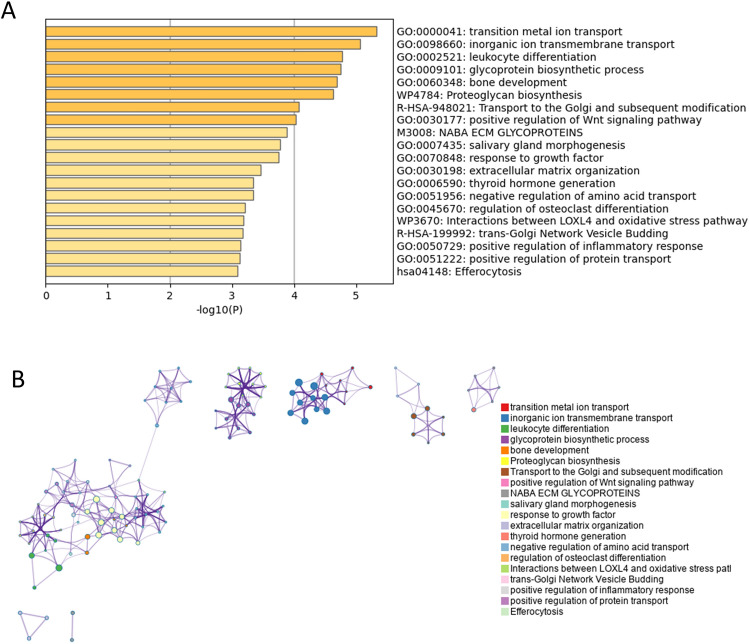
Functional enrichment analysis, conducted via Metascape, revealed that DEGs between young and old corneal endothelial cells were markedly enriched in several biological processes. These processes include endomembrane system organization, the nuclear receptors meta pathway, efferocytosis, and the positive regulation of cellular component biogenesis. Additionally, significant enrichment was observed in the cellular response to abiotic stimuli, positive regulation of aspartic-type endopeptidase activity—which plays a critical role in the amyloid precursor protein catabolic process—proteoglycan biosynthesis, positive regulation of stress fiber assembly, and peroxisomal membrane transport (p < 0.05; see Fig. 2 and Table 2).
Fig. 2.
Enrichment analysis of total differentially expressed genes (DEGs) by Metascape (http://metascape.org/gp/index.html#/main/step1). (A) Bar graph of enriched terms of total DEGs (colored by p-values). (B) Network of enriched terms of total DEGs, colored by cluster identity, where nodes that share the same cluster identity are typically close to each other.
Table 2.
Pathway and process enrichment analysis of total differentially expressed genes (Metascape, Access 2023.12.15).
| GO | Category | Description | Count | % | Log10(P) | Log10(q) |
|---|---|---|---|---|---|---|
| GO:0010256 | GO Biological Processes | Endomembrane system organization | 26 | 4.91 | − 5.19 | − 0.85 |
| WP2882 | WikiPathways | Nuclear receptors meta pathway | 17 | 3.21 | − 4.36 | − 0.45 |
| hsa04148 | KEGG Pathway | Efferocytosis | 11 | 2.08 | − 4.00 | − 0.37 |
| GO:0044089 | GO Biological Processes | Positive regulation of cellular component biogenesis | 22 | 4.16 | − 3.99 | − 0.37 |
| GO:0071214 | GO Biological Processes | Cellular response to abiotic stimulus | 16 | 3.02 | − 3.77 | − 0.37 |
| GO:1902961 | GO Biological Processes | Positive regulation of aspartic-type endopeptidase activity involved in amyloid precursor protein catabolic process | 3 | 0.57 | − 3.75 | − 0.37 |
| GO:0002521 | GO Biological Processes | Leukocyte differentiation | 19 | 3.59 | − 3.75 | − 0.37 |
| WP4784 | WikiPathways | Proteoglycan biosynthesis | 4 | 0.76 | − 3.64 | − 0.36 |
| GO:0051496 | GO Biological Processes | Positive regulation of stress fiber assembly | 6 | 1.13 | − 3.59 | − 0.36 |
| GO:0062197 | GO Biological Processes | Cellular response to chemical stress | 14 | 2.65 | − 3.44 | − 0.35 |
| GO:0051345 | GO Biological Processes | Positive regulation of hydrolase activity | 20 | 3.78 | − 3.38 | − 0.35 |
| GO:0015919 | GO Biological Processes | Peroxisomal membrane transport | 4 | 0.76 | − 3.28 | − 0.34 |
| WP5103 | WikiPathways | Progeria associated lipodystrophy | 4 | 0.76 | − 3.28 | − 0.34 |
| WP3915 | WikiPathways | Angiopoietin like protein 8 regulatory pathway | 9 | 1.70 | − 3.27 | − 0.34 |
| GO:0006873 | GO Biological Processes | Intracellular monoatomic ion homeostasis | 18 | 3.40 | − 3.17 | − 0.27 |
| GO:0071476 | GO Biological Processes | Cellular hypotonic response | 3 | 0.57 | − 3.10 | − 0.27 |
| R-HSA-9696264 | Reactome Gene Sets | RND3 GTPase cycle | 5 | 0.95 | − 3.10 | − 0.27 |
| WP474 | WikiPathways | Endochondral ossification | 6 | 1.13 | − 3.09 | − 0.27 |
| GO:0001837 | GO Biological Processes | Epithelial to mesenchymal transition | 7 | 1.32 | − 3.08 | − 0.27 |
| GO:0061024 | GO Biological Processes | Membrane organization | 26 | 4.91 | − 3.03 | − 0.27 |
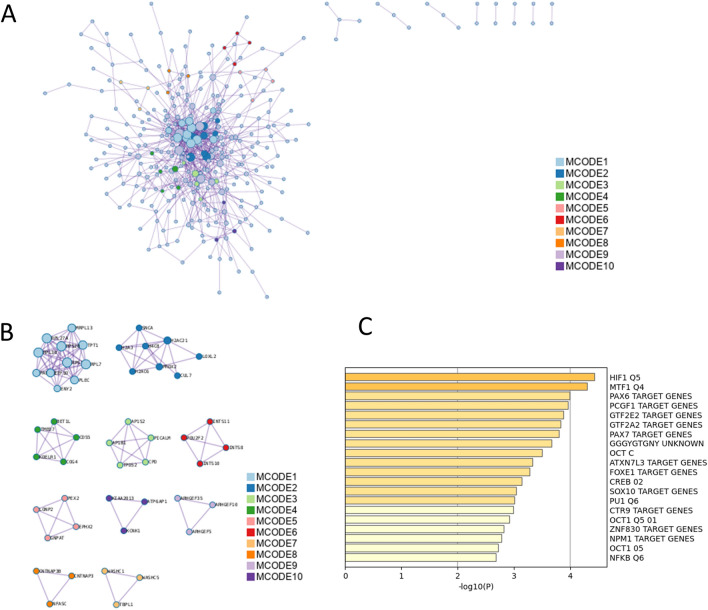
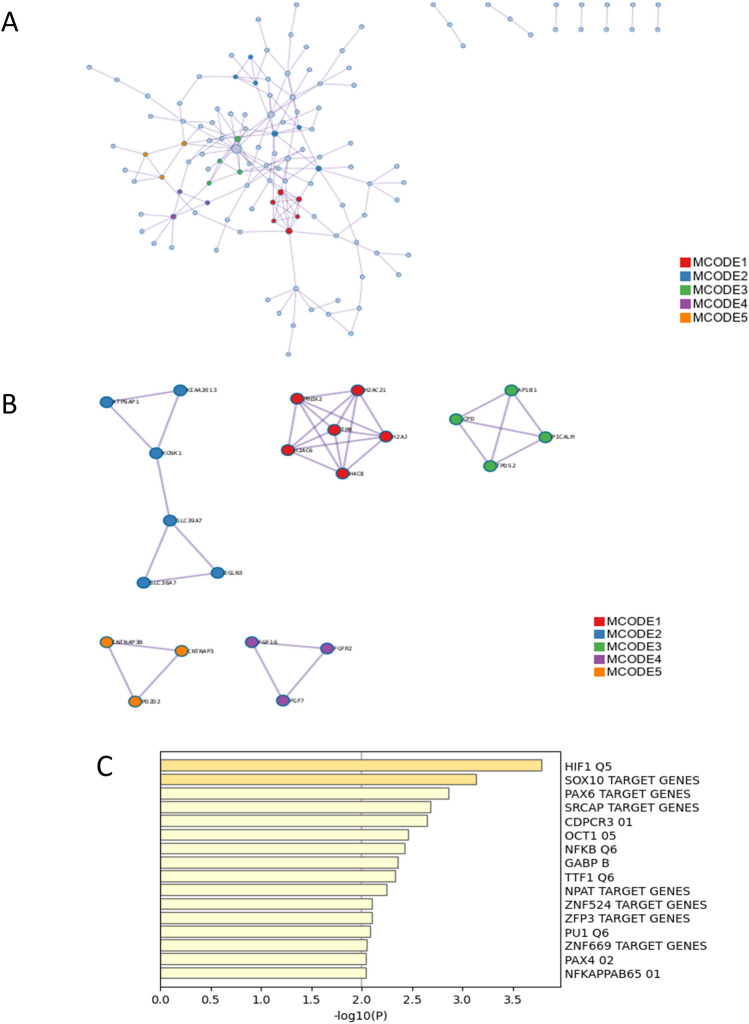
The enrichment analysis of PPI among the total DEGs is presented in Table 3 and Fig. 3. The MCODE plugin, a tool designed for the identification of functional modules within PPI networks, was employed for this analysis. Top-scored modules were translation, eukaryotic translation elongation, nonsense mediated decay (NMD) independent of the exon junction complex (EJC), RMTs methylate histone arginines, diseases of programmed cell death, heterochromatin organization, Golgi associated vesicle biogenesis, trans-Golgi network vesicle budding, membrane trafficking, COPI-mediated anterograde transport, ER to Golgi anterograde transport, transport to the Golgi and subsequent modification, peroxisomal protein import, protein localization, peroxisome, RNA polymerase II transcribes snRNA genes, DSS1 complex, integrator complex, NRAGE signals death through JNK, cell death signaling via NRAGE, NRIF and NADE, and G alpha (12/13) signaling events. Enrichment analysis in transcription factor targets of total DEGs was performed (Table 4 and Fig. 3C) and led to the enrichment of HIF1 Q5, MTF1 Q4, PAX6 TARGET GENES, PCGF1 TARGET GENES, GTF2E2 TARGET GENES, GTF2A2 TARGET GENES, PAX7 TARGET GENES, GGGYGTGNY UNKNOWN, OCT C, ATXN7L3 TARGET GENES, FOXE1 TARGET GENES, CREB 02, SOX10 TARGET GENES and NFKB Q6.
Table 3.
Protein–protein interaction enrichment analysis of total differentially expressed genes (Metascape, Access 2023.12.15).
| MCODE | GO | Description | Log10(P) |
|---|---|---|---|
| MCODE_1 | R-HSA-72766 | Translation | − 14.0 |
| MCODE_1 | R-HSA-156842 | Eukaryotic Translation Elongation | − 12.5 |
| MCODE_1 | R-HSA-975956 | Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) | − 12.4 |
| MCODE_2 | R-HSA-3214858 | RMTs methylate histone arginines | − 8.5 |
| MCODE_2 | R-HSA-9645723 | Diseases of programmed cell death | − 8.0 |
| MCODE_2 | GO:0070828 | Heterochromatin organization | − 7.8 |
| MCODE_3 | R-HSA-432722 | Golgi Associated Vesicle Biogenesis | − 13.7 |
| MCODE_3 | R-HSA-199992 | Trans-Golgi Network Vesicle Budding | − 13.2 |
| MCODE_3 | R-HSA-199991 | Membrane Trafficking | − 8.4 |
| MCODE_4 | R-HSA-6807878 | COPI-mediated anterograde transport | − 12.4 |
| MCODE_4 | R-HSA-199977 | ER to Golgi Anterograde Transport | − 11.5 |
| MCODE_4 | R-HSA-948021 | Transport to the Golgi and subsequent modification | − 11.1 |
| MCODE_5 | R-HSA-9033241 | Peroxisomal protein import | − 10.8 |
| MCODE_5 | R-HSA-9609507 | Protein localization | − 9.1 |
| MCODE_5 | hsa04146 | Peroxisome | − 7.1 |
| MCODE_6 | R-HSA-6807505 | RNA polymerase II transcribes snRNA genes | − 10.5 |
| MCODE_6 | CORUM:1154 | DSS1 complex | − 9.7 |
| MCODE_6 | CORUM:1153 | Integrator complex | − 9.7 |
| MCODE_9 | R-HSA-193648 | NRAGE signals death through JNK | − 8.2 |
| MCODE_9 | R-HSA-204998 | Cell death signalling via NRAGE, NRIF and NADE | − 7.8 |
| MCODE_9 | R-HSA-416482 | G alpha (12/13) signalling events | − 7.8 |
Fig. 3.
Protein–protein interaction (PPI) enrichment analysis of total differentially expressed genes (DEGs). (A) PPI interaction network of total DEGs. MCODE algorithm was applied to clustered enrichment ontology terms to identify neighborhoods where proteins are densely connected. Each MCODE network is assigned a unique color. (B) PPI MCODE component associated with total DEGs. GO enrichment analysis was applied to each MCODE network to assign “meanings” to the network component. (C) Summary of enrichment analysis in transcription factor targets of total differentially expressed genes.
Table 4.
Summary of enrichment analysis in transcription factor targets of total differentially expressed genes (Metascape, Access 2023.12.15).
| GO | Description | Count | % | Log10(p) | Log10(q) |
|---|---|---|---|---|---|
| M5320 | HIF1 Q5 | 15 | 2.80 | − 4.40 | − 1.10 |
| M2463 | MTF1 Q4 | 15 | 2.80 | − 4.30 | − 1.10 |
| M40719 | PAX6 TARGET GENES | 29 | 5.50 | − 4.00 | − 0.97 |
| M30115 | PCGF1 TARGET GENES | 23 | 4.30 | − 4.00 | − 0.96 |
| M29984 | GTF2E2 TARGET GENES | 19 | 3.60 | − 3.90 | − 0.92 |
| M40742 | GTF2A2 TARGET GENES | 22 | 4.20 | − 3.80 | − 0.91 |
| M30110 | PAX7 TARGET GENES | 27 | 5.10 | − 3.80 | − 0.90 |
| M9645 | GGGYGTGNY UNKNOWN | 26 | 4.90 | − 3.70 | − 0.82 |
| M4238 | OCT C | 14 | 2.60 | − 3.50 | − 0.72 |
| M40770 | ATXN7L3 TARGET GENES | 14 | 2.60 | − 3.30 | − 0.64 |
| M29968 | FOXE1 TARGET GENES | 26 | 4.90 | − 3.30 | − 0.61 |
| M6342 | CREB 02 | 13 | 2.50 | − 3.10 | − 0.54 |
| M30173 | SOX10 TARGET GENES | 14 | 2.60 | − 3.00 | − 0.48 |
| M14376 | PU1 Q6 | 12 | 2.30 | − 3.00 | − 0.46 |
| M29934 | CTR9 TARGET GENES | 6 | 1.10 | − 3.00 | − 0.45 |
| M9638 | OCT1 Q5 01 | 13 | 2.50 | − 2.90 | − 0.44 |
| M30396 | ZNF830 TARGET GENES | 13 | 2.50 | − 2.80 | − 0.40 |
| M34465 | NPM1 TARGET GENES | 15 | 2.80 | − 2.80 | − 0.40 |
| M5708 | OCT1 05 | 12 | 2.30 | − 2.70 | − 0.37 |
| M11921 | NFKB Q6 | 12 | 2.30 | − 2.70 | − 0.36 |
GO functional and KEGG pathway analyses of DEGs
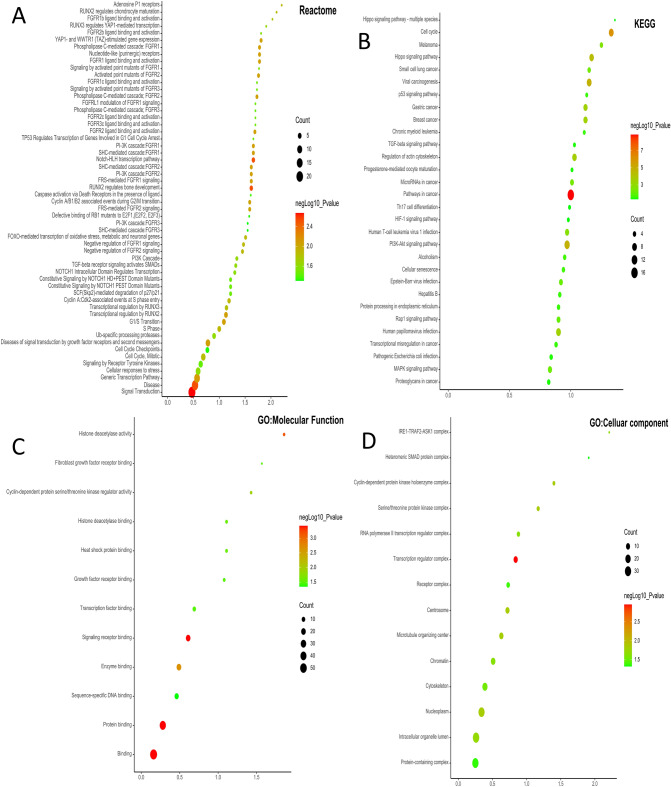
Both GO functional and KEGG pathway analyses of DEGs were performed using ShinyGo 0.80 and STRING database. In terms of Reactome, the DEGs were mainly enriched in pathways involved in RUNX2, FGFR, YAP1- and TAZ-stimulated gene expression, and cell cycle pathway (Fig. 4A), In terms of KEGG pathways (www.kegg.jp/kegg/kegg1.html), the DEGs were mainly enriched in pathways involved in Hippo signaling pathway, cell cycle, p53 signaling pathway, TGF-β signaling pathway, regulation of actin cytoskeleton and HIF1 signaling pathway (Fig. 4B and 4C). For GO MF analysis, the DEGs were mainly enriched in histone deacetylase activity, FGFR binding, CDK regulator activity, growth factor receptor binding and transcription factor binding (Fig. 4D and 4E). The GO analysis showed that the DEGs were significantly involved in cellular components, such as SMAD protein complex, transcription regulator complex centrosome, and nucleoplasm (Fig. 4F and 4G).
Fig. 4.
Dot plots and network diagram of gene ontology using ShinyGO 0.80. Reactome (A), KEGG pathway analysis (www.kegg.jp/kegg/kegg1.html) (B), molecular functions of GO enrichment analysis (C), and cellular components of GO enrichment analysis (D) in young vs old corneal endothelial cells. Nodes represent enriched molecular functions. Size of node represents the number of genes involved in a function.
Enrichment analysis of up-regulated differentially expressed genes
Pathway and process enrichment analysis of up-regulated DEGs is presented in Table 5, Fig. 5. Functional enrichment analysis with Metascape showed that up-regulated DEGs in old corneal endothelial cells compared to young corneal endothelial cells were significantly enriched in transition metal ion transport, inorganic ion transmembrane transport, glycoprotein biosynthetic process, transport to the Golgi and subsequent modification, positive regulation of Wnt signaling pathway, extracellular matrix organization and efferocytosis.
Table 5.
Pathway and process enrichment analysis of up-regulated differentially expressed genes (Metascape, Access 2023.12.15).
| GO | Category | Description | Count | % | Log10(P) | Log10(q) |
|---|---|---|---|---|---|---|
| GO:0000041 | GO Biological Processes | Transition metal ion transport | 8 | 2.74 | − 5.33 | − 1.07 |
| GO:0098660 | GO Biological Processes | Inorganic ion transmembrane transport | 21 | 7.19 | − 5.06 | − 1.07 |
| GO:0002521 | GO Biological Processes | Leukocyte differentiation | 15 | 5.14 | − 4.77 | − 1.07 |
| GO:0009101 | GO Biological Processes | Glycoprotein biosynthetic process | 12 | 4.11 | − 4.75 | − 1.07 |
| GO:0060348 | GO Biological Processes | Bone development | 10 | 3.42 | − 4.69 | − 1.07 |
| WP4784 | WikiPathways | Proteoglycan biosynthesis | 4 | 1.37 | − 4.63 | − 1.07 |
| R-HSA-948021 | Reactome Gene Sets | Transport to the Golgi and subsequent modification | 9 | 3.08 | − 4.08 | − 0.88 |
| GO:0030177 | GO Biological Processes | Positive regulation of Wnt signaling pathway | 8 | 2.74 | − 4.02 | − 0.88 |
| M3008 | Canonical Pathways | NABA ECM GLYCOPROTEINS | 9 | 3.08 | − 3.89 | − 0.88 |
| GO:0007435 | GO Biological Processes | Salivary gland morphogenesis | 4 | 1.37 | − 3.78 | − 0.88 |
| GO:0070848 | GO Biological Processes | Response to growth factor | 15 | 5.14 | − 3.76 | − 0.87 |
| GO:0030198 | GO Biological Processes | Extracellular matrix organization | 10 | 3.42 | − 3.46 | − 0.68 |
| GO:0006590 | GO Biological Processes | Thyroid hormone generation | 3 | 1.03 | − 3.34 | − 0.66 |
| GO:0051956 | GO Biological Processes | Negative regulation of amino acid transport | 3 | 1.03 | − 3.34 | − 0.66 |
| GO:0045670 | GO Biological Processes | Regulation of osteoclast differentiation | 5 | 1.71 | − 3.21 | − 0.58 |
| WP3670 | WikiPathways | Interactions between LOXL4 and oxidative stress pathway | 3 | 1.03 | − 3.19 | − 0.58 |
| R-HSA-199992 | Reactome Gene Sets | Trans-Golgi Network Vesicle Budding | 5 | 1.71 | − 3.18 | − 0.58 |
| GO:0050729 | GO Biological Processes | Positive regulation of inflammatory response | 7 | 2.40 | − 3.13 | − 0.58 |
| GO:0051222 | GO Biological Processes | Positive regulation of protein transport | 9 | 3.08 | − 3.13 | − 0.58 |
| hsa04148 | KEGG Pathway | Efferocytosis | 7 | 2.40 | − 3.08 | − 0.57 |
Fig. 5.
Enrichment analysis of up-regulated differentially expressed genes (DEGs) by Metascape (http://metascape.org/gp/index.html#/main/step1). (A) Bar graph of enriched terms of the up-regulated genes (colored by p-values). (B) Network of enriched terms of up-regulated DEGs, colored by cluster identity, where nodes that share the same cluster identity are typically close to each other.
PPI enrichment analysis of up-regulated DEGs were shown in Table 6 and Fig. 6. It led to the enrichment of RMTs methylate histone arginines, diseases of programmed cell death, transcriptional regulation by small RNAs, inorganic cation transmembrane transport, monoatomic cation transmembrane transport, inorganic ion transmembrane transport, Golgi associated vesicle biogenesis, trans-Golgi network vesicle budding, membrane trafficking, activated point mutants of FGFR2, phospholipase C-mediated cascade FGFR2 and FGFR2 ligand binding and activation. Enrichment analysis in transcription factor targets of up-regulated DEGs was performed (Table 7 and Fig. 6C). It showed the enrichment of HIF1 Q5, SOX10 TARGET GENES, PAX6 TARGET GENES, SRCAP TARGET GENES, CDPCR3 01, OCT1 05, NFKB Q6 and GABP B.
Table 6.
Protein–protein interaction enrichment analysis of up-regulated differentially expressed genes (Metascape, Access 2023.12.15).
| MCODE | GO | Description | Log10(P) |
|---|---|---|---|
| MCODE_1 | R-HSA-3214858 | RMTs methylate histone arginines | − 9.2 |
| MCODE_1 | R-HSA-9645723 | Diseases of programmed cell death | − 8.7 |
| MCODE_1 | R-HSA-5578749 | Transcriptional regulation by small RNAs | − 8.7 |
| MCODE_2 | GO:0098662 | Inorganic cation transmembrane transport | − 5.6 |
| MCODE_2 | GO:0098655 | Monoatomic cation transmembrane transport | − 5.5 |
| MCODE_2 | GO:0098660 | Inorganic ion transmembrane transport | − 5.3 |
| MCODE_3 | R-HSA-432722 | Golgi Associated Vesicle Biogenesis | − 11.0 |
| MCODE_3 | R-HSA-199992 | Trans-Golgi Network Vesicle Budding | − 10.5 |
| MCODE_3 | R-HSA-199991 | Membrane Trafficking | − 6.7 |
| MCODE_4 | R-HSA-2033519 | Activated point mutants of FGFR2 | − 9.8 |
| MCODE_4 | R-HSA-5654221 | Phospholipase C-mediated cascade FGFR2 | − 9.8 |
| MCODE_4 | R-HSA-190241 | FGFR2 ligand binding and activation | − 9.6 |
Fig. 6.
Enrichment analysis in protein–protein interaction (PPI) and transcription factor targets of up-regulated differentially expressed genes (DEGs). (A) PPI network construction of up-regulated genes. (B) The essential modules identified by MCODE from the PPI network of upregulated DEGs. Ingenuity pathway analysis of genes in each sub-network to obtain the biological pathways. (C) Summary of enrichment analysis in transcription factor targets of up-regulated differentially expressed genes.
Table 7.
Summary of enrichment analysis in transcription factor targets of up-regulated differentially expressed genes (Metascape, Access 2023.12.15).
| GO | Description | Count | % | Log10(P) | Log10(q) |
|---|---|---|---|---|---|
| M5320 | HIF1 Q5 | 10 | 3.40 | − 3.80 | − 0.68 |
| M30173 | SOX10 TARGET GENES | 10 | 3.40 | − 3.10 | − 0.46 |
| M40719 | PAX6 TARGET GENES | 17 | 5.80 | − 2.90 | − 0.35 |
| M40790 | SRCAP TARGET GENES | 13 | 4.50 | − 2.70 | − 0.27 |
| M7737 | CDPCR3 01 | 4 | 1.40 | − 2.70 | − 0.25 |
| M5708 | OCT1 05 | 8 | 2.70 | − 2.50 | − 0.16 |
| M11921 | NFKB Q6 | 8 | 2.70 | − 2.40 | − 0.15 |
| M6985 | GABP B | 8 | 2.70 | − 2.40 | − 0.11 |
| M6331 | TTF1 Q6 | 8 | 2.70 | − 2.30 | − 0.10 |
| M30096 | NPAT TARGET GENES | 8 | 2.70 | − 2.20 | − 0.06 |
| M30246 | ZFP3 TARGET GENES | 9 | 3.10 | − 2.10 | − 0.00 |
| M30339 | ZNF524 TARGET GENES | 9 | 3.10 | − 2.10 | − 0.00 |
| M14376 | PU1 Q6 | 7 | 2.40 | − 2.10 | 0.00 |
| M30374 | ZNF669 TARGET GENES | 5 | 1.70 | − 2.00 | 0.00 |
| M2315 | NFKAPPAB65 01 | 7 | 2.40 | − 2.00 | 0.00 |
| M8816 | PAX4 02 | 7 | 2.40 | − 2.00 | 0.00 |
Enrichment analysis of down-regulated differentially expressed genes
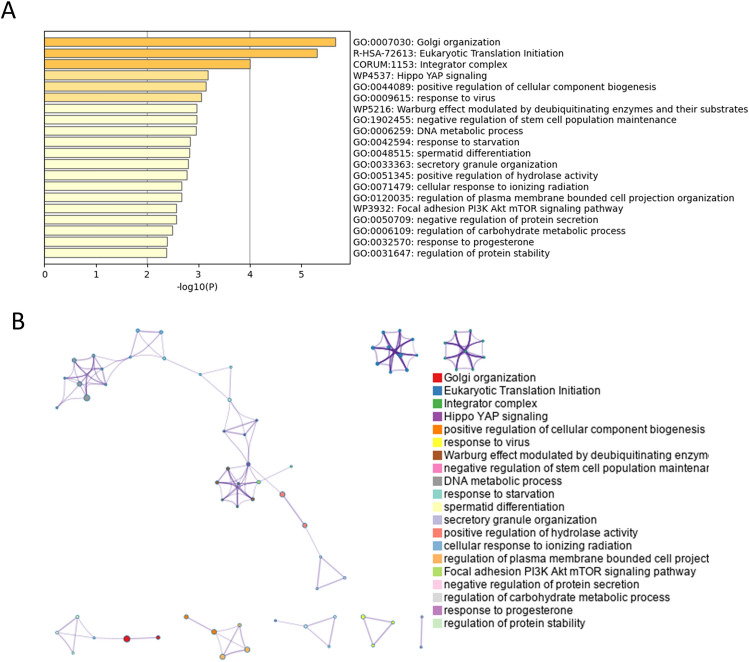
Pathway and process enrichment analysis of down-regulated DEGs was shown in Table 8 and Fig. 7. Functional enrichment analysis with Metascape showed that down-regulated DEGs in old corneal endothelial cells compared to young corneal endothelial cells were significantly enriched in Golgi organization, eukaryotic translation initiation, integrator complex, Hippo YAP signaling, positive regulation of cellular component biogenesis, response to virus, Warburg effect modulated by deubiquitinating enzymes and their substrates, negative regulation of stem cell population maintenance, DNA metabolic process, response to starvation, secretory granule organization, positive regulation of hydrolase activity, cellular response to ionizing radiation, regulation of plasma membrane bounded cell projection organization, focal adhesion PI3K Akt mTOR signaling pathway, negative regulation of protein secretion and regulation of carbohydrate metabolic process.
Table 8.
Pathway and process enrichment analysis of down-regulated differentially expressed genes (Metascape, Access 2023.12.15).
| GO | Category | Description | Count | % | Log10(p) | Log10(q) |
|---|---|---|---|---|---|---|
| GO:0007030 | GO Biological Processes | Golgi organization | 9 | 3.80 | − 5.67 | − 1.44 |
| R-HSA-72613 | Reactome Gene Sets | Eukaryotic Translation Initiation | 8 | 3.38 | − 5.31 | − 1.44 |
| CORUM:1153 | CORUM | Integrator complex | 3 | 1.27 | − 4.01 | − 0.73 |
| WP4537 | WikiPathways | Hippo YAP signaling | 3 | 1.27 | − 3.19 | − 0.21 |
| GO:0044089 | GO Biological Processes | Positive regulation of cellular component biogenesis | 12 | 5.06 | − 3.14 | − 0.21 |
| GO:0009615 | GO Biological Processes | Response to virus | 10 | 4.22 | − 3.05 | − 0.17 |
| WP5216 | WikiPathways | Warburg effect modulated by deubiquitinating enzymes and their substrates | 3 | 1.27 | − 2.97 | − 0.13 |
| GO:1902455 | GO Biological Processes | Negative regulation of stem cell population maintenance | 3 | 1.27 | − 2.97 | − 0.13 |
| GO:0006259 | GO Biological Processes | DNA metabolic process | 15 | 6.33 | − 2.95 | − 0.13 |
| GO:0042594 | GO Biological Processes | Response to starvation | 7 | 2.95 | − 2.83 | − 0.06 |
| GO:0048515 | GO Biological Processes | Spermatid differentiation | 7 | 2.95 | − 2.82 | − 0.06 |
| GO:0033363 | GO Biological Processes | Secretory granule organization | 4 | 1.69 | − 2.79 | − 0.06 |
| GO:0051345 | GO Biological Processes | Positive regulation of hydrolase activity | 11 | 4.64 | − 2.77 | − 0.05 |
| GO:0071479 | GO Biological Processes | Cellular response to ionizing radiation | 4 | 1.69 | − 2.67 | − 0.01 |
| GO:0120035 | GO Biological Processes | Regulation of plasma membrane bounded cell projection organization | 13 | 5.49 | − 2.67 | − 0.01 |
| WP3932 | WikiPathways | Focal adhesion PI3K Akt mTOR signaling pathway | 8 | 3.38 | − 2.56 | 0.00 |
| GO:0050709 | GO Biological Processes | Negative regulation of protein secretion | 4 | 1.69 | − 2.56 | 0.00 |
| GO:0006109 | GO Biological Processes | Regulation of carbohydrate metabolic process | 6 | 2.53 | − 2.50 | 0.00 |
| GO:0032570 | GO Biological Processes | Response to progesterone | 3 | 1.27 | − 2.39 | 0.00 |
| GO:0031647 | GO Biological Processes | Regulation of protein stability | 8 | 3.38 | − 2.37 | 0.00 |
Fig. 7.
Enrichment analysis of down-regulated differentially expressed genes (DEGs) by Metascape (http://metascape.org/gp/index.html#/main/step1). (A) Bar graph of enriched terms of the down-regulated genes (colored by p-values). (B) Network of enriched terms of down-regulated DEGs, colored by cluster identity, where nodes that share the same cluster identity are typically close to each other.
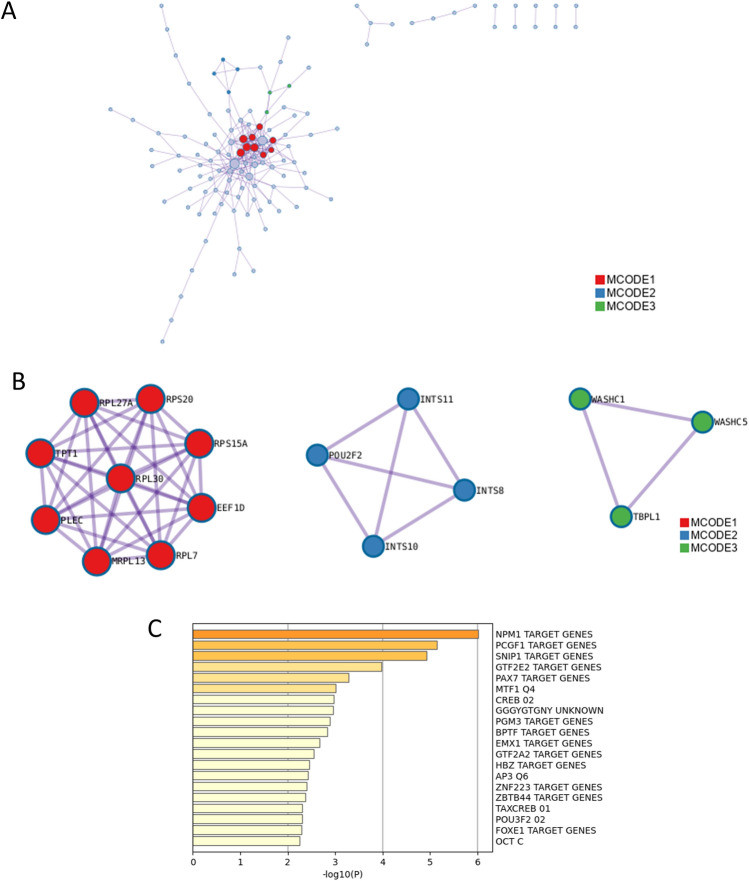
PPI enrichment analysis of down-regulated DEGs were performed (Table 9 and Fig. 8). It led to the enrichment of eukaryotic translation elongation, translation, RNA polymerase II transcribes snRNA genes, DSS1 complex and integrator complex. Enrichment analysis in transcription factor targets of down-regulated DEGs was shown in Table 10 and Fig. 8C. It showed the enrichment of NPM1 TARGET GENES, PCGF1 TARGET GENES, SNIP1 TARGET GENES, GTF2E2 TARGET GENES, PAX7 TARGET GENES, MTF1 Q4 and CREB 02.
Table 9.
Protein–protein interaction enrichment analysis of down-regulated differentially expressed genes (Metascape, Access 2023.12.15).
| MCODE | GO | Description | Log10(P) |
|---|---|---|---|
| MCODE_1 | R-HSA-156842 | Eukaryotic Translation Elongation | − 13.2 |
| MCODE_1 | R-HSA-72766 | Translation | − 12.6 |
| MCODE_1 | GO:0006412 | Translation | − 11.7 |
| MCODE_2 | R-HSA-6807505 | RNA polymerase II transcribes snRNA genes | − 10.5 |
| MCODE_2 | CORUM:1154 | DSS1 complex | − 9.7 |
| MCODE_2 | CORUM:1153 | Integrator complex | − 9.7 |
Fig. 8.
Enrichment analysis in protein–protein interaction (PPI) and transcription factor targets of down-regulated differentially expressed genes (DEGs). (A) PPI network construction of down-regulated genes. (B) The essential modules identified by MCODE from the PPI network of down-regulated DEGs. Ingenuity pathway analysis of genes in each sub-network to obtain the biological pathways. (C) Summary of enrichment analysis in transcription factor targets of down-regulated differentially expressed genes.
Table 10.
Summary of enrichment analysis in transcription factor targets of down-regulated differentially expressed genes (Metascape, Access 2023.12.15).
| GO | Description | Count | % | Log10(P) | Log10(q) |
|---|---|---|---|---|---|
| M34465 | NPM1 TARGET GENES | 14 | 5.90 | − 6.00 | − 1.80 |
| M30115 | PCGF1 TARGET GENES | 16 | 6.80 | − 5.10 | − 1.30 |
| M30170 | SNIP1 TARGET GENES | 19 | 8.00 | − 4.90 | − 1.20 |
| M29984 | GTF2E2 TARGET GENES | 12 | 5.10 | − 4.00 | − 0.71 |
| M30110 | PAX7 TARGET GENES | 15 | 6.30 | − 3.30 | − 0.27 |
| M2463 | MTF1 Q4 | 8 | 3.40 | − 3.00 | − 0.11 |
| M6342 | CREB 02 | 8 | 3.40 | − 3.00 | − 0.07 |
| M9645 | GGGYGTGNY UNKNOWN | 14 | 5.90 | − 3.00 | − 0.07 |
| M34464 | PGM3 TARGET GENES | 8 | 3.40 | − 2.90 | − 0.03 |
| M40764 | BPTF TARGET GENES | 15 | 6.30 | − 2.80 | − 0.01 |
| M29957 | EMX1 TARGET GENES | 7 | 3.00 | − 2.70 | 0.00 |
| M40742 | GTF2A2 TARGET GENES | 11 | 4.60 | − 2.50 | 0.00 |
| M40709 | HBZ TARGET GENES | 14 | 5.90 | − 2.50 | 0.00 |
| M498 | AP3 Q6 | 7 | 3.00 | − 2.40 | 0.00 |
| M30281 | ZNF223 TARGET GENES | 8 | 3.40 | − 2.40 | 0.00 |
| M40815 | ZBTB44 TARGET GENES | 8 | 3.40 | − 2.40 | 0.00 |
| M5608 | TAXCREB 01 | 5 | 2.10 | − 2.30 | 0.00 |
| M14960 | POU3F2 02 | 7 | 3.00 | − 2.30 | 0.00 |
| M29968 | FOXE1 TARGET GENES | 13 | 5.50 | − 2.30 | 0.00 |
| M4238 | OCT C | 7 | 3.00 | − 2.30 | 0.00 |
Discussion
Ageing has a significant effect on corneal endothelial cells, leading to reduced cell density, altered cell morphology and reduced regenerative capacity20. Indeed, understanding the changes that occur in corneal endothelial cells as a result of ageing is crucial to suggesting new therapeutic strategies for corneal endothelial cell regeneration. This study provides valuable insights into the effects of aging on corneal endothelial cells by identifying DEGs between young and old corneal endothelial cells. The key areas impacted by aging included metabolism, cell death, cellular component biogenesis, proteoglycan biosynthesis, and membrane transport. These results underscore the complex nature of aging on cellular functions, especially within the corneal endothelium, which plays a crucial role in maintaining corneal clarity and visual acuity through its barrier and pump functions2. The identification of DEGs in these specific biological processes suggests that aging lead to significant changes in cellular metabolism, potentially affecting energy production and the synthesis of vital components. Changes in cell death mechanisms, including apoptosis, may influence cell turnover and tissue health21. The impact on cellular component biogenesis indicates alterations in the ability to maintain and renew its structural components, essential for cellular integrity and function22. The findings related to proteoglycan biosynthesis are particularly relevant to the corneal endothelium, given the importance of proteoglycans in maintaining the extracellular matrix and corneal hydration23. Lastly, alterations in membrane transport mechanisms could affect the function of corneal endothelial cells to regulate ion and fluid balance, critical for corneal dehydration and transparency2.
Corneal endothelial cells from old donors can proliferate more slowly than cells from young donors in the presence of fetal bovine serum and FGF, although cells from old donors can enter and complete the cell cycle8. Corneal endothelial cells from older donors may respond differently to EGF, media and other environmental conditions, emphasizing the need to develop treatments that consider the elderly population as a primary target for these diseases6,9. Protein expression of corneal endothelial cells with age has been reported. Human corneal endothelial cells from older donors show reduced expression of proteins that support important cellular functions such as metabolism, antioxidant protection, protein folding, and protein degradation7. Corneal endothelial cells have been reported to show heterogeneous expression of senescence markers such as MT2A, CDKN2A (p16)24. and TAGLN, and an increase in the senescence marker CDKN2A and fibrosis marker ACTA2 with passage25. Additionally, it was suggested that after converting to senescent cells, there was a transition to the fibrotic cells25. a-SMA, COL8A1, and CD44 were suggested as fibrotic markers26,27 and ZO-1 and CD166 were suggested as corneal endothelial cell marker and had a concomitant decrease in transition to fibrotic cells25. However, in this study, there was no statistical difference in corneal endothelial cell markers such as ZO-1 and CD166 and in fibrosis markers such as a-SMA, COL8A1, and CD44 between senescent and young cells.
Molecular mechanisms of aging include genomic instability, telomere attrition, epigenetic alteration, loss of proteostasis, deregulation of nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and alteration of intercellular communication28. In this study, we found 308 up-regulated and 260 down-regulated DEGs in old corneal endothelial cells. The expression of aging-related molecules such as TGFB1, FGF7, and IGFBP7 and functional molecules of ATP6AP1 and ATP1B3 increased in old corneal endothelial cells, which is consistent with the previous study evaluating mitochondria and oxidative stress in relation to aging29–31. The increase in expression of up-regulated genes in old corneal endothelial cells suggests two possibilities: these genes may directly contribute to the aging process, or they could be up-regulated in an attempt to compensate for the detrimental changes that accompany aging. Identifying these up-regulated DEGs provides a valuable data to target these genes for therapeutic intervention. By inhibiting the action of these genes, it may slow down or even reverse some aspects of the aging process in corneal endothelial cells. This approach could involve suppressing aging-induced transcription factor expression, which may maintain or rejuvenate the corneal endothelial cells by counteracting the molecular mechanisms that drive aging. Conversely, the genes that are down-regulated in old corneal endothelial cells may represent a decline in essential cellular functions due to aging. These could be involved in critical pathways necessary for maintaining cellular health, integrity, and function. Strategies aimed at reinforcing or supplementing these decreased DEGs could offer another therapeutic avenue to combat aging. This could involve enhancing the expression of core transcription factors that have been disrupted by aging, potentially rejuvenating the corneal endothelial cells by restoring the transcriptional regulatory networks that are essential for their function. In this study, down-regulated DEGs included proliferation genes such as CDKL432, CDK2AP2P133, VEGFA34, SINHCAF35, and CCDC144A36 and DNA repair genes such as PARP437 and POLG238. Proteostasis-associated genes such as UBXN2B39, PSMG340, PSD341, and ERLIN242 were also down-regulated.
We found transcription factors targets which were up-regulated and down-regulated by aging. By targeting these molecular changes, either by inhibiting the action of up-regulated DEGs or enhancing the expression of down-regulated DEGs, it may be possible to develop targeted therapies that address the root causes of aging at the molecular level43. Such interventions could not only improve the health and function of corneal endothelial cells but also have broader implications for aging research and therapeutic development. HIF1 plays a significant role in the cellular response to hypoxia by activating signaling pathway involved in energy metabolism, angiogenesis, and other processes, which influence senescence44–46. MTF1, metal response element-binding transcription factor 1, regulates the expression of genes in response to heavy metals like zinc, copper, and cadmium, playing a crucial role in metal metabolism and detoxification processes in cells47. It may have an effect on senescence by regulating metallothioneins involved in metal detoxification and ROS scavenging and by regulating genes involved in detoxification and antioxidant responses48. NPM1, nucleophosmin 1, is a multifunctional protein and impacts on senescence by regulating p53 pathway, centrosome function, ribosome biogenesis and response to oxidative stress49,50. PCGF1 is a component of polycomb repressive complex 1 (PRC1), which modifies chromatin to maintain the genes in an inactive state51. By influencing chromatin structure and gene expression, PCGF1 affects cellular aging and senescence and is involved in stem cell renewal and differentiation52,53. SNIP1, smad nuclear interacting protein 1, is implicated in TGF-β signaling, the activity of p53, cellular stress responses, and cell cycle regulation54. Reversal and modulation of cellular senescence55 may be useful in suppressing aging and regenerating corneal endothelial cells, in which TFs may play an important role.
In conclusion, our study has unveiled pivotal genes contributing to the aging process of corneal endothelial cells, alongside an in-depth exploration of relevant biological pathways. The identification of key genes and transcription factors involved in aging provides a solid foundation for the development of targeted therapies. These therapies may prevent the aging on corneal endothelial cells and may pave the way for innovative approaches to corneal endothelial cell rejuvenation.
Supplementary Information
Author contributions
HJK, and YJS contributed to the study’s conception and design. HJK and YJS conceived and designed the experiments; JSH, YKR, and YJS performed the experiments; HJK,YJS, JHS, and YL analyzed the data; JSH, JHS, and YJS wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Research Foundation (NRF) grant (NRF-2023R1A2C2002674) funded by the Korea government and Hallym University Research Fund funded by Hallym University.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jin Sun Hwang and Je Hyun Seo have contribute equally to this work.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82423-6.
References
- 1.Feizi, S. Corneal endothelial cell dysfunction: etiologies and management. Ther. Adv. Ophthalmol.10, 2515841418815802 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catala, P. et al. Approaches for corneal endothelium regenerative medicine. Prog. Retin. Eye Res.87, 100987 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Vercammen, H. et al. Corneal endothelial wound healing: understanding the regenerative capacity of the innermost layer of the cornea. Transl. Res.248, 111–127 (2022). [DOI] [PubMed] [Google Scholar]
- 4.McHugh, D. & Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol.217, 65–77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheerin, A. N. et al. Characterization of cellular senescence mechanisms in human corneal endothelial cells. Aging Cell11, 234–240 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu, C. & Joyce, N. C. Proliferative response of corneal endothelial cells from young and older donors. Invest. Ophthalmol. Vis. Sci.45, 1743–1751 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Zhu, C., Rawe, I. & Joyce, N. C. Differential protein expression in human corneal endothelial cells cultured from young and older donors. Mol. Vis.14, 1805–1814 (2008). [PMC free article] [PubMed] [Google Scholar]
- 8.Senoo, T. & Joyce, N. C. Cell cycle kinetics in corneal endothelium from old and young donors. Invest. Ophthalmol. Vis. Sci.41, 660–667 (2000). [PubMed] [Google Scholar]
- 9.Merra, A., Maurizi, E. & Pellegrini, G. Impact of culture media on primary human corneal endothelial cells derived from old donors. Exp. Eye Res.240, 109815 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Satam, H. et al. Next-generation sequencing technology: Current trends and advancements. Biology (Basel)12, 997 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava, A. et al. Tissue-specific gene expression changes are associated with aging in mice. Genomics Proteomics Bioinform.18, 430–442 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engler, C., Kelliher, C., Chang, S., Meng, H. & Jun, A. S. Cryopreservation and long-term culture of transformed murine corneal endothelial cells. Graefes Arch. Clin. Exp. Ophthalmol.250, 103–110 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol.33, 290–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao, X., Cai, T., Olyarchuk, J. G. & Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics21, 3787–3793 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun.10, 1523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, X. et al. Identification of differentially expressed genes between mucinous adenocarcinoma and other adenocarcinoma of colorectal cancer using bioinformatics analysis. J. Int. Med. Res.48, 300060520949036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taurone, S. et al. Age related changes seen in human cornea in formalin fixed sections and on biomicroscopy in living subjects: A comparison. Clin. Anat.33, 245–256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park, W. et al. Diversity and complexity of cell death: a historical review. Exp. Mol. Med.55, 1573–1594 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker, C., Mojares, E. & Del Rio Hernandez, A. Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci.19, 3028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies, Y. et al. Proteoglycans on normal and migrating human corneal endothelium. Exp. Eye. Res.68, 303–311 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Malavolta, M. et al. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Exp. Gerontol.99, 35–45 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Catala, P., Groen, N., LaPointe, V. L. S. & Dickman, M. M. A single-cell RNA-seq analysis unravels the heterogeneity of primary cultured human corneal endothelial cells. Sci. Rep.13, 9361 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamuro, J. et al. Metabolic plasticity in cell state homeostasis and differentiation of cultured human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci.57, 4452–4463 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Rao, K. B., Malathi, N., Narashiman, S. & Rajan, S. T. Evaluation of myofibroblasts by expression of alpha smooth muscle actin: a marker in fibrosis, dysplasia and carcinoma. J. Clin. Diagn. Res.8, ZC14–ZC17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo, J. et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Target Ther.7, 391 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joo, H. J., Ma, D. J., Hwang, J. S. & Shin, Y. J. SIRT1 activation using CRISPR/dCas9 promotes regeneration of human corneal endothelial cells through inhibiting senescence. Antioxidants (Basel)9, 1085 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tubita, A. et al. Latent-transforming growth factor beta-binding protein 1/transforming growth factor beta1 complex drives antitumoral effects upon erk5 targeting in melanoma. Am. J. Pathol.10.1016/j.ajpath.2024.03.015 (2024). [DOI] [PubMed] [Google Scholar]
- 31.He, J. et al. Single-cell transcriptomics identifies senescence-associated secretory phenotype (SASP) features of testicular aging in human. Aging (Albany NY)16, 3350–3362 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker, S. J., Poulikakos, P. I., Irie, H. Y., Parekh, S. & Reddy, E. P. CDK4: a master regulator of the cell cycle and its role in cancer. Genes Cancer13, 21–45 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong, D. T., Kim, J. J., Khalid, O., Sun, H. H. & Kim, Y. Double edge: CDK2AP1 in cell-cycle regulation and epigenetic regulation. J. Dent. Res.91, 235–241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernatchez, P. N., Rollin, S., Soker, S. & Sirois, M. G. Relative effects of VEGF-A and VEGF-C on endothelial cell proliferation, migration and PAF synthesis: Role of neuropilin-1. J. Cell. Biochem.85, 629–639 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Holley, J. M. et al. Characterization of gene expression profiles in the mouse brain after 35 days of spaceflight mission. NPJ Microgravity8, 35 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Farsi, H. et al. Discovery of new therapeutic targets in ovarian cancer through identifying significantly non-mutated genes. J. Transl. Med.20, 244 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richard, I. A., Burgess, J. T., O’Byrne, K. J. & Bolderson, E. Beyond PARP1: The potential of other members of the poly (ADP-Ribose) polymerase family in DNA repair and cancer therapeutics. Front. Cell Dev. Biol.9, 801200 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wojtaszek, J. L. et al. Structure-specific roles for PolG2-DNA complexes in maintenance and replication of mitochondrial DNA. Nucleic Acids Res51, 9716–9732 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrobel, L., Hoffmann, J. L., Li, X. & Rubinsztein, D. C. p37 regulates VCP/p97 shuttling and functions in the nucleus and cytosol. Sci. Adv.10, eadl6082 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh, Y. C. et al. Person-specific differences in ubiquitin-proteasome mediated proteostasis in human neurons. Alzheimers Dement.20, 2952–2967 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasenjager, S., Bologna, A., Essen, L. O., Spadaccini, R. & Taxis, C. C-terminal sequence stability profiling in Saccharomyces cerevisiae reveals protective protein quality control pathways. J. Biol. Chem.299, 105166 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, J. et al. A novel autosomal dominant ERLIN2 variant activates endoplasmic reticulum stress in a Chinese HSP family. Ann. Clin. Transl. Neurol.10, 2139–2148 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maldonado, E., Morales-Pison, S., Urbina, F. & Solari, A. Aging hallmarks and the role of oxidative stress. Antioxidants (Basel)12, 651 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alique, M. et al. Hypoxia-inducible factor-1alpha: the master regulator of endothelial cell senescence in vascular aging. Cells9, 195 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen, S. & Sang, N. Hypoxia-inducible factor-1: A critical player in the survival strategy of stressed cells. J. Cell. Biochem.117, 267–278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao, H. et al. Role of hypoxia in cellular senescence. Pharmacol. Res.194, 106841 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Olea-Flores, M. et al. ZIP11 regulates nuclear zinc homeostasis in HeLa cells and is required for proliferation and establishment of the carcinogenic phenotype. Front. Cell. Dev. Biol.10, 895433 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swindell, W. R. Metallothionein and the biology of aging. Ageing Res. Rev.10, 132–145 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Box, J. K. et al. Nucleophosmin: from structure and function to disease development. BMC Mol. Biol.17, 19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, K. et al. A redox mechanism underlying nucleolar stress sensing by nucleophosmin. Nat. Commun.7, 13599 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takano, J. et al. PCGF1-PRC1 links chromatin repression with DNA replication during hematopoietic cell lineage commitment. Nat. Commun.13, 7159 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dupret, B., Volkel, P., Le Bourhis, X. & Angrand, P. O. The Polycomb group protein Pcgf1 is dispensable in zebrafish but involved in early growth and aging. PLoS One11, e0158700 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji, G. et al. PCGF1 promotes epigenetic activation of stemness markers and colorectal cancer stem cell enrichment. Cell Death Dis.12, 633 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen, Y., Guo, W., Guo, X., Wanqing, Q. & Yin, Z. The clinical utilization of SNIP1 and its pathophysiological mechanisms in disease. Heliyon10, e24601 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubin de Celis, M. F. & Bonner-Weir, S. Reversing and modulating cellular senescence in beta cells, a new field of opportunities to treat diabetes. Front Endocrinol (Lausanne)14, 1217729 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.