Abstract
This study aims to compare the incidences of neurological deterioration (ND) and poor outcome (a modified Ranking scale > 2 points at discharge) among patients with different atherosclerotic stroke patterns. A total of 688 participants were categorized into 4 groups according to atherosclerotic stroke pattern: multiple small infarcts (MSI), single subcortical infarction (SSI), borderzone infarct (BZI) and large infarct groups. Among the 4 groups, MSI group had the lowest incidences of ND and poor outcome (13.5% and 16.2%, respectively). In multivariable analyses, for BZI patients, the risks of ND [odds ratio (OR) = 3.90, 95% confidence interval (CI) = 2.10–7.22, p < 0.001] and poor outcome (OR = 3.45, 95% CI = 1.67–7.14, p = 0.001) both significantly increased compared to MSI, both of which were the highest among the 4 stroke patterns. The neutrophil to lymphocyte ratio in BZI and large infarct groups were higher than in MSI and SSI groups [3.35 (2.28, 5.04) and 3.36 (2.53, 4.94) vs. 2.64 (1.89, 4.06) and 2.71 (1.93, 3.91), p < 0.001]. BZI group had the highest risks of ND and poor outcome among atherosclerotic stroke patients. BZI and large infarct patients had stronger poststroke inflammation than MSI and SSI patients.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82862-1.
Keywords: Atherosclerotic stroke, Stroke pattern, Borderzone infarct, Neurological deterioration, Poor outcome
Subject terms: Diseases, Medical research, Neurology
Introduction
Atherosclerosis is one of the common pathologies of ischemic stroke1. In clinical practice, ischemic stroke caused by atherosclerosis could generally be categorized into 4 major patterns: multiple small infarcts (MSI) in cortical and (or) subcortical areas but not involving borderzone areas, single subcortical infarction (SSI) which was traditionally termed as “lacunar stroke”, borderzone infarct (BZI) with or without cortical infarct, and a large infarct involving cortical and (or) subcortical area2,3. Mechanistically, MSI is likely to be involved with artery-to-artery embolism; SSI seems to be derived from the blockage of the orifice of the perforating artery by a parental arterial atheroma; BZI is associated with the hypoperfusion introduced by a significant atherosclerotic stenosis; a large infarct maybe caused by in situ thrombo-occlusion on a major vessel2,4. Some scholars has suggested that the pathogenesis of a proportion of MSI may resemble to lacunar stroke and the differentiation between these two subtypes are essential5. In our classification, the diagnosis of MSI pattern is based on a significant stenosis on the culprit vessel and the lesion could extend to the cortical area, whereas the significant stenosis is not necessary and the lesion is limited to the deep white matter or basal ganglia in the diagnosis of SSI.
Neurological deterioration (ND) occurs within a range of 2.2% to 37.5% in patients with acute ischemic stroke6, and is majorly adverse to the clinical outcomes of these patients7,8. The pathogenies of ND are heterogeneous, including hypoperfusion, clot progression, recurrence of stroke, cerebral edema, hemorrhagic transformation and so on9, and are likely to be different among patients with various atherosclerotic stroke patterns. For patients with MSI, ND seems to be attributed to the recurrence of stroke which is caused by a second-time rupture of a vulnerable plaque or thrombus; SSI patients’ ND is likely to be caused by the progression of plaque which blocks the orifice of the penetrating artery10; ND taking place in patients with BZI maybe due to the persistent hypoperfusion and the consequent impaired clearance of the emboli11; ND of patients with a large infarct appears to correlate with edema and/or hemorrhagic transformation in the lesion. Because of the diversity of ND pathogenies in different atherosclerotic stroke patterns, we speculate that the incidences of ND are also different. Further, the clinical outcomes of patients with various stroke patterns probably also differ due to the tight relationship between ND and poor outcome.
The present study aims to compare the risks of ND and poor outcome among patients with different atherosclerotic stroke patterns, in order to early predict the occurence of ND and poststroke disability in these patients, and to guide the clinical managements of those with high risks of ND and poststroke disability.
Methods.
Study population
This single-center cross sectional study adhered to STROBE guidelines and was in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The Scientific Research Ethics Committee of Affiliated Hospital of Jiangsu University approved this study (protocol code. KY2023K1003). The informed consent was obtained from all participants and/or their legal guardians. Patients with acute ischemic stroke who were consecutively admitted to the stroke unit from 2021.08.01 to 2023.07.31 were screened. The inclusion criteria were as follows: (1) with an age ≥ 18 years; (2) within 7-day of onset; (3) with ischemic stroke caused by atherosclerosis, i.e., an atherosclerotic stenosis was found on the artery supplying the territory where the infarct located in. The exclusion criteria were as follows: (1) with shortage of neuroimaging evidence, or poor neuroimaging quality, or diffusion-weighed image negative stroke; (2) with stroke of other determined etiology, such as cardioembolism, or of undetermined etiology; (3) with a SSI but without an atherosclerotic stenosis on the parental artery; (4) with intravenous thrombolysis and (or) endovascular thrombectomy; (5) with a prestroke modified Ranking Scale (mRS) > 2 points.
Baseline data
On admission, patients’ baseline data, including age, sex, height, weight, a history of hypertension and diabetes mellitus, smoking, alcohol consumption, and a history of previous stroke (including both ischemic and hemorrhagic stroke) were collected through a face-to-face interview. A neurologist evaluated the neurological deficit severity of participants according to the National Institute of Health Stroke Scale (NIHSS): 0 point was categorized as normal, 1–4 points as minor stroke, 5–15 points as moderate stroke and more than 15 points as severe stroke. All participants received medical administrations in accordance with the guidelines during hospitalization, such as antiplatelet and statins therapies, blood pressure and blood glucose control12.
Evaluation of the stroke mechanisms and the topography of stroke
All participants underwent the cranial computed tomography or nuclear magnetic resonance to ascertain the atherosclerotic stroke patterns. The definitions of various stroke patterns were as follows: (1) MSI was defined as multiple small infarcts located in the cortical and (or) subcortical territory, which was accompanied by a stenosis of any degree on relevant artery; (2) a single subcortical infarct located in cerebrum, brainstem or cerebellum, which was accompanied by a stenosis of any degree on the parental artery was defined as SSI; (3) a large ischemic lesion involved with cortical and (or) subcortical territories which was accompanied by a significant (≥ 50%) stenosis on relevant artery was categorized as large infarct; (4) BZI consisted of external cortical border-zone and internal border-zone infarcts. External cortical border-zone infarct was represented as linear or wedge-shaped lesions in the regions between the cortical territories of anterior cerebral artery and middle cerebral artery or between anterior cerebral artery and posterior cerebral artery. Internal border-zone infarct referred to cigar-shaped or linear lesions in the white matter along and above the lateral ventricles. Meanwhile, these lesions should be accompanied by a significant stenosis on relevant artery1,4 (Fig. 1).
Fig. 1.
Graphical representation of four major atherosclerotic stroke patterns. MSI: multiple small infarcts in the cortical and (or) subcortical territory (A) which are accompanied by a stenosis on relevant artery (B, red arrow); SSI: a single subcortical infarct (C) which is accompanied by a stenosis on the parental artery (D, red arrow); large infarct: a large infarct involved with cortical and (or) subcortical territories (E) which is accompanied by a significant stenosis on relevant artery (F, red arrow); BZI: a linear infarct located in the border zone between middle cerebral artery and posterior cerebral artery (G) which is accompanied by a significant stenosis on middle cerebral artery (H, red arrow). MSI indicates multiple small infarcts; SSI, single subcortical infarct; BZI, borderzone infarct.
Two independent neurologists evaluated the stroke patterns of every participant, respectively. Cohen’s kappa test was used to analyze the inter-observer agreement value of the stroke patterns evaluation, and the κ value was 0.970. When there was disagreement between the 2 neurologists, a superior practioner made the final decision.
A previous work reported that the distribution of infarct lesions may affect the prognosis of ischemic stroke13. Therefore, we evaluated the distribution of infarct lesions in this study. According to the distribution territory of the main body of the infarct, we divided the subjects into four groups: anterior cerebral artery infarction, middle cerebral artery infarction, posterior cerebral artery infarction, and brainstem and/or cerebellar infarction.
Evaluation of the atherosclerotic stenosis
After admission, all participants underwent time-of-flight angiography of nuclear magnetic resonance or computed tomography angiography to evaluate the intracranial atherosclerosis. The intracranial arteries under evaluation consisted of bilateral anterior cerebral (A1/A2 segments), middle cerebral (M1/M2 segments), posterior cerebral (P1/P2 segments), intracranial internal carotid, intracranial vertebral arteries, and the basilar artery. The stenotic degree of these arteries was evaluated in accordance with Warfarin-Aspirin Symptomatic Intracranial Disease Study Trial method14. In the subgroup of SSI, the parental artery was defined as the artery directly supplying the territory where the SSI lesion was located in15.
All participants underwent cervical computed tomography angiography to evaluate the extracranial vessels, including bilateral common carotid, extracranial internal carotid, and extracranial vertebral arteries. The stenotic degree of these vessels was evaluated in accordance with North American Symptomatic Carotid Endarterectomy Trial method, as a previous study16.
The atherosclerotic stenosis on intra and extra-cranial vessels were evaluated by two radiologists who were blinded to the clinical information of the participants, respectively. A third superior practitioner would make the definite diagnosis when there was disagreement between these two radiologists.
The definition of ND
ND was defined as an increase of ≥ 2 points in the NIHSS total score, or an increase of ≥ 1point in the consciousness subscore, or an increase of ≥ 1point in the motor subscore, or any new neurological deficit during hospitalization. The definition of ND was in line with previous researches7,17.
The definition of poor clinical outcome
At discharge, the daily activity of every participant was evaluated by a neurologist according to mRS score (0 to 5 points, death was recorded as 6 points). The poor outcome was categorized as a mRS > 2 points at discharge.
Statistical analysis
All Statistical analyses were performed by using SPSS software 25.0 (IBM). The comparisons of categorical variables among multiple groups were performed by using chi-squared test or Fisher’s exact test. The Shapiro‒Wilk test was used to test the data distribution. Normally distributed continuous variables were described by the mean ± standard deviation (SD) and were compared by using one-way analysis of variance test. Nonnormally distributed continuous variables were described by the median (interquartile range, IQR) and were compared by using Kruskal–Wallis test. Bonferroni adjustment were used in pairwise comparison of continuous variables among multiple groups.
Age, sex and all factors with a p < 0.1 in the univariable analyses were included into a binary logistic regression model. In this study, due to the lowest incidences of ND and poor outcomes in MSI group, this group was used as the reference in the multivariable analyses. The logistic regression analysis was used to calculate the odds ratio (OR) and 95% confidence interval (CI) of the risks of ND and poor outcome in SSI, large infarct and BZI groups by comparing to MSI group, respectively. The linear regression was used to analyze the mediating effect of the initial NIHSS between large infarct and poor outcomes. All tests were two-sided, and a p-value of < 0.05 was considered statistically significant.
Results
A total of 2302 patients with acute ischemic stroke within 7-day of onset were screened, and 126 patients were excluded because of the absence of neuroimaging evidence, or poor neuroimaging quality, or diffusion-weighed image negative stroke. Among the remaining 2176 patients, 548 patients were diagnosed with cardioembolism, 310 patients with stroke of undetermined etiology and 96 patients with stroke of other determined etiology. These patients were also excluded. Of the remaining 1222 patients, 412 were excluded because of a SSI without an atherosclerotic stenosis on the parental artery. Then, 810 patients were with a definite diagnosis of atherosclerotic stroke. Of these patients, 85 accepted intravenous thrombolysis and (or) endovascular thrombectomy and 37 had a prestroke mRS > 2 points. After the exclusion of these patients, 688 participants were ultimately enrolled in this study (Fig. 2). Among these patients, 1 (0.15%) with BZI died during hospitalization due to the recurrence of ischemic stroke.
Fig. 2.
Flow chart of the participants selection. Abbreviations: DWI indicates diffusion weighed image; mRS, modified Ranking Scale; NIHSS, National Institute of Health Stroke Scale.
The baseline data of all participants
The median age was 69.0 (61.0, 76.0) years, and 245 (35.6%) participants were female. The median of NIHSS score on admission was 2.0 (1.0, 4.0) points. Of all participants, 229 (33.3%) were diagnosed with MSI, 244 (35.5%) with SSI, 135 (19.6%) with large infarct and 80 (11.6%) with BZI. 154 (22.4%) participants experienced ND during hospitalization, and 184 (26.7%) participants suffered poor clinical outcomes at discharge (Table 1).
Table 1.
Baseline data of all participants.
| Clinical characteristics | All participants (n = 688) |
|---|---|
| Female, n (%) | 245 (35.6) |
| Age (year), median (IQR) | 69.0 (61.0, 76.0) |
| Hypertension, n (%) | 532 (77.3) |
| Diabetes mellitus, n (%) | 252 (36.6) |
| Smoking, n (%) | 281 (40.8) |
| Alcohol consumption, n (%) | 223 (32.4) |
| Previous stroke, n (%) | 165 (24.0) |
| Time of onset (hour), median (IQR) | 24.0 (10.0, 72.0) |
| BMI, median (IQR) | 24.58 (22.49, 27.34) |
| SBP (mmHg), mean ± SD | 156.3 ± 23.5 |
| DBP (mmHg), median (IQR) | 82.0 (75.0, 92.0) |
| TG (mmol/L), median (IQR) | 1.45 (1.04, 2.00) |
| TC (mmol/L), median (IQR) | 4.64 (3.87, 5.32) |
| HDL-C (mmol/L), median (IQR) | 1.02 (0.85, 1.25) |
| LDL-C (mmol/L), median (IQR) | 2.66 (2.06, 3.28) |
| HbA1c (%), median (IQR) | 6.30 (5.80, 8.08) |
| Homocysteine (mmol/L), median (IQR) | 12.23 (9.44, 15.60) |
| Hs-CRP (mg/L), median (IQR) | 1.65 (0.50, 5.88) |
| NLR, median (IQR) | 2.92 (2.05, 4.28) |
| Initial NIHSS (point), median (IQR) | 2.0 (1.0, 4.0) |
| Stroke mechanism | |
| MSI, n (%) | 229 (33.3) |
| SSI, n (%) | 244 (35.5) |
| Large infarct, n (%) | 135 (19.6) |
| BZI, n (%) | 80 (11.6) |
| Stenotic degrees of culprit vessel, n (%) | |
| Mild stenosis (< 50%) | 87 (12.6) |
| Moderate stenosis (50%-69%) | 251 (36.5) |
| Severe stenosis to occlusion (70%-100%) | 350 (50.9) |
| The territory of infarcts, n (%) | |
| Anterior cerebral artery | 41 (6.0) |
| Middle cerebral artery | 434 (63.1) |
| Posterior cerebral artery | 51 (7.4) |
| Brainstem or cerebellum | 162 (23.5) |
| ND, n (%) | 154 (22.4) |
| Poor outcome at discharge, n (%) | 184 (26.7) |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; hs-CRP, high-sensitivity C-reactive protein; NLR, neutrophil to lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale; MSI, multiple small infarcts; SSI, single subcortical infarct; BZI, borderzone infarct; ND, neurological deterioration.
The comparisons of baseline data among 4 subgroups
All participants were classified into 4 groups according to the stroke pattern: MSI (n = 229), SSI (n = 244), large infarct (n = 135) and BZI (n = 80) groups. Among the 4 groups, the systolic blood pressure on admission (p = 0.011), the proportion of female (p = 0.041), levels of homocysteine (p < 0.001), neutrophil count (p = 0.001), neutrophil to lymphocyte ratio (NLR) (p < 0.001), the proportion of posterior lesion (p < 0.001), initial NIHSS (p = 0.032), the stenotic degree of culprit vessel (p < 0.001) and the distribution of infarcts (p < 0.001) were significantly different. The incidence of ND in group of BZI was higher than in other 3 groups [41.3% vs. 13.5% (MSI), 23.8% (SSI) and 23.7% (large infarct), p < 0.001], and large infarct and BZI groups had higher proportions of poor outcome than other 2 groups [39.3% (large infarct) and 37.5% (BZI) vs. 16.2% (MSI) and 26.2% (SSI), p < 0.001] (Table 2).
Table 2.
Univariable analyses of baseline data among patients with different stroke mechanisms.
| Clinical characteristics | MSI (n = 229) | SSI (n = 244) | Large infarct (n = 135) | BZI (n = 80) | P-value |
|---|---|---|---|---|---|
| Female, n (%) | 69 (30.1) | 102 (41.8) | 43 (31.9) | 31 (38.8) | 0.041* |
| Age (year), median (IQR) | 69.0 (60.0, 76.0) | 69.0 (63.0, 76.0) | 68.0 (61.0, 77.0) | 69.0 (59.0, 75.8) | 0.57 |
| Hypertension, n (%) | 173 (75.5) | 191 (78.3) | 105 (77.8) | 63 (78.8) | 0.89 |
| Diabetes mellitus, n (%) | 80 (34.9) | 96 (39.3) | 51 (37.8) | 35 (31.3) | 0.55 |
| Smoking, n (%) | 99 (43.2) | 92 (37.7) | 61 (45.2) | 29 (36.3) | 0.35 |
| Alcohol consumption, n (%) | 85 (37.1) | 71 (29.1) | 42 (31.1) | 25 (31.3) | 0.30 |
| Previous stroke, n (%) | 56 (24.5) | 63 (25.8) | 30 (22.2) | 16 (20.0) | 0.70 |
| Time of onset (hour), median (IQR) | 24.0 (8.8, 72.0) | 24.0 (10.3, 48.0) | 24.0 (7.0, 48.0) | 24.0 (20.5, 96.0) | 0.054 |
| BMI, median (IQR) | 24.60 (22.97, 27.34) | 24.91 (23.14, 27.34) | 24.22 (22.23, 27.34) | 23.91 (22.22, 27.34) | 0.37 |
| SBP (mmHg), median (IQR) | 153.9 ± 22.1 | 158.0 ± 23.4 | 159.1 ± 22.6 | 153.1 ± 28.1 | 0.07 |
| DBP (mmHg), median (IQR) | 80.0 (74.0, 90.0) | 83.5 (75.0,. 94.0) | 83.0 (75.0, 92.0) | 80.0 (71.5, 91.8) | 0.12 |
| TG (mmol/L), median (IQR) | 1.48 (1.04, 2.09) | 1.48 (1.08, 2.21) | 1.38 (1.07, 1.73) | 1.36 (0.97, 1.89) | 0.085 |
| TC (mmol/L), median (IQR) | 4.67 (3.82, 5.36) | 4.62 (3.95, 5.36) | 4.61 (3.93, 5.42) | 4.69 (3.45, 5.19) | 0.84 |
| HDL-C (mmol/L), median (IQR) | 1.02 (0.85, 1.26) | 1.02 (0.85, 1.26) | 1.04 (0.86, 1.34) | 0.99 (0.84, 1.20) | 0.60 |
| LDL-C (mmol/L), median (IQR) | 2.65 (2.05, 3.25) | 2.68 (2.11, 3.32) | 2.65 (2.06, 3.38) | 2.64 (7.18, 3.15) | 0.85 |
| HbA1c (%), median (IQR) | 6.20 (5.70, 7.80) | 6.65 (5.90, 8.50) | 6.50 (5.80, 8.10) | 6.20 (5.90, 7.58) | 0.05 |
| Homocysteine (mmol/L), median (IQR) | 12.45 (9.74, 15.59) | 11.45 (8.78, 14.27) | 13.56 (10.28, 17.46) | 12.35 (9.85, 16.92) | < 0.001* |
| Hs-CRP (mg/L), median (IQR) | 1.60 (0.50, 5.55) | 1.50 (0.50, 5.38) | 2.10 (0.50, 6.00) | 1.70 (0.50, 7.24) | 0.72 |
| NLR, median (IQR) | 2.64 (1.89, 4.06) | 2.71 (1.93, 3.91) | 3.36 (2.53, 4.94) | 3.35 (2.28, 5.04) | < 0.001* |
| Initial NIHSS (point), median (IQR) | 2.0 (1.0, 3.0) | 2.0 (1.0, 4.0) | 2.0 (1.0, 7.0) | 2.0 (1.0, 4.0) | 0.032* |
| Stenotic degrees of culprit vessel | < 0.001* | ||||
| Mild | 0 | 87 (37.5) | 0 | 0 | |
| Moderate | 112 (48.9) | 73 (29.9) | 37 (27.4) | 29 (36.3) | |
| Severe to occlusion | 117 (51.1) | 84 (34.4) | 98 (72.6) | 51 (63.7) | |
| The territory of infarcts | < 0.001* | ||||
| Anterior cerebral artery | 9 (3.9) | 4 (1.6) | 5 (3.7) | 23 (28.7) | |
| Middle cerebral artery | 156 (68.1) | 158 (64.8) | 67 (49.6) | 53 (66.3) | |
| Posterior cerebral artery | 21 (9.2) | 14 (5.7) | 15 (11.1) | 1 (1.3) | |
| Brainstem or cerebellum | 43 (18.8) | 68 (27.9) | 48 (35.6) | 3 (3.8) | |
| ND, n (%) | 31 (13.5) | 58 (23.8) | 32 (23.7) | 33 (41.3) | < 0.001* |
| Poor outcome at discharge, n (%) | 37 (16.2) | 64 (26.2) | 53 (39.3) | 30 (37.5) | < 0.001* |
MSI, multiple small infarcts; SSI, single subcortical infarct; BZI, borderzone infarct; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; hs-CRP, high-sensitivity C-reactive protein; NLR, neutrophil to lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale; ND, neurological deterioration.
*p < 0.05 was considered statistically significant.
The comparisons of the risk of ND in the multivariable analyses
Due to the lowest incidences of ND and poor outcome in MSI group (13.5% and 16.2%, respectively), this group was used as a reference in the multivariable analyses. After adjusting for age, stroke pattern and clinical factors with a p < 0.1 in the univariable analyses, including sex, time of onset, systolic blood pressure on admission, triglyceride, glycosylated hemoglobin, homocysteine, NLR, initial NIHSS, the stenotic degrees of culprit vessel and the distribution of the infarcts, the BZI group had the highest incidence of ND (OR = 3.90, 95% CI = 2.10–7.22, p < 0.001) by comparing to the MSI group, followed by SSI (OR = 2.24, 95% CI = 1.33–3.75, p = 0.002) and large infarct groups (OR = 1.81, 95% CI = 1.02–3.22, p = 0.044). (Table 3).
Table 3.
The correlations of stroke mechanisms with neurological deterioration.
| Stroke mechanisms | Neurological deterioration | |||
|---|---|---|---|---|
| Crude OR (95% CI) | P-value | Adjusted OR§ (95% CI) | P-value | |
| MSI | Ref | Ref | ||
| SSI | 1.99 (1.23–3.22) | 0.005* | 2.24 (1.33–3.75) | 0.002* |
| Large infarct | 1.98 (1.15–3.43) | 0.014* | 1.81 (1.02–3.22) | 0.044* |
| BZI | 4.49 (2.50–8.04) | < 0.001* | 3.90 (2.10–7.22) | < 0.001* |
MSI, multiple small infarcts; SSI, single subcortical infarct; BZI, borderzone infarct.
*p < 0.05 was considered statistically significant.
§Adjusted for age, sex, time of onset, systolic blood pressure on admission, triglyceride, glycosylated hemoglobin, homocysteine, neutrophil to lymphocyte ratio, initial NIHSS, the stenotic degrees of culprit vessel and the distribution of the infarcts.
The comparisons of the risk of poor outcome in the multivariable analyses
MSI group was still used as reference. In the logistic regression model, after adjusting for forementioned confounders other than initial NIHSS, compared to MIS group, large infarct group (OR = 3.13, 95% CI = 1.82–5.37, p < 0.001) had an approximate twofold increased risk of poor outcome, whereas BZI group (OR = 2.52, 95% CI = 1.35–4.69, p = 0.004) and SSI group (OR = 2.28, 95% CI = 1.38–3.76, p = 0.001) had an approximate 1.5-fold increased risk. After including initial NIHSS into the model, compared to MIS group, BZI group had an approximate 2.5-fold increased risk of poor outcome (OR = 3.45, 95% CI = 1.67–7.14, p = 0.001), and large infarct group had only a 1.6-fold increased risk (OR = 2.61, 95% CI = 1.31–5.18, p = 0.006) (Table 4).
Table 4.
The correlations of stroke mechanisms with poor outcome at discharge.
| Stroke mechanisms | Poor outcome at discharge | |||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| Adjusted OR§ (95%CI) | P-value | Adjusted OR†(95%CI) | P-value | |
| MSI | Ref | Ref | ||
| SSI | 2.28 (1.38–3.76) | 0.001* | 2.36 (1.30–4.28) | 0.005* |
| Large infarct | 3.13 (1.82–5.37) | < 0.001* | 2.61 (1.31–5.18) | 0.006* |
| BZI | 2.52 (1.35–4.69) | 0.004* | 3.45 (1.67–7.14) | 0.001* |
MSI indicates multiple small infarcts; SSI, single subcortical infarct; BZI, borderzone infarct, NIHSS, National Institutes of Health Stroke Scale.
*p < 0.05 was considered statistically significant.
§Adjusted for age, sex, time of onset, systolic blood pressure on admission, triglyceride, glycosylated hemoglobin, homocysteine, neutrophil to lymphocyte ratio, the stenotic degrees of culprit vessel and the distribution of the infarcts.
†Adjusted for confounders included into model 1 plus initial NIHSS.
The proportions of various stroke severity among different stroke patterns
Among the 4 groups, the proportion of moderate-severe stroke was the lowest in MSI group (14.4%), followed by BZI (20.0%) and SSI (20.5%) groups. Large infarct group had the highest proportion of participants with moderate-severe stroke (31.9%) (Fig. 3).
Fig. 3.
The distribution of various stroke severity levels in four stroke patterns. Abbreviations: MSI indicates multiple small infarcts; SSI, single subcortical infarct; BZI, borderzone infarct.
The mediating effect of the initial NIHSS between large infarct and poor outcomes
After adjusting for age, sex and the significantly different factors among large infarct and non-large infarct groups, including time of onset, homocysteine, neutrophil to lymphocyte ratio, the stenotic degrees of culprit vessel and the distribution of the infarcts (Supplementary Table 1), the direct effect coefficient between large infarct and poor outcomes was 0.128 (p = 0.001), and the indirect effect coefficients between large infarct and initial NIHSS and between initial NIHSS and poor outcomes were 0.252 (p = 0.001) and 0.186 (p = 0.013), respectively (Table 5).
Table 5.
The mediating effect of the initial NIHSS between large infarct and poor outcomes.
| Clinical factors | Poor outcome at discharge | |||||||
|---|---|---|---|---|---|---|---|---|
| Direct effect | Indirect effect | |||||||
| Crude | Adjusted† | Crude | Adjusted† | |||||
| Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | |
| Stroke pattern | 0.243 | 0.012* | 0.128 | 0.001* | 0.115 | 0.003* | 0.252 | 0.001* |
| Initial NIHSS | – | – | – | – | 0.495 | < 0.001* | 0.186 | 0.013* |
NIHSS, National Institutes of Health Stroke Scale.
*p < 0.05 was considered statistically significant.
†Adjusted for age, sex, time of onset, homocysteine, neutrophil to lymphocyte ratio, the stenotic degrees of culprit vessel and the distribution of the infarcts.
The comparisons of NLR among different stroke patterns.
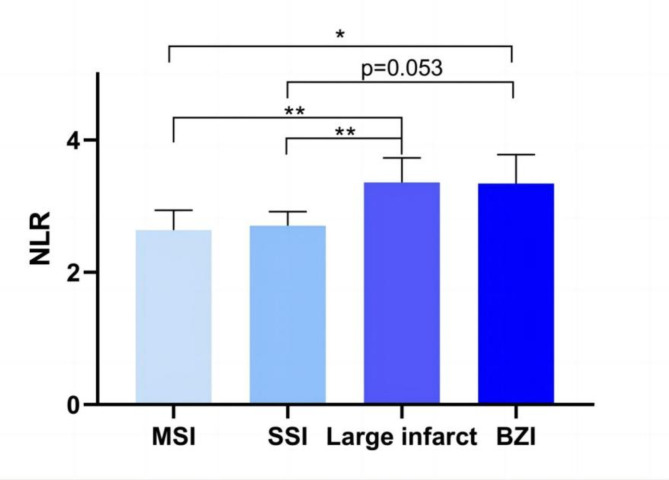
The NLR of BZI and large infarct groups were higher than that of other 2 groups [3.35 (2.28, 5.04) (BZI) and 3.36 (2.53, 4.94) (large infarct) vs. 2.64 (1.89, 4.06) (MSI) and 2.71 (1.93, 3.91) (SSI), p < 0.001]. In the pairwise comparisons, the NLR in large infarct group was higher than in MSI and SSI groups [3.36 (2.53, 4.94) vs. 2.64 (1.89, 4.06), p = 0.001; 3.36 (2.53, 4.94) vs. 2.71 (1.93, 3.91), p = 0.001], respectively. In BZI group, the NLR was significantly higher than in MSI group [3.35 (2.28, 5.04) vs. 2.64 (1.89, 4.06), p = 0.046], and was marginal significantly higher than in SSI group [3.35 (2.28, 5.04) vs. 2.71 (1.93, 3.91), p = 0.053] (Fig. 4).
Fig. 4.
Comparisons of NLR between different atherosclerotic stroke patterns. * p < 0.05; ** p < 0.01. p < 0.05 was considered statistically significant. Abbreviations: MSI indicates multiple small infarcts; SSI, single subcortical infarct; BZI, borderzone infarct; NLR, neutrophil to lymphocyte ratio.
According to the median value of NLR (2.92), all participants were categorized into low NLR (< 2.92) and high NLR (≥ 2.92) groups. In multivariable logistic regression analysis, after adjusting for age, sex, time of onset, systolic blood pressure on admission, triglyceride, glycosylated hemoglobin, homocysteine, initial NIHSS, the stenotic degrees of culprit vessel and the distribution of the infarcts, compared to MSI group, the odds of high NLR significantly increased in large infarct (OR = 1.87, 95% CI = 1.17–2.99, p = 0.009) and BZI groups (OR = 2.16, 95% CI = 1.24–3.75, p = 0.006). The odds of high NLR were comparable between MSI and SSI groups (Table 6).
Table 6.
The correlation of stroke patterns with high NLR in logistic regression model.
| Stroke patterns | High NLR | |||
|---|---|---|---|---|
| Crude OR (95% CI) | P-value | Adjusted OR† (95% CI) | P-value | |
| MSI | Ref | Ref | ||
| SSI | 0.97 (0.68–1.40) | 0.88 | 0.92 (0.61–1.39) | 0.70 |
| Large infarct | 2.15 (1.39–3.32) | 0.001* | 1.87 (1.17–2.99) | 0.009* |
| BZI | 2.04 (1.21–3.44) | 0.007* | 2.16 (1.24–3.75) | 0.006* |
NLR, neutrophil to lymphocyte ratio; MSI, multiple small infarcts; SSI, single subcortical infarct; BZI, borderzone infarct, NIHSS, National Institutes of Health Stroke Scale.
*p < 0.05 was considered statistically significant.
†Adjusted for age, sex, time of onset, systolic blood pressure on admission, triglyceride, glycosylated hemoglobin, homocysteine, neutrophil to lymphocyte ratio, initial NIHSS, the stenotic degrees of culprit vessel and the distribution of the infarcts.
Discussion
In this study, we found that compared with MSI patients, BZI patients have an approximately threefold increased risk of developing ND and post stroke disability, both of which were the highest among the 4 stroke patterns. The levels of NLR in BZI and large infarct groups were higher than in MSI and SSI groups.
Some previous studies explored the associations between the stroke etiology and ND. Siegler JE et al. classified the participants into cardioembolic, large vessel, small vessel, other, unknown source, and multiple possible stroke etiologies, and reported that group with unknown source had the lowest risk of ND whereas the group of other source had the highest one18. Some other researches reported that non-cardioembolism had a higher risk of ND than cardioembolism, and the risk of ND was higher in lacunar stroke than in non-lacunar stroke19,20.
These studies merely analyzed the correlations between the occurence of ND and the stroke etiologies which were categorized according to the Trial of Org 10172 in Acute Stroke Treatment method, without further analysis of the relationship between different stroke patterns and ND in subpopulation with atherosclerotic stroke. Because of the susceptibility to intracranial atherosclerosis in Asian population21, we focused on the impact of the atherosclerotic stroke patterns on the incidence of ND in this study. We found that BZI patients had the highest risk of ND among 4 atherosclerotic stroke patterns, and the underlying reason was likely to be correlated with the nature of BZI which was associated with the impaired intracranial blood flow and perfusion delay attributed to the significant stenosis on major vessels22. Alawneh JA et al. considered that hypoperfusion played a pivotal role in the occurence of ND because that it could lead to the infarction of the oligemic tissue surrounding the penumbra and consequent deterioration of stroke severity23.
Approximate to the rate of ND, the incidence of poor outcome in participants with BZI was also more than 1/3. The multivariable analyses corroborated that the risk of poor outcome in BZI group was also the highest among 4 stroke patterns. However, up to 80% participants with BZI were categorized as normal or minor stroke on admission. Because that all BZI patients had a significant stenosis or occlusion on the relevant artery of infarct territory, this category of patients were relatively consistent with the concept of “minor stroke with steno-occlusive arterial disease”. Several studies reported that a significant proportion of these patients had unstable clinical status and a high risk of ND, thereby leading to major physical disability24–26. This conclusion was in line with the present study. Some scholars suggested that endovascular treatments may restore the blood flow thus lower the incidence of ND in patients with minor stroke with steno-occlusive arterial disease27.
In addition to hypoperfusion, inflammation was also a pivotal factor mediating the occurence of ND and poor outcomes in patients with stroke28,29. NLR was one of indicators reflecting systemic inflammatory status30, and was correlated with the increased risk of poststroke disability and mortality31. In this study, we found the NLR in BZI and large infarct groups were higher than in other 2 patterns, suggesting that participants in these 2 groups had a relatively strong poststroke inflammatory reaction. This was likely to be a reason of high risk of poor outcome in BZI and large infarct groups. According to our clinical experiences, the BZI and large infarct groups seemed to have larger ischemic core and penumbra than MSI and SSI groups. A previous study reported that the poststroke inflammation was significantly associated with the infarct size32. This was a possible rationale for why poststroke inflammation was greater in the BZI and large infarct groups than in the MSI and SSI groups.
Due to the relatively strong poststroke inflammation, the anti-inflammation therapy might be applicable to the clinical managements for patients with BZI or large infarct. A recent study reported that a combination of low-dose methylprednisolone and endovascular treatment could decrease the incidence of mortality and symptomatic intracranial hemorrhage in patients with large artery occlusive stroke33, indicating that the anti-inflammation therapy was effective to improve the clinical outcomes. For BZI or large infarct patients with a minor stroke but without indications for endovascular treatment, low-dose methylprednisolone might be also favourable to their clinical outcomes. A further clinical trail could be conducted to verify this speculation.
The incidence of poor outcome in large infarct patients resembled to that in BZI group (39.3% and 37.5%), both of which were at the top level among 4 stroke patterns. In multivariable analysis, after adjusting for confounders other than initial NIHSS, the risks of poor outcome in these 2 groups were still approximate. Interestingly, after initial NIHSS included into the regression model, compared to the MSI group, the risk of poor outcome in large infarct group increased only 1.6-fold, lower than that in BZI group which increased approximately threefold. This might be because the proportion of moderate-severe stroke in large infarct group was significantly higher than that in MSI group (31.9% vs. 14.4%), which but resembled to BZI group (14.4% vs. 20.0%). Consequently, the risks of poor outcome in large infarct and BZI groups differed after adjusting for initial NIHSS. We performed the mediating effect of the initial NIHSS between large infarct and poor outcomes, revealing that initial NIHSS significantly effects the association between large infarct and poor outcome.
For SSI patients, the risk of poor outcome was also higher than MSI patients, although increased less than onefold. The SSI patients enrolled in this study were all with parental arterial atheroma, which could block the the orifice of the penetrating artery34. Because that the penetrating artery was an end-artery without collateral supply, SSI patients with parental arterial atheroma were likely to be susceptible to ND and subsequent poor outcome15. For these patients, intensive managements for atherosclerotic disease might be essential.
Patients with MSI also had more favorable prognoses than patients with BZI and large infarct. There were two underlying reasons as follows. Firstly, the infarct size of MSI was far smaller than that of BZI and large infarct. Due to the significant prognostic value of infarct size for the outcomes of patients with ischemic stroke35, patients with small infarcts probably had better outcomes than those with relatively larger infarcts. Secondly, the comprehensive stenotic degrees of culprit vessels in MSI group were better than in BZI and large infarct groups (Table 2). This indicated that the perfusion for the ischemic parenchyma in MSI group was likely to be more sufficient than in BZI and large infarct groups. This hypothesis would be validated in our future study. Thirdly, the poststroke inflammation of MSI was more slight than BZI and large infarct. As mentioned above, strong poststroke inflammation mediated the occurence of ND and poor outcomes in patients with ischemic stroke.
The present topic has several research directions. Firstly, the association between the BZI pattern and the perfusion imaging is needed to be validated. Secondly, we found that the stroke pattern caused by some certain vessel could be heterogeneous despite with the same stenotic degree. A future study would be conducted to explore the mechanisms underlying this interesting phenomenon.
This study had some limitations. Firstly, this was a single center study and the majority of participants were Chinese Han population. The generalizations of this study to other populations or regions should be cautious. Secondly, a proportion of participants had experienced suspicious ND before admission according to their complaints. However, these patients were not recorded as having ND because that their NDs were not confirmed by a professional neurologist. Therefore, the rate of ND in patients with atherosclerotic stroke might be underestimated. Thirdly, the pathogenesis of SSI patients without parental arterial atheroma were likely to be correlated with lipohyalinosis or fibrinoid degeneration of the perforating artery36, and these patients were excluded. However, the pathogenesis of these patients may also be caused by microatheroma in the wall of the perforating artery, which yet could not be ascertained by current neuroimaging methods. The exclusion of these patients might have an impact on the results of this study.
Conclusion
Among the 4 patterns of atherosclerotic stroke, MSI group had the lowest incidences of ND and poor outcome. Compared to MSI group, the risks of ND and poststroke disability both increased approximately threefold in BZI group, which were highest in 4 patterns. The levels of poststroke NLR in BZI and large infarct groups were higher than in MSI and SSI groups, suggesting that BZI and large infarct patients had a relatively strong poststroke inflammatory reaction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from Zhenjiang Jinshan Talent Training Program (NO. JSYCBS202202).
Author contributions
The study was designed by Y.H. and Y.Y. Y.H. wrote the first draft of the manuscript, Y.Y. performed all statistical analyses. Y.H., Z.T., W.W, and K.N. vouches for the data and analyses. All of the authors participated in data collection, analyses and interpretation. All authors vouch for the accuracy and completeness of the data and analyses and made the decision to submit the manuscript for publication. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Data availability
All data generated or analyzed during this study could be acquired from the corresponding author through e-mail.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campbell, B. C. V. et al. Ischaemic stroke. Nat. Rev. Dis. Primers5, 70 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Tekle, W. G. & Hassan, A. E. Intracranial atherosclerotic disease: Current concepts in medical and surgical management. Neurology97, S145–S157 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Woo, H. G. et al. Blood viscosity associated with stroke mechanism and early neurological deterioration in middle cerebral artery atherosclerosis. Sci. Rep.13, 9384 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng, X. et al. Stroke mechanisms in symptomatic intracranial atherosclerotic disease: Classification and clinical implications. Stroke50, 2692–2699 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Arboix, A. et al. Clinical predictors of lacunar syndrome not due to lacunar infarction. BMC. Neurol.10, 31 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji, X. et al. A systematic review of body fluids biomarkers associated sith early neurological deterioration following acute ischemic stroke. Front. Aging. Neurosci.14, 918473 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park, T. H. et al. Neurologic deterioration in patients with acute ischemic stroke or transient ischemic attack. Neurology95, 2178–2191 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Seners, P. et al. MINOR-STROKE Collaborators: Prediction of early neurological deterioration in individuals with minor stroke and large vessel occlusion intended for intravenous thrombolysis alone. JAMA. Neurol.78, 321–328 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thanvi, B., Treadwell, S. & Robinson, T. Early neurological deterioration in acute ischaemic stroke: Predictors, mechanisms and management. Postgrad. Med. J.84, 412–417 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Jiang, J. et al. Total MRI burden of cerebral vessel disease correlates with the progression in patients withacute single small subcortical strokes. Brain. Behav.9, e01173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caplan, L. R. Worsening in ischemic stroke patients: Is it time for a new strategy?. Stroke33, 1443–1445 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018: Chinese Society of Neurology, Chinese Stroke Society. Chin. J. Neurol.51, 666–682 (2018).
- 13.Arboix, A. et al. Infarctions in the vascular territory of the posterior cerebral artery: Clinical features in 232 patients. BMC. Res. Notes4, 329 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuels, O. B., Joseph, G. J., Lynn, M. J., Smith, H. A. & Chimowitz, M. I. A standardized method for measuring intracranial arterial stenosis. AJNR. Am. J. Neuroradiol.21, 643–646 (2000). [PMC free article] [PubMed] [Google Scholar]
- 15.Nam, K. W., Kwon, H. M. & Lee, Y. S. Different predictive factors for early neurological deterioration based on the location of single subcortical infarction: Early prognosis in single subcortical infarction. Stroke52, 3191–3198 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Trial, N. A. S. C. E. Methods, patient characteristics, and progress. Stroke22, 711–720 (1991). [DOI] [PubMed] [Google Scholar]
- 17.Yang, Y. et al. Clinical factors associated with functional outcomes in patients with single subcortical infarction with neurological deterioration. Front. Neurol.14, 1129503 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegler, J. E., Samai, A., Semmes, E. & Martin-Schild, S. Early neurologic deterioration after stroke depends on vascular territory and stroke etiology. J. Stroke18, 203–210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tei, H. et al. Deteriorating ischemic stroke in 4 clinical categories classified by the Oxfordshire Community Stroke Project. Stroke31, 2049–2054 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Weimar, C. et al. Neurologic worsening during the acute phase of ischemic stroke. Arch. Neurol.62, 393–397 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Jia, B. et al. EAST Study Group: Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J. Neurointerv. Surg.10, 746–750 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Das, S. et al. Borderzone infarcts and recurrent cerebrovascular events in symptomatic intracranial arterial stenosis: A systematic review and meta-analysis. J. Stroke25, 223–232 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alawneh, J. A., Moustafa, R. R. & Baron, J. C. Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke40, e443–e450 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Yaghi, S. et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol.73, 572–578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, J. T. et al. Internal border zone lesions as a predictor of early neurological deterioration in minor stroke patients with severe arterial steno-occlusion. J. Neuroimaging21, 173–176 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Mazya, M. V. et al. Minor stroke due to large artery occlusion. When is intravenous thrombolysis not enough? Results from the SITS International Stroke Thrombolysis Register. Eur. Stroke. J.3, 29–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He, L. et al. The length of susceptibility vessel sign predicts early neurological deterioration in minor acute ischemic stroke with large vessel occlusion. BMC. Neurol.21, 421 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia, W. L. et al. Associations between admission levels of multiple biomarkers and subsequent worse outcomes in acute ischemic stroke patients. J. Cereb. Blood. Flow Metab.17, 271678X231214831 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar, A. D. et al. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J. Stroke Cerebrovasc. Dis.22, e111–e117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahorec, R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy.102, 5–14 (2001). [PubMed] [Google Scholar]
- 31.Song, S. Y. et al. Clinical significance of baseline neutrophil-to-lymphocyte ratio in patients with ischemic stroke or hemorrhagic stroke: An updated Meta-analysis. Front. Neurol.10, 1032 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buck, B. H. et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke39, 355–360 (2008). [DOI] [PubMed] [Google Scholar]
- 33.MARVEL Trial Authors for the MARVEL Investigators; Yang, Q. et al. Methylprednisolone as adjunct to endovascular thrombectomy for large-vessel occlusion stroke: The MARVEL Randomized Clinical Trial. JAMA331, 840–849 (2024). [DOI] [PMC free article] [PubMed]
- 34.Caplan, L. R. Lacunar infarction and small vessel disease: Pathology and pathophysiology. J. Stroke.17, 2–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan, S. L., Wu, S. C., Wu, T. H., Lee, T. K. & Chen, T. H. Location and size of infarct on functional outcome of noncardioembolic ischemic stroke. Disabil. Rehabil.28, 977–983 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Fisher, C. M. Lacunes: Small, deep cerebral infarcts. Neurology77, 2104 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study could be acquired from the corresponding author through e-mail.