Abstract
In this study, we aimed to assess the effects of enhanced external counterpulsation (EECP) and individual shear rate therapy (ISRT) on peripheral artery function in patients with lower extremity atherosclerotic disease (LEAD). We randomly assigned 45 LEAD patients to receive 35 sessions of 45 min of EECP (n = 15), ISRT (n = 15), or sham-control (n = 15). Flow-mediated dilation in the brachial artery (brachial-FMD); 6-min walk distance; blood flow in the popliteal, posterior tibial, anterior tibial, and dorsalis pedis arteries; and plasma levels were measured before and after the 7 weeks treatment. 36-item Short Form Health Survey [SF-36] was analyzed before, after 7 weeks, and 3-month follow-ups. EECP treatment significantly improved brachial-FMD and quality of life, increased walking distance, and increased blood flow and the diameters of the popliteal artery and posterior tibial artery (all P < 0.01). Conversely, ISRT markedly increased blood flow in the anterior tibial artery (P < 0.05). EECP and ISRT decreased the endothelin-1 and asymmetrical dimethylarginine levels in patients with LEAD (both P < 0.01). Additionally, sVCAM-1 was significantly reduced after EECP intervention (P = 0.004). Our findings demonstrate that EECP and ISRT have beneficial effects on walking distance, quality of life, flow-mediated dilation, endothelial-derived vasoactive agents, and inflammatory and oxidative stress in LEAD patients.
Date of registration: 2021-06-21. Trial registration: ChiCTR2100048086.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82485-6.
Keywords: Enhanced external counterpulsation, Individual shear rate therapy, Lower extremity atherosclerotic disease, Vascular hemodynamics, Walking distance
Subject terms: Physiology, Diseases, Health care
Introduction
Enhanced external counterpulsation (EECP) is a Food and Drug Administration-approved non-invasive circulation-assisting treatment recommended as a clinical guideline for cardiovascular and cerebrovascular diseases by the American College of Cardiology/American Heart Association, European Society of Cardiology, and the Chinese Medical Association1–3. The EECP involves sequential inflation and deflation of compressible cuffs wrapped around the patients’ calves, lower thighs, and upper thighs. Compressed air pressure is applied by cuffs to the lower extremities in a sequence synchronized with the cardiac cycle via microprocessor-interpreted ECG signals.
EECP alleviates angina symptoms and reduces myocardial ischemia in patients with coronary artery disease (CAD)4,5. It significantly increases myocardial perfusion and coronary collateral flow in patients with CAD6,7. While the pathogenesis and progression of CAD are influenced by the behavior of the coronary arteries and other peripheral systemic vessels, little is known about whether EECP can affect lower-extremity atherosclerotic disease (LEAD)8. CAD is often associated with atherosclerotic lesions in peripheral vascular beds9, and 12.1% of all patients with CAD have asymptomatic LEAD9,10. One study reported that EECP appears to be an effective therapy in patients with peripheral artery disease (PAD); however, they suggested that future studies on EECP should actively include individuals with PAD defined by anatomy (infra popliteal disease) and ankle-brachial index (ABI) severity. Evaluation of PAD-specific clinical outcomes, including maximal walking distance and peripheral vascular and blood flow characteristic variables, may provide additional evidence of the benefits for patients with LEAD11.
Some studies have investigated the protective effects of EECP on peripheral blood vessels12,13. Braith et al. showed that 35 1-h sessions of EECP improve peripheral artery flow-mediated dilation (FMD) and endothelial-derived vasoactive agents (including endothelin-1 [ET-1], 6-keto-prostaglandin PGF1α[6-keto-PGF1α], and 8-isoprostane [8-iso-PGF2α]) evaluated by plasma levels12. Bonetti et al. described an improvement in myocardial perfusion and decrease in peripheral resistance are needed to achieve the clinical benefits associated with EECP14. Gurovich and Braith et al. demonstrated that EECP acutely improves endothelium-dependent vasodilation in both the femoral and brachial arteries, as measured using FMD15. Endothelial dysfunction can be assessed based on impaired vasoreactivity of the brachial artery after an ischemic stimulus using FMD. They demonstrated that peripheral arterial function could be considered another therapeutic target for chronic EECP treatment16.
Although the mechanism underlying the beneficial effect of EECP is unclear, evidence suggests that the improvement of endothelial function is a crucial mechanism for the favorable clinical effect of EECP17. However, Hashemi et al. found that FMD is not the main mechanism of long-term EECP treatment and that other mechanisms should be considered18. Werner et al. observed that EECP significantly reduced the flow volumes of the posterior tibial artery on ultrasound measurement19. Dockery et al. reported that arterial stiffness measured using carotid-radial pulse wave velocity (cr-PWV) and aortic augmentation index (AI) did not change after EECP intervention20. Martin et al. reported that the EECP did not significantly improve resistance to arterial function in the calf21.
Based on EECP technology, Buschman et al. proposed individual shear rate therapy (ISRT) for lower extremity PAD, which changes different counterpulsation modes under low counterpulsation pressure, monitors lower-extremity arteries using ultrasound technology, and calculates individualized optimal shear rate10,22. Compared with EECP, ISRT added Doppler measurements and removed the calves during treatment. It was evaluated using real-time Doppler-derived variables of calf perfusion during counterpulsation22. ISRT reportedly increases lower limb walking distance and exercise capacity, improves endothelial function and the degree of peripheral arteriosclerosis, and reduces arterial blood pressure10,23. However, the ABI and PWV were not reduced after the ISRT intervention24. Currently, the clinical evidence is insufficient, and the hemodynamic mechanism remains unclear11,19,25.
Therefore, in this pilot clinical trial, we aimed to investigate and compare the effects of 35 45-min session of EECP and ISRT on the peripheral blood flow indicators mentioned above (brachial-FMD, walking distance, ultrasound blood flow, plasma levels, and Health-related Quality of Life [HRQoL]) in patients with LEAD.
Methods
Study design
The protocol of this prospective randomized sham-controlled clinical trial was approved by the Institutional Review Board of the Eighth Affiliated Hospital of Sun Yat-sen University (NO.2021-020-02). All the participants provided written informed consent. The study was first registered on June 21, 2021, with registration number ChiCTR2100048086 (https://www.chictr.org.cn/searchproj.html). This study was conducted in accordance with the Interim Measures for Guidelines on the Ethical Review of Biomedical Research Involving Human Subjects.
Participants
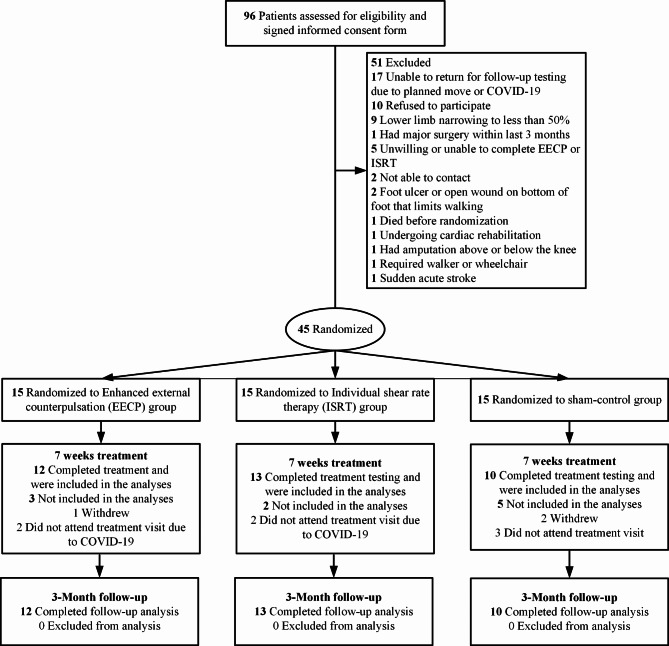
Forty-five patients with LEAD were randomized into either EECP (n = 15), ISRT (n = 15) or sham-control (n = 15) group. Among them, EECP group (n = 12), ISRT group (n = 13) and control group (n = 10) completed the entire treatment, and no adverse events occurred. 10 were dropped out due to several reasons (4 did not attend treatment visit due to COVID-19, 3 withdrew from the study midway, and 3 in the control group did not attend treatment visit). The participants’ flow diagram can be seen in Fig. 1.
Fig. 1.
Flowchart of study participants.
Inclusion criteria
We recruited outpatients, inpatients, and individuals from community health service centers aged 45–75 years old at the Eighth Affiliated Hospital of Sun Yat-sen University. The inclusion criteria included patients with ABI ≤ 0.9; any arterial (popliteal artery, anterior tibial artery, posterior tibial artery, or dorsalis pedis artery) stenosis > 50% diagnosed using ultrasound examination; and stage II LEAD according to the Fontaine classification.
Exclusion criteria
Patients were excluded if they had an amputation of the lower limbs, walking disorders not caused by vascular stenosis of the lower limbs or iliofemoral disease. Additionally, patients with stents in the lower limbs, arterial thrombosis or unstable plaques, or critical limb ischemia were excluded. Furthermore, patients were excluded if they had contraindications for EECP, such as moderate-to-severe aortic regurgitation, dissecting aneurysm, various bleeding disorders or bleeding tendencies, valve disease, congenital heart disease, cardiomyopathy, active phlebitis, venous thrombosis, infection of the lower extremities, hypertension (> 170/110 mmHg), frequent premature beats (> 10 beats/min), severe left heart failure, severe lower extremity arterial occlusive disease, and pregnancy.
Randomization
The patients were randomized to undergo either the EECP, ISRT, or sham-control; they were randomized between December 10, 2020 and June 10, 2022. A simple randomization procedure (computer random number generator) was used for the randomization, and a statistician generated the allocation sequence and assigned the patients. A clinical research assistant enrolled the participants, and the nurses and doctors who operated the EECP were blinded to the participant allocation.
Sample size calculation
No previous studies have reported the effect of EECP, ISRT and sham-control on the peripheral artery functions in LEAD patients. The sample size was estimated according to the brachial-FMD results of the three groups in our pre-experiment results. The sample size was calculated based on Analysis of Covariance Power Analysis, which was performed by mean with standard deviation of brachial-FMD with power fixed at 90% (alpha = 0.05 and k [number of groups] = 3).
Intervention and outcomes
EECP
EECP and ISRT were delivered using a Pushikang P-ECP/TM Oxygen Saturation Monitoring EECP Instrument (Chongqing, China). It involved sequential inflation and deflation of compressible cuffs wrapped around the participants’ calves, lower thighs, and upper thighs. Compressed air was applied through these cuffs to the lower extremities in a sequence synchronized with the cardiac cycle via microprocessor-explicated ECG signals. During diastole, the increased pressure facilitated the return of blood from the periphery to the heart. During systole, the decreased pressure aided the perfusion of blood from the heart to the extremities. The experiment was conducted at the EECP center with a unified operation of the nurses. All participants received a standard treatment of three-stage EECP, with 35 sessions, each lasting 45 min, once daily, 5 days weekly, for a total duration of 7 weeks. The treatment pressure was set between 0.021 and 0.033 MPa according to the change of the ratio of diastolic pressure (D) to systolic pressure (S) (D/S ratio) in photoplethysmography, which is a critical indicator for evaluating the effectiveness of EECP26.
ISRT
During ISRT, the participants had inflatable cuffs wrapped only around their buttocks and thighs. Simultaneously, to determine the optimal treatment pressure, we applied different pressures (0, 80, 120, 160, 200, and 220 mmHg) to the patients and used ultrasonography to measure the maximum peak velocity and acceleration of the anterior tibial artery during systole. The pressure for each treatment was maintained for 4 min. Doppler flow parameters were assessed within the 3rd min. The pressure was adjusted thrice throughout the treatment cycle, specifically during the 2nd, 12th, and 22nd sessions. Treatment pressure under optimal conditions was applied during different courses of the intervention.
Sham-control
Patients in the sham-control group received 35 sessions, each lasting 45 min, of EECP with cuff inflation pressures of 80 mmHg, which were insufficient to change the blood pressure in the lower limbs27.
Outcomes
The primary endpoints of this study were the 6-min walk distance and brachial-FMD. The secondary endpoints included arterial stiffness indicators (brachial ankle [ba]-PWV and ABI), ultrasonography parameters (peak systolic velocity [PSV], area, and flow rate [FR]), biochemical criteria (including ET-1, soluble vascular cell adhesion molecule-1 [sVCAM-1], 8-iso-PGF2α, and asymmetrical dimethylarginine [ADMA]), and the HRQoL Assessment Scale.
Data collection and analysis
Patient characteristics
Basic characteristics, including waistline, HR, BP, smoking, drinking (≥ 3 times/week), family history, exercise habits (≥ 3 times/week), and sleep disorders (inability to sleep for 3–5 consecutive days recently), were collected in this study. Cardiovascular disease and risk factors, including CAD, hypertension, diabetes, hyperlipidemia, high-sensitivity C-reactive protein, glycosylated hemoglobin, glucose, total cholesterol, low-density lipoprotein, and uric acid, were also analyzed.
Brachial-FMD
Brachial artery FMD was evaluated on the right arm using a high-resolution ultrasonography machine (Omron, Japan) equipped with a 10.5-MHz transducer. Briefly, baseline end-diastolic brachial diameters at rest were obtained by placing the transducer 3–5 cm above the antecubital fossa. Reactive hyperemia was induced by inflating a blood pressure cuff placed on the upper forearm at 200 mmHg for 5 min, followed by rapid deflation. Subsequently, the brachial artery was imaged and recorded for 3 min after cuff deflation. The time required to measure the brachial-FMD for each participant was 7 min.
6-Minute walking test
The 6-min walk test is an important method for evaluating peripheral arterial disease and various treatments for cardiovascular rehabilitation28. All participants underwent the assessment under a standardized protocol conducted by a trained and certified research assistant who was blinded to the random assignment. The participants walked up and down a 100-foot hallway for 6 min, and the distance covered was recorded.
Measurement of arterial stiffness
An arteriosclerosis detector (Omron, Japan) was used to measure blood pressure (systolic, diastolic, and mean pressure) in the right and left brachial arteries, dorsalis pedis, left and right ba-PWV, and ABI. These parameters were calculated by dividing the mean of the two continuous waveform acquisitions obtained at 3 min.
Ultrasonic hemodynamic detection
The hemodynamic parameters in the left infra-popliteal arteries (popliteal, anterior tibial, posterior tibial, and dorsalis pedis arteries) were measured using Color Doppler Ultrasound (TOSHIBA APLIO 500 TUS-A500, Japan) at baseline and after 7 weeks of EECP, ISRT and sham-control. Parameters such as PSV, area, and FR were continuously recorded for 5 s, and the mean values were calculated. The mean FR was calculated using the vessel diameter, cardiac period, and velocity-time integral as follows:
| 1 |
,
where VTI is the averaged velocity-time integral, T is the average cardiac cycle time, and S is the vascular area.
Blood collection and biochemical assays
A nurse performed venipuncture to collect blood samples before and after 7 weeks of EECP, ISRT, or sham-control treatment in the Second Ward of the Cardiology Department from 7:30 am to 8:00 am. The blood samples were centrifuged at 3,000 × g for 10 min and stored at -80 °C for subsequent analysis. Plasma levels of ET-1, sVCAM-1, 8-iso-PGF2α, and ADMA were quantified using enzyme-linked immunosorbent assay kits (MEIMIAN, China). The samples and standards were incubated at 37 °C for 30 min according to the manufacturer’s instructions. After incubation, the plates were washed, and the enzyme-labeled reagent was added, followed by another 30 min of incubation. Subsequently, a color developer was added, and the mixture was incubated for 10 min. Finally, the optical density was measured at 450 nm within 15 min of adding the stop solution. All tests were conducted in the laboratory of the Eighth Affiliated Hospital of Sun Yat-sen University by the researcher. The patients maintained a diet diary and adhered to the National Institutes of Health’s low-nitrate diet guidelines for a minimum of 48 h before each blood collection.
HRQoL assessment scale
HRQoL scores have been shown to predict long-term survival in individuals with PAD, highlighting the significance of HRQoL as a predictor of long-term survival in patients with PAD29,30. We used the 36-item Short Form Health Survey (SF-36) to assess the HRQoL of patients. This scale includes physical function (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social function (SF), role-emotional (SE), and mental health (MH). The score for each dimension ranges from 0 to 100, with a higher score representing a more convenient state. The research assistant administered the SF-36 questionnaire before treatment, after 7 weeks of treatment, and after 3 months of follow-up. In the first two sessions, face-to-face interviews were conducted in the treatment room, and in the last session, telephone follow-ups were conducted due to COVID-19 restrictions. Scores for the eight aspects of the questionnaire were calculated and analyzed.
Parameters measurement at each time point
The brachial-FMD, 6-min walk distance, blood flow in the popliteal artery, posterior tibial artery, anterior tibial artery, dorsalis pedis artery, plasma biochemical factor levels, and SF-36 were measured before and after 7 weeks of treatment. Additionally, the SF-36 score was analyzed before and after 3 months of follow-up for EECP/ISRT. The parameter measurements at each time point are shown in Fig. 2. The selection of time points was based mainly on the results of our preliminary experiments and the effectiveness of these time points in previous studies12,13,31.
Fig. 2.
A flow diagram of outcomes at each testing time point.
We also described the detailed measurement times for each parameter. Patients’ fasting blood was collected and processed before and after treatment in the Second Ward of the Cardiology Department from 7:30 am to 8:00 am. The brachial-FMD and 6-min walk test were performed in an exercise-based cardiac rehabilitation center from 10 am to 11 am. Ultrasonic hemodynamic detection was conducted at the ultrasound examination center from 5:00 pm to 6:00 pm. The SF-36 score was recorded in the exercise-based cardiac rehabilitation center before and after 7 weeks of treatment and via telephone for the follow-up after 3 months.
Statistical analysis
All statistical analyses were conducted using SPSS software (version 22.0; SPSS, Chicago, Ill, USA). Considering the small sample size, all the outcomes were tested for normal distribution with the Kruskal-Wallis test. Categorical variables were analyzed using the chi-square test. An α level of P < 0.05 was considered statistically significant.
Indicators that did not satisfy the normal distribution were conducted by Kruskal-Wallis test for comparing multiple independent samples. Conversely, repeated-measures analysis of variance (ANOVA) was used to evaluate continuous dependent variables with normal distribution. When the main effect of significant difference among the EECP, ISRT, and sham-control groups was determined, within-group repeated-measures ANOVAs were performed for each variable to analyze the time point mean differences from baseline for each group and to determine within-group time point significance. Furthermore, Tukey’s post hoc analysis was performed for intergroup comparisons using ANOVA.
Results
In this study, 12, 13, and 10 patients in the EECP, ISRT, and control groups completed the entire treatment regimen, and no adverse events occurred. Overall, 19 and 16 patients were classified as stage IIa and IIb on Fontaine classification, respectively). Table 1 shows the baseline characteristics of the participants. There were no significant differences in basic characteristics or cardiovascular risk factors among the EECP, ISRT, and sham-control groups. Table 2 lists the drug regimens used in the three groups. There were significant differences in the use of β-blocker (P = 0.048) and biguanide (P = 0.043) among patients in the EECP, ISRT, and sham control groups.
Table 1.
Baseline characteristics and cardiovascular risk factors of participants among EECP、ISRT and Control groups.
| EECP (n = 12) | ISRT (n = 13) | Control (n = 10) | P-value | |
|---|---|---|---|---|
| Male (percentage/n) | 11 (91.67%) | 8 (61.54%) | 6 (60.00%) | 0.396 |
| Age (year) | 63.67 ± 7.19 | 67.00 ± 6.01 | 69.90 ± 6.74 | 0.105 |
| Height (cm) | 169.25 ± 7.31 | 165.46 ± 6.04 | 163.20 ± 6.76 | 0.114 |
| Weight (kg) | 72.96 ± 7.66 | 66.77 ± 10.04 | 63.85 ± 10.78 | 0.084 |
| BMI (kg/m2) | 25.47 ± 2.23 | 24.27 ± 2.42 | 23.93 ± 3.51 | 0.372 |
| Waistline (cm) | 94.17 ± 8.82 | 89.92 ± 7.65 | 93.30 ± 6.07 | 0.358 |
| Heart rate (bpm) | 78.69 ± 23.85 | 76.54 ± 13.72 | 76.80 ± 9.07 | 0.943 |
| SBP (mmHg) | 123.00 ± 23.72 | 133.92 ± 18.28 | 137.90 ± 15.80 | 0.181 |
| DBP (mmHg) | 74.46 ± 6.01 | 79.15 ± 7.50 | 77.60 ± 6.43 | 0.209 |
| Smoking(percentage/n) | 3 (25.00%) | 3 (23.08%) | 2 (20.00%) | 0.926 |
| Drinking (percentage/n) | 1 (8.33%) | 3 (23.08%) | 0 (0.00%) | 0.207 |
| Family history(percentage/n) | 5 (41.67%) | 3 (23.08%) | 2 (20.00%) | 0.458 |
| Exercise habits(percentage/n) | 9 (75.00%) | 7 (53.85%) | 3 (30.00%) | 0.329 |
| Sleep disorders(percentage/n) | 0 (0.00%) | 1 (7.69%) | 3 (30.00%) | 0.067 |
| Coronary artery disease (percentage/n) | 6 | 10 | 5 | 0.721 |
| Claudication | 5 | 7 | 6 | 0.557 |
| Hypertension(percentage/n) | 11 (84.62%) | 11 (84.62%) | 8 (80.00%) | 0.946 |
| II diabetes (percentage/n) | 10 (76.92%) | 13 (100.00%) | 6 (60.00%) | 0.051 |
| Hyperlipidemia(percentage/n) | 3 (7.69%) | 4 (23.08%) | 6 (60.00%) | 0.309 |
| hs-CRP | 3.03 ± 5.88 | 0.48 ± 0.46 | 1.05 ± 1.26 | 0.549 |
| Glycosylated hemoglobin | 7.81 ± 2.02 | 6.87 ± 1.86 | 7.48 ± 2.07 | 0.672 |
| Glucose | 6.86 ± 2.36 | 6.23 ± 1.61 | 7.92 ± 4.02 | 0.543 |
| Total cholesterol | 4.16 ± 0.61 | 3.78 ± 1.79 | 4.86 ± 1.11 | 0.291 |
| Low density lipoprotein | 2.41 ± 0.50 | 2.13 ± 1.13 | 3.19 ± 0.88 | 0.087 |
| Uric acid | 370.49 ± 95.57 | 412.39 ± 99.94 | 442.30 ± 65.92 | 0.338 |
Table 2.
Medication of participants among three groups.
| EECP (n = 12) |
ISRT (n = 13) |
Control (n = 10) |
P-value | |
|---|---|---|---|---|
| Diuretic | 1 (8.33%) | 2 (15.38%) | 1 (10.00%) | 0.816 |
| Antiplatelet aggregation | 7(58.33%) | 10 (76.92%) | 7 (70.00%) | 0.695 |
| β-blocker | 4 (33.33%) | 10 (76.92%) | 4 (40.00%) | 0.048 |
| Statins | 11 (91.67%) | 12 (92.31%) | 7 (70.00%) | 0.223 |
| Ezetimibe | 4 (33.33%) | 5 (38.46%) | 6 (60.00%) | 0.355 |
| ACEI/ARB | 9 (75.00%) | 10 (76.92%) | 5 (50.00%) | 0.386 |
| Calcium channel blocker | 4 (33.33%) | 3 (23.08%) | 3 (30.00%) | 0.893 |
| GLP-1 receptor agonist | 0 (0.00%) | 1 (7.69%) | 0 (0.00%) | 0.403 |
| Protamine zinc insulin | 5 (41.67%) | 4 (30.77%) | 4 (40.00%) | 0.879 |
| Regular insulin | 1 (8.33%) | 4 (30.77%) | 4 (40.00%) | 0.173 |
| Sulfonylureas | 1 (8.33%) | 0 (0.00%) | 0 (0.00%) | 0.403 |
| Biguanides | 5 (41.67%) | 6 (46.15%) | 0 (0.00%) | 0.043 |
| Glinides | 0 (0.00%) | 0 (0.00%) | 1 (10.00%) | 0.263 |
| Glycosidase inhibitor | 2 (16.67%) | 1 (7.69%) | 1 (10.00%) | 0.686 |
| Dipeptide kininase-4 | 0 (0.00%) | 1 (7.69%) | 1 (10.00%) | 0.534 |
| Sodium glucose cotransporter 2 | 6 (50.00%) | 4 (30.77%) | 1 (11.11%) | 0.175 |
EECP and ISRT improved brachial artery FMD and 6-min walk distance
Compared with baseline values, EECP and ISRT treatment significantly improved brachial-FMD (3.43 ± 0.64% vs. 7.44 ± 1.44% and 3.33 ± 1.02% vs. 6.20 ± 1.46%, respectively; both P < 0.001, Fig. 3a) and increased 6-min walk distance (376.67 ± 130.52 m vs. 436.17 ± 138.81 m and 362.67 ± 81.38 m vs. 418.67 ± 81.24 m, respectively; both P < 0.001, Fig. 3b).
Fig. 3.
Comparison of brachial-FMD and 6-min walk test before and after intervention in. EECP, ISRT and control group (“*” indicates the comparison within each group, p < 0.01, “+” indicates the difference between groups, p < 0.01).
At baseline, resting brachial-FMD showed no significant difference among the groups, whereas after EECP and ISRT treatment, the brachial-FMD was significantly higher than that in the sham-control group (P = 0.001 and P = 0.015 in EECP and ISRT vs. sham, respectively; Fig. 3a).
At baseline, the left and right ba-PWVs and ABI demonstrated no significant difference between the groups. After ISRT and EECP, there were still no significant differences in these parameters (all P > 0.05, Table 3). There were no significant differences in brachial-FMD, 6-min walk distance, right and left ba-PWVs, or right and left ABI between the EECP and ISRT groups (all P > 0.05).
Table 3.
Significant analysis of arterial stiffness pre and post 7 weeks treatment among EECP、ISRT、Control group.
| EECP (n = 12) | ISRT (n = 13) | Control (n = 10) | P-value | |
|---|---|---|---|---|
| Pre | ||||
| RbaPWV | 1783.25 ± 471.60 | 1878.00 ± 461.12 | 2238.00 ± 595.13 | 0.109 |
| LbaPWV | 1876.33 ± 544.71 | 2113.25 ± 479.15 | 2292.30 ± 506.41 | 0.182 |
| RABI | 1.14 ± 0.17 | 1.03 ± 0.23 | 1.12 ± 0.12 | 0.326 |
| LABI | 1.09 ± 0.18 | 1.03 ± 0.17 | 0.98 ± 0.19 | 0.365 |
| Post 7 weeks | ||||
| RbaPWV | 1650.83 ± 393.51 | 1931.75 ± 589.73 | 1961.30 ± 323.78 | 0.214 |
| LbaPWV | 1706.92 ± 424.45 | 2194.58 ± 597.11 | 2178.00 ± 587.02 | 0.054 |
| RABI | 1.16 ± 0.11 | 1.04 ± 0.13 | 1.12 ± 0.12 | 0.060 |
| LABI | 1.13 ± 0.14 | 1.03 ± 0.11 | 1.08 ± 0.15 | 0.236 |
RbaPWV right ankle brachial pulse wave velocity, LbaPWV left ankle brachial pulse wave velocity, RABI right ankle brachial index, LABI left ankle brachial index.
EECP increased blood flow of the popliteal artery and posterior tibial artery
At baselined, the FR of the popliteal and posterior tibial arteries showed no significant differences among the three groups. EECP therapy significantly increased FR in the popliteal artery (171.33 ± 84.69 mL/min to 233.33 ± 141.21 mL/min and 165.70 ± 89.49 mL/min to 123.10 ± 45.51 mL/min in the EECP and sham-control groups, respectively; P < 0.01; Fig. 4(a)). Meanwhile, EECP treatment also elevated FR in the posterior tibial artery (67.42 ± 42.98 mL/min to 117.17 ± 90.78 mL/min and 75.56 ± 49.06 mL/min to 52.22 ± 21.90 mL/min in the EECP and sham-control groups, respectively; P < 0.01; Fig. 4(b)).
Fig. 4.
Flow rate changes of popliteal artery (a), posterior tibial artery (b), anterior. tibial artery (c) and dorsalis pedis artery (d) before and after intervention in EECP, ISRT and control groups (“*” indicates the significant comparison within each group. “*” and “**” denote p < 0.5 and < 0.01 respectively. “+” and “++” denote p-values of the difference between groups < 0.5 and < 0.01 respectively).
ISRT increased blood flow and diameter of the anterior tibial artery
At baseline, the FR and diameter of the anterior tibial artery were not significantly different among the three groups. ISRT therapy significantly increased FR (46.54 ± 34.45 mL/min to 67.34 ± 39.66 mL/min and 52.75 ± 49.11 mL/min to 24.38 ± 10.99 mL/min in the ISRT and sham-control groups, respectively; P < 0.05; Fig. 4c.
EECP and ISRT increased blood flow and diameter of the dorsalis pedis artery
At baseline, the FR and diameter of the dorsalis pedis artery were not significantly different between the EECP, ISRT, and sham-control groups. EECP and ISRT treatment significantly increased FR and diameter of the dorsalis pedis artery compared with the sham control (both P < 0.05; Fig. 4d). However, there was no significant difference in FR in the popliteal, posterior tibial, anterior tibial, and dorsalis pedis arteries between the EECP and ISRT groups (all P > 0.05).
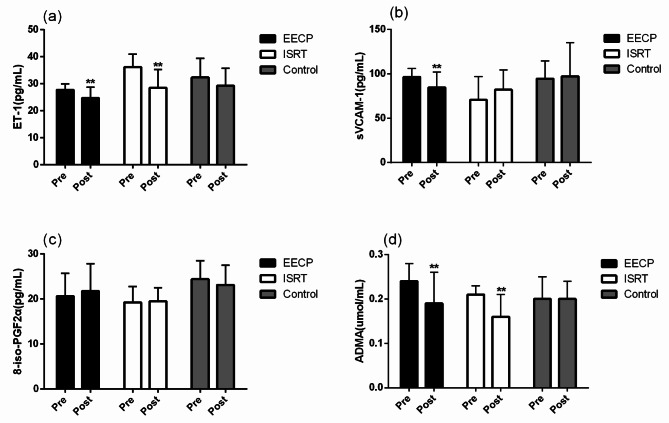
EECP and ISRT decreased ET-1, sVCAM-1, and ADMA
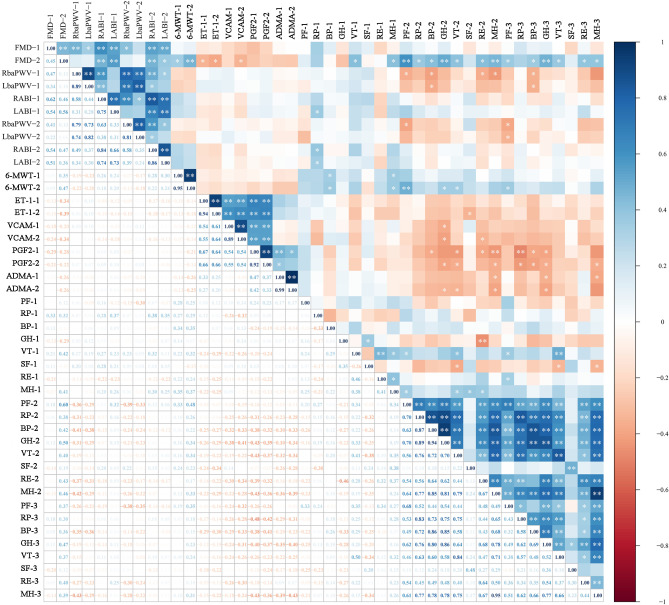
At baseline, there were no significant differences in ET-1, sVCAM-1, 8-iso-PGF2α, and ADMA among the three groups, and there were still no significant differences after EECP and ISRT intervention (all P > 0.05, Fig. 5a-d). Compared with baseline values, only EECP significantly decreased sVCAM-1 (84.66 ± 17.42 vs. 96.54 ± 9.41 pg/mL, P = 0.004, Fig. 5b). EECP and ISRT treatment decreased ET-1 levels (27.79 ± 2.19 pg/mL vs. 24.69 ± 3.99 pg/mL and 36.16 ± 4.73pg/mL vs. 28.51 ± 6.76 pg/mL in the EECP and ISRT groups, respectively; both P < 0.01, Fig. 5a). ADMA levels were concurrently decreased (0.19 ± 0.07 vs. 0.24 ± 0.04 µmol/L, P = 0.001; 0.16 ± 0.05 vs. 0.21 ± 0.02 µmol/L, P = 0.001 in the EECP and ISRT groups, respectively; Fig. 5d). However, there was no significant difference in 8-iso-PGF2α levels after EECP and ISRT treatment (21.76 ± 6.11 vs. 20.64 ± 5.10pg/mL and 19.51 ± 3.01 pg/mL vs. 19.25 ± 3.54 pg/mL in the EECP and ISRT groups, respectively; both P > 0.05, Fig. 5c). There were also no significant differences in ET-1, sVCAM-1, 8-iso-PGF2α, and ADMA between the EECP and ISRT groups (all P > 0.05). Additionally, brachial-FMD after 7 weeks of treatment was significantly associated with ET-1 before and after 7 weeks of treatment, as well as sVCAM-1 after 7 weeks of treatment (Fig. 6).
Fig. 5.
Comparison of biochemical assays before and after intervention in EECP, ISRT and control group (“*” indicates the comparison within each group, p < 0.01, “+” indicates the difference between groups, p < 0.01).
Fig. 6.
Associations between clinical indices. Heat map of the Spearma’s rank correlation coefficient between 44 clinical indices. n = 35; *P < 0.05; **P < 0.01; Spearman’s rank correlation.
EECP improved PF
PF were significantly improved after 7 weeks and 3-month follow-up EECP or ISRT treatment compared with baseline (P < 0.05). Additionally, PF was not significantly different between the EECP, ISRT, and sham-control groups before the intervention. Compared with that in the ISRT group, PF significantly improved after the EECP intervention (P < 0.01; Table 4). However, the significant difference in PF between EECP and ISRT intervention did not last for 3-month.
Table 4.
Comparison of SF-36 life scale pre and post 7 weeks treatment, 3 months follow-up in three groups.
| EECP | ISRT | Control | P-value | |
|---|---|---|---|---|
| Pre | ||||
| Physical Function (PF) | 72.5(70.0,75.0) | 65.0(57.5,75.0) | 72.5(70.0,75.0) | 0.070 |
| Role-Physical (RP) | 50.0(50.0,50.0) | 50.0(50.0,50.0) | 50.0(50.0,50.0) | 1.000 |
| Bodily Pain (BP) | 62.0(51.0,62.0) | 62.0(56.5,62.0) | 51.0(51.0,62.0) | 0.137 |
| General Health (GH) | 40.0(37.5,47.5) | 40.0(40.0,43.7) | 45.0(45.0,45.0) | 0.049 |
| Vitality (VT) | 65.0(56.3,65.0) | 55.0(50.0,65.0) | 55.0(48.7,60.0) | 0.044 |
| Social Function (SF) | 87.5(78.1,87.5) | 87.5(75.0,87.5) | 87.5(87.5,90.6) | 0.297 |
| Role Emotional (RE) | 66.6(41.6,66.6) | 66.6(50.0,66.6) | 66.6(58.3,66.6) | 0.881 |
| Mental Health (MH) | 64.0(64.0,74.0) | 60.0(56.6,70.0) | 60.0(59.0,65.0) | 0.122 |
| Post-7 W | ||||
| Physical Function (PF) | 92.5(85.0,95.0)#* | 80.0(75.0,85.0)&* | 70.0(70.0,75.0) | < 0.001 |
| Role-Physical (RP) | 100.0(75.0,100.0)#* | 100.0(75.0,100.0)##* | 50.0(43.7,50.0) | < 0.001 |
| Bodily Pain (BP) | 90.0(82.5,90.0)#* | 90.0(90.0,90.0)##* | 51.0(48.5,51.0) | < 0.001 |
| General Health (GH) | 69.5(67.0,72.0)#* | 67.0(66.0,72.0)##* | 45.0(44.2,45.0) | < 0.001 |
| Vitality (VT) | 80.0(76.25,80.0)#* | 70.0(70.0,77.5)##* | 57.5(55.0,65.0) | < 0.001 |
| Social Function (SF) | 100.0(100.0,100.0)* | 100.0(87.5,100.0)* | 100.0(87.5,100.0) | 0.218 |
| Role Emotional (RE) | 100.0(100.0,100.0)#* | 100.0(100.0,100.0)##* | 66.6(33.3,66.6) | < 0.001 |
| Mental Health (MH) | 80.0(77.0,84.0)#* | 80.0(74.0,82.0)##* | 58.0(56.0,69.0) | < 0.001 |
| Post-3 M | ||||
| Physical Function (PF) | 87.5(80.0,90.0)#* | 80.0(77.5,87.5)* | 75.0(70.0,80.0) | 0.002 |
| Role-Physical (RP) | 87.5(75.0,100.0)#* | 75.0(75.0,100.0)##* | 50.0(43.7,75.0)*+ | 0.001 |
| Bodily Pain (BP) | 80.0(72.0,87.5)#* | 80.0(72.0,90.0)##* | 56.5(51.0,62.0)+ | < 0.001 |
| General Health (GH) | 67.0(63.2,72.0)#* | 72.0(66.0,72.0)##* | 45.0(41.5,47.0)*+ | < 0.001 |
| Vitality (VT) | 80.0(70.0,83.7)#* | 75.0(70.0,80.0)##* | 62.5(53.7,66.2) | 0.001 |
| Social Function (SF) | 100.0(87.5,100.0)* | 100.0(87.5,100.0)* | 87.5(87.5,100.0) | 0.283 |
| Role Emotional (RE) | 100.0(100.0,100.0)#* | 100.0(66.6,100.0)* | 66.6(33.3,75.0) | 0.002 |
| Mental Health (MH) | 80.0(77.0,87.0)#* | 76.0(76.0,84.0)##* | 62.0(59.0,69.0) | < 0.001 |
Values are presented as mean ± sd. “*” indicates significant difference between intervention in each group and baseline (P < 0.05); “+” indicates significant difference between post-3 m and post-7 w (P < 0.05). “&” indicates significant difference between EECP and ISRT (P < 0.05). “#” indicates significant difference between EECP and control (P < 0.05). “##” indicates significant difference between ISRT and control (P < 0.05).
Discussion
In this study, we investigated the effects of EECP and ISRT on peripheral blood flow indicators in patients with LEAD. We found that among patients with LEAD, EECP treatment significantly improved brachial-FMD and QoL and markedly increased walking distance. EECP also increased the blood flow and diameter of the popliteal and posterior tibial arteries. Compared with the other groups, the ISRT group showed markedly increased blood flow to the anterior tibial artery. Furthermore, EECP significantly decreased sVCAM-1 levels, while EECP and ISRT therapy decreased ET-1 and ADMA levels in patients with LEAD.
In this study, 7 weeks of EECP and ISRT significantly improved the brachial-FMD (Fig. 3). Braith et al. reported that 35 sessions of EECP increased FMD in the brachial and femoral arteries by 51% and 30%, respectively12. These arterial adaptations are attributed to decreased vasoconstrictor ET-1 levels and dramatic increases in nitric oxide (NO)x and Prostaglandin F1 levels12. The improved brachial-FMD in our study also aligns with the collective result of our observed significant decreases in ET-1 and sVCAM-1 levels. Moreover, we observed that brachial-FMD after 7 weeks treatment was significantly associated with ET-1 before and after the 7 weeks treatment, as well as sVCAM-1 level after the 7 weeks treatment.
The brachial FMD has emerged as an attractive non-invasive technique for the study of endothelial function as a marker of subclinical atherosclerosis32. There are three potential explanations for the changes in these indices after EECP and ISRT. First, the extrusion of inflation and deflation of the outer cuffs lead to elevated shear stress, promoting the release of NO31,33, which serves an anti-inflammatory role by reducing VCAM-1 expression34. Zhang et al. demonstrated that the peak diastolic arterial wall shear stress increased by more than 2-fold during EECP31. Second, inflation of EECP cuffs can create high-pressure retrograde blood flow in the lower extremity artery and simultaneous moderate-pressure antegrade flow in the upper limb arteries15,31,35. Pulsatile and oscillatory flow after EECP intervention may provide a form of “message” on the endothelium and then improve their functions12,36,37. Third, systolic unloading and diastolic augmentation induced by the systolic deflation and diastolic inflation sequence of EECP or ISRT increase blood flow in the lower extremity arteries in a pulsatile manner31.
PAD-related arterial blockages limit blood flow to the lower extremity potentially leading to devastating lower-extremity amputation and acute limb ischemia. This underscores the importance of improving lower limb blood flow38,39. We observed that EECP or ISRT treatment significantly increased blood flow in the infra-popliteal arteries. Blood flow in the popliteal and posterior tibial arteries and in the anterior tibial artery were significantly increased after EECP and ISRT, respectively. Few studies have reported the effects of EECP or ISRT therapy on the popliteal, posterior tibial, anterior tibial, and dorsalis pedis arteries. Our previous study indicated that EECP creates an acute increase in blood flow and PSV of the anterior tibial artery, whereas ISRT produces an acute decrease in blood flow in the posterior tibial artery40. Werner et al. reported that acute EECP significantly reduced flow volume of the posterior tibial artery19. Martin et al. reported that 35 1-h EECP sessions did not significantly improve resistance arterial function in the calf21. However, Avery et al. showed that 35 1-h EECP sessions markedly improved endothelium-dependent vasodilation in calf resistance arteries36. Many studies have focused on the effects of EECP or ISRT on the entire lower limb or peripheral blood vessels. Braith et al. found that EECP treatment has a beneficial effect on femoral artery FMD12. Nichols et al. reported that EECP treatment reduced peripheral arterial stiffness13, whereas Dockery et al. demonstrated that EECP treatment did not reduce peripheral arterial stiffness20. Hashemi et al. reported that EECP did not improve endothelial function18.
In view of these differing therapeutic effects, Buschmann et al. proposed an improved method, ISRT, in which two cuffs are wrapped around the hips and thighs to ensure adequate calf perfusion. Buschmann et al. reported that 35-h ISRT increases lower limb walking distance10, and Picard et al. demonstrated that 35-h ISRT treatment can improve the degree of peripheral arteriosclerosis and exercise capacity and reduce BP23,24. However, previous studies have reported that ISRT cannot markedly reduce the ABI or PWV10,23. To compare the effectiveness of EECP and ISRT, Soubh et al. conducted a cross-over design of EECP versus ISRT, and investigated the blood flow via micro-lightguide spectrophotometer (O2C) of EECP versus ISRT9. They found that blood flow and rHb increased significantly during ISRT (p-values: 0.001, and 0.048, respectively), while there were no statistical changes in blood flow and rHb during EECP when compared to baseline values. However, the O2C index mainly showed the local capillary-venous-system (microcirculation) of the skin during EECP and ISRT in that study9.
To further clarify and compare the blood flow of EECP and ISRT in patients with LEAD, the present study, for the first time, demonstrated the long-term clinical benefits of EECP and ISRT on infra-popliteal arterial blood flow. We tested the changes in blood flow in multiple parts such as the popliteal artery, anterior tibial artery, posterior tibial artery, and dorsalis pedis artery. More importantly, we also investigated related plasma levels. A dramatic increase in shear stress after EECP or ISRT intervention can lead to robust NO production, resulting in vessel relaxation and a significant increase in the blood flow of the infra-popliteal arteries26,41. We observed that ADMA levels significantly decreased after EECP and ISRT. The levels of ADMA, a key modulator of oxidative stress that inhibits NO formation and decreases NO bioavailability, are reportedly reduced after EECP or exercise training in patients with CAD12,42,43, similar to our findings.
In the present study, the EECP and ISRT treatments also produced different changes in the arteries regarding blood flow. There are at least four possible explanations for this discrepancy. First, the ISRT comprises two cuffs wrapped around the hips and thighs for adequate calf perfusion. Low treatment pressure reduces blood flow returning to the heart and concurrently increases blood flow in the lower extremities. Conversely, EECP involves a traditional three-level sequential mode (hip, thigh, and lower limb) and applies high treatment pressure. Upon deflation, the lower limb produces a dramatic “suction force” generated by the final inflation in the diastolic stage. Second, ISRT can increase fluid shear stress, resulting in the activation of endothelial function and vascular remodeling44,45. In comparison, EECP significantly decreases tumor necrosis factor alpha levels, which participates in the atherogenic process by a decrease in NO synthase production and an increase in endothelial cell activation12,46. The most significant benefit important contribution of EECP for arterial function in the lower extremities is the expansion of blood vessels due to the shear rate under a large treatment pressure. Third, EECP contributes to collateral artery growth around occluded arteries in patients with PAD47. Fourth, the EECP also improves the the score of SF-36 and increases walking distance. In the present study, both the EECP and ISRT groups had significantly increased walking distances, and the EECP improved the PF of SF-36. SF-36 is considered a crucial criterion for health outcomes in CAD or PAD48. Although the survival rates for CAD and PAD have declined globally, PAD still poses a high individual burden regarding QoL and related disabilities49. Manchanda et al. showed that patients with angina experienced the beneficial effects of EECP on their QoL for long periods after treatment50. Abbottsmith et al. reported that EECP increased exercise duration and improved patient functioning and QoL without affecting peak oxygen consumption51. Generally, both EECP and ISRT provide potential options for patients with PAD to improve local blood perfusion to a certain extent, although the mechanism requires further elucidation.
Study limitation
This study had some limitations. First, although our study demonstrated important findings from different perspectives, the overall sample size was relatively small. Second, conducting a subgroup analysis of the specific location of stenosis in patients was challenging owing to the limited sample size. Increasing the sample size in future studies could provide a stronger experimental basis for clinical randomized controlled trials. Third, the inclusion criteria lacked specificity, as Rutherford’s grading was not included in this study. We anticipate that further research will provide more comprehensive analyses. Fourth, this study did not investigate the molecular mechanisms underlying the clinical benefits; further experiments are needed in the future.
Conclusion
This study demonstrates that 35 sessions of 45 min of EECP and ISRT improved brachial artery FMD and increased walking distance in patients with LEAD. EECP and ISRT also increased blood flow and diameter in the dorsalis pedis artery. Meanwhile, EECP and ISRT reduced ET-1 and ADMA levels in patients with LEAD. EECP also improves the PF of patients with PAD and decreases sVCAM-1 levels.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was, in part, supported by the National Natural Science Foundation of China [Grant No. 82202292, C0054822], and the National Key Research and Development Program of China (No.2020YFC2004400). Part of this research was supported by Shenzhen Key Clinical Discipline Funds (ZDXKJF-01002), Guangdong Medical Science and Technology Research Foundation (No. A2022383) and Guangdong Basic and Applied Basic Research Foundation (No.2021A1515110738).
Author contributions
YZ and GW proposed the scientific problems. YZ and CZ designed and collected the experimental data. YZ processed and calculated the data. YZ wrote the draft manuscript. GW, YW and WW contributed to the revision and final version of the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yaqin Wang, Email: wangyaqin@uor.edu.cn.
Wenbin Wei, Email: 13688808973@139.com.
Guifu Wu, Email: wuguifu@mail.sysu.edu.cn.
References
- 1.Fihn, S. D. et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of cardiology foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and interventions, and Society of thoracic surgeons. J. Am. Coll. Cardiol.60 (24), e44–e164 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Montalescot, G. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J.34 (38), 2949–3003 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Cardiovascular disease prevention and control research center. Ministry of Health. China Cardiovascular Disease Report: China Cardiovascular Disease Report ([M], 2014).
- 4.Kim, M. C., Kini, A. & Sharma, S. K. Refractory angina pectoris: mechanism and therapeutic options. J. Am. Coll. Cardiol.39 (6), 923–934 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Lawson, W. E. et al. Efficacy of enhanced external counterpulsation in the treatment of angina pectoris. Am. J. Cardiol.70 (9), 859–862 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Masuda, D. et al. Enhanced external counterpulsation improved myocardial perfusion and coronary flow reserve in patients with chronic stable angina. Evaluation by13N-ammonia positron emission tomography. Eur. Heart J.22 (16), 1451–1458 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Gloekler, S. et al. Coronary collateral growth by external counterpulsation: a randomised controlled trial. Heart96 (3), 202–207 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Hirai, T., Sasayama, S., Kawasaki, T. & Yagi, S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation80 (1), 78–86 (1989). [DOI] [PubMed] [Google Scholar]
- 9.Soubh, N., Hillmeister, P., Buschmann, E., Klaproth, C. & Buschmann, I. Tolerability safety and effectiveness of enhanced external counterpulsation versus individual shear rate therapy in patients with lower extremity atherosclerotic disease: a prospective pilot clinical trial. Acta Physiol. (Oxford, England). 237 (3), e13913 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Buschmann, E. E. et al. Adaptation of external counterpulsation based on individual shear rate therapy improves endothelial function and claudication distance in peripheral artery disease. Vasa45, 317–324 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Thakkar, B. V. et al. The efficacy and safety of enhanced external counterpulsation in patients with peripheral arterial disease. Vasc Med.15 (1), 15–20 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Braith, R. W. et al. Enhanced external counterpulsation improves peripheral Artery Flow-mediated dilation in patients with chronic angina. Circulation122, 1612–1620 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols, W. W., Estrada, J. C., Braith, R. W., Owens, K. & Conti, C. R. Enhanced external counterpulsation treatment improves arterial wall properties and wave reflection characteristics in patients with refractory angina. J. Am. Coll. Cardiol.48, 1208–1214 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Bonetti, P. O., Holmes, D. R., Lerman, A. & Barsness, G. W. Enhanced external counterpulsation for ischemic heart disease. J. Am. Coll. Cardiol.41 (11), 1918–1925 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Gurovich, A. N. & Braith, R. W. Enhanced external counterpulsation creates acute blood flow patterns responsible for improved flow-mediated dilation in humans. Hypertens. Res.36 (4), 297–305 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Cheng, C. et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation113 (23), 2744–2753 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Shechter, M. et al. External counterpulsation therapy improves endothelial function in patients with refractory angina pectoris. J. Am. Coll. Cardiol.42 (12), 2090–2095 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Hashemi, M., Hoseinbalam, M. & Khazaei, M. Long-term effect of enhanced external counterpulsation on endothelial function in the patients with intractable angina. Heart Lung Circ.17 (5), 383–387 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Werner, D. et al. Impact of enhanced external counterpulsation on peripheral circulation. Angiology58 (2), 185–190 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Dockery, F., Rajkumar, C., Bulpitt, C. J., Hall, R. J. & Bagger, J. P. Enhanced external counterpulsation does not alter arterial stiffness in patients with angina. Clin. Cardiol.27 (12), 689–692 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin, J. S., Beck, D. T. & Braith, R. W. Peripheral resistance artery blood flow in subjects with abnormal glucose tolerance is improved following enhanced external counterpulsation therapy. Appl. Physiol. Nutr. Me. 39 (5), 596–599 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Buschmann, I. et al. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Dev. (Cambridge England). 137, 2187–2196 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Picard, F. et al. Usefulness of individual shear rate therapy, New treatment option for patients with symptomatic coronary artery disease. Am. J. Cardiol.121 (4), 416–422 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Picard, F. et al. Individual shear rate therapy (ISRT)-further development of external counterpulsation for decreasing blood pressure in patients with symptomatic coronary artery disease (CAD). Hypertens. Res.43 (3), 186–196 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Cai, D., Wu, R. & Shao, Y. Experimental study of the effect of external counterpulsation on blood circulation in the lower extremities. Clin. Invest. Med.23 (4), 239–247 (2000). [PubMed] [Google Scholar]
- 26.Michaels, A. D., Accad, M., Ports, T. A. & Grossman, W. Left ventricular systolic unloading and augmentation of intracoronary pressure and doppler flow during enhanced external counterpulsation. Circulation106 (10), 1237–1242 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Arora, R. R. et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J. Am. Coll. Cardiol.33, 1833–1840 (1999). [DOI] [PubMed] [Google Scholar]
- 28.McDermott, M. M. et al. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J. Am. Coll. Cardiol.57 (8), 962–970 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parmenter, B. J., Dieberg, G., Phipps, G. & Smart, N. A. Exercise training for health-related quality of life in peripheral artery disease: a systematic review and meta-analysis. Vasc Med. (London England). 20 (1), 30–40 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Jain, A. et al. The walking impairment questionnaire stair-climbing score predicts mortality in men and women with peripheral arterial disease. J. Vasc Surg.55 (6), 1662–1673e1662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, Y. et al. Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation116, 526–534 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Weiner, S. D. et al. Systemic inflammation and brachial artery endothelial function in the multi-ethnic study of atherosclerosis (MESA). Heart (British Cardiac Society). 100 (11), 862–866 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Rudic, R. D. et al. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Invest.101, 731–736 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison, D. G. et al. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J. Intern. Med.259 (4), 351–363 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Ozawa, E. T., Bottom, K. E., Xiao, X. & Kamm, R. D. Numerical simulation of enhanced external counterpulsation. Ann. Biomed. Eng.29, 284–297 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Avery, J. C., Beck, D. T., Casey, D. P., Sardina, P. D. & Braith, R. W. Enhanced external counterpulsation improves peripheral resistance artery blood flow in patients with coronary artery disease. Appl. Physiol. Nutr. Me.39, 405–408 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Klein-Nulend, J. et al. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem. Bioph Res. Co.250, 108–114 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Criqui, M. H. et al. Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: A scientific statement from the American heart association. Circulation144 (9), e171–e191 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gornik, H. L. & Beckman, J. A. Cardiology patient page. Peripheral arterial disease. Circulation111 (13), e169–172 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Zhang, Y. et al. Effects of enhanced external counterpulsation with different sequential levels on lower extremity hemodynamics. Front. Cardiovasc. Med.8, 795697 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niebauer, J. & Cooke, J. P. Cardiovascular effects of exercise: role of endothelial shear stress. J. Am. Coll. Cardiol.28, 1652–1660 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Richter, B. et al. Endurance training reduces circulating asymmetric dimethylarginine and myeloperoxidase levels in persons at risk of coronary events. Thromb. Haemost.94 (6), 1306–1311 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Sydow, K. & Münzel, T. ADMA and oxidative stress. Atherosclerosis Supplements. 4 (4), 41–51 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Chatzizisis, Y. S. et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol.49, 2379–2393 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Davies, P. F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Card. 6, 16–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker, P. M. et al. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation101, 2149–2153 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Schirmer, S. H. et al. Exercise promotes collateral artery growth mediated by monocytic nitric oxide. Arterioscl Throm Vas. 35 (8), 1862–1871 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Tomás, C. C. et al. May. Proceedings of the 3rd IPLeiria’s international health congress: Leiria, Portugal. 6–7 BMC Health Serv Res. 2016;16 Suppl 3(Suppl 3):200. (2016). [DOI] [PMC free article] [PubMed]
- 49.Bonetti, P. O. et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J. Am. Coll. Cardiol.41 (10), 1761–1768 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Manchanda, A. & Soran, O. Enhanced external counterpulsation and future directions: step beyond medical management for patients with angina and heart failure. J. Am. Coll. Cardiol.50 (16), 1523–1531 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Abbottsmith, C. W. et al. Enhanced external counterpulsation improves exercise duration and peak oxygen consumption in older patients with heart failure: a subgroup analysis of the PEECH trial. Congest Heart Fail.12 (6), 307–311 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.