Abstract
This study aimed to discuss the effects of extreme temperatures and ozone on the incidence of varicella in Shijiazhuang City from 2014 to 2022, which provides new ideas for preventing public health events. METHODS We collected varicella cases in Shijiazhuang, China, from 2014 to 2022 and evaluated the relationship between temperature extremes and ozone on varicella incidence by building distributional lag nonlinear models. The analysis was stratified by age and sex, with 19,188 varicella cases reported. A nonlinear “J”-shaped relationship emerged between mean daily temperature and varicella incidence, where colder temperatures heightened risk, while hotter ones reduced it, particularly affecting females and adolescents. Additionally, ozone concentration displayed an “S”-shaped correlation, with low levels posing a risk and high levels protective against varicella, notably among females and adults. Our study results show a significant correlation between extreme temperatures and ozone concentrations on varicella incidence, and this study may help prevention and control authorities to create timely warnings of high-risk outbreaks, and assist the public in responding to varicella outbreaks.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82306-w.
Subject terms: Climate sciences, Environmental sciences, Environmental social sciences, Risk factors, Diseases, Infectious diseases, Viral infection
Introduction
Infectious diseases are diseases caused by pathogens that can be transmitted between humans, between animals, or between humans and animals. Pathogenic microorganisms that cause infectious diseases include viruses, rickettsiae, mycoplasmas, bacteria, fungi and parasites1. Since the 1970s, more than 40 new infectious diseases have been identified globally, and infectious diseases are characterized by widespread uncertainty and spread. Therefore, the impact of infectious diseases on the global health and economy must be taken seriously2. Varicella, one of the infectious diseases, poses a significant challenge for prevention and control. Varicella, caused by the varicella-zoster virus (VZV), is a highly contagious infectious disease3. The varicella virus can lurk in the ganglia, which can cause a range of complications such as cardiovascular disease and eye problems4–6. Many studies in Mexico, Colombia, Denmark, Finland, Australia, Wuhan and Hong Kong, China, have confirmed that varicella outbreaks are distinctly seasonal, reaching a peak in winter and spring7–11. The Varicella vaccine has been effective in reducing the incidence of varicella, but the protective effect of the vaccine decreases with the time of vaccination12. In China, the incidence of varicella has been showing an upward trend since 2014, peaking in 2018–2019, and with the outbreak of COVID-19, the incidence has gradually declined in the last three years, but the number of illnesses is still high13. Thus, varicella has remained a globally recognized and relatively prominent public health problem over the past decade or so14.
Under the conditions of global warming and economic development, the problem of environmental pollution is becoming increasingly severe, which posing a significant threat to human health15. The prevalence of varicella is directly associated with environmental factors. Research in Japan revealed a bimodal pattern in varicella incidence curves amid significant annual temperature fluctuations6; in Mexico, it has also been pointed out that the incidence of varicella is higher in temperate climates than in tropical climates7; and Hoseini SG concluded from his study that the regional temperature may be the only determinant of the outbreak of the VZV virus16.
In China, correlation analysis between environmental factors and varicella incidence has also been reported. Qingdao, Guangzhou, and Shanghai have reported that the risk of varicella incidence decreases with increasing temperature17–19; Guijie Luan reported that in Jinan, China, low temperature is a risk factor for varicella incidence, while high temperature is a protective factor20. Yan Wang concluded from her investigation that the increased risk of varicella in Lanzhou, China, was associated with both low and high temperatures, with the effect of low temperature is more substantial than that of high temperature21. A Huai’an, China report indicated that the overall effect of mean daily temperature on varicella showed a W-curve22. Zixuan Wang pointed out that high concentrations of NO2 showed a protective effect on varicella incidence, and PM2.5could lead to varicella23. Hongjie Yu showed in his study that high PM10concentration increases the risk of varicella transmission24. From the above reports, we can see that the effect of temperature on the incidence of varicella is controversial, and there are limited studies on the effect of air pollutants on the incidence of varicella.
The purpose of this study was to discuss the effects of temperature extremes and ozone on the incidence of varicella in the population of Shijiazhuang, China, and to assist the local health authorities in better preventing and controlling varicella outbreaks.
Methods and materials
Study site and climatic characteristics
Our study site is based in Shijiazhuang City, Hebei Province, China, which is located in North China, in the south-central part of Hebei Province. Specifically, Shijiazhuang City is located on the alluvial fan of the Taihang Mountains, with the topography that is slightly inclined to the southeast. As a result of this unique topography, Shijiazhuang has a flat surface, fertile land, and rich and high-quality underground water reserves. Shijiazhuang borders Hengshui City to the east, Xingtai City to the south, Shanxi Province to the west by the Taihang Mountains, and Baoding City to the north.
Shijiazhuang is strategically located in the center of the Beijing-Tianjin-Hebei economic circle, and is an important transportation hub connecting North China, East China, Northeast China, and Northwest China. As one of the main hubs of China’s railroad transportation, it has a perfect railway and highway transportation network, which is convenient and well-connected.
According to the Köppen-Geiger climate classification, Shijiazhuang belongs to the BSk subtype25, which has four distinct seasons, each with its own unique climatic characteristics. Temperatures gradually rise in spring, summer is hot and humid, fall is crisp and clear, and winter is cold and dry.
Shijiazhuang is located in the eastern foothills of the Taihang Mountains, and the terrain is high in the west and low in the east, which makes Shijiazhuang among the most severe cities in the country in terms of “incendiary wind effect”, resulting in Shijiazhuang temperatures being higher than those in neighboring cities. Shijiazhuang enjoys northwesterly winds in winter, but the barrier of the western mountains makes it difficult for cold air to penetrate deep into the city, causing pollutants to accumulate at the bottom of the atmosphere. At the same time, industrial emissions in the city can also cause temperatures in the upper atmosphere to be higher than those in the lower atmosphere, creating an inversion. The inversion causes suspended particles in the low air to be affected by the heat of the high air in the vertical direction, making it difficult for them to spread.
Study population and data source
In China, varicella is a non-statutory infectious disease. However, the General Office of the Ministry of Health of China has included the varicella as an infectious disease in the “National Public Health Emergency Information Reporting Management Standard (Trial)” and reported it to the higher-level health department within two hours26. Some provinces and cities directly include varicella into the local category C infectious disease, requiring local departments to report27. In this study, data on varicella cases in Shijiazhuang City, including personal sex, gender, age, and date of onset, were collected from Shijiazhuang Center for Disease Control and Prevention (CDC) from 2014 to 2022 (https://wsjk.sjz.gov.cn/). We obtained meteorological exposures, including average temperature (℃), average wind speed (m/s), average barometric pressure (hPa), and average relative humidity (%) from the China Meteorological Data Sharing Service System (http://data.cma.cn/). Based on the data provided by the China Air Quality Online Monitoring and Analysis Platform (CAQAMAP), we obtained the daily average concentrations of PM10, PM2.5, NO2, SO2, and the maximum 8-h moving average ozone concentration (O3-8 h) above the exposures from 2014 to 2022 (https://www.aqistudy.cn/).
Statistical analysis
Varicella incidence is generally viewed as a small probability event following a quasi-Poisson distribution, in addition to previous studies showing delayed associations between the health effects of meteorological factors and air pollutants, and the effects are usually nonlinear in outcome. Therefore, we combine the generalized linear model (GLM) and the distributed lag nonlinear model (DLNM) to explore the effects of air pollutants on varicella incidence. Natural spline functions (ns) allowed meteorological factors, seasonal and long-term trends, weekly effects, and other confounding factors to enter the model as covariates. The Akaike Informativeness Criterion (AIC) was used to select the maximum lag for entry into the model. However, given the incubation period of 10–21 days for varicella, we chose 21 days as the maximum lag for the study28. Spearman’s correlation coefficient was also used to assess the correlation between meteorological factors and air pollutants. To avoid multicollinearity, variables with correlation coefficients less than 0.7 and VIF coefficients less than 10 were included in the model. Single meteorological factor/pollutant modeled as:
 |
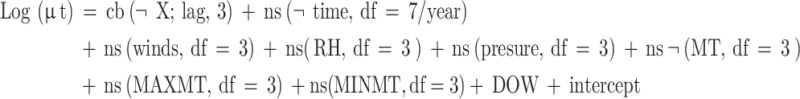 |
In Eq. µt denotes the expected number of varicella cases on day t, and cb denotes the established cross-basis function of meteorological factors or air pollutants. Exposure lags were fitted using a natural cubic spline function with logarithmic equally spaced node position settings. ns is the natural cubic spline function, df is the degrees of freedom, time is the time variable, and DOW is a dummy variable controlling for the days of the week effect.
The median mean temperature of 14 °C was set as the reference temperature during the study. In order to analyze the hot and cold effects of temperature, the 5th percentile of the average daily temperature (−3℃) was defined as extreme low temperature, and the 95th percentile of the average daily temperature (26℃) was defined as extreme high temperature. According to China’s current Ambient Air Quality Standards (GB 3095 − 2012), which set daily maximum 8-hour average concentration limits for ozone, the primary standard is 100 µg/m3, and the secondary standard is 160 µg/m329. When ozone concentrations exceed these limits, they may have adverse effects on human health and the environment. In this study, the median of the collected ozone data was used as the reference standard, with the fifth percentile of the data (15 µg/m3) as the very low concentration of ozone and the 95th percentile (204 µg/m3) as the very high concentration of ozone. We introduced a one-day lag (lag 0 to lag 21) and a moving average exposure lag (lag 0–1 to lag 0–21) into the model to explore the effects of meteorological factors and air pollutants on daily visits for varicella. The maximum number of lag days was set to 21.
R (version4.2.3, www.R-project.org/) software was used for statistical analysis, and the “splines (Gasparrini 2014)” and “dlnm (version 2.4.7)” toolkits were utilized to build the distribution lag nonlinear model, and “ggplot2 (version 2.0.0)” is used to plot 2D and 3D plots. P-value < 0.05, the difference is statistically significant.
Results
Generalization of varicella cases, meteorological factors, and air pollutants
From 2014 to 2022, the number of varicella visits for Shijiazhuang residents was 19,188 cases. Meanwhile, in this study, we stratified gender and age separately, in which 8792 cases of females accounted for 45.82% of the total number of cases, and 10,369 cases of males accounted for 54.18% of the total number of cases; varicella visits among children accounted for 30.58% of the total number of cases, and the number of varicella visits among adolescents was as high as 49.86%. In contrast, adults accounted for only 19.56% of all visits. Also, Table S11 summarizes the descriptive statistics of four meteorological factors (i.e., mean temperature, mean pressure, relative humidity, and wind speed) and three air pollutants (SO2, NO2, and O3) in Shijiazhuang City from 2014 to 2022.
Relationship between daily varicella outpatients volume and temperature
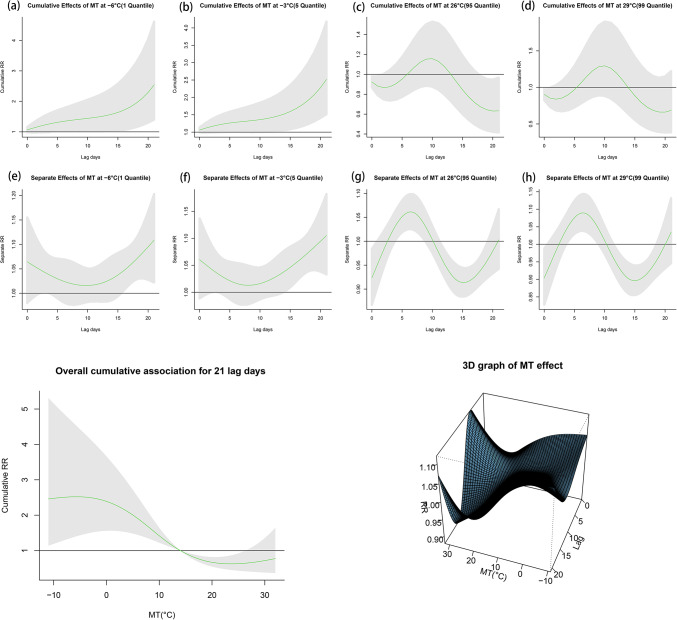
First, we analyzed the relationship between daily mean temperature, lag time, and varicella incidence. We used 14 °C as the reference temperature for the daily mean temperature. According to the time trend of varicella incidence risk and the exposure-response curve, we can find that the relationship between mean daily temperature and varicella incidence is nonlinear, with a general trend roughly in the shape of “J” (Fig. 1). The cumulative relative risk was 2.516 (1.514, 4.183) for low temperatures (−3 °C, quintile 5) and 0.636 (0.415, 0.974) for high temperatures (26 °C, quintile 95). When the temperature was at 24 °C, the cumulative risk was minimized to 0.625 (0.446, 0.878).
Fig. 1.
Effect of mean temperatures to varicella, all case.
Secondly, under extreme conditions, temperature had different effects on varicella incidence on different lag days (Fig. 1a−h). The hot effect of mean daily temperature on varicella incidence (26 °C, 95th percentile) compared with the reference temperature of 14 °C reached a maximum of 1.059 (1.025,1.094) on the fifth lag day. It subsequently began to decline, down to a relative risk of 0.911 (0.883, 0.941) on the 16th lag day. The cumulative relative risk peaked at 1.140 (0.863, 1.506) on the following day and decreased to 0.778 (0.546, 1.109) on the 21st day. The cold effect of mean daily temperature on varicella incidence (−3 °C, fifth quintile) had a relative risk value of 1.076 (0.993, 1.167) on the 0th lag day, declined to 1.010 (0.976, 1.046) on the seventh lag day, and then began to increase, rising to a maximum value of 1.095 (1.013–1.183) on the 21st lag day. Cumulative relative risk increases with lag time. The value of cumulative relative risk is 1.076 (0.993, 1.167) on the day of lagging, and the maximum value of cumulative relative risk is 2.516 (1.514, 4.183) on the 21st lag day. Details can be found in Table S2-3.
Finally, we investigated the relative risk and cumulative relative risk of varicella incidence by temperature with different lag days, stratified by sex and age groups (Figure S1-S5). The cumulative relative risk of varicella incidence was 2.061 (1.073, 3.957) for males and 3.126 (1.535, 6.366) for females at low temperatures. The relative risk of varicella in males reached a minimum value of 0.997 (0.952, 1.044) on the eighth lag day and increased to 1.071 (1.016, 1.129) on the 19th lag day. The relative risk of varicella in females decreased to 1.013 (0.965, 1.064) on the 13th lag day and then increased to 1.146 (1.042, 1.261) on the 21st lag day. Varicella incidence in males and females was insensitive to the hot effect of temperature, with a cumulative relative risk value of 0.721 (0.412, 1.261) for males and 0.566 (0.316, 1.015) for females. For different age groups, the cold effect of temperature had no effect on the incidence of varicella in children (0–6 years) with a cumulative relative risk of 1.054 (0.430, 2.582). Meanwhile, the hot effect of temperature did not affect the incidence of varicella in children. The cumulative risk of thermal effects in children was 1.497 (0.767, 2.920), and the relative risk was statistically significant at lag days 3–9, with a maximum value of 1.123 (1.064, 1.186) at lag day 6. There was a significant hot/cold effect on varicella incidence in adolescents (7–17 years) and adults (≥ 18 years), and it was more significant in adolescents. Similar to the general situation, adolescents and adults had a higher cumulative relative risk at low temperatures and a lower cumulative relative risk at high temperatures. In addition, we also investigated the extreme cold effect (−6 °C, 1st quartile) and extreme hot effect (29 °C, 99th quartile) of mean daily temperature on varicella incidence, which were consistent with these results. Details can be found in Table S4-5.
Relationship between daily varicella outpatients volume and ozone
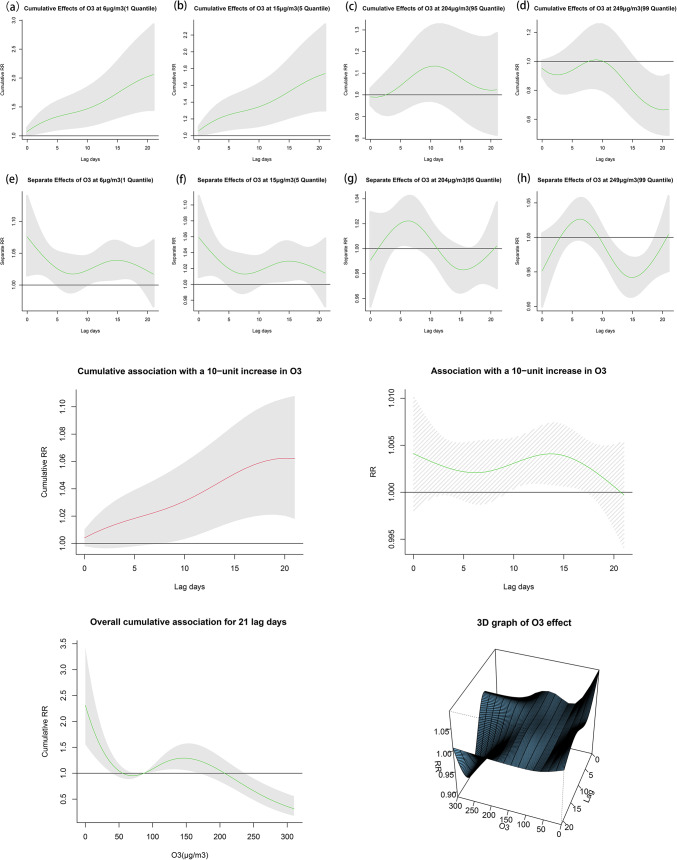
According to the time trend and exposure-response curve of ozone concentration and varicella incidence risk, it was found that the two were nonlinear, and the trend was “S”-shaped (Fig. 2). Using the median ozone concentration of 87 µg/m3 as the reference value, there was a significant correlation between every 10 µg/m3 increase in ozone concentration and the incidence of varicella. The cumulative relative risk showed a positive correlation with lag time, with a cumulative relative risk of 1.062 (1.018.1.108) at lag days 0–21. The relative risk was statistically significant at lag days 11–15(Fig. 2).
Fig. 2.
Effect of ozone concentrations to varicella, all case.
The relative and cumulative risk of varicella incidence on different lag days were observed at low and high concentrations(Fig. 2a−h). The risk of varicella incidence on the day of lag was 1.059 (1.009, 1.110) at the low concentration (15 µg/m3, 5th quintile), and the cumulative risk increased with lag time, peaking at 1.740 (1.301, 2.328) on the 21st lag day. The relative risk decreased to 1.013 (0.990, 1.036) on the eighth lag day and then increased. At high concentrations (204 µg/m3, 95th quintile), the risk of varicella on the 0th lag day was 0.991 (0.954, 1.029), and the cumulative risk peaked at 1.133 (0.968, 1.326) on the 11th lag day, then began to decline, falling to 1.021 (0.815, 1.286) on the 21st lag day. The relative risk increased from 1.020 (1.003, 1.038) on the 5th lag day up to 1.022 (1.002, 1.042) on the seventh lag day. Details can be found in Table S7-8.
The effect of ozone concentration on the incidence of varicella varied for different sexes and age groups (Figure S6-S10). At low concentrations, the cumulative risk was statistically significant for males and females, and the cumulative relative risk increased with lag time. The cumulative relative risk was lower for males at 1.575 (1.081, 2.296) compared to 1.974 (1.313, 2.969), and females were more sensitive to low concentrations of ozone. The relative risk for males at low ozone concentrations was statistically significant at lag days 0–4, with a maximum value of 1.077 (1.011, 1.146) at the 0th lag day. The relative risk for females under low ozone concentrations was statistically significant at lag days 9–18, with a maximum value of 1.045 (1.010–1.081) at the 14th lag day. For different age groups, the cumulative relative risk for adults at low ozone concentrations was as high as 2.164 (1.194, 3.992), followed by children at 1.928 (1.123, 3.310), and adolescents at low ozone concentrations had a lower cumulative relative risk value of 1.484 (0.990, 2.226). At high concentrations, only the incidence of varicella in adults was significantly correlated with high concentrations of ozone; no correlation was found for other age groups or gender stratification. Details can be found in Table S9-10.
In addition, we also studied the relative risk and cumulative relative risk of varicella incidence with different lag days at extremely high concentrations (249 µg/m3, 99th percentile) and extremely low concentrations (6 µg/m3, 1st percentile). The extremely low and low concentrations were consistent with our findings. The cumulative risk value for varicella incidence was 0.668 (0.492, 0.907) at the extremely high concentration. The extremely high concentration had a significant effect on varicella incidence at lag days 12–18, reaching a maximum value of 0.961 (0.937, 0.897) at the 18th lag day.
Discussion
This study used a DLNM to investigate the lagged effects of temperature extremes and ozone on varicella incidence from 2014 to 2022. We found a general “J” shaped trend between mean daily temperature and varicella incidence. Low-temperatures increased the risk of varicella incidence, and high-temperatures decreased the risk of varicella incidence. This conclusion is consistent with a previous report from Jinan, China, where the effect of temperature on varicella incidence was negatively correlated20. This result may be due to the fact that varicella virus is mainly transmitted through direct contact, respiratory droplets and airborne transmission, and in a low-temperature environment, people are more inclined to gather indoors, which increases the risk of varicella virus transmission; in addition, low temperature may lead to a decrease in human immunity, which makes the individual more susceptible to varicella virus infection. Moreover, the varicella virus is weak enough to survive in high-temperature environments30; as the temperature increases, the human body’s metabolism becomes faster31, which also helps to resist varicella virus infection. Meanwhile, this study found that the effect of temperature on the incidence of varicella in females is more sensitive than in males, which may be due to the physiological differences between females and males, which make females more susceptible to the virus at particular times. Secondly, we found no significant correlation between temperature and the incidence of chickenpox in children; at the same temperature, adolescents are more susceptible to varicella than adults. Because China recommends varicella vaccination for children at the ages of 1 and 4 years, it has been reported that varicella vaccine can effectively prevent the varicella virus from invading children’s bodies32, which has vastly reduced the incidence of varicella in children. However, the validity of the varicella vaccine will be weakened with the prolongation of the vaccination time12, coupled with the fact that adolescents will spend most of their time in the school group life, which increases the risk of adolescents’ varicella virus infection.
The present study also found that the overall trend between ozone concentration and varicella incidence showed an “S” shape, with low ozone concentration being a risk factor for varicella incidence and high ozone concentration being a protective factor for varicella incidence. Ozone has oxidizing solid and bactericidal properties, which can kill some of the varicella viruses in the air3334;, so that the incidence of varicella can be reduced. With the improvement of medical treatment, ozone has also been used as a complementary therapy for treating skin diseases, and much medical evidence has verified the feasibility of this therapy35–37. So we can suspect that a high concentration of ozone has an inhibitory effect on the varicella virus. A number of studies show that temperature may have some effect on the concentration of pollutants. Ozone is also of increasing concern as a secondary pollutant. A U.S. report notes that ozone typically rises with temperature38; X. Pu reported that in China, heat waves can increase surface ozone concentrations39; From the study of Romer, Paul S, it is concluded that temperature promotes NOx production by soil microorganisms, which leads to an increase in surface ozone with increasing temperature40. So we suspect that atmospheric temperatures are also lower at low ozone concentrations, leading to the fact that low concentrations of ozone increase the risk of varicella development. In response to the fact that adults have a higher risk of contracting varicella than children and adolescents under conditions of low ozone concentration, This is explained by the fact that the immune system of adults is weakened due to work stress, late nights, excessive fatigue, and with age, more and more adults are in a state of sub-health and suffer from underlying illnesses, which can lower the immune system and thus increase the prevalence of varicella in adults4142;. The use of varicella attendance data from only one city is a limitation of our present study, and more urban studies are needed to confirm the results. Furthermore, to avoid model complexity, we only analyzed the effects of temperature extremes and ozone on varicella incidence. Other social and environmental factors were not included in our model. Finally, the effect of meteorological factors and air pollutants on varicella incidence requires more complex studies to explore their interaction.
Conclusions
In conclusion, extreme temperatures and ozone concentrations were significantly correlated with the incidence of varicella. In Shijiazhuang, females and adolescents aged 7 to 17 years were more likely to be infected with varicella due to extreme temperatures; females and adults (≥ 18 years) were more likely to be infected with varicella due to ozone concentration. The study results will help improve public health services under extreme temperatures and different concentrations of ozone and to implement more targeted interventions according to the characteristics of different populations to prevent varicella outbreaks.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
[J.Z.]: Led the overall research design and methodology, developed the experimental protocols, and supervised the data collection process.[B. W.]: Conducted the primary experiments and data analysis, interpreted the results, and prepared the initial figures and tables.[Z. L.]: Assisted in data collection, performed secondary data analysis to validate the findings, and provided critical insights into the interpretation of results.[W. Z.]: Managed the literature review, compiled relevant background information, and contributed to the discussion of the results in the context of previous studies.[S. Y.]: Oversaw the statistical analysis, ensured the rigor of the analysis methods, and reviewed the statistical interpretations.[Q.G.]: Contributed to the writing of the manuscript, revised the sections for clarity and conciseness, and polished the language.[X.G.]: Assisted in the preparation of the experimental setup, maintained the equipment, and ensured the reproducibility of the experiments.[Z.Z.]: Participated in the initial discussions of the research goals and objectives, provided input on the experimental design, and offered guidance throughout the project.[J.C.]: Secured funding for the research (if applicable), managed the project budget, and coordinated resources for the study.[L.L.]: Oversaw the ethical aspects of the research, ensured compliance with relevant regulations, and provided guidance on ethical considerations.[X.Z.]: Provided overall supervision and guidance for the research, helped resolve challenges and obstacles, and offered strategic direction for the project.
Data availability
The datasets generated and analysed during the current study are not publicly available due the data involves the privacy of personnel but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Statement of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juan Zhang, Binhao Wang and Zixuan Li contributed equally to this work.
Contributor Information
Lijuan Liu, Email: 57701236@hebmu.edu.cn.
Xiaolin Zhang, Email: 17700862@hebmu.edu.cn.
References
- 1.Lobato, G. M. et al. Contributions of the International Plant Science Community to the Fight Against Human infectious diseases - Part 1: Epidemic and Pandemic diseases. Plant. Biotechnol. J.19, 1901–1920 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olliaro, P. & Torreele, E. Global challenges in preparedness and response to Epidemic Infectious diseases. Mol. Ther.30, 1801–1809 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvin, A. M. Varicella-Zoster Virus. Clin. Microbiol. Rev.9, 361–381 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cersosimo, A. et al. Varicella Zoster Virus and Cardiovascular diseases. Monaldi Arch. Chest Dis.93, 2414 (2022). [DOI] [PubMed]
- 5.Tofade, T. O. & Chwalisz, B. K. Neuro-Ophthalmic complications of Varicella-Zoster Virus. Curr. Opin. Ophthalmol.34, 470–475 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Harigane, K., Sumi, A., Mise, K. & Kobayashi, N. The role of temperature in reported chickenpox cases from 2000 to 2011 in Japan. Epidemiol. Infect.143, 2666–2678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergara-Castañeda, A. et al. Epidemiology of Varicella in Mexico. J. Clin. Virol.55, 51–57 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Barrero, G. L. et al. Delineating the seasonality of Varicella and its Association with Climate in the Tropical Country of Colombia. J. Infect. Dis.228, 674–683 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korostil, I. A. & Regan, D. G. Varicella-Zoster Virus in Perth, Western Australia: seasonality and reactivation. Plos One. 11, e151319 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumi, A. Role of temperature in reported chickenpox cases in Northern European Countries: Denmark and Finland. Bmc Res. Notes. 11, 377 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, B., Sumi, A., Wang, L., Zhou, W. & Kobayashi, N. Role of Meteorological conditions in reported chickenpox cases in Wuhan and Hong Kong, China. Bmc Infect. Dis.17, 538 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro, E. D. & Marin, M. The effectiveness of Varicella Vaccine: 25 years of Postlicensure Experience in the United States. J. Infect. Dis.226, S425–S430 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Wang, M. et al. Epidemiological characteristics of Varicella outbreaks - China, 2006–2022. China Cdc Wkly.5, 1161–1166 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varicella and Herpes Zoster Vaccines. Who position paper, June 2014. Wkly. Epidemiol. Rec. 89, 265–287 (2014). [PubMed] [Google Scholar]
- 15.Landrigan, P. J. & Fuller, R. Global Health and Environmental Pollution. Int. J. Public. Health. 60, 761–762 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Hoseini, S. G. et al. Seroprevalence and risk factors of Varicella Zoster infection in Iranian adolescents: a multilevel analysis; the Caspian-III Study. Plos One. 11, e158398 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, Z. et al. Association between Ambient Temperature and varicella among adults in Qingdao, China during 2008–2019. Int. J. Environ. Health Res.33, 629–638 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Lu, J. Y., Zhang, Z. B., He, Q., Ma, X. W. & Yang, Z. C. Association between Climatic Factors and varicella incidence in Guangzhou, Southern China, 2006–2018. Sci. Total Environ.728, 138777 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Yang, Y., Geng, X., Liu, X., Wang, W. & Zhang, J. Association between the Incidence of Varicella and Meteorological conditions in Jinan, Eastern China, 2012–2014. Bmc Infect. Dis.16, 179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luan, G. et al. Associations between Air temperature and daily varicella cases - Jinan City, Shandong Province, China, 2019–2021. China Cdc Wkly.6, 36–39 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, J. Y. et al. [Research on the Relationship between the Daily Mean temperature and the daily cases of Varicella during 2008–2016 in Lanzhou, China]. Zhonghua Yu Fang Yi Xue Za Zhi. 52, 842–848 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Zhang, T., Qin, W., Nie, T., Zhang, D. & Wu, X. Effects of Meteorological factors on the incidence of Varicella in Lu’an, Eastern China, 2015–2020. Environ. Sci. Pollut Res.30, 10052–10062 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Wang, Z. et al. Influence of Air pollutants on Varicella among adults. Sci. Rep.11, 21020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu, H. et al. Influence of Coarse Particulate Matter on Chickenpox in Jiading District, Shanghai, 2009–2018: a distributed lag Non-linear Time Series Analysis. Environ. Res.190, 110039 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Beck, H. E. et al. High-resolution (1 km) Köppen-Geiger maps for 1901–2099 based on constrained Cmip6 projections. Sci. Data. 10, 724 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Circular of the General Office of the Ministry of Health on. Printing and Distributing the Norms for the Management of Reporting and Management of Information Related to National Public Health Emergencies (for Trial Implementation). MoH P.R.China. 44–60 (2006).
- 27.Qiu, L. et al. The epidemiology of Varicella and Effectiveness of Varicella Vaccine in Ganyu, China: a long-term community Surveillance Study. Bmc Public. Health. 23, 1875 (2023). [DOI] [PMC free article] [PubMed]
- 28.Ayoade, F. & Kumar, S. Varicella-Zoster Virus (Chickenpox). (2024). [PubMed]
- 29.Kutlar, J. M., Eeftens, M., Gintowt, E., Kappeler, R. & Künzli, N. Time to Harmonize National Ambient Air Quality standards. Int. J. Public. Health. 62, 453–462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VACZI, L., GEDER, L., KOLLER, M. & JENEY, E. Influence of temperature on the multiplication of Varicella Virus. Acta Microbiol. Acad. Sci. Hung.10, 109–115 (1963). [PubMed] [Google Scholar]
- 31.Schulte, P. M. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol.218, 1856–1866 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Hao, B. et al. Efficacy, Safety and Immunogenicity of Live attenuated Varicella Vaccine in Healthy Children in China: Double-Blind, randomized, placebo-controlled clinical trial. Clin. Microbiol. Infect.25, 1026–1031 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Cai, Y. et al. Ozone based inactivation and disinfection in the Pandemic Time and Beyond: taking Forward what has been learned and best practice. Sci. Total Environ.862, 160711 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epelle, E. I. et al. Bacterial and fungal disinfection Via Ozonation in Air. J. Microbiol. Methods. 194, 106431 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Liu, L., Zeng, L., Gao, L., Zeng, J. & Lu, J. Ozone therapy for skin diseases: Cellular and Molecular mechanisms. Int. Wound J.20, 2376–2385 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng, J. & Lu, J. Mechanisms of action involved in Ozone-Therapy in skin diseases. Int. Immunopharmacol.56, 235–241 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Borges, G. Á. et al. In Vitro evaluation of Wound Healing and Antimicrobial potential of ozone therapy. J. Craniomaxillofac. Surg.45, 364–370 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Camalier, L., Cox, W. & Dolwick, P. 41, 7127–7137 (2007).
- 39.Pu, X. et al. 603, 807–816 (2017).
- 40.Romer, P. S. et al. 18, 2601–2614 (2018).
- 41.Downes, L., St, H. H. & Mays, T. A-Smart Lifestyle Behaviors Model for Health, Wellbeing, and Immune System Enhancement. Nurse Pract.46, 31–39 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Macia, L., Galy, O., Nanan, R. & Editorial Modern lifestyle and health: how changes in the Environment impacts Immune function and physiology. Front. Immunol.12, 762166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due the data involves the privacy of personnel but are available from the corresponding author on reasonable request.