Abstract
Background
NK-1 receptor antagonists (NK-1RAs) are proven to be successful in preventing chemotherapy-induced nausea and vomiting (CINV). The safety profile of NK-1RAs has not been systematically analyzed in the real world. This pharmacovigilance study investigated the differences in adverse events (AEs) between NK-1RAs.
Methods
Adverse events (AEs) associated with NK-1RAs were gathered and standardized using data from the FAERS database spanning from the first quarter of 2009 to the fourth quarter of 2023. Various disproportionality techniques were employed for data analysis, such as the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS).
Results
A total of 5434AE reports listing NK-1RAs as the primary suspected drugs were identified. The System Organ Classes (SOC) appeared as significant safety signals were found. Among NK-1RAs, the most frequently reported AEs were related to general disorders and administration site conditions. In terms of PT level, the strong signals were mainly injection site reactions associated with aprepitant and fosaprepitant. Moreover, toxic encephalopathy and encephalopathy of the aprepitant were all positive with four algorithms. A significant finding was the recognition of adverse events linked to endocrine disorders, which were not previously mentioned in the medication instructions.
Conclusion
The safety profile of NK-1RAs has been reported to be variable.If intravenous formulations were used in the clinic, injection site reactions should be a concern. In addition, more attention should be paid to the management of encephalopathy toxicity in patients treated with aprepitant in combination with ifosfamide. Besides known AEs, we have identified several new high-risk AEs, such as inappropriate antidiuretic hormone secretion, adrenal insufficiency and hyponatraemia. Overall, clinicians should closely monitor the occurrence of NK-1RA-related AEs in clinical applications.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82575-5.
Keywords: NK-1 receptor antagonists, Adverse events, FAERS database, Pharmacovigilance, Drug safety
Subject terms: Epidemiology, Drug safety, Pharmacology
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a prevalent and distressing side effect of cancer therapy, impacting a substantial proportion of patients1. In 2022, the global incidence of malignant tumors reached nearly 20 million cases, resulting in approximately 9.7 million fatalities2. Concurrently, China reported nearly 4.82 million new cases of malignant tumors, leading to approximately 2.57 million deaths3. Chemotherapy continues to serve as the primary modality for treating tumors, with CINV representing a frequently encountered adverse reaction. CINV impacted a considerable number of patients receiving moderately or highly emetogenic chemotherapy, with up to 61% experiencing symptoms despite prophylactic measures4. The pathophysiology of CINV involves a complex interplay of various neurotransmitters and receptors5. In addition to causing electrolyte and nutritional imbalances, severe cases of CINV can result in esophageal mucosal lacerations, leading to decreased patient compliance with treatment regimens or even refusal of anti-neoplastic therapies. Severe cases of CINV may result in decreased or postponed delivery of chemotherapeutic medications, potentially diminishing the efficacy of treatment and shortening patients’ survival duration6.
Commonly used medications for treating CINV consist of 5-HT3 receptor antagonists, neurokinin-1 receptor antagonists (NK-1RAs), dexamethasone, atypical antipsychotics, and dopamine receptor blockers. NK-1RAs are a pharmacological class of agents with diverse therapeutic properties, including antiemetic, antidepressant, anxiolytic, and antipruritic effects. By attaching to NK-1 receptors in both the central nervous system and periphery, these agents work to prevent the release of substance P (SP), thus exerting their effects7. Aprepitant (APR) was the first NK-1RA approved by the US Food and Drug Administration (FDA) for treating nausea and vomiting caused by chemotherapy8. Fosaprepitant (FAP), a prodrug of APR designed for patients with challenges in oral intake, was subsequently approved by the FDA in 2008. Rolapitant (RP), which was granted approval by the FDA in September 2015, is an oral, long-acting, highly selective NK-1RA with a half-life of up to 180 h. The combination of NK-1RAs with standard anti-emetic therapy had been documented in literature as an efficacious treatment for preventing CINV and was widely used in clinical practice9. The U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) database, a valuable tool for collecting and analyzing drug-related adverse events, especially with the widespread use of three medications and increasing awareness of their side effects. It helps evaluate the safety and effectiveness of these drugs and address issues like incomplete and delayed reporting10. This study aimed to use data mining methods to identify and evaluate adverse event signals associated with common NK-1RAs medications after they have been released to the market, in order to improve understanding of the adverse event profiles related to NK-1RAs.
Materials and methods
Data source and collection
In order to thoroughly investigate the various adverse events associated with NK-1RAs, a retrospective pharmacovigilance study was conducted utilizing the FAERS database. The NK-1RAs examined in this study include aprepitant (APR), fosaprepitant (FAP), and rolapitant (RP). Data from reports submitted between the first quarter of 2009 (2009 Q1) and the fourth quarter of 2023 (2023 Q4) for APR and FAP, and between the third quarter of 2015 (2015 Q3) and the fourth quarter of 2023 (2023 Q4) for RP were included. A PubMed search was conducted using MeSH terms and keywords “aprepitant”, “fosaprepitant”, and “rolapitant” to identify all the relevant terms. The ultimate target terms for these NK-1RAs were determined as follows: for APR, the terms included “Aprepitant”, “MK0869”, “MK0517”, and “Emend”; for FAP, the terms included “Fosaprepitant” and “Fosaprepitant dimeglumine”; for RP, the terms included “Rolapitant” and “Varubi”. In accordance with recommendations from the FDA, data extraction was performed, with duplicate reports being removed.
Data mining
AEs in the FAERS database were categorized and normalized using utilizing the Medical Dictionary for Regulatory Activities (MedDRA version 26.1). Within the FAERS, each AE report underwent coding and description based on the Preferred Term (PT) and subsequent classification by System Organ Class (SOC). To assess potential AEs associated with NK-1RAs, disproportionality methods such as Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN) and Multi-item Gamma Poisson Shrinker(MGPS) were employed for data analysis11. Each of four methods has its own advantages.ROR can adjust for bias when the number of AE reports is small.Compared to ROR, PRR has higher specificity because it is less affected by missing of AEs.BCPNN and MGPS are Bayesian algorithms. BCPNN is excellent for integration of data from multiple sources and cross-validation, while MGPS has the advantage that it can detect signals from rare events.Therefore, by combining the four algorithms, this study mitigates the risk of false-positive signals, expands the detection range and enhances the credibility of the study’s objectives12.
The number of reports of NK-1RA related AEs was not less than three and the vaule of the 95% CI of the ROR should be greater than one.The BCPNN method further categorised ADR signals into four groups (no, weak, medium, and strong signals) based on the vaule of IC02513.Higher values indicated the strength of stronger signal, which suggested a more reliable association between the target drug and the adverse event.The calculation formulas and threshold values for these four methods were provided in Tables S1 and S2 in the supplementary materials. The data analyses were carried out using R 4.2.3.
Results
Basic information
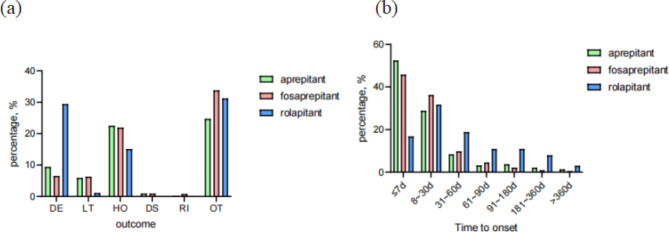
From the first quarter of 2009 to the fourth quarter of 2023, a total of 5434 AE reports identified NK-1RAs as the primary suspected drugs. Among these AE reports, 3596 cases were associated with APR, 989 cases were related to FAP and 849 cases were linked to RP. The basic characteristics of these adverse reports were presented in Table 1. Adverse events were more commonly reported in females than in males for these three drugs. The 18 to 64.9 age group had the highest proportion in APR at 41.0% and in FAP at 49.9%, whereas the missing age group made up the majority in RP at 68.1%. The majority of AEs were reported by consumers and doctors, with a significant proportion originating from the United States (APR 50.3%, FAP 42.0%, RP 98.5%). In addition to unspecified serious AEs, the primary serious outcome of RP AEs was death (29.4%), while hospitalization or prolonged hospitalization was the main outcome for the other two NK-1RAs (APR 22.5%, FAP 21.9%) (Fig. 1a). Despite a lack of information on AE occurrences and medication dates, it was evident that APR and FAP had higher occurrences within 0–7 days compared to other time periods, whereas RP had higher occurrences at 8–30 days (Fig. 1b).
Table 1.
Basic characteristics of NK-1RAs in FAERS.
| Categories | APR, % | FAP, % | RP, % |
|---|---|---|---|
| Cases | 3596 | 989 | 849 |
| Gender | |||
| Female | 1893 (52.6) | 498 (50.4) | 377 (44.4) |
| Male | 1192 (33.1) | 372 (37.6) | 254 (29.9) |
| Missing | 511 (14.2) | 119 (12.0) | 218 (25.7) |
| Age, years | |||
| < 18 | 98 (2.7) | 20 (2.0) | 1 (0.1) |
| 18-64.9 | 1475 (41.0) | 494 (49.9) | 151 (17.8) |
| 65–85 | 745 (20.7) | 239 (24.2) | 117 (13.8) |
| > 85 | 13 (0.4) | 4 (0.4) | 2 (0.2) |
| Missing | 1265 (35.2) | 232 (23.5) | 578 (68.1) |
| Weight, kg | |||
| < 50 | 135 (3.8) | 62 (6.3) | 8 (0.9) |
| 50–100 | 908 (25.3) | 399 (40.3) | 96 (11.3) |
| > 100 | 83 (2.3) | 39 (3.9) | 12 (1.4) |
| Reporters | |||
| Consumer | 1075 (29.9) | 193 (19.5) | 400 (47.1) |
| Health Professional | 129 (3.6) | 52 (5.3) | 16 (1.9) |
| Doctor | 1051 (29.2) | 421 (42.6) | 97 (11.4) |
| Pharmacist | 615 (17.1) | 206 (20.8) | 103 (12.1) |
| Others | 673 (18.7) | 102 (10.3) | 230 (27.1) |
| Report countries | |||
| United States | 1810 (50.3) | 416 (42.0) | 836 (98.5) |
| France | 478 (13.3) | 3 (0.3) | 1 (0.1) |
| Korean | 349 (9.7) | 339 (34.3) | NA |
| Japan | 294 (8.2) | 125 (12.6) | NA |
| Serious outcomes | |||
| Death | 338 (9.4) | 65 (6.5) | 250 (29.4) |
| Hospitalization-initial or prolonged | 810 (22.5) | 217 (21.9) | 129 (15.1) |
| Life-threatening | 214 (5.9) | 63 (6.3) | 10 (1.1) |
| Disability | 35 (0.9) | 9 (0.9) | 0 |
| Other serious outcomes | 888 (24.7) | 334 (33.8) | 265 (22.8) |
Fig. 1.
Basic characteristics of NK-1RAs. a Serious outcomes. DE for death. LT for Life-threatening. HO for hospitalization-initial or prolonged. DS for disability. RI for required intervention to prevent permanent impairment/damage. OT for other serious outcomes. b Adverse event occurrence time-medication date (days).
Signal mining results
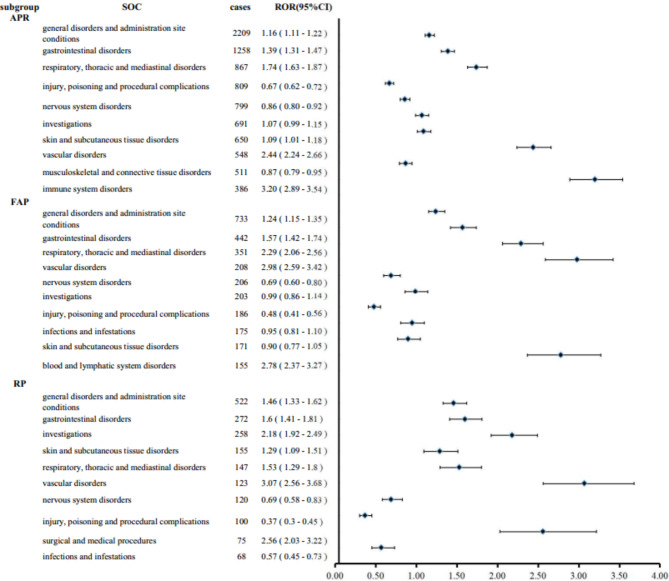
This study presented an analysis of the top ten most frequently reported AEs associated with NK-1RAs at SOC level, as depicted in Fig. 2. The whole findings indicated that APR encompassed 27 SOCs, with 9 positive signals identified (Table S3). The most commonly reported AEs related to APR were general disorders and administration site conditions (n = 2209), gastrointestinal disorders (n = 1258), and respiratory, thoracic and mediastinal disorders (n = 867). The three most significant signals were immune system disorders (ROR = 3.20), vascular disorders (ROR = 2.44), and blood and lymphatic system disorders (ROR = 2.01). The analysis of AEs of FAP revealed that out of 26 SOCs, 7 exhibited positive signals, all the information was shown in Table S3. The general disorders and administration site conditions had the highest number of AEs (n = 733), followed by gastrointestinal disorders(n = 442), and respiratory, thoracic and mediastinal disorders(n = 351). Vascular disorders exhibited the strongest signal with an ROR of 2.98, followed by immune system disorders (ROR = 2.80) and blood and lymphatic system disorders (ROR = 2.78). Given that FAP was the injectable prodrug of AP, it was not surprising that they share many similar AEs. In comparison, RP involved 25 SOCs, with 8 positive signals identified (Table S3). Similarly to the two medications previously discussed, the highest number of AEs related to RP was general disorders and administration site conditions (n = 522), followed by gastrointestinal disorders (n = 272) and investigations (n = 258). Vascular disorders (ROR = 3.07), surgical and medical procedures (ROR = 2.56), and investigations (ROR = 2.18) were the three strongest signals identified.
Fig. 2.
Top ten most frequently reported AEs of NK-1RAs at the SOC level.
In terms of the PT level, we utilized four algorithms to analyze the AEs linked to NK-1RAs and evaluate their conformity with the diverse screening criteria. From the positive signals given by four algorithms, the top ten most frequently reported AEs of NK-IRAs were shown in Table 2. For the most common clinical symptoms associated with AEs, the most frequently reported AE of APR was dyspnea(n = 378). The same was true for FAP (n = 139) and the most frequently reported AE of RP was flushing (n = 92). The signal profiles of the top 8 induced by NK-1RAs in the SOC of general disorders and administration site conditions, nervous system disorders, endocrine disorders and musculoskeletal and connective tissue disorders were listed in Table 3. The strong signals were mainly injection site reactions associated with APR and FAP. Interestingly, we found that toxic encephalopathy and encephalopathy of APR were both positive with four algorithms. Furthermore, we discovered new and valuable AE signals that were not explicitly identified in the prescribing information, such as adrenal insufficiency, inappropriate antidiuretic hormone secretion, and hypothyroidism in the SOC for endocrine disorders, which should be taken into account in clinical practice.
Table 2.
Top ten most frequently reported AEs of NK-1RAs at the PT level.
| Rank | APR | FAP | RP | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTs | n | ROR (95%CI lower limit) |
χ2 | IC (IC025) |
EBGM (EBGM05) |
PTs | n | ROR(95%CI lower limit) | χ2 | IC (IC025) |
EBGM (EBGM05) |
PTs | n | ROR (95%CI lower limit) |
χ2 | IC (IC025) |
EBGM (EBGM05) |
|
| 1 | Dyspnoea | 378 | 3.46 | 763.93 | 0.23 | 3.43 | Dyspnoea | 139 | 3.80 | 363.06 | 0.46 | 3.78 | Death | 241 | 7.59 | 1453.00 | 1.30 | 6.99 |
| 2 | Flushing | 267 | 12.64 | 3205.79 | 2.13 | 12.57 | Flushing | 112 | 15.85 | 1859.81 | 2.54 | 15.82 | Flushing | 92 | 29.53 | 3026.10 | 3.45 | 29.23 |
| 3 | Erythema | 180 | 4.06 | 515.54 | 0.55 | 4.10 | Pyrexia | 87 | 3.70 | 237.47 | 0.50 | 3.76 | Dyspnoea | 73 | 3.06 | 150.11 | 0.25 | 3.10 |
| 4 | Back pain | 163 | 3.31 | 340.28 | 0.27 | 3.35 | Infusion related reaction | 75 | 17.52 | 1470.91 | 2.76 | 17.79 | Infusion related reaction | 64 | 22.51 | 1668.18 | 3.14 | 22.74 |
| 5 | Hypersensitivity | 138 | 3.52 | 327.42 | 0.38 | 3.58 | Erythema | 54 | 3.42 | 143.21 | 0.48 | 3.53 | Erythema | 56 | 5.64 | 299.48 | 1.18 | 5.76 |
| 6 | Infusion related reaction | 119 | 9.10 | 1056.00 | 1.76 | 9.26 | Neutropenia | 54 | 5.59 | 289.65 | 1.18 | 5.76 | Feeling hot | 47 | 18.73 | 1058.15 | 2.94 | 19.19 |
| 7 | Chest discomfort | 114 | 5.40 | 523.22 | 1.02 | 5.51 | Chest discomfort | 54 | 7.49 | 419.97 | 1.61 | 7.71 | Hospitalisation | 35 | 4.04 | 131.52 | 0.81 | 4.21 |
| 8 | Adverse event | 113 | 5.70 | 559.55 | 1.10 | 5.82 | Back pain | 52 | 2.97 | 110.87 | 0.28 | 3.07 | Back pain | 32 | 2.79 | 69.71 | 0.30 | 2.92 |
| 9 | Anaphylactic reaction | 108 | 10.12 | 1100.57 | 1.93 | 10.32 | Constipation | 49 | 3.18 | 118.77 | 0.40 | 3.30 | Chest discomfort | 30 | 6.29 | 211.02 | 1.49 | 6.59 |
| 10 | Infusion site pain | 79 | 28.68 | 2632.54 | 3.47 | 29.29 | Febrile neutropenia | 44 | 9.24 | 456.65 | 1.95 | 9.58 | Abdominal discomfort | 29 | 3.14 | 78.67 | 0.50 | 3.30 |
Table 3.
The top 8 signal strength of AEs refered to four SOCs.
| SOC | APR | FAP | RP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| General disorders and administration site conditions | PTs | n | Intensity | PTs | n | Intensity | PTs | n | Intensity |
| Injection site vasculitis | 13 | +++ | Injection site vasculitis | 12 | +++ | Feeling hot | 47 | ++ | |
| Injection site phlebitis | 29 | +++ | Injection site phlebitis | 26 | +++ | Chest discomfort | 30 | + | |
| Infusion site phlebitis | 6 | +++ | Infusion site irritation | 8 | +++ | Death | 241 | + | |
| Infusion site irritation | 18 | +++ | Infusion site reaction | 8 | +++ | Chest pain | 28 | + | |
| Infusion site urticaria | 8 | +++ | Infusion site discomfort | 3 | +++ | Disease progression | 17 | + | |
| Infusion site rash | 11 | +++ | Infusion site pain | 32 | +++ | Chills | 8 | — | |
| Therapeutic producteffective for unapproved indication | 3 | +++ | Infusion site induration | 3 | +++ | Adverse drug reaction | 6 | — | |
| Infusion site reaction | 17 | +++ | Injection site atrophy | 4 | +++ | Illness | 6 | — | |
| Nervous system disorders | Tonic clonic movements | 4 | ++ | Seizure like phenomena | 3 | +++ | Unresponsive to stimuli | 3 | + |
| Encephalopathy | 69 | ++ | Unresponsive to stimuli | 11 | + | Cerebral haemorrhage | 4 | + | |
| Seizure like phenomena | 4 | ++ | Presyncope | 6 | + | Dizziness | 45 | — | |
| Toxic encephalopathy | 8 | ++ | Sensory disturbance | 3 | + | Loss of consciousness | 10 | — | |
| Clonus | 4 | + | Depressed level of consciousness | 6 | — | Hypoaesthesia | 9 | — | |
| Muscle contractions involuntary | 4 | + | Loss of consciousness | 15 | — | Neuropathy peripheral | 6 | — | |
| Peripheral sensory neuropathy | 6 | + | Paraesthesia | 17 | — | Seizure | 8 | — | |
| Unresponsive to stimuli | 20 | + | Aphasia | 3 | — | Burning sensation | 3 | — | |
| Endocrine disorders | Inappropriate antidiuretic hormone secretion | 15 | ++ | Inappropriate antidiuretic hormone secretion | 3 | + | |||
| Adrenal insufficiency | 5 | — | Adrenal insufficiency | 3 | + | ||||
| Hypothyroidism | 3 | — | |||||||
| Musculoskeletal and connective tissue disorders | Joint deposit | 3 | +++ | Back pain | 52 | + | Back pain | 32 | + |
| Facet joint syndrome | 4 | ++ | Muscle twitching | 4 | — | Bone pain | 3 | — | |
| Rheumatic fever | 5 | ++ | Musculoskeletal pain | 6 | — | Muscle spasms | 5 | — | |
| Hand deformity | 12 | + | Neck pain | 4 | — | Arthralgia | 4 | — | |
| Back pain | 163 | + | Muscle spasms | 10 | — | ||||
| Synovitis | 11 | + | Pain in extremity | 14 | — | ||||
| Joint range of motion decreased | 6 | — | Myalgia | 7 | — | ||||
| Flank pain | 4 | — | Arthralgia | 8 | — | ||||
Strong signal(+++)IC025>3,Medium signal(++)1.5< IC025 ≤ 3,Weak signal(+)0< IC025 ≤ 1.5,No signal(—)IC025 ≤ 0. IC025 the lower limit of the 95% confidence interval of IC.
Discussion
CINV can occur at various points during chemotherapy. Acute CINV happens within the first 24 h of receiving chemotherapy, with vomiting primarily caused by 5-HT3. On the other hand, delayed CINV occurs between 24 and 120 h after chemotherapy and is mainly mediated by substance P (SP) binding to NK-1R in the central nervous system14. As reported in the literature15,16, the prophylactic use of NK-1RAs can significantly reduce CINV. This study compared and analyzed the similarities and differences of NK-1RAs from the FAERS database in the real world. Four main discoveries were explored: (1) NK-1RAs associated AEs were linked to multiple SOCs, such as gastrointestinal disorders, general disorders and administration site conditions, vascular disorders, immune system disorders, and respiratory, thoracic and mediastinal disorders; (2) although the reporters of injection site reactions linked to RP were low, but the ROR of it was the highest of the three drugs;3) the likelihood of encephalopathy toxicity associated with APR was greater than the other two medications; 4) in addition to established AEs, novel AEs were identified, such as inappropriate antidiuretic hormone secretion, adrenal insufficiency, hypothyroidism and hyperthyroidism.
Table 1 indicated that females experienced a greater number of adverse events than males. Consistent with previous reports, women were at a higher risk for CINV1,16,17. Apart from the missing age, the age group of 18-64.9 had a higher proportion of AE reporters, suggesting that there may be widespread use of NK-1RAs in this age group. This finding was consistent with the existing literature, which reported the highest tumor risk in people over 5018.
In this study, injection site vasculitis and phlebitis showed strong signals for APR and FAP. Injection site reactions (ISRs) were a prevalent type of side effect associated with NK-1RAs, and may lead to adjustment, pauses, or discontinuation. Furthermore, several studies have indicated a correlation between the use of FAP and ISRs19–21, with reported rates varying from 7% to as high as 67% reported in retrospective studies. These ISRs included injection site vasculitis, phlebitis, irritation, infusion-related hypersensitivity reaction and other conditions.A study comparing the use of APR and FAP in the treatment of gynecological tumors showed that intravenous administration of FAP and age less than 65 years were risk factors for ISRs22.Furthermore, ISRs tended toward a higher incidence with shorter infusion times(less than 15 min)and higher concentration(0.6 mg/L,1.0 mg/L,1.5 mg/L)23.Among breast cancer patients undergoing doxorubicin-/cyclophosphamide chemotherapy, the occurrence of infusion site reactions linked to FAP was 34.7%, significantly higher than the 2.3% seen with APR ISRs20. In addition, the simultaneous administration of anthracycline-based regimens increased the risk of FAP-related ISRs by around six times21. Currently, the RP-related ISRs were less commonly reported, but the ROR of them in our study was the highest of the three drugs, suggesting that we still need to be vigilant in clinical therapy. A previous study found that out of 60 patients who used RP to prevent CINV prophylactically, there were 6 cases of ISRs with intravenous RP24. This incidence was higher than the 2.8% reported in its Phase I clinical trial25. In January 2018, the U.S. FDA issued a Health Care Provider Letter noting that anaphylaxis, anaphylactic shock, and other serious hypersensitivity reactions were associated with the use of RP Injectable Emulsion in the post-marketing setting24. In order to reduce the incidence of ISRs, infusion of FAP via a central venous catheter had been reported in previous literature26, diluting the concentration to 0.6 mg/ml and infusing over 30 min27.
This study found that several PTs in nervous system disorders had positive signals, particularly in relation to APR, such as encephalopathy, tonic clonic movements, seizure like phenomena and so on. Sano T et al.28. reported a case of ifosfamide-induced encephalopathy(IIE) in a patient treated with a combination of APR and ifosfamide (IFO). Furthermore, Shimada K et al.. believed that APR might be associated with a higher risk of IIE29 and the incidence was as high as 26%30. The mechanisms of APR and IIE were that APR may interfere with the metabolism of IFO via CYP3A4 inhibition, resulting in an increased concentration of IFO in the blood and its metabolites, such as 2-chloroacetaldehyde and acrolein. Both of these substance can cross the blood-brain barrier and induce encephalopathy31. However, the results of a systematic review indicated a positive enhanced trend between neurotoxicity and concomitant use of IFO and APR or FAP, but the association was not statistically significant32. Baseline albumin < 3.5 g/dl33 ,age ≥ 60 years and IFO dose ≥ 2000mg/m229 were risk factors for IIE. The association of NK-1RAs with IIE was not clear, but vigilance was required for IIE when combined with IFO, which can be reduced by prolonged titration or administration of methylene blue34 .
Furthermore, our study revealed novel AEs related to endocrine disorders, including inappropriate antidiuretic hormone secretion, adrenal insufficiency, hypothyroidism and hyperthyroidism in both APR and FAP. Despite no new adverse events regarding endocrine disorders being found with RP, hyponatremia induced by RP was still considered to be connected to either inappropriate antidiuretic hormone secretion or adrenal insufficiency.Studies had indicated that the NK-1R was present in the nervous system and peripheral tissues, participating in endocrine and paracrine processes. SP was also related to various physiological and pathological processes in the human body35. Additionally, a prospective clinical trial found that APR decreased aldosterone production by approximately 30% in healthy male participants by inhibiting the vasorelaxant effect of SP, leading to a decrease in adrenal blood flow36.Isorna and colleagues had found that both SP and NK-1R were expressed in normal thyroid tissue and thyroid cancer37.In summary, we speculated that the endocrine disorders were related to the antagonism of NK-1R to SP, however, the more precise mechanisms still require further research and exploration.The signal strength of back pain linked to all three medications found to be high at the PT level (APR: N = 163, ROR 3.86; FAP: N = 52, ROR 3.91; RP: N = 32, ROR 3.96), indicating the need for further attention.Furthermore, continuous exposure to SP could be associated with the development of tendinopathy38. Therefore, it was suggested39 that the NK-1RA showed promise in treating tendinopathy, rheumatoid arthritis and osteoarthritis. However, in our study, apart from the commonly reported AEs, we found some new AEs in musculoskeletal and connective tissue disorders, such as musculoskeletal stiffness, osteoarthritis, systemic lupus erythematosus and so on. The mechanism was considered to possibly involve the SP-NK1R pathway in inflammatory processes, as suggested by the aberrant expression of this pathway in various inflammatory diseases40.
This research offers trustworthy scientific data for evaluating the safety of NK-1RAs from various angles. Nevertheless, the FAERS database is not without its drawbacks, given its reliance on voluntary reporting that could introduce bias anf result in incomplete data. Some reports were submitted by consumers, which may lack the reliability and depth of analysis seen in reports from medical professors. Moreover, the database’s incomplete nature means that a definitive causal relationship between product exposure and NK-1RA-related AEs cannot be proven. More cases and clinical trials are necessary in the future to establish scientific conclusions.
Conclusion
This study not only confirms the known AEs of NK-1RAs listed on the drug label but also identifies several new high-risk AEs, such as inappropriate antidiuretic hormone secretion, adrenal insufficiency and hyponatremia. In addition, our study also shows that the number of reactions at the site of infection associated with NK-1RAs was high. Furthermore, the encephalopathy toxicity associated with APR was higher than with the other two drugs. The analysis of AEs associated with NK-1RAs in the real world suggests the need to be vigilant for the occurrence of ISRs when using intravenous NK-1RAs.Secondly, it suggests the need for further research into new AEs and potential drug interactions to provide safety recommendations for clinical medication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
LS designed the study. XS and JQW download and clean the data. LS and YGJ analyzed the data and generated the figures. LS, PH, and JQW wrote the manuscript. XS and YGJ supervised the details of the study. All authors reviewed the manuscript.
Funding
This study was supported by grants from the 2021 Suzhou High-tech Zone Health Talent Project.
Data availability
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.The datasets supporting the conclusions of this study are publicly accessible. All data we used in this study is available on the website:https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong Pan, Xiang Shi and Yiguo Jiang share first authorship.
Contributor Information
Jiaqiang Wu, Email: 18115846916@163.com.
Li Shen, Email: lishen_clic@163.com.
References
- 1.Dranitsaris, G. et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann. Oncol.28, 1260–1267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229–263 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Zheng, R. S. et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 46, 221–231 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Rojas, C. & Slusher, B. S. Mechanisms and latest clinical studies of new NK1 receptor antagonists for chemotherapy-induced nausea and vomiting: Rolapitant and NEPA (netupitant/palonosetron). Cancer Treat. Rev.41, 904–913 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Frame, D. G. Best practice management of CINV in oncology patients: I. Physiology and treatment of CINV. Multiple neurotransmitters and receptors and the need for combination therapeutic approaches. J. Support Oncol.8, 5–9 (2010). [PubMed] [Google Scholar]
- 6.Navari, R. M. & Schwartzberg, L. S. Evolving role of neurokinin 1-receptor antagonists for chemotherapy-induced nausea and vomiting. Onco Targets Ther.11, 6459–6478 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam, M., Buddenkotte, J., Ahmad, F. & Steinhoff, M. Neurokinin 1 receptor antagonists for Pruritus. Drugs81, 621–634 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schöppe, J. et al. Crystal structures of the human neurokinin 1 receptor in complex with clinically used antagonists. Nat. Commun.10, 17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piechotta, V. et al. Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis. Cochrane Database Syst. Rev.11, Cd012775 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, Y. et al. Safety assessment of Brexpiprazole: real-world adverse event analysis from the FAERS database. J. Affect. Disord. 346, 223–229 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Zhou, Q. et al. Adverse events of epidiolex: a real-world drug safety surveillance study based on the FDA adverse event reporting system (FAERS) database. Asian J. Psychiatr. 90, 103828 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Zou, F. et al. A real-world pharmacovigilance study of mepolizumab in the FDA adverse event reporting system (FAERS) database. Front. Pharmacol.14, 1320458 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, G., Wang, J., Du, R., Wang, Y. & Li, Z. Toxicity spectrum of Anti-GD2 immunotherapy: a real-world study leveraging the US Food and Drug Administration adverse event reporting system. Paediatr. Drugs. 26, 175–185 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Aapro, M. CINV: still troubling patients after all these years. Support Care Cancer. 26, 5–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weibel, S. et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: an abridged Cochrane network meta-analysis. Anaesthesia76, 962–973 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Wang, D. S. et al. Effect of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in women: a randomized clinical trial. JAMA Netw. Open.4, e215250 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osoba, D. et al. Determinants of postchemotherapy nausea and vomiting in patients with cancer. Quality of life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol.15, 116–123 (1997). [DOI] [PubMed] [Google Scholar]
- 18.Lin, L. et al. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990–2019. J. Hematol. Oncol.14, 197 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dranitsaris, G., Moezi, M., Dobson, K., Phelan, R. & Blau, S. A real-world study to evaluate the safety and efficacy of three injectable neurokinin-1 receptor antagonist formulations for the prevention of chemotherapy-induced nausea and vomiting in cancer patients. Support Care Cancer. 30, 6649–6658 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leal, A. D. et al. Fosaprepitant-induced phlebitis: a focus on patients receiving doxorubicin/cyclophosphamide therapy. Support Care Cancer. 22, 1313–1317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii, T. et al. Differential impact of fosaprepitant on infusion site adverse events between cisplatin- and anthracycline-based chemotherapy regimens. Anticancer Res.35, 379–383 (2015). [PubMed] [Google Scholar]
- 22.Nishibe-Toyosato, S. et al. Comparing injection site reactions of aprepitant and fosaprepitant in gynecologic cancer chemotherapy. Vivo38, 2374–2382 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azuma, J. & Fukase, H. Pharmacokinetics of a single 150-mg intravenous infusion of Fosaprepitant: effects of concentration and infusion time in healthy Japanese men. Clin. Pharmacol. Drug Dev.2, 394–399 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Cass, A. S., Odinet, J. S., Valgus, J. M. & Crona, D. J. Infusion reactions following administration of intravenous rolapitant at an academic medical center. J. Oncol. Pharm. Pract.25, 1776–1783 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Wang, X. et al. Bioequivalence of intravenous and oral rolapitant: results from a randomized, open-label pivotal study. J. Clin. Pharmacol.57, 1600–1606 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Tsuda, T. et al. Infusion site adverse events in breast cancer patients receiving highly emetic chemotherapy with prophylactic anti-emetic treatment with aprepitant and fosaprepitant: a retrospective comparison. Mol. Clin. Oncol.4, 603–606 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chau, E., Lundberg, J., Phillips, G., Berger, M. & Wesolowski, R. Updated report on incidence of infusion-site reactions associated with peripheral intravenous administration of fosaprepitant. J. Oncol. Pharm. Pract.25, 1053–1057 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Sano, T., Setsu, N., Kobayashi, E. & Kawai, A. A case of Encephalopathy caused by Drug Interaction between Ifosfamide and Aprepitant. Yakugaku Zasshi. 143, 541–544 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Shimada, K. et al. Adverse event profiles of ifosfamide-induced encephalopathy analyzed using the Food and Drug Administration adverse event reporting system and the Japanese adverse drug event report databases. Cancer Chemother. Pharmacol.84, 1097–1105 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Jordan, K. et al. The NK-1 receptor-antagonist aprepitant in high-dose chemotherapy (high-dose melphalan and high-dose T-ICE: paclitaxel, ifosfamide, carboplatin, etoposide): efficacy and safety of a triple antiemetic combination. Bone Marrow Transpl.46, 784–789 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Durand, J. P., Gourmel, B., Mir, O. & Goldwasser, F. Antiemetic neurokinin-1 antagonist aprepitant and ifosfamide-induced encephalopathy. Ann. Oncol.18, 808–809 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Vazirian, F., Samadi, S., Rahimi, H., Sadeghi, M. & Mohammadpour, A. H. Aprepitant, fosaprepitant and risk of ifosfamide-induced neurotoxicity: a systematic review. Cancer Chemother. Pharmacol.90, 1–6 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Modi, J. N. & Cimino, S. K. Incidence of ifosfamide induced encephalopathy in patients receiving concomitant fosaprepitant. J. Oncol. Pharm. Pract.27, 1891–1895 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Idle, J. R. & Beyoğlu, D. Ifosfamide - History, efficacy, toxicity and encephalopathy. Pharmacol. Ther.243, 108366 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Recio, S. & Gascón, P. Biological and Pharmacological aspects of the NK1-Receptor. Biomed. Res. Int.495704 2015 (2015). [DOI] [PMC free article] [PubMed]
- 36.Wils, J. et al. The neuropeptide substance P regulates aldosterone secretion in human adrenals. Nat. Commun.11, 2673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isorna, I., Esteban, F., Solanellas, J., Coveñas, R. & Muñoz, M. The substance P and neurokinin-1 receptor system in human thyroid cancer: an immunohistochemical study. Eur. J. Histochem.64, (2020). [DOI] [PMC free article] [PubMed]
- 38.Oh, S. Y. et al. Sustained exposure of substance P causes tendinopathy. Int. J. Mol. Sci.21, (2020). [DOI] [PMC free article] [PubMed]
- 39.Ko, K. R. et al. Substance P, a promising therapeutic target in musculoskeletal disorders. Int. J. Mol. Sci.23, (2022). [DOI] [PMC free article] [PubMed]
- 40.Muñoz, M. & Coveñas, R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids. 46, 1727–1750 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.The datasets supporting the conclusions of this study are publicly accessible. All data we used in this study is available on the website:https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html