Abstract
Limited knowledge exists regarding biomarkers that predict treatment response in Lupus nephritis (LN). We aimed to identify potential molecular biomarkers to predict treatment response in patients with LN. We enrolled 66 patients with active LN who underwent renal biopsy upon enrollment. Serum and urine samples were collected longitudinally, and we measured 12 biomarkers in each sample using a multiplex immunofluorescence assay. These biomarkers included monocyte chemoattractant protein-1 (MCP-1), interferon gamma-induced protein 10 (IP-10), interferon-γ (IFN-γ), interleukin 6 (IL-6), interleukin 16 (IL-16), interleukin 17 (IL-17), interleukin 23 (IL-23), tumor necrosis factor receptor II (TNF-RII), vascular cell adhesion molecule 1 (VCAM-1), retinol-binding protein 4 (RBP 4), vitamin D binding protein (VDBP), and neutrophil gelatinase-associated lipocalin (NGAL). Patients were categorized into two groups based on their 1-year treatment response to Mycophenolate mofetil (MMF)-based therapy: 50 responders and 16 non-responders. Only urine IL-17 (uIL-17) showed baseline level differences between the two groups, with higher in responders. In ROC curve analyses assessing the predictive performance of biomarkers, baseline uIL-17 and changes in uIL-6 and uIL-23 levels at 3 months could predict the 1-year treatment response, showing AUC values of 0.70 (95% CI 0.54–0.87), 0.70 (0.54–0.86), and 0.71 (0.57–0.85), respectively. Combining uIL-6 and uIL-23 into a model improved predictability, achieving an AUC of 0.75 (0.61–0.90). Baseline uIL-17 levels and early changes in uIL-6 and uIL-23 could serve as potential biomarkers to predict 1-year treatment response in lupus nephritis patients receiving MMF-based therapy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83057-4.
Keywords: Lupus nephritis, Biomarker, Treatment response
Subject terms: Molecular biology, Rheumatology
Introduction
Lupus nephritis (LN) is one of the most severe organ manifestations in systemic lupus erythematosus (SLE), affecting 40–69% of patients with SLE1,2. As per the American College of Rheumatology (ACR) guidelines, all patients exhibiting clinical evidence of active LN are recommended to undergo renal biopsy. This procedure is recognized as the gold standard for diagnosing LN3. Additionally, conventional clinical parameters such as proteinuria, creatinine (Cr), anti-double stranded DNA antibody (anti-dsDNA), complement 3 (C3), and complement 4 (C4) are utilized to diagnose and assess LN disease activity. However, these biomarkers have significant limitations. For instance, proteinuria cannot distinguish between active and chronic lesions or differentiate glomerular from tubular origin of protein leakage, while complement levels and anti-dsDNA titers do not consistently correlate with pathologic processes. For these reasons, the need for appropriate biomarkers that can reflect disease activity and pathological findings has been increasingly emphasized. Additionally, in cases of active proliferative or membranous LN, the commonly recommended induction therapy primarily involves mycophenolate mofetil (MMF), with the combination of MMF and tacrolimus (TAC) being considered as an alternative treatment approach3–7. Nevertheless, treatment response varies among patients, with only approximately 30% achieving a complete response (CR) following standard induction therapy8,9. These challenges further highlight the importance of identifying more precise and clinically applicable biomarkers to improve LN management and patient outcomes.
Recent research has focused on identifying non-invasive and convenient biomarker assays that are expected to outperform conventional biomarkers. Over the past decade, several new biomarkers, including serum and urinary chemokines, cytokines, adhesion molecules, and growth factors, have been evaluated for their potential in predicting treatment response. At the baseline before induction treatment, elevated levels of serum interleukin 17 (sIL-17), interleukin 23 (sIL-23), urine monocyte chemoattractant protein-1 (uMCP-1), and neutrophil gelatinase-associated lipocalin (uNGAL) have been associated with an unfavorable response to treatment10–16. Regarding treatment-related changes, a significant decrease in serum interleukin 6 (sIL-6), interferon-γ (sIFN-γ), tumor necrosis factor receptor II (sTNF-RII), vascular cell adhesion molecule 1 (sVCAM-1), and urine retinol-binding protein (uRBP4) levels have been observed in clinical remission cases17–21. Certain biomarkers, including interferon gamma-induced protein 10 (IP-10), demonstrated a significant association with treatment response in serum and urine, with a significant decrease observed following therapy22. In a juvenile cohort, responders exhibited a significant decrease in urine levels of vitamin D binding protein (uVDBP) from 3 to 12 months than non-responders23. A recent study conducted by the Accelerating Medicines Partnership (AMP) group, utilizing proteomics-based approaches, revealed that urinary levels of interleukin 16 (uIL-16) decreased over time in patients responding to 1-year treatment24. However, these studies have pointed out issues such as incomplete datasets and relatively insufficient longitudinal follow-up regarding treatment response11,12,15–17,22. We have addressed these issues by collecting comprehensive datasets from all patients at baseline and at 0, 3, 6, and 12 months. Additionally, to increase homogeneity, we used proliferative LN and an MMF-based treatment. Furthermore, this study collected urine samples before the biopsy, in contrast to clinical trials where urine samples for biomarker discovery are usually collected after the biopsy and initial pre-randomization treatment.
Therefore, this study aims to identify potential biomarkers that can predict treatment response in patients with LN. We conducted a prospective study focusing on a relatively homogeneous group of patients with LN regarding histopathology and treatment regimens. The main goal was to identify potential biomarkers from a pool of 12 candidates selected based on prior studies. To accomplish this, we utilized longitudinally collected serum and urine samples from the prospective Korean Unlimited multi-Dimensional Omics research in SLE (KUDOS) cohort25.
Methods
Study design and participants
The study participants are part of the KUDOS cohort in South Korea, a prospective cohort consisting of active biopsy-proven patients with LN enrolled between March 2018 and July 202025. All patients (N = 66) were aged ≥ 18 years old and met either the 2012 Systemic Lupus International Collaborating Clinics (SLICC) clinical classification criteria or 2019 European League Against Rheumatism (EULAR)/ACR classification criteria26,27. Patients presenting with persistent proteinuria of ≥ 500 mg/day (measured by urine protein to creatinine ratio [UPCR] or 24-hour urine collection) and/or active sediment (more than five red or white blood cells per high-power field [HPF] and/or the presence of ≥ 1 cellular cast) underwent renal biopsy. Serum and urine samples for biomarker analysis were collected at the time of renal biopsy (baseline) and then longitudinally at 3, 6, and 12 months. All patients underwent induction treatment following the current LN treatment guidelines from the ACR and EULAR recommendations3,28. In the initial induction regimen, MMF was administered at a dosage of 2 g/day. Patients who were intolerant to MMF were given a combination of low-dose MMF (0.5–1 g/day) and TAC (1–3 mg/day). At enrollment, all patients were treated with either prednisolone at a dosage of 0.5–1 mg/kg/day or intravenous methylprednisolone pulse therapy followed by oral prednisolone. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of the Hanyang University Hospital (IRB No: HYUH2017-08-035). All study participants provided written informed consent.
Data collection at baseline and during 1-year follow-up
In this study, baseline and follow-up clinical data on renal function were collected, including serum creatinine, estimated glomerular filtration rate (eGFR), blood urea nitrogen, and urine UPCR obtained from either a spot urine sample or a 24-hour urine collection. Additionally, SLE-related autoantibodies such as anti-dsDNA antibodies, anti-Smith antibodies (anti-Sm), anti-ribonucleoprotein antibodies (anti-RNP), anti-Ro/La antibodies, and antiphospholipid antibodies (including lupus anticoagulant, anti-cardiolipin antibody, and β2-glycoprotein 1 [β2-GP1] IgM/IgG antibody), and complement levels (C3, C4, and CH50) were assessed. The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and renal SLEDAI (rSLEDAI) were also assessed29. Renal SELDAI is defined as the sum of scores of four components of urine examination, including proteinuria, urinary casts, hematuria, and pyuria. The classification of LN, along with the activity index (AI) and chronicity index (CI), adhered to the revised 2018 International Society of Nephrology/Renal Pathology Society (ISN/RPS) guidelines30.
Biomarker selection
We searched for research papers on LN-associated biomarkers published between 2011 and 2021 via PubMed, identifying 45 potential biomarkers. From these, we selected 16 biomarkers based on the following specific criteria: (1) specificity to nephritis among patients with SLE, (2) strong correlation with renal activity, (3) capability for serial monitoring to track disease activity, treatment response, and clinical remission, and (4) ability to predict renal treatment response31. After excluding biomarkers lacking commercially available kits and those that cannot be analyzed through multiplex immunofluorescence assay, we ultimately selected 12 biomarkers for analysis in serum and urine samples (MCP-1, IP-10, IFN-r, IL-6, IL-16, IL-17, IL-23, TNF-RII, VCAM-1, RBP4, VDBP, and NGAL).
Biomarker assay
The serum and urine samples were centrifuged, and the resulting supernatant was aliquoted and stored at -80 °C until subsequent analysis. The concentrations of 12 biomarkers (MCP-1, IP-10, IFN-r, IL-6, IL-16, IL-17, IL-23, TNF-RII, VCAM-1, RBP4, VDBP, NGAL) in the serum and urine were assessed using multiplex immunofluorescence assay (Luminex® 100/200™) kits from R&D Systems (LXSAHM-01, LXSAHM-01, LXSAHM-09, and LXSAHM-11) through LABIS KOMA Inc., following the instructions of the manufacturer. Before the experiment, the samples were thawed, vortexed, and centrifuged at 10,000 g for 2 min at a refrigerated temperature to obtain the supernatant. The supernatant was subsequently diluted, with most assays using a 1:2 dilution for both serum and urine samples. However, serum NGAL was diluted using 1:50, and serum RBP4 and VDBP were diluted using 1:4,000. All analytes were measured in duplicate, with a single sample on each of two separate plates, and the results were averaged. Urinary biomarker levels were normalized via urine creatinine to account for variations in urine concentration. Urine creatinine was measured using commercial ELISA kits from R&D Systems (Catalog number KGE005), which were diluted 1:20 and processed according to the instructions of the manufacturer. Each sample was analyzed twice, and the protein concentration was determined using a 5-parameter logistic curve to fit the standard curve.
Definition of treatment response
The treatment response was assessed based on CR, partial response (PR), and non-response (NR) using criteria from both the ACR and Kidney Disease Improving Global Outcomes (KDIGO) guidelines32,33. CR is defined as having a “UPCR (or 24-hour urine collection) of 500 mg/day or less, and if the serum creatinine (and/or eGFR) was abnormal, it had to be 25% lower than baseline”. PR was defined as achieving at least a 50% reduction in UPCR (or 24-hour urine collection) compared to baseline. However, if the patient had nephrotic-range proteinuria (≥ 3000 mg/day) at baseline, achieving a UPCR of < 3000 mg/day was also required, and if the serum creatinine (and/or eGFR) was abnormal, it had to be 25% lower than baseline”. NR is defined as “neither CR nor PR”. Subsequently, patients in the CR/PR group were classified as responders, while those in the NR group were classified as non-responders.
Statistical analysis
Continuous variables were presented as median ± interquartile ranges (IQR), while categorical data were analyzed using either the Pearson chi-square test or Fisher’s exact test. To identify biomarkers with differing baseline levels between the responder and non-responder groups and to examine variations in the patterns of serum and urine biomarker changes after treatment between them, comparison of variables between two groups at baseline and each time point (3, 6, and 12 months) was performed using the non-parametric Mann-Whitney U test. To assess differences between variables at different time points (3, 6, 12 months) and baseline, the non-parametric Wilcoxon signed-rank test was utilized. To determine the association of LN disease activity markers with potential biomarkers, Spearman’s rank correlation was used for correlation analysis between 24 molecular biomarkers and traditional activity markers. The validity of molecular biomarkers in predicting treatment response for LN was assessed using receiver operating characteristic (ROC) curve analyses, including calculation of the corresponding area under the curve (AUC) with 95% confidence intervals (CI). All p-values were calculated as two-tailed. A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using Statistical Analysis System (SAS) version 9.4 statistical software (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of patients
This study enrolled 66 patients with biopsy-proven active LN, consisting of 50 responders and 16 non-responders categorized based on their 1-year treatment response. The patients had a mean age of 34.0 years at baseline. And the majority (90.9%) were female. The mean LN duration was 5.7 years, and 42.4% of patients were newly diagnosed. All patients exhibited proliferative LN (Table 1), with class IV being the most common (47.0%), followed by classes III, III + V, and IV + V at 22.7%, 16.7%, and 13.6%, respectively. Pure membranous LN cases were not included. All patients were treated with MMF or a combination of MMF and TAC for induction therapy. Among responders, 86.0% were treated with MMF alone and 14.0% with the combination, while among non-responders, 68.8% received MMF alone and 31.3% received the combination, with a p-value of 0.14. No significant differences were observed in the baseline characteristics between the two groups.
Table 1.
Baseline characteristics of study participants.
| Variable* | Total (N = 66) | Responders (N = 50) | Non-responders (N = 16) | p-value |
|---|---|---|---|---|
| Female sex, n (%) | 60 (90.9) | 46 (92.0) | 14 (87.5) | 0.63 |
| Age at enrollment, mean (± SD) | 34.0 (1.2) | 35.0 (1.4) | 30.8 (2.0) | 0.16 |
| LN duration, mean (± SD) | 5.7 (0.8) | 5.9 (1.0) | 4.8 (1.6) | 0.99 |
| Lupus nephritis | 0.65 | |||
| New-onset LN, n (%) | 28 (42.4) | 22 (44.0) | 6 (37.5) | |
| Relapse LN, n (%) | 38 (57.6) | 28 (56.0) | 10 (62.5) | |
| Laboratory findings | ||||
| Creatinine (mg/dl) | 0.6 (0.5, 0.8) | 0.61 (0.5, 0.8) | 0.60 (0.6, 0.9) | 0.61 |
| eGFR (ml/min/1.73m2) | 120.0 (102.2, 130.8) | 120.0 (106.5, 128.2) | 117.5 (95.8, 132.0) | 0.81 |
| C3 (mg/dl) | 50.0 (40.0, 60.0) | 49.5 (41.0, 60.0) | 51.0 (38.5, 58.5) | 0.85 |
| C4 (mg/dl) | 9.1 (5.3, 12.0) | 8.0 (5.3, 12.0) | 9.0 (5.5, 11.3) | 0.76 |
| Proteinuria (g/day) | 1.8 (1.2, 3.0) | 1.9 (1.2, 3.8) | 1.6 (1.1, 2.0) | 0.21 |
| SLEDAI | 16 (12, 18) | 16 (12.3, 18) | 16 (12, 17.3) | 0.78 |
| Renal SLEDAI | 12 (8, 12) | 12 (8, 12) | 12 (11, 12) | 0.37 |
| SDI | 0 (0, 1) | 0 (0, 1) | 1 (0, 1.5) | 0.29 |
| Autoantibody | ||||
| Anti-dsDNA, n (%) | 52 (78.8) | 42 (84.0) | 10 (62.5) | 0.09 |
| Anti-Sm, n (%) | 24 (36.4) | 16 (32.0) | 8 (50.0) | 0.32 |
| Anti-Ro, n (%) | 40 (60.6) | 27 (54.0) | 13 (81.3) | 0.10 |
| Anti-La, n (%) | 12 (18.2) | 8 (16.0) | 4 (25.0) | 0.46 |
| Anti-RNP, n (%) | 32 (48.5) | 26 (52.0) | 6 (37.5) | 0.47 |
| Anti-APL, n (%)** | 14 (21.2) | 11 (22.0) | 3 (18.8) | 1.00 |
| Biopsy class | 0.26 | |||
| III, n (%) | 15 (22.7) | 13 (26.0) | 2 (12.5) | |
| IV, n (%) | 31 (47.0) | 23 (46.0) | 8 (50.0) | |
| III + V, n (%) | 11 (16.7) | 6 (12.0) | 5 (31.3) | |
| IV+V, n (%) | 9 (13.6) | 8 (16.0) | 1 (6.3) | |
| Renal histology (ISN/RPS) | ||||
| AI, /24 | 7 (5, 10) | 8 (5.3, 10) | 5 (3.8, 8.5) | 0.20 |
| CI, /12 | 2 (0.3, 3) | 2 (0, 3) | 1.5 (1, 3) | 0.63 |
| Induction treatment | 0.14 | |||
| MMF, n (%) | 54 (81.8) | 43 (86.0) | 11 (68.8) | |
| MMF + TAC, n (%) | 12 (18.2) | 7 (14.0) | 5 (31.3) | |
LN lupus nephritis, eGFR estimated glomerular filtration rate, C3 complement 3, C4 complement 4, Anti-dsDNA anti-double-stranded deoxyribonucleic acid, Anti-Sm anti-Smith, Anti-RNP anti-ribonucleoprotein, APL antibody anti-phospholipid antibody, SLEDAI systemic lupus erythematosus disease activity index, SDI SLICC/ACR damage index, AI Activity index, CI chronicity index, MMF mycophenolate mofetil, TAC tacrolimus.
*Values are the median (Q1, Q3) unless otherwise indicated.
**Have at least one antiphospholipid antibody.
Correlation between molecular biomarkers with traditional activity markers of LN
Table 2 shows the correlation between 12 serum and urine molecular biomarkers and traditional LN activity markers at baseline levels. Among these, proteinuria showed a significant positive correlation with several biomarkers, significantly demonstrating a strong correlation with uVDBP (rho = 0.62, p < 0.001). SLEDAI score, reflecting lupus activity, correlated positively with five urine biomarkers: uMCP-1 (rho = 0.36, p = 0.003), uIL-16 (rho = 0.30, p = 0.01), uIL-23 (rho = 0.33, p = 0.008), uVCAM-1 (rho = 0.30, p = 0.01), and uNGAL (rho = 0.35, p = 0.004). Moreover, all these biomarkers correlated positively with the rSLEDAI. Urine biomarkers including uMCP-1 (rho = 0.49, p < 0.001), uIP-10 (rho = 0.44, p < 0.001), uIL-6 (rho = 0.27, p = 0.03), uIL-16 (rho = 0.25, p = 0.04), uVDBP (rho = 0.35, p = 0.004), and uNGAL (rho = 0.34, p = 0.006) and serum biomarker, sTNF-RII (rho = 0.38, p = 0.002) showed a positive correlation with renal AI. Urine IL-6 (rho = 0.26, p = 0.04) was the only biomarker that demonstrated a positive correlation with renal CI.
Table 2.
Correlations between 12 biomarkers and traditional lupus nephritis activity markers in the baseline.
| eGFR | C3 | C4 | SLEDAI | rSLEDAI | Proteinuria | Renal AI | Renal CI | Anti-dsDNA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum ρ | Urine ρ | Serum ρ | Urine ρ | Serum ρ | Urine ρ | Serum ρ | Urine ρ | Serum ρ | Urine ρ | Serum ρ | Urine ρ | Serum ρ | Urine ρ | Serum ρ | Urine ρ | Serum ρ | Urine ρ | |
| MCP-1 | − 0.29* | − 0.25* | − 0.27* | 0.36** | 0.45*** | 0.46*** | 0.49*** | |||||||||||
| IP-10 | − 0.28* | − 0.25* | 0.44*** | 0.30* | ||||||||||||||
| IFN-γ | 0.30* | 0.24* | ||||||||||||||||
| IL-6 | − 0.31* | 0.47*** | 0.27* | 0.26* | ||||||||||||||
| IL-16 | − 0.28* | 0.30* | 0.47*** | 0.39** | 0.25* | |||||||||||||
| IL-17 | 0.26* | 0.37** | ||||||||||||||||
| IL-23 | 0.33** | 0.34** | ||||||||||||||||
| TNF-RII | − 0.30* | − 0.25* | − 0.30* | 0.38** | ||||||||||||||
| VCAM-1 | − 0.27* | 0.30* | 0.37** | 0.41*** | ||||||||||||||
| RBP-4 | − 0.30* | |||||||||||||||||
| VDBP | − 0.41*** | 0.62*** | 0.35** | |||||||||||||||
| NGAL | − 0.28* | 0.35** | 0.53*** | 0.47*** | 0.34** | |||||||||||||
MCP-1 monocyte chemoattractant protein-1, IP-10 interferon-gamma inducible protein-10, IFN-γ interferon-gamma, IL-6 interleukin-6, IL-16 interleukin-16, IL-17 interleukin-17, IL-23 interleukin-23, TNF-RII tumor necrosis factor receptor II, VCAM-1 vascular cell adhesion molecule-1, RBP4 retinol binding protein 4, VDBP vitamin D binding protein, NGAL neutrophil gelatinase-associated lipocalin, ρ Spearman’s rank correlation coefficient, eGFR estimated glomerular filtration rate, C3 complement 3, C4 complement 4, SLEDAI systemic lupus erythematosus disease activity index, rSLEDAI renal domain score of SLEDAI, AI activity index, CI chronicity index, Anti-dsDNA anti-double-stranded deoxyribonucleic acid titer.
*p-value ≤ 0.05, **p-value ≤ 0.01, ***p-value ≤ 0.001.
Comparison of molecular biomarkers levels between responders and non-responders
In the analysis whether there were differences in baseline levels of biomarkers between responder and non-responder groups, only urine IL-17 showed a significant distinction, with responders exhibiting higher levels than in non-responders (12.8 versus 4.6 pg/mg creatinine, p = 0.02) (Table 3).
Table 3.
Baseline levels of 12 biomarkers of patients with lupus nephritis based on treatment response.
| Variable* | Total (N = 66) | Responders (N = 50) | Non-responders (N = 16) | p-value |
|---|---|---|---|---|
| Serum | ||||
| MCP-1 (pg/ml) | 389.1 (272.1, 566.5) | 382.0 (253.4, 560.2) | 389.1 (345.7, 547.2) | 0.37 |
| IP-10 (pg/ml) | 61.1 (36.7, 142.1) | 64.7 (37.0, 144.4) | 54.0 (34.1, 124.7) | 0.61 |
| IFN-γ (pg/ml) | 1.5 (0.9, 1.5) | 1.5 (0.9, 1.5) | 1.2 (0.9, 1.8) | 0.80 |
| IL-6 (pg/ml) | 1.8 (0.8, 4.6) | 2.0 (0.8, 6.4) | 1.2 (0.1, 2.7) | 0.18 |
| IL-16 (pg/ml) | 111.0 (77.9, 168.9) | 99.9 (75.8, 178.1) | 132.5 (102.8, 155.7) | 0.34 |
| IL-17 (pg/ml) | 1.1 (0.4, 3.4) | 1.1 (0.4, 3.4) | 1.1 (0.4, 2.7) | 0.91 |
| IL-23 (pg/ml) | 259.3 (185.1, 446.1) | 270.7 (185.1, 528.0) | 259.3 (194.8, 374.3) | 0.76 |
| TNF-RII (ng/ml) | 4.4 (3.0, 6.3) | 4.4 (2.9, 6.3) | 4.5 (3.1, 6.5) | 0.68 |
| VCAM-1 (ng/ml) | 1966.5 (1094.9, 2454.0) | 1727.1 (1083.5, 2442.0) | 2262.3 (1232.9, 2402.1) | 0.33 |
| RBP4 (µg/ml) | 31.3 (17.7, 52.4) | 26.8 (17.3, 51.2) | 42.0 (30.4, 81.0) | 0.09 |
| VDBP (µg/ml) | 250.5 (112.7, 200.8) | 88.0 (64.8, 200.8) | 130.3 (71.8, 253.3) | 0.33 |
| NGAL (ng/ml) | 16.6 (11.8, 23.1) | 15.8 (11.8, 22.6) | 20.4 (13.0, 26.2) | 0.47 |
| Urine | ||||
| MCP-1 (pg/mg creatinine) | 480.7 (282.4, 853.5) | 519.3 (277.4, 867.8) | 368.4 (288.5, 788.9) | 0.44 |
| IP-10 (pg/mg creatinine) | 31.6 (21.7, 54.6) | 32.6 (24.6, 55.7) | 24.5 (15.8, 47.3) | 0.24 |
| IFN-γ (pg/mg creatinine) | 30.4 (17.9, 45.1) | 31.1 (20.0, 53.0) | 25.8 (17.1, 43.7) | 0.52 |
| IL-6 (pg/mg creatinine) | 8.6 (5.1, 14.6) | 11.4 (5.4, 19.6) | 6.6 (5.0, 9.0) | 0.14 |
| IL-16 (pg/mg creatinine) | 107.1 (63.8, 160.2) | 112.1 (62.8, 166.0) | 81.3 (64.3, 128.6) | 0.32 |
| IL-17 (pg/mg creatinine) | 10.2 (4.9, 20.6) | 12.8 (5.8, 20.8) | 4.6 (3.6, 12.0) | 0.02 |
| IL-23 (pg/mg creatinine) | 694.8 (458.3, 1053.7) | 775.9 (504.3, 1099.9) | 589.5 (400.2, 766.6) | 0.12 |
| TNF-RII (ng/mg creatinine) | 12.9 (6.3, 24.0) | 12.3 (6.4, 22.4) | 14.9 (5.9, 26.1) | 0.75 |
| VCAM-1 (ng/mg creatinine) | 13.3 (8.8, 19.3) | 13.7 (9.3, 19.6) | 9.5 (7.0, 16.7) | 0.12 |
| RBP4 (ng/mg creatinine) | 203.2 (122.0, 1052.7) | 195.0 (129.5, 755.4) | 237.1 (113.3, 1304.7) | 0.98 |
| VDBP (ng/mg creatinine) | 263.1 (90.6, 764.5) | 263.1 (84.9, 749.9) | 293.9 (91.5, 828.6) | 0.89 |
| NGAL (ng/mg creatinine) | 18.3 (11.8, 27.2) | 18.6 (12.4, 27.1) | 16.0 (6.1, 28.0) | 0.29 |
MCP-1 monocyte chemoattractant protein-1, IP-10 interferon-gamma inducible protein-10, IFN-γ interferon-gamma, IL-6 interleukin-6, IL-16 interleukin-16, IL-17 interleukin-17, IL-23 interleukin-23, TNF-RII tumor necrosis factor receptor II, VCAM-1 vascular cell adhesion molecule-1, RBP4 retinol binding protein 4, VDBP vitamin D binding protein, NGAL neutrophil gelatinase-associated lipocalin.
*Values are the median (Q1, Q3) unless otherwise indicated.
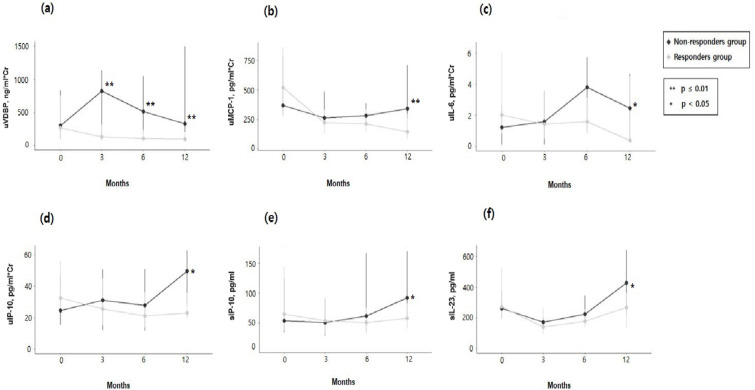
To examine variations in the patterns of serum and urine biomarker changes after treatment between responders and non-responders, we investigated the longitudinal pattern of biomarker levels at 3, 6, and 12 months following induction therapy. At 12 months, among the 12 biomarkers, each obtained from both serum and urine, analyzed in both serum and urine samples, six biomarkers (uVDBP, uMCP-1, uIL-6, uIP-10, sIP-10, and sIL-23) showed elevated levels in the non-responders compared to the responders (Fig. 1; Supplementary Table S1, and Supplementary Fig. S1). Their levels for uVDBP were 154.4 versus 109.4 ng/mg creatinine (p = 0.001), uMCP-1 levels were 342.7 versus 144.7 pg/mg creatinine (p = 0.001), uIL-6 levels measured 7.9 versus 2.4 pg/mg creatinine (p = 0.02), uIP-10 levels were 49.6 versus 23.0 pg/mg creatinine (p = 0.01), sIP-10 had values of 92.3 versus 57.9 pg/ml (p = 0.03), and sIL-23 levels were at 425.9 versus 266.3 pg/ml (p = 0.01). Additionally, uVDBP levels were significantly higher in non-responders compared to responders at both 3 and 6 months after induction therapy. At 3 months, the levels were 819.4 ng/mg creatinine in non-responders versus 128.1 ng/mg creatinine in responders (p = 0.04), and at 6 months, they were 511.0 ng/mg creatinine in non-responders versus 102.6 ng/mg creatinine in responders (p = 0.01).
Fig. 1.
Longitudinal patterns of six biomarkers between responders and non-responders. (a) Urine vitamin D binding protein (uVDBP). (b) Urine monocyte chemoattractant protein-1 (uMCP-1). (c) Urine interleukin-6 (uIL-6). (d) Urine interferon-gamma inducible protein-10 (uIP-10). (e) Serum interferon-gamma inducible protein-10 (sIP-10). (f) Serum interleukin-23 (sIL-23). Asterisk represents the p-value comparing the levels of responders and non-responders at each time point using the non-parametric Wilcoxon signed-rank test.
Comparison of the changes of molecular biomarkers following induction therapy between responders and non-responders
Upon assessing the differences between the baseline levels and those each time point, all six biomarkers (uVDBP, uMCP-1, uIL-6, uIP-10, sIP-10, and sIL-23) demonstrated significant changes in the delta (Δ) 12-month values (levels at 12-month minus baseline levels), as shown in Supplementary Table S1 and Supplementary Fig. S1. Among these six biomarkers, uVDBP, uIL-6, uIP-10, and uIL-23 also showed significant changes at Δ3 months or Δ6 months (Supplementary Table S1 and Supplementary Fig. S1).
ROC curve analyses for predictive biomarkers for 1-year treatment response
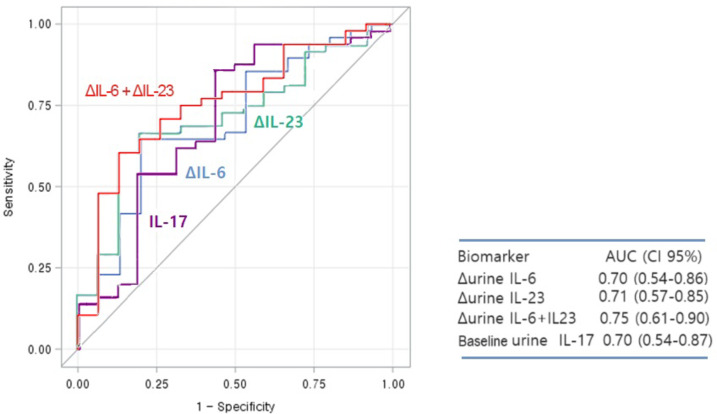
We performed additional ROC curve analyses to assess the predictive performance of biomarkers for treatment response at 1-year after induction treatment. uIL-17 levels at baseline significantly predicted the 1-year treatment response, with an AUC of 0.70 (95% CI 0.54–0.86, p = 0.022, Fig. 2). In contrast, other baseline clinical markers such as proteinuria, C3, C4, ds-DNA, renal AI and CI did not predict the 1-year treatment response [0.61 (95% CI 0.46–0.76, p = 0.154), 0.52 (0.34–0.69, p = 0.849), 0.53 (0.36–0.69, p = 0.759), 0.56 (0.38–0.74, p = 0.524), 0.61 (95% CI 0.43–0.78, p = 0.233) and 0.54 (95% CI 0.38–0.70, p = 0.623), respectively] (Supplementary Table S3).
Fig. 2.
Receiver operating characteristic (ROC) curves illustrate the predictive abilities of baseline urine interleukin-17 (uIL-17) and the changes in urine interleukin-6 (uIL-6), urine Interleukin-23 (uIL-23), and their combination at 3 months for predicting a 1-year response after induction therapy in patients with LN.
We further performed ROC curve analyses using the early changes at 3 months in each serum and urine biomarker and found that the changes in uIL-6 and uIL-23 levels at 3 months (Δ3-month values) could significantly predict the treatment response at 1-year, with AUC values of 0.70 (95% CI 0.54–0.86) and 0.71 (95% CI 0.57–0.85), respectively. When considering the combination of Δ3-month values for both uIL-6 and uIL-23, the predictive performance improved, with AUC values of 0.75 (95% CI 0.61–0.90) (Fig. 2). We performed subgroup analyses for the two groups: new-onset LN and relapsed LN (Supplementary Table S4). In the new-onset LN patient group, Δ3 uIL-23, combination of Δ3 uIL-6 and Δ3 uIL-23, and baseline IL-17 were all predictive of treatment response at 1 year, with baseline uIL-17 showing the highest predictive performance (AUC 0.79, 95% CI 0.60–0.97). In the relapsed LN patient group, Δ3 uIL-6 and combination of Δ3 uIL-6 and Δ3 uIL-23 demonstrated statistical significance, with combination of Δ3 uIL-6 and Δ3 uIL-23 exhibiting the best predictive performance (AUC 0.74, 95% CI 0.55–0.92).
Discussion
Using a prospective LN cohort, we conducted the study to identify molecular biomarkers capable of predicting treatment response after MMF-based induction therapy in patients with LN. Our investigation identified two significant findings: First, among the 12 molecular biomarkers analyzed in both serum and urine, six demonstrated significantly higher levels at 12 months in non-responders compared to responders. These biomarkers included uVDBP, uMCP-1, uIL-6, uIP-10, sIP-10, and sIL-23. Moreover, the Δ12-month values of these biomarkers showed greater increases in non-responders. Second, we identified potential predictive biomarkers for treatment response at 1-year, including baseline uIL-17 levels and changes in uIL-6 and uIL-23 levels at the 3 months after induction treatment.
We investigated the same panel of 12 biomarkers in both serum and urine samples; however, the biomarkers in urine showed a closer relationship to treatment response and disease activity than those in serum. Among the six molecular biomarkers showing significant changes during the 12 months after induction treatment, four were urine biomarkers (uVDBP, uMCP-1, uIL-6, and uIP-10). Additionally, potential predictive biomarkers for treatment response at 1-year were mainly urine biomarkers (uIL-17, uIL-6, and uIL-23). Furthermore, most biomarkers that positively correlated with conventional LN clinical parameters, including proteinuria, eGFR and renal histopathology (AI and CI), were urine biomarkers. These findings imply that urine biomarkers, possibly due to their stronger correlation with renal inflammation or chronic damage compared to serum biomarkers, hold promise for future clinical application as a non-invasive diagnostic tool.
Among the urine biomarkers, uIL-6, correlating with both AI and CI in renal histopathology, was predictive for renal response, showing a significant gradual decrease at 3, 6, and 12 months following therapy in responders compared to non-responders. Renal resident cells, including podocytes and tubular epithelial cells, produce IL-6, which is associated with various kidney conditions, including IgA nephropathy, diabetic nephropathy, chronic kidney disease, acute renal injury, and LN34. In addition, in an in vitro study of SLE, the neutralizing anti-IL-6 antibody inhibited spontaneous IgG production, a characteristic of SLE, while exogenous IL-6 restored it, suggesting its role in SLE pathogenesis35. Significantly, El-Shereef et al. found that the high levels of uIL-6 were associated with active LN rather than sIL-636. Singh et al. also showed a decrease in uIL-6 levels in the CR and PR group after treatment17. Consistent with these findings, our research suggests that a longitudinal decrease in uIL-6 levels was observed in responders to immunosuppressive therapy, in contrast to non-responders.
IL-23 is a pro-inflammatory cytokine known for driving the differentiation and activation of T helper 17 (Th17) cells, a process observed across various autoimmune diseases37. Previous studies have underscored the significance of the IL-23/IL-17 pathway in SLE38,39. Elevated levels of IL-23 have been consistently observed in both serum and urine samples of individuals with active LN compared to those with inactive LN or healthy controls, with a subsequent decrease after treatment10,14,40. In our study, sIL-23 levels showed a tendency to decrease more in responders than in non-responders after induction therapy, with statistical significance observed at the 12-month after induction treatment. This finding is consistent with those of previous studies. Similarly, uIL-23 levels also showed a tendency for greater reduction in responders than in non-responders after induction treatment, with significant differences revealed in uIL-23 levels at 3 months after induction treatment. Furthermore, the changes at 3 months after treatment were able to predict the treatment response at 1-year, suggesting a potential predictive biomarker as in uIL-6.
IL-17 is recognized as a pro-inflammatory activator, evidenced by increased serum levels in active LN, expansion of IL-17-producing T cells in the peripheral blood, and infiltration of Th 17 cells in the kidney in SLE41–44. Th17 cells migrating to the kidney play an important role in renal pathogenesis in LN. Galil et al. identified serum IL-17 levels as predictors of disease activity and remission, correlating with markers such as proteinuria, anti-dsDNA titers, and C3 levels45. Existing literature indicates that in patients with active LN, there is an expansion of IL-17-producing T cells in peripheral blood and migration of Th17 cells to the kidney. Th17 cells migrating to the kidney play an important role in renal pathogenesis in LN46, and both uIL-17 and sIL-17 have been shown to correlate with disease activity markers such as SLEDAI and BILAG200447,48. In our study, uIL-17 levels at baseline were significantly higher in responders compared to non-responders, consistent with the findings of Nordin et al.48. Furthermore, higher baseline levels of uIL-17 could predict treatment response in LN. Kwan et al. reported increased expression of IL-17-related genes in the urine of patients with SLE49. It is also known that IL-17-expressing T cells are present in glomeruli50. Thus, it can be speculated that IL-17-expressing T cells are a possible target of LN treatment and may be effectively suppressed by MMF-based treatment51,52. For this reason, LN patients with higher baseline levels of uIL-17, which indirectly reflects the status of IL-17-expressing T cells in the glomeruli, are more likely to respond well to treatment, as shown in our study. However, more research is needed to confirm this, and therefore caution is required in interpreting these results.
VDBP serves the essential function of transporting vitamin D metabolites in the bloodstream. After filtration by the glomerulus in the kidneys, VDBP is normally reabsorbed by proximal tubular cells through receptor-mediated uptake. However, in cases of tubulointerstitial inflammation, this process may be disrupted, leading to increased levels of VDBP excretion in the urine. uVBDP was increased with the severity of renal damage and associated with tubular and inflammatory markers independently of albuminuria53. VDBP has been also recognized to play a role in inflammation by contributing to macrophage activation and enhanced the chemotactic function of C5a54,55. These findings indicate that uVDBP has the potential to be developed as a non-invasive urinary biomarker to monitor tubulointerstitial inflammation and fibrosis. VDBP is involved in intricate interactions with the immune system, suggesting its potential role in immune-related processes. These observations are further supported by previous studies that highlight the increased clinical significance of uVDBP levels in active LN56,57. Our study showed that uVDBP levels were correlated with proteinuria, eGFR, and renal AI in active LN. In addition, uVDBP levels were significantly lower from 3 months to 12 months in the responders compared to non-responders. In a study conducted by Brunner et al. on children and young adults with LN, similar results were observed. The study found that the level of uVDBP significantly decreased (p < 0.01) in the response group after 3 months of treatment23. We were able to identify, for the first time in an adult population, the association between treatment response and uVDBP levels. However, this association was not observed in sVDBP levels, suggesting that sVDBP may not effectively reflect the urinary loss of VDBP caused by compensatory enhanced production58.
Another biomarker, IL-16, was identified in immune cells within the kidneys in LN through proteomics by the AMP group. They specifically focused on locating IL-16-producing cells at sites of kidney injury24. Moreover, their study revealed significant differences in urine IL-16 levels based on treatment response and its correlation with renal AI. In our study, a correlation between urine IL-16 and renal AI (r = 0.25, p = 0.04) was identified. However, no association with treatment response was observed, suggesting the need for further research.
The study had some limitations. The sample size was relatively small, with only 16 patients in the non-responder group, potentially reducing the statistical power of our findings. However, the strengths of our study lie in the systematic collection of serum and urine samples at 0, 3, 6, and 12-month intervals in a prospective cohort. Furthermore, we enhanced the homogeneity of the study by exclusively enrolling patients with proliferative LN treated with MMF or MMF/TAC as the induction treatment. The second point is that a healthy control group was not included in this study, so we were unable to assess the biomarker levels in healthy individuals.
Conclusions
This study revealed significantly higher levels of uVDBP, uMCP-1, uIL-6, uIP-10, sIP-10, and sIL-23 in non-responders compared to responders after 1-year of MMF-based treatment. Additionally, baseline uIL-17 levels and changes in uIL-6 and uIL-23 at 3 months were identified as potential predictors for 1-year response to LN treatment. The study provided insights into the pathophysiological mechanisms underlying LN and its response to treatment. Furthermore, our findings could lead to the development of predictive biomarkers for non-responder group to the current standard treatment, necessitating targeted therapies and personalized treatment approaches, with further validation through larger prospective cohort studies.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We express our gratitude to the study nurses Eun-Kyoung Joo, Myung Joo Lee, and Namhee Kim from the Hanyang University Institute for Rheumatology Research.
Abbreviations
- AUC
Under the curve
- CR
Complete response
- IFN-r
Interferon-r
- IL-6
Interleukin 6
- IL-16
Interleukin 16
- IL-17
Interleukin 17
- IP-10
Interferon gamma-induced protein 10
- LN
Lupus nephritis
- MCP-1
Monocyte chemoattractant protein-1
- MMF
Mycophenolate mofetil
- NGAL
Neutrophil gelatinase-associated lipocalin
- NR
Non-response
- PR
Partial response
- RBP-4
retinol-binding protein 4
- ROC
Receiver operation characteristics
- SLE
Systemic lupus erythematosus
- TAC
Tacrolimus
- TNF-RII
Tumor necrosis factor receptor II
- VCAM-1
Vascular cell adhesion molecule 1
- VDBP
Vitamin D binding protein
Author contributions
SCB conceived and designed the study and conducted data collection. DJP, YBJ, SYB, and HSL analyzed the data and contributed to writing the paper. EWN and JYL also performed data analysis. All authors read and approved the final manuscript.
Funding
Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A1A03038899).
NFEC 2023R1A6C101A009.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Human samples were collected in accordance with approved guidelines set by the human ethics committee (Hanyang University Medical Center, Approval Number: HYUH2017-08-035). Informed consent was obtained from all participants before participation. This study adhered to the ethical principles outlined in the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all participants.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dae Jin Park and Young Bin Joo contributed equally to this work.
Contributor Information
Hye-Soon Lee, Email: lhsberon@hanyang.ac.kr.
Sang-Cheol Bae, Email: scbae@hanyang.ac.kr.
References
- 1.Hanly, J. G. et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford)55, 252–262. 10.1093/rheumatology/kev311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastian, H. M. et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus11, 152–160 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Hahn, B. H. et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. (Hoboken)64, 797–808. 10.1002/acr.21664 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalloo, S., Aggarwal, N., Mohan, P. & Radhakrishnan, J. Lupus nephritis: treatment of resistant disease. Clin. J. Am. Soc. Nephrol.8, 154–161. 10.2215/CJN.05870612 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Liu, Z. et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann. Intern. Med.162, 18–26. 10.7326/M14-1030 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Fanouriakis, A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis.78, 736–745. 10.1136/annrheumdis-2019-215089 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes Lupus Nephritis Work Group. KDIGO 2024 Clinical Practice Guideline for the management of LUPUS NEPHRITIS. Kidney Int.105, S1–S69. 10.1016/j.kint.2023.09.002 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Appel, G. B. et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J. Am. Soc. Nephrol.20, 1103–1112. 10.1681/ASN.2008101028 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginzler, E. M. et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N. Engl. J. Med.353, 2219–2228 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Zickert, A. et al. IL-17 and IL-23 in lupus nephritis—association to histopathology and response to treatment. BMC Immunol.16. 10.1186/s12865-015-0070-7 (2015). [DOI] [PMC free article] [PubMed]
- 11.Davies, J. C. et al. A panel of urinary proteins predicts active lupus nephritis and response to rituximab treatment. Rheumatology (Oxford)60, 3747–3759. 10.1093/rheumatology/keaa851 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta, R. & Yadav, A. Longitudinal assessment of monocyte chemoattractant protein-1 in lupus nephritis as a biomarker of disease activity. Clin. Rheumatol.35, 2707–2714. 10.1007/s10067-016-3404-9 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Singh, R. G., Usha, Rathore, S. S., Behura, S. K., NK, Urinary, S. & MCP-1 as diagnostic and prognostic marker in patients with lupus nephritis flare. Lupus11, 1214–1218 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Dedong, H., Feiyan, Z., Jie, S. & Xiaowei, L. Analysis of interleukin-17 and interleukin-23 for estimating disease activity and predicting the response to treatment in active lupus nephritis patients. Immunol. Lett.210, 33–39. 10.1016/j.imlet.2019.04.002 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Liu, L. et al. The utility of urinary biomarker panel in predicting renal pathology and treatment response in Chinese lupus nephritis patients. PLoS ONE15, e0240942. 10.1371/journal.pone.0240942 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satirapoj, B., Kitiyakara, C., Avihingsanon, L. A., Supasyndh, Y. & Urine neutrophil gelatinase-associated lipocalin to predict renal response after induction therapy in active lupus nephritis. BMC Nephrol.18, 263. 10.1186/s12882-017-0678-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijay, P. S., Deba, P. K., Bandita, D. & NM. Urinary Interleukin 6: A prognostic bio marker in lupus nephritis. Acad. J. Med.2, 81–85 (2019). [Google Scholar]
- 18.Zickert, A., Oke, V., Parodis, I. & Svenungsson, E. Interferon (IFN)-lambda is a potential mediator in lupus nephritis. Lupus Sci. Med.3, e000170. 10.1136/lupus-2016-000170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenig, K. F., Groeschl, I., Pesickova, S. S., Tesar, V. & Eisenberger, U. Serum cytokine profile in patients with active lupus nephritis. Cytokine60, 410–416. 10.1016/j.cyto.2012.07.004 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Whittall-Garcia, L. et al. Identification and validation of a urinary biomarker panel to accurately diagnose and predict response to therapy in lupus nephritis. Front. Immunol.13, 889931. 10.3389/fimmu.2022.889931 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal, A. et al. Urinary haptoglobin, alpha-1 anti-chymotrypsin and retinol binding protein identified by proteomics as potential biomarkers for lupus nephritis. Clin. Exp. Immunol.188, 254–262. 10.1111/cei.12930 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abujam, B., Cheekatla, S. & Urinary, A. A. CXCL-10/IP-10 and MCP-1 as markers to assess activity of lupus nephritis. Lupus22, 614–623 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Brunner, H. I. et al. Urine biomarkers to predict response to lupus nephritis therapy in children and young adults. J. Rheumatol.44, 1239–1248. 10.3899/jrheum.161128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fava, A. et al. Urine proteomics and renal single-cell transcriptomics implicate interleukin-16 in lupus nephritis. Arthritis Rheumatol.74, 829–839. 10.1002/art.42023 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park, D. J. et al. Predictive factors for renal response in lupus nephritis: A single-center prospective cohort study. J. Rheum. Dis.29, 223–231. 10.4078/jrd.22.0006 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petri, M. et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum.64, 2677–2686. 10.1002/art.34473 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aringer, M. et al. European league against rheumatism/American College of Rheumatology Classification Criteria for systemic lupus erythematosus. Arthritis Rheumatol.71, 1400–1412. 10.1002/art.40930 (2019). [DOI] [PMC free article] [PubMed]
- 28.Fanouriakis, A. et al. 2019 update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann. Rheum. Dis.79, 713–723. 10.1136/annrheumdis-2020-216924 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Gladman, D. D. & Ibañez, D. MB, U. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol.2, 288–291 (2002). [PubMed] [Google Scholar]
- 30.Umeda, R. et al. Comparison of the 2018 and 2003 International Society of Nephrology/Renal Pathology Society classification in terms of renal prognosis in patients of lupus nephritis: a retrospective cohort study. Arthritis Res. Ther.22, 260. 10.1186/s13075-020-02358-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mok, C. C. Biomarkers for lupus nephritis: A critical appraisal. J. Biomed. Biotechnol. 638413. 10.1155/2010/638413 (2010). [DOI] [PMC free article] [PubMed]
- 32.Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum.54, 421–432. 10.1002/art.21625 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int. Suppl.2, 139–274 (2012). [Google Scholar]
- 34.Su, H., Lei, C. T. & C, Z. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front. Immunol.8, 405. 10.3389/fimmu.2017.00405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linker-Israeli, M. et al. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J. Immunol.147, 117–123. 10.4049/jimmunol.147.1.117 (1991). [PubMed] [Google Scholar]
- 36.El-Shereef, R. R., Lotfi, A. & Abdel-Naeam, E. A. Serum and urinary interleukin-6 in assessment of renal activity in Egyptian patients with systemic lupus erythematosus. Clin. Med. Insights Arthritis Musculoskelet. Disord.9, 29–36. 10.4137/CMAMD.S32269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schinocca, C. et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: An overview. Front. Immunol.12, 637829. 10.3389/fimmu.2021.637829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, Z., Kyttaris, V. C. & GC. The role of IL-23/IL-17 axis in lupus nephritis. J. Immunol.183, 3160–3169. 10.4049/jimmunol.0900385 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai, H., He, F., Tsokos, G. C. & VC. IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J. Immunol.199, 903–910. 10.4049/jimmunol.1700418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia, L. P., Li, B. F. & Shen, H. Interleukin-27 and interleukin-23 in patients with systemic lupus erythematosus: possible role in lupus nephritis. Scand. J. Rheumatol.44, 200–205. 10.3109/03009742.2014.962080 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Paquissi, F. C. The Th17/IL-17 axis and kidney diseases, with focus on lupus nephritis. Front. Med. (Lausanne)8, 654912. 10.3389/fmed.2021.654912 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med.201, 233–240. 10.1084/jem.20041257 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong, C. K. et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin. Immunol.127, 385–393. 10.1016/j.clim.2008.01.019 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Apostolidis, S. A., Crispín, J. C. & GC. IL-17-producing T cells in lupus nephritis. Lupus20, 120–124 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Abdel Galil, S. M., Ezzeldin, N. & ME. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine76, 280–287. 10.1016/j.cyto.2015.05.007 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Alunno, A. et al. Balance between regulatory T and Th17 cells in systemic lupus erythematosus: the old and the new. Clin. Dev. Immunol. 823085. 10.1155/2012/823085 (2012). [DOI] [PMC free article] [PubMed]
- 47.Nayera, Z., Saber, Sherif, H., Maroof, Dina, A., Soliman & Fathi, M. S. Expression of T helper 17 cells and interleukin 17 in lupus nephritis patients. Egypt. Rheumatol.39, 151–157. 10.1016/j.ejr.2017.01.005 (2017). [Google Scholar]
- 48.Nordin, F. et al. Serum and urine interleukin-17A levels as biomarkers of disease activity in systemic lupus erythematosus. Int. J. Rheum. Dis.22, 1419–1426. 10.1111/1756-185X.13615 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Kwan, B. C. et al. The gene expression of type 17 T-helper cell-related cytokines in the urinary sediment of patients with systemic lupus erythematosus. Rheumatology (Oxford)48, 1491–1497. 10.1093/rheumatology/kep255 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Chen, D. Y., Wen, C. Y., Hsieh, M. C., Hung, T. Y. & Lan, W. T. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis. Lupus21, 1385–1396. 10.1177/0961203312457718 (2012). [DOI] [PubMed] [Google Scholar]
- 51.von Vietinghoff, S., Ouyang, H. & Ley, K. Mycophenolic acid suppresses granulopoiesis by inhibition of interleukin-17 production. Kidney Int.78, 79–88. 10.1038/ki.2010.84 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Slight-Webb, S. et al. Mycophenolate mofetil reduces STAT3 phosphorylation in systemic lupus erythematosus patients. JCI Insight4, 575. 10.1172/jci.insight.124575 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirkovic, K. et al. Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis. PLoS ONE8, e55887. 10.1371/journal.pone.0055887 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delanghe, J. R., Speeckaert, R. & Speeckaert, M. M. Behind the scenes of vitamin D binding protein: more than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab.29, 773–786. 10.1016/j.beem.2015.06.006 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Swamy, N., Dutta, A. & Ray, R. Roles of the structure and orientation of ligands and ligand mimics inside the ligand-binding pocket of the vitamin D-binding protein. Biochemistry36, 7432–7436 (1997). [DOI] [PubMed] [Google Scholar]
- 56.Go, D. J. et al. Urinary vitamin D-binding protein, a novel biomarker for lupus nephritis, predicts the development of proteinuric flare. Lupus27, 1600–1615 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Morell, M. & Pérez-Cózar, F. Immune-related urine biomarkers for the diagnosis of lupus nephritis. Int. J. Mol. Sci.22, 143. 10.3390/ijms22137143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalousova, M. et al. Vitamin D binding protein is not involved in vitamin D deficiency in patients with chronic kidney disease. Biomed. Res. Int. 492365. 10.1155/2015/492365 (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.