Abstract
This study aimed to develop and validate a predictive model for failure to collect oocytes in the Patient-Oriented Strategies Encompassing Individualized Oocyte Number (POSEIDON) Groups 3 and 4 during their first in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) cycle. A retrospective analysis was conducted on patients in POSEIDON Groups 3 and 4 who underwent their first IVF/ICSI cycle at our center from January 2016 to December 2023. A total of 2,373 patients were randomly assigned to the training or validation cohort at a ratio of 6:4. Univariate analysis, the least absolute shrinkage and selection operator (LASSO) regression and multivariate logistic regression analysis were used to identify the risk factors. It revealed that the anti-Müllerian hormone (AMH) concentration, controlled ovarian stimulation (COS) protocols, the number of follicles ≥ 14 mm on the day of trigger, and the change in estradiol level between the day before trigger and the trigger day (ΔE2) were the independent predictors. A nomogram was constructed accordingly. The areas under the receiver operating characteristic curves (ROC) of the training and the validation cohorts were 0.868 (95% CI: 0.835–0.902) and 0.860 (95% CI: 0.823–0.897), respectively. The calibration curve showed that the predicted risk of the model was in good agreement with the actual results. Decision curve analysis (DCA) demonstrated the clinical value of this nomogram. Our nomogram provides a practical and user-friendly tool for clinical decision-making.
Keywords: Failure to collect oocytes, Expected poor ovarian responder, POSEIDON criteria, Nomogram, Prediction model, In vitro fertilization
Subject terms: Endocrine reproductive disorders, Infertility
Introduction
It has been reported that one in six couples will encounter personal fertility difficulties in their lifetime, and with the increasing popularity and success of fertility treatment, an increasing number of couples are seeking the help of assisted reproductive technology (ART) to produce offspring1. It is estimated that more than 9 million babies are born through ART treatment worldwide2. The management of patients with poor ovarian response (POR) has always been a challenge for ART therapy. The definitions of POR have varied among studies over the past decades. In 2016, the Patient-Oriented Strategies Encompassing Individualized Oocyte Number (POSEIDON) group proposed a novel and comprehensive classification system3. According to the POSEIDON criteria, patients were divided into two groups: unexpected poor responders (Groups 1 and 2) and expected poor responders (Groups 3 and 4). Compared with unexpected poor responders and non-POSEIDON patients, expected poor responders (POSEIDON Groups 3 and 4) had fewer oocytes retrieved and worse pregnancy outcomes4,5. Patients in POSEIDON Groups 3 and 4 were more likely to have adverse outcomes, such as a lower ovarian response, a higher cycle cancellation rate, a lower live birth rate and a lower cumulative live birth rate6–8.
Failure to collect oocytes after successful ovarian stimulation is rare9. The overall incidence of failure to collect oocytes (including a minimum controlled ovarian stimulation protocol with clomiphene citrate) was 0.045%-7%10. However, the likelihood of failure to collect oocytes even after an adequate pre-retrieval human chorionic gonadotropin (HCG) or agonist trigger is still heightened in patients with POR. A previous study showed that oocyte retrieval failed in 8.6% of patients with only a single follicle11. At present, research on patients in POSEIDON Groups 3 and 4 has focused mostly on the refinement of the controlled ovarian stimulation (COS) protocol or medication12–14. However, for such patients, all COS protocols and medications are limited by an insufficient number of retrieved oocytes, difficulty in collecting oocytes, and failure to collect oocytes.
Clinical prediction models have been widely used in many disciplines15,16. In the field of ART, it has been reported that prediction models can be used to predict the risks of a thin endometrium17, ovarian hyperstimulation syndrome (OHSS)18, live birth8 and fertilization failure19. However, less emphasis has been placed on the failure to collect oocytes in POSEIDON Groups 3 and 4. There is still a lack of comprehensive, intuitive and individualized prediction models for the failure to collect oocytes from such patients. Although rare, adverse outcomes may be devastating to the individual, both economically and psychologically. To our knowledge, our study is the first to construct a nomogram model for the failure to collect oocytes in POSEIDON Groups 3 and 4 based on logistic regression analysis. Our model can help clinicians provide better clinical consultation and decisions together with patients. In this manner, the patient’s economic burden and psychological pressure caused by failure to collect oocytes will be minimized, and the patient’s interests will be maximized.
Results
Demographics and general characteristics
The baseline demographics and general characteristics are summarized in Table 1. Our study included a total of 2,373 patients according to the inclusion and exclusion criteria. Among them, 137 patients were in the no-oocyte-acquired (NOA) group. The overall incidence of NOA was 5.77%. Based on the need to establish and evaluate the prediction model, all patients were randomly divided into a training cohort (n = 1,424) and a validation cohort (n = 949) at a ratio of 6:4. There was no significant difference in the baseline demographics between the two cohorts (P > 0.05). The incidence of NOA was 5.69% and 5.90% in the training and validation cohorts, respectively.
Table 1.
Characteristics of the training and validation cohorts.
| Characteristics | Training Cohort (n = 1,424) |
Validation Cohort (n = 949) |
P |
|---|---|---|---|
| Age (year) | 38.00 (33.00, 42.00) | 37.00 (33.00, 42.00) | 0.344 |
| Infertility type, n (%) | 0.172 | ||
| Primary infertility | 448 (31.46) | 324 (34.14) | |
| Secondary infertility | 976 (68.54) | 625 (65.86) | |
| Infertility duration (year) | 3.00 (1.00, 5.00) | 3.00 (1.00, 6.00) | 0.169 |
| Gravidity | 1.00 (0.00, 3.00) | 1.00 (0.00, 2.00) | 0.092 |
| Parity | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 0.214 |
| BMI (kg/m2), n (%) | 0.341 | ||
| < 25 | 1022 (71.77) | 698 (73.55) | |
| ≥ 25 | 402 (28.23) | 251 (26.45) | |
| Basal FSH (mIU/ml) | 9.73 (7.24, 13.12) | 9.70 (7.32, 13.24) | 0.616 |
| Basal LH (mIU/ml) | 4.58 (3.37, 6.33) | 4.60 (3.39, 6.39) | 0.981 |
| Basal E2 (pg/ml) | 38.53 (25.29, 54.99) | 38.56 (24.84, 54.97) | 0.766 |
| Basal P (ng/ml) | 0.31 (0.20, 0.47) | 0.31 (0.18, 0.48) | 0.950 |
| AMH (ng/ml) | 0.49 (0.27, 0.74) | 0.48 (0.27, 0.77) | 0.620 |
| TSH (μIU/ml) | 2.20 (1.51, 3.10) | 2.21 (1.55, 3.08) | 0.410 |
| AFC | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | 0.883 |
| Infertility diagnosis, n (%) | 0.122 | ||
| Tubal factor | 642 (45.08) | 380 (40.04) | |
| Anovulatory | 5 (0.35) | 2 (0.21) | |
| Endometriosis | 101 (7.09) | 65 (6.85) | |
| Male factor | 95 (6.67) | 74 (7.80) | |
| Unexplained | 581 (40.80) | 428 (45.10) | |
| COS protocol, n (%) | 0.501 | ||
| Agonist | 820 (57.58) | 526 (55.43) | |
| Antagonist | 441 (30.97) | 322 (33.93) | |
| PPOS | 117 (8.22) | 72 (7.59) | |
| Mild stimulation/natural cycle | 46 (3.23) | 29 (3.06) | |
| Total dose of Gn | 3600.00 (2700.00, 4200.00) | 3350.00 (2700.00, 4200.00) | 0.399 |
| Duaration of stimulation | 12.00 (9.00, 14.00) | 12.00 (9.00, 14.00) | 0.229 |
| Fertilization method, n (%) | 0.305 | ||
| IVF | 1160 (81.46) | 757 (79.77) | |
| ICSI | 264 (18.54) | 192 (20.23) | |
| No. of follicles ≥ 14 mm on trigger | 3.00 (1.00, 4.00) | 3.00 (1.00, 4.00) | 0.263 |
| ∆E2 (pg/ml), n (%) | 0.806 | ||
| ≥ 150 | 834 (58.57) | 566 (59.64) | |
| < 150 and ≥ 0 | 495 (34.76) | 325 (34.25) | |
| < 0 | 95 (6.67) | 58 (6.11) | |
| ∆LH (mIU/ml) | 0.02 (-0.38, 0.59) | 0.06 (-0.30, 0.79) | 0.067 |
| ∆P (ng/ml) | 0.09 (0.00, 0.21) | 0.09 (0.00, 0.21) | 0.828 |
| Group, n (%) | |||
| OA | 1343 (94.31) | 893 (94.10) | |
| NOA | 81 (5.69) | 56 (5.90) |
Variables were presented as median (interquartile range), or n (%).
BMI = body mass index; FSH = follicle-stimulating hormone; LH = luteinizing hormone; E2 = estradiol; P = progesterone; AMH = anti-Müllerian hormone; TSH = thyroid stimulating hormone; AFC = antral follicle count; COS = controlled ovarian stimulation; PPOS = progestin-primed ovarian stimulation; Gn = gonadotropin; IVF = in vitro fertilization; ICSI = intracytoplasmic sperm injection; ∆E2 = change in E2 level between the day before trigger and the trigger day; ∆LH = change in LH level between the day before trigger and the trigger day; ∆P = change in P level between the day before trigger and the trigger day; OA = oocytes acquired; NOA = no oocytes acquired.
Univariate analysis of failure to collect oocytes in POSEIDON Groups 3 and 4
In the training cohort, there were 81 patients in the NOA group and 1,343 patients in the oocyte acquisition (OA) group. There were significant differences in basal follicle-stimulating hormone (bFSH), basal luteinizing hormone (bLH), anti-Müllerian hormone (AMH), antral follicle count (AFC), COS protocol, total dose of gonadotropin (Gn), duaration of stimulation, number of follicles ≥ 14 mm on the day of trigger, change in estradiol (E2) level between the day before trigger and the trigger day (ΔE2), change in LH level between the day before trigger and the trigger day (ΔLH) and change in progesterone (P) level between the day before trigger and the trigger day (ΔP) between the two groups (P < 0.05; Table 2). The progestin-primed ovarian stimulation (PPOS) protocol accounted for 29.63% and 6.92% of the patients in the NOA and OA groups, respectively. The proportions of mild stimulation/natural cycle in the NOA and OA groups were 14.81% and 2.53%, respectively (Table 2). In the NOA group, the percentages of patients with 0 pg/ml ≤ E2 < 150 pg/ml and E2 < 0 pg/ml were 64.20% and 16.05%, respectively (Table 2).
Table 2.
Univariate analysis of influencing factors of failure to collect oocytes in training cohort.
| Characteristics | OA (n = 1,343) |
NOA (n = 81 ) |
P |
|---|---|---|---|
| Age (year) | 38.00 (33.00, 42.00) | 39.00 (33.00, 43.00) | 0.457 |
| Infertility type, n (%) | 0.386 | ||
| Primary infertility | 419 (31.20) | 29 (35.80) | |
| Secondary infertility | 924 (68.80) | 52 (64.20) | |
| Infertility duration (year) | 3.00 (1.00, 5.00) | 3.00 (1.00, 5.00) | 0.920 |
| Gravidity | 1.00 (0.00, 3.00) | 1.00 (0.00, 3.00) | 0.886 |
| Parity | 0.00 (0.00, 1.00) | 1.00 (0.00, 1.00) | 0.682 |
| BMI (kg/m2), n (%) | 0.973 | ||
| < 25 | 964 (71.78) | 58 (71.60) | |
| ≥ 25 | 379 (28.22) | 23 (28.40) | |
| Basal FSH (mIU/ml) | 9.57 (7.09, 12.91) | 12.03 (9.91, 19.60) | < 0.001 |
| Basal LH (mIU/ml) | 4.56 (3.31, 6.27) | 5.51 (4.15, 7.83) | < 0.001 |
| Basal E2 (pg/ml) | 38.83 (25.70, 54.92) | 35.14 (20.58, 56.91) | 0.425 |
| Basal P (ng/ml) | 0.31 (0.20, 0.48) | 0.31 (0.19, 0.41) | 0.446 |
| AMH (ng/ml) | 0.50 (0.29, 0.76) | 0.23 (0.10, 0.37) | < 0.001 |
| TSH (μIU/ml) | 2.20 (1.52, 3.09) | 2.14 (1.45, 3.21) | 0.982 |
| AFC | 3.00 (2.00, 4.00) | 2.00 (1.00, 3.00) | 0.001 |
| Infertility diagnosis, n (%) | 0.328 | ||
| Tubal factor | 608 (45.27) | 34 (41.98) | |
| Anovulatory | 5 (0.37) | 0 (0.00) | |
| Endometriosis | 91 (6.78) | 10 (12.35) | |
| Male factor | 88 (6.55) | 7 (8.64) | |
| Unexplained | 551 (41.03) | 30 (37.04) | |
| COS protocol, n (%) | < 0.001 | ||
| Agonist | 805 (59.94) | 15 (18.52) | |
| Antagonist | 411 (30.60) | 30 (37.04) | |
| PPOS | 93 (6.92) | 24 (29.63) | |
| Mild stimulation/natural cycle | 34 (2.53) | 12 (14.81) | |
| Total dose of Gn | 3600.00 (2700.00, 4200.00) | 2700.00 (1800.00, 3600.00) | < 0.001 |
| Duaration of stimulation | 12.00 (10.00, 14.00) | 10.00 (7.00, 12.00) | < 0.001 |
| Fertilization method, n (%) | 0.996 | ||
| IVF | 1094 (81.46) | 66 (81.48) | |
| ICSI | 249 (18.54) | 15 (18.52) | |
| No. of follicles ≥ 14 mm on trigger | 3.00 (2.00, 5.00) | 1.00 (1.00, 1.00) | < 0.001 |
| ∆E2 (pg/ml), n (%) | < 0.001 | ||
| ≥ 150 | 818 (60.91) | 16 (19.75) | |
| < 150 and ≥ 0 | 443 (32.99) | 52 (64.20) | |
| < 0 | 82 (6.11) | 13 (16.05) | |
| ∆LH (mIU/ml) | 0.01 (-0.39, 0.55) | 0.34 (-0.21, 2.40) | 0.004 |
| ∆P (ng/ml) | 0.09 (0.00, 0.22) | 0.04 (-0.04, 0.15) | 0.003 |
Variables were presented as median (interquartile range), or n(%).
BMI = body mass index; FSH = follicle-stimulating hormone; LH = luteinizing hormone; E2 = estradiol; P = progesterone; AMH = anti-Müllerian hormone; TSH = thyroid stimulating hormone; AFC = antral follicle count; COS = controlled ovarian stimulation; PPOS = progestin-primed ovarian stimulation; Gn = gonadotropin; IVF = in vitro fertilization; ICSI = intracytoplasmic sperm injection; ∆E2 = change in E2 level between the day before trigger and the trigger day; ∆LH = change in LH level between the day before trigger and the trigger day; ∆P = change in P level between the day before trigger and the trigger day; OA = oocytes acquired; NOA = no oocytes acquired.
Preliminary screening of predictors for failure to collect oocytes in POSEIDON Groups 3 and 4
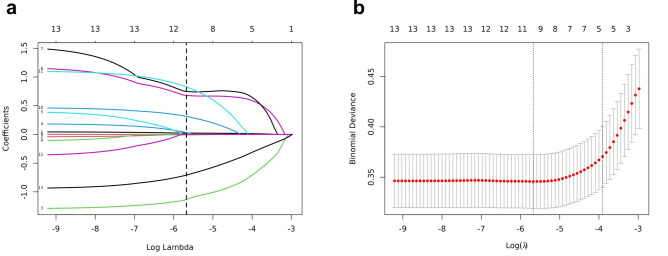
The least absolute shrinkage and selection operator (LASSO) regression was applied to analyze the 11 factors mentioned above further to minimize potential collinearity and overfitting of variables. The coefficient track diagram is shown in Fig. 1A. Figure 1B shows the cross-validation error curve of the LASSO regression model. By controlling the optimal parameter λ, the cross-validation error of the model is minimized (logλ min). The variables with nonzero coefficients were screened by LASSO regression. The best matching predictors were as follows: bFSH, AMH, AFC, COS protocol (including PPOS, and mild stimulation/natural cycle), duaration of stimulation, ∆E2 (including 0 pg/ml ≤ ∆E2 < 150 pg/ml and ∆E2 < 0 pg/ml) and number of follicles ≥ 14 mm on the day of trigger. The receiver operating characteristic curve (ROC) analysis of the above variables revealed that all the area under the curve (AUC) values were greater than 0.5 (Supplementary Fig. 1).
Fig. 1.
Best match factor screening by LASSO regression model. (a) is the LASSO regression path diagram. Each continuous variable is shown as a coloured line; the classification variables are split to dummy variables, and each dummy variable is shown as a coloured line. The vertical dotted line represents the optimal λ, with which eight variables with non-zero coefficients were screened out. (b) is the plot of the best matching factors screened by the tenfold cross validation method, and the best matching factors were selected using lambda.min as the criterion.
Multivariate logistic regression analysis of failure to collect oocytes in POSEIDON Groups 3 and 4
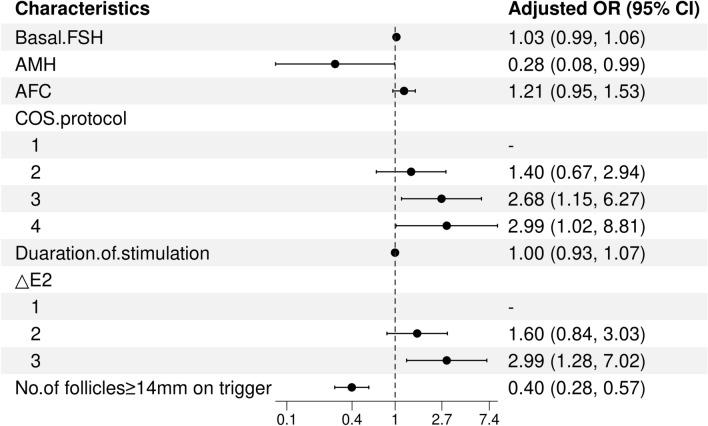
The above variables were further substituted into multivariate logistic regression analysis. AMH (OR = 0.28, 95% CI: 0.08–0.99, P = 0.048), PPOS protocol (OR = 2.68, 95% CI: 1.15–6.27, P = 0.023), mild stimulation/natural cycle (OR = 2.99, 95% CI: 1.02–8.81, P = 0.047), ∆E2 < 0 pg/ml (OR = 2.99, 95% CI: 1.28–7.02, P = 0.012) and number of follicles ≥ 14 mm on the day of trigger (OR = 0.40, 95% CI: 0.28–0.57, P < 0.001) were identified as independent risk factors for failure to collect oocytes in POSEIDON Groups 3 and 4 (Fig. 2). Among these factors, AMH and the number of follicles ≥ 14 mm on the day of trigger were determined to be independent protective factors. Compared with the agonist protocol, the PPOS and mild stimulation/natural cycle were determined to be independent risk factors. A decrease in the serum E2 concentration on the trigger day compared with the previous day was a risk factor.
Fig. 2.
Forest plots of independent influencing factors for failure to collect oocytes by multivariate analysis. bFSH = basal follicle-stimulating hormone; AMH = anti-Müllerian hormone; COS = controlled ovarian stimulation; ∆E2 = change in E2 level between the day before trigger and the trigger day; COS protocol (1: Agonist; 2: Antagonist; 3: PPOS; 4: Mild stimulation/natural cycle); ∆E2 (1: ≥ 150 pg/ml; 2: < 150 pg/ml and ≥ 0 pg/ml; 3: < 0 pg/ml); OR = odds ratio; CI = confidence interval.
Construction and validation of a nomogram model for predicting failure to collect oocytes in POSEIDON Groups 3 and 4
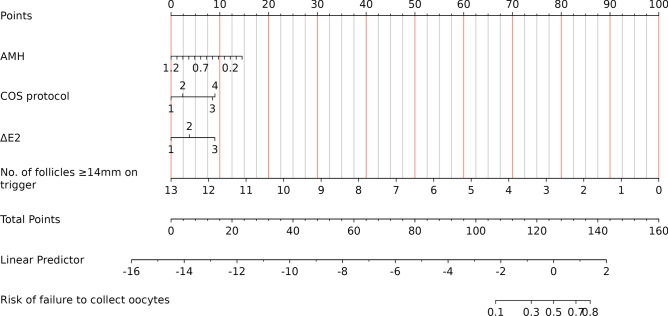
According to the multivariate logistic regression findings, the logistic regression equation was as follows: log(Y) = -1.261–1.403 × AMH + 0.980 × (COS protocol = PPOS)/1.040 × (COS protocol = mild stimulation/natural cycle) + 1.039 × (∆E2 < 0 pg/ml)—0.889 × number of follicles ≥ 14 mm on the day of trigger. A nomogram that integrates all significant independent factors for the failure to collect oocytes in POSEIDON Groups 3 and 4 is shown in Fig. 3. Moreover, the longer the length of the line is, the greater the effect of these factors on the risk of developing failed oocyte retrieval. According to the nomogram, the number of follicles ≥ 14 mm on the day of trigger had the greatest effect on the occurrence of failed oocyte retrieval. The top line of the nomogram corresponded to the score for each factor. Scores for each parameter were pooled, with higher scores indicating a greater risk of developing failed oocyte retrieval.
Fig. 3.
Nomogram of the prediction model for failure to collect oocytes in POSEIDON Groups 3 and 4. AMH = anti-Müllerian hormone; COS = controlled ovarian stimulation; ∆E2 = change in E2 level between the day before trigger and the trigger day; COS protocol (1: Agonist; 2: Antagonist; 3: PPOS; 4: Mild stimulation/natural cycle); ∆E2 (1: ≥ 150 pg/ml; 2: < 150 pg/ml and ≥ 0 pg/ml; 3: < 0 pg/ml).
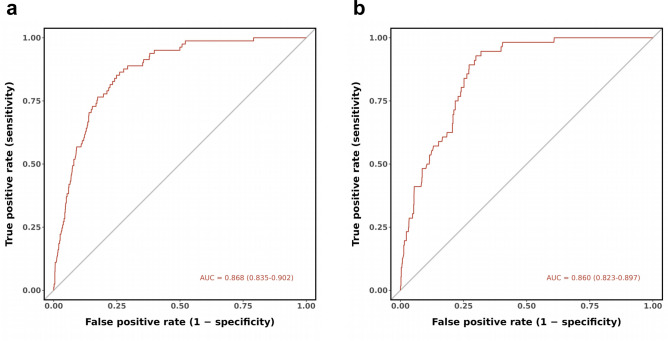
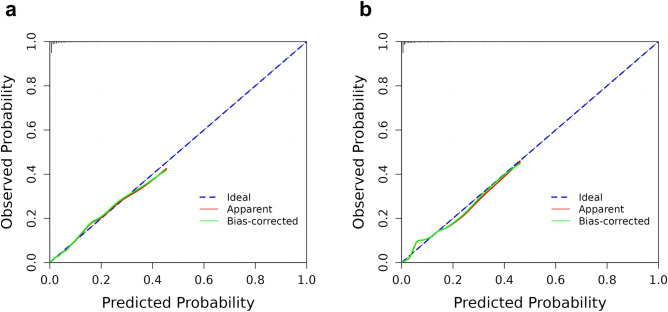
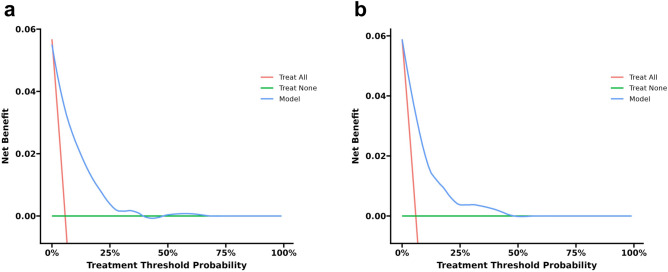
In the training cohort, the AUC was 0.868 (95% CI: 0.835–0.902), indicating good performance with 75.1% sensitivity and 84.0% specificity. The validation cohort had similar results, with an AUC of 0.860 (95% CI: 0.823–0.897), 72.7% sensitivity, and 89.3% specificity (Fig. 4). The calibration curves in the training and validation cohorts demonstrated good agreement between the predicted and ideal lines. The Hosmer–Lemeshow test revealed no statistically significant difference between the predicted and observed probabilities in either the training cohort (χ2 = 8.886, P = 0.352 > 0.05) or the validation cohort (χ2 = 11.551, P = 0.172 > 0.05), suggesting that the model accurately predicted the probability of failure to collect oocytes in POSEIDON Groups 3 and 4 (Fig. 5). Decision curve analysis (DCA) was used to analyze the clinical efficacy of the model. The DCA of the training and validation cohorts proved the potential clinical value of the model (Fig. 6).
Fig. 4.
Discriminative power of the nomogram for failure to collect oocytes in POSEIDON Groups 3 and 4. (a) shows the receiver operating curve of the training cohort. The area under the curve was 0.868 (95% CI 0.835–0.902). (b) shows the receiver operating curve of the validation cohort. The area under the curve was 0.860 (95% CI 0.823–0.897).
Fig. 5.
Calibration curve of the nomogram for failure to collect oocytes in POSEIDON Groups 3 and 4. (a) and (b) are calibration curves of the training cohort and the validation cohort, respectively.
Fig. 6.
Decision curve analysis of the nomogram for failure to collect oocytes in POSEIDON Groups 3 and 4. (a) and (b) are decision curves of the training cohort and the validation cohort, respectively.
Discussion
Our team is the first to successfully construct a quantifiable and comprehensive nomogram for the failure to collect oocytes in POSEIDON Groups 3 and 4 based on multivariate logistic regression. After internal validation, it was suggested that the model has good discrimination ability and good calibration. Using this prediction model, clinicians can identify risk groups with lower AMH levels as early as possible according to their baseline characteristics before patients start COS treatment. After entering COS treatment, clinicians can also identify the high-risk population for which oocytes cannot be retrieved according to the COS protocol, the number of follicles ≥ 14 mm on the day of trigger and the ∆E2. This model can provide patients with a visual consulting tool for determining oocyte retrieval prognosis and guide clinical decision-making.
Although the incidence of failure to collect oocytes in the whole population who received ART treatment was less than 1%9. However, in fact, failure to collect oocytes is more likely to occur in expected poor responders classified by the POSEIDON criteria. Moreover, failure to collect oocytes results in the cancellation of treatment, which can cause economic losses and mental stress to patients. Although no specific factors were directly related to the inability to retrieve oocytes in Driscoll et al.’s study, poor ovarian reserve preceded 80% of these occurrences9. Poor ovarian reserve is associated with abnormalities in folliculogenesis, ovulation and oocyte structure and performance20. Previous studies have confirmed that in women with a diminished ovarian reserve (DOR), the steroidogenic potential of ovarian granulosa cells decreases, proliferation decreases and apoptosis increases21,22. The adverse influence of increasing basal FSH on cumulative granulosa cell viability was independent of patient age23. In this study, we found that the incidence of failure to collect oocytes in POSEIDON Groups 3 and 4 was 5.77%, which was much greater than that in the general population. This finding is consistent with previous research11.
To date, the most sensitive markers of ovarian reserve have been identified as AMH and AFC24,25. AMH is a predictor of ovarian response in the in vitro fertilization (IVF) cycle26. In a mouse model with low AMH, primordial follicles are recruited at a faster rate, which leads to the depletion of the primordial follicular pool in younger mice27. Wang et al. reported that even if the number of mature follicles on the day of HCG trigger was similar, patients with DOR were more likely to have difficulty obtaining oocytes than patients with a normal ovarian reserve28. Similar to the above study, we found that AMH is an independent predictor of failure to collect oocytes in POSEIDON Groups 3 and 4. With a lower AMH and fewer dominant follicles on the day of trigger, the possibility of failure to collect oocytes is greater. This may be related to the deficit of maturity and/or healthiness of oocytes, cumulus cells, and mural granulosa cells29. Leung et al. also reported that cumulus–oocyte complexes in patients with POR exhibit reduced luteinizing hormone receptor responsiveness and that compared with those in normal responders, oocytes may not be released from the follicle wall as easily30.
COS plays a critical role in the success of in vitro fertilization-embryo transfer (IVF-ET). It enables the recruitment of enough healthy fertilizable oocytes and, thereby, high-quality embryos to improve the cumulative live birth rate31. Although the COS protocol has made great progress, the modified protocol does not show overwhelming advantages for patients with POR32. Thus, the management of patients with POR is still challenging. There is controversy in previous studies on the effects of antagonist and agonist protocols on the outcome of oocyte retrieval. Most studies suggest that it is less difficult to collect oocytes via an agonist protocol28. In the antagonist protocol, the incidence of failure to collect oocytes is greater33. Some studies also consider that there is no difference in the cycle cancellation rate between the two protocols34. This may be related to the fact that the pituitary is not down-regulated in the antagonist protocol group; thus, the endogenous LH level in the antagonist protocol group was greater than that in the agonist protocol group, which is much closer to the physiological environment of follicular development. The PPOS protocol has been widely used in IVF/intracytoplasmic sperm injection (ICSI) treatment for POR in recent years because it can effectively inhibit spontaneous ovulation35 and increase the percentage of high-quality embryos36–38. Turkgeldi et al. confirmed that the flexible PPOS protocol was as effective as the flexible GnRH antagonist protocol in preventing premature ovulation and oocyte yield in DOR women39. In our study, we found that the PPOS protocol and mild stimulation/natural cycle have a greater risk of oocyte collection failure than does the antagonist protocol. In recent years, several researchers have proposed the STOP-START protocol and Stop GnRH-agonist/GnRH-antagonist protocol, which may become effective, feasible and time-saving management options for patients in POSEIDON Groups 3 and 440,41. This still requires a large-scale randomized controlled trial (RCT) for further verification.
Choosing the appropriate time to administer HCG to trigger ovulation is necessary for successful oocyte retrieval. Oocyte maturity parallels both progressive antral cavity enlargement and the production of E2 by granulosa cells. Generally, the E2 level on the day of trigger during COS treatment is regarded as the peak concentration of E2. A continuous increase in E2 before the trigger indicates a good IVF outcome42. Our results showed that in POSEIDON Groups 3 and 4, the decrease (∆E2 < 0 pg/ml) in E2 on the trigger day compared with that of the previous day, which was a risk factor for the failure to collect oocytes. This finding is consistent with those of previous studies. Decreasing E2 levels on the day of trigger or the day after trigger are predictive of more atretic oocytes and a greater polyspermic fertilization rate43. Previous studies have confirmed that a spontaneous reduction in E2 leads to a decrease in the number of retrieved oocytes44,45. This may be due to a decrease in the absolute number of granulosa cells or a decrease in aromatase activity in follicles, resulting in a reduction in E2 production. Before HCG administration, a plateau or decrease in E2 may indicate that the proliferation of granulosa cells is stagnant or that apoptosis is increased, thus negatively affecting oocyte retrieval.
Most of the previous studies on the failure to collect oocytes were observational studies or correlation analyses. Moreover, none of them restricted the study population33,46. Our study focused on expected low responders who are more prone to adverse pregnancy outcomes. In addition to this, we excluded all patients with premature ovulation of dominant follicles before oocyte pick-up (OPU), as they may have a different pathogenesis, which made our conclusions more rigorous. The clinical baseline characteristics and COS-related indicators of the patients were comprehensively considered. Through multivariate logistic regression, we constructed a nomogram based on 4 independent factors: AMH, the COS protocol, ∆E2 and the number of follicles ≥ 14 mm on the day of trigger. In addition, the prediction result is more accurate. The AUCs of this model were greater than 0.8 in both the training cohort and validation cohort, indicating a high discriminative ability and good predictive accuracy and specificity. Our study is a real-world retrospective study based on clinical cases with a large sample size, which provides a more comprehensive reference for the formulation of clinical guidelines and medical decisions.
Our study has several limitations that should be acknowledged. First, the retrospective nature of the study may introduce some selection bias. Second, the exact diameter of follicles on the day of trigger and the time interval from trigger to OPU were not included in our study, which may also affect the results of oocyte retrieval. Finally, our model currently lacks effective external validation. Further research needs to include more possible influencing factors, and external validation should be performed with data from multiple regions and medical centers.
In conclusion, we found that AMH, the COS protocols, ∆E2 and the number of follicles ≥ 14 mm on the day of trigger were 4 independent factors for predicting failure to collect oocytes in POSEIDON Groups 3 and 4. Compared with traditional logistic regression models, nomograms are simpler, more intuitive, and more practical. However, it has greater value in clinical application. To improve the stability and universality of the model, prospective research and external validation are needed in the future.
Methods
Study population
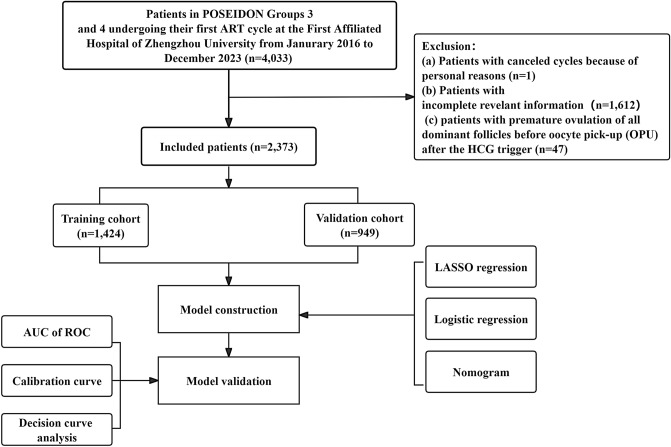
This was a retrospective case‒control study of patients receiving assisted reproduction treatments. We included female patients who underwent their first IVF/ICSI cycle at the Reproductive Center of the 1st Affiliated Hospital of Zhengzhou University from January 2016 to December 2023. All patients met the following criteria for POSEIDON Groups 3 or 4: AFC < 5 and AMH < 1.2 ng/ml. The exclusion criteria were: (i) incomplete COS cycles for personal reasons; (ii) incomplete clinical data and (iii) patients with premature ovulation of all dominant follicles before OPU after the HCG trigger. Patients whose oocytes could not be retrieved were defined as NOA, which included patients with no oocytes retrieved. Patients with > 0 oocytes were defined as the OA group. The flowchart of patient screening is shown in Fig. 7. This study was approved by the Ethics Committee of the 1st Affiliated Hospital of Zhengzhou University (Approval number: 2024-KY-0386–001). Written informed consent was obtained from all patients before IVF treatment. The research methods were carried out in accordance with relevant guidelines and regulations.
Fig. 7.
Flowchart of patient screening.
Data collection
Clinical data were collected from the Clinical Reproductive Medicine Management System/Electronic Medical Record Cohort Database (CCRM/EMRCD) of the Reproductive Medicine Center of the 1st Affiliated Hospital of Zhengzhou University. The clinical indicators included age, body mass index (BMI), type of infertility, infertility diagnosis, duration of infertility, gravidity, parity, AMH, bFSH, bLH, basal estradiol (bE2), basal progesterone (bP), thyroid stimulating hormone (TSH), AFC, COS protocol, total dose of Gn, duaration of stimulation, fertilization method, hormone levels (LH, E2, P) one day before HCG trigger and on the day of trigger, number of follicles ≥ 14 mm on the day of trigger, and number of oocytes retrieved.
Controlled ovarian stimulation protocols
COS protocols include GnRH agonist (GnRH-a) protocols, the GnRH antagonist (GnRH-ant) protocol, the PPOS protocol, mild stimulation and the natural cycle.
(1) GnRH agonist protocols: Agonist protocols included the early-follicular phase long-acting GnRH-a long (EFLL) protocol and the luteal phase short-acting GnRH-a (LPS) protocol. The EFLL protocol and LPS protocol were performed according to our previous research from our team47.
In EFLL protocol, patients underwent transvaginal ultrasound and serum sex hormone assessment on the 2nd-3rd day of their menstrual cycles. If no substantial follicular growth, cysts, or abnormalities in hormone levels were observed, long-acting GnRH-a (Diphereline, Ipsen) was administered at a dose of 3.75 mg for down-regulation. After 28 days, patients returned to the hospital for repeat transvaginal ultrasound and serum hormone evaluation. Ovarian stimulation was started by recombinant FSH (Gonal F, Merck Serono) when the pituitary down-regulation criteria were met (no functional cysts in the ovaries, follicle diameter 3–5 mm, E2 < 30 pg/ml, FSH < 5 mIU/ml, LH < 5 mIU/ml, endometrial thickness < 5 mm). The exogenous Gn dose was adjusted as needed, based on patient age, baseline AMH, and BMI, to facilitate follicular growth. When the dominant follicle reached a size of ≥ 20 mm, with another follicle ≥ 18 mm or more than 2/3 of the follicles had a size of ≥ 16 mm, HCG (Zhuhai Lizhu Group, Lizhu Pharmaceutical Factory) was administered to trigger ovulation. Oocyte retrieval was then scheduled 37 h after HCG trigger.
In LPS protocol, patients underwent transvaginal ultrasound and serum progesterone tests on the 19th-21st day of their menstrual cycles. If the serum progesterone level > 3 ng/ml, short-acting GnRH-a (Decapeptyl, Ferring Pharmaceuticals) was administered for 14 days for down-regulation. Ovarian stimulation was started by recombinant FSH (Gonal F, Merck Serono) when the pituitary down-regulation criteria were met (FSH < 5 mIU/ml, LH < 5 mIU/ml, E2 < 30 pg/ml, follicle diameter 4–7 mm, and endometrial thickness < 5 mm). Regular transvaginal ultrasound and serum hormone assessments were performed to adjust the Gn dose. The remaining procedure was performed as in the EFLL protocol.
(2) GnRH antagonist protocol: Ovarian stimulation was started by administering exogenous Gn on the 2nd-4th day of menstruation. Based on the personal experience of physicians, a fixed or flexible GnRH-ant protocol was performed using 0.25 mg daily of GnRH-ant (Cetrotide, Merck Serono) from Day 6 of stimulation or as soon as the diameter of the leading follicle reached 12–14 mm. When two dominant follicles reached a diameter of ≥ 18 mm or three follicles reached a diameter of ≥ 17 mm, HCG (Zhuhai Lizhu Group, Lizhu Pharmaceutical Factory) was administered to trigger ovulation. Oocytes were retrieved 36 h after HCG injection.
(3) PPOS: From the 2nd-4th day of menstruation, patients were orally administered 10 mg/d medroxyprogesterone acetate (Zhejiang Xianju Pharmaceutical Co., Ltd.) and 225–300 IU/d HMG (Zhuhai Lizhu Group, Lizhu Pharmaceutical Factory) until the trigger day. When at least one dominant follicle reached a diameter of ≥ 18 mm or two follicles reached a diameter of ≥ 17 mm, 0.1 mg of the trigger medicine triptorelin (Decapeptyl, Ferring Pharmaceuticals) and 1,000 IU of HCG (Zhuhai Lizhu Group, Lizhu Pharmaceutical Factory) were administered. Oocytes were retrieved 34–36 h after the trigger.
(4) Mild stimulation: From the 2nd-4th day of menstruation, patients were orally administered 2.5–5 mg/d letrozole (Jiangsu Hengrui Pharmaceutical Co., Ltd.) and injected with 150–225 IU/d HMG (Zhuhai Lizhu Group, Lizhu Pharmaceutical Factory). When the dominant follicle reached a diameter of > 15 mm, daily follicle tracking and serum hormone assessment were performed. 10,000 IU HCG (Zhuhai Lizhu Group, Lizhu Pharmaceutical Factory) was administered when appropriate. Oocytes were retrieved 33 h after triggering.
(5) Natural cycle: From the 6th-8th day of menstruation, patients returned periodically for cycle monitoring with an assessment of serum hormones and transvaginal sonography to monitor follicular growth. 10,000 IU HCG (Zhuhai Lizhu Group, Lizhu Pharmaceutical Factory) was administered when appropriate. Oocytes were retrieved 33 h after triggering. The oocytes were retrieved the day after the LH peak appeared on the trigger day.
Sex hormone assessment
A validated electrochemiluminescence immunoassay (Cobas 12,145,383) was used to detect the hormone. The detection limit and sensitivity of the method were 0.03 ng/ml and 0.15 ng/ml, respectively. The intra-assay and interassay coefficients of variation were 3.0 and 5.5%, respectively. The same detection method was utilized throughout the study, and the data were calibrated regularly to reduce unnecessary errors.
Oocyte collection
The cumulus-oocyte complexes (COCs) were collected by vaginal aspiration after the ovulatory trigger with a 30 cm, 16- or 17- gauge oocyte aspiration needle. Before OPU, transvaginal ultrasonography was performed. Oocyte retrieval was performed under transvaginal ultrasound guidance, with a suction pressure of 120–140 mmHg. A 2–3 ml flush with culture medium (G-MOPS, Vitrolife Sweden AB Göteborg) was used each time if no oocyte was retrieved via direct aspiration. If no oocyte was retrieved at the first flush, further flushes were performed up to a maximum of 6 flushes before moving to the next follicle28. Oocytes were picked up under a microscope by two experienced embryologists. The total number of oocytes retrieved was recorded.
Statistical analysis
The classified data are expressed as the frequency and percentage (%), and the median (interquartile distance) was used for continuous data that did not conform to a normal distribution. Overall, patients were randomly divided into a training cohort and a validation cohort at a ratio of 6:4. In the training cohort, the Mann‒Whitney U test or χ2 test was used for univariate analysis. Covariates with P <0.05 were included in the LASSO regression. The covariates with nonzero regression coefficients were screened for further analysis via multivariate logistic regression. A nomogram was constructed based on the independent influencing factors with P < 0.05 in the multivariate logistic regression. The ROC curve was drawn, and the AUC was calculated to test the discrimination of the nomogram in the training and validation cohorts. Calibration curves were used to assess the consistency of the actual and predicted results. To evaluate the net benefit threshold of the prediction, DCA was conducted. All the statistical analyses were performed with R (version 4.3.2) and MSTATA software. P < 0.05 was considered to indicate statistical significance in general situations.
Supplementary Information
Acknowledgements
We are very grateful to all the patients included in this study. This study was supported by grant 24A320033 from the Key Research Projects of Henan Higher Education Institutions.
Author contributions
TY was responsible for study design, data collection, data analysis, and manuscript drafting, revision and submission. WF and LD contributed to figures and manuscript revision and submission. ZB and JL contributed to data collection and statistical analysis. HK contributed to study design, manuscript revision and funding acquisition.
Funding
The Key Research Projects of Henan Higher Education Institutions,24A320033
Data availability
The data utilized and analyzed in the current study is accessible from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study involving human participants was reviewed and approved by the Ethics Committee of the 1st Affiliated Hospital of Zhengzhou University (Approval number: 2024-KY-0386–001). The procedures used in this study followed the principles of the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82783-z.
References
- 1.Wijs, L. A., Fusco, M. R., Doherty, D. A., Keelan, J. A. & Hart, R. J. Asthma and allergies in offspring conceived by ART: a systematic review and meta-analysis. Hum. Reprod. Update.28, 132–148. 10.1093/humupd/dmab031 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Abdullah, K., Atazhanova, T., Chavez-Badiola, A. & Shivhare, S. B. Automation in ART: Paving the way for the future of infertility treatment. Reprod. Sci.30, 1006–1016. 10.1007/s43032-022-00941-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alviggi, C. et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil. Steril.105, 1452–1453. 10.1016/j.fertnstert.2016.02.005 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Esteves, S. C. et al. Low Prognosis by the POSEIDON criteria in women undergoing assisted reproductive technology: a multicenter and multinational prevalence study of over 13,000 patients. Front. Endocrinol.12, 630550. 10.3389/fendo.2021.630550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudendijk, J. F., Yarde, F., Eijkemans, M. J., Broekmans, F. J. & Broer, S. L. The poor responder in IVF: is the prognosis always poor?: a systematic review. Hum Reprod. Update.18, 1–11. 10.1093/humupd/dmr037 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Massin, N. et al. The BISTIM study: a randomized controlled trial comparing dual ovarian stimulation (duostim) with two conventional ovarian stimulations in poor ovarian responders undergoing IVF. Hum. Reprod.38, 927–937. 10.1093/humrep/dead038 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, H. J., Noh, H. K. & Joo, J. K. Comparison of ART outcome in patients with poor ovarian response according to POSEIDON criteria. Sci. Rep.12, 17723. 10.1038/s41598-022-22859-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong, X. et al. Development and validation of a live birth prediction model for expected poor ovarian response patients during IVF/ICSI. Front. Endocrinol.14, 1027805. 10.3389/fendo.2023.1027805 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driscoll, G. L. et al. Failure to collect oocytes in assisted reproductive technology: a retrospective. Hum Reprod.13, 84–87. 10.1093/humrep/13.1.84 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Kim, J. H. & Jee, B. C. Empty follicle syndrome. Clin. Exp. Reprod. Med.-Cerm.39, 132–137. 10.5653/cerm.2012.39.4.132 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichman, D. E. et al. In vitro fertilization versus conversion to intrauterine insemination in the setting of three or fewer follicles: how should patients proceed when follicular response falls short of expectation?. Fertil. Steril.100, 94–99. 10.1016/j.fertnstert.2013.02.049 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Xu, Y. et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod. Biol. Endocrinol.16, 29. 10.1186/s12958-018-0343-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, Y. et al. Comparison of cumulative live birth rates between flexible and conventional progestin-primed ovarian stimulation protocol in poor ovarian response patients according to POSEIDON criteria: a cohort study. J. Clin. Med.12, 5775. 10.3390/jcm12185775 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaiarelli, A. et al. Double stimulation in the same ovarian cycle (duostim) to maximize the number of oocytes retrieved from poor prognosis patients: a multicenter experience and SWOT analysis. Front. Endocrinol.9, 317. 10.3389/fendo.2018.00317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, F. et al. Predictive nomogram for deep brain stimulation-related infections. Neurosurg. Focus.53, E8. 10.3171/2022.9.FOCUS21558 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Zhang, L., Wang, P., Li, K. & Xue, S. A novel nomogram for identifying high-risk patients among active surveillance candidates with papillary thyroid microcarcinoma. Front. Endocrinol.14, 1185327. 10.3389/fendo.2023.1185327 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo, Z. et al. Nomogram to predict an endometrial thickness above 7.5 mm in the frozen embryo transfer cycle of women with a thin endometrium. Reprod. Biomed. Online.44, 324–332. 10.1016/j.rbmo.2021.10.022 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Cao, M. et al. A personalized management approach of OHSS: development of a multiphase prediction model and smartphone-based app. Front. Endocrinol.13, 911225. 10.3389/fendo.2022.911225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xingnan, L. & Na, Z. Development and validation of a clinical prediction model of fertilization failure during routine IVF cycles. Front. Endocrinol.14, 1331640. 10.3389/fendo.2023.1331640 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battaglia, D. E., Goodwin, P., Klein, N. A. & Soules, M. R. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum. Reprod.11, 2217–2222. 10.1093/oxfordjournals.humrep.a019080 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Chin, K. V., Seifer, D. B., Feng, B., Lin, Y. & Shih, W. C. DNA microarray analysis of the expression profiles of luteinized granulosa cells as a function of ovarian reserve. Fertil. Steril.77, 1214–1218. 10.1016/s0015-0282(02)03114-x (2002). [DOI] [PubMed] [Google Scholar]
- 22.Nakahara, K. et al. Incidence of apoptotic bodies in membrana granulosa of the patients participating in an in vitro fertilization program. Fertil. Steril.67, 302–308. 10.1016/S0015-0282(97)81915-2 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Greenseid, K. et al. Declining ovarian reserve adversely influences granulosa cell viability. Fertil. Steril.91, 2611–2615. 10.1016/j.fertnstert.2008.03.065 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Broekmans, F. J. et al. The antral follicle count: practical recommendations for better standardization. Fertil. Steril.94(1044–10), 51. 10.1016/j.fertnstert.2009.04.040 (2010). [DOI] [PubMed] [Google Scholar]
- 25.La Marca, A. & Sunkara, S. K. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum. Reprod. Update.20, 124–140. 10.1093/humupd/dmt037 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Broer, S. L. et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum. Reprod. Update.19, 26–36. 10.1093/humupd/dms041 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Durlinger, A. L. et al. Control of primordial follicle recruitment by anti-mullerian hormone in the mouse ovary. Endocrinology.140, 5789–5796. 10.1210/endo.140.12.7204 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Wang, Y. et al. Causes and effects of oocyte retrieval difficulties: a retrospective study of 10,624 cycles. Front. Endocrinol.12, 564344. 10.3389/fendo.2021.564344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumann, K. & Griesinger, G. Follicular flushing in patients with poor ovarian response: a systematic review and meta-analysis. Reprod. Biomed. Online.36, 408–415. 10.1016/j.rbmo.2017.12.014 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Leung, A. S., Dahan, M. H. & Tan, S. L. Techniques and technology for human oocyte collection. Expert Rev. Med. Devices.13, 701–703. 10.1080/17434440.2016.1205485 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Garrido, N., Bellver, J., Remohi, J., Simon, C. & Pellicer, A. Cumulative live-birth rates per total number of embryos needed to reach newborn in consecutive in vitro fertilization (IVF) cycles: a new approach to measuring the likelihood of IVF success. Fertil. Steril.96, 40–46. 10.1016/j.fertnstert.2011.05.008 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Shanbhag, S., Aucott, L., Bhattacharya, S., Hamilton, M. A. & McTavish, A. R. Interventions for “poor responders” to controlled ovarian hyperstimulation (COH) in in-vitro fertilisation (IVF). Cochrane Database Syst. Rev.10.1002/14651858.CD004379.pub2 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa, A. et al. Predictive factors for oocyte retrieval failure in controlled ovarian hyperstimulation protocols: a retrospective observational cohort study. Reprod. Biol. Endocrinol.13, 53. 10.1186/s12958-015-0052-x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal, A. et al. Effectiveness of GnRH agonist short protocol versus gnrh antagonist protocol in POSEIDON Groups 3 and 4: a retrospective cohort study. Reprod. Sci.30, 2481–2488. 10.1007/s43032-023-01196-x (2023). [DOI] [PubMed] [Google Scholar]
- 35.Wikstrom, A., Green, B. & Johansson, E. D. The plasma concentration of medroxyprogesterone acetate and ovarian function during treatment with medroxyprogesterone acetate in 5 and 10 mg doses. Acta Obstet. Gynecol. Scand.63, 163–168. 10.3109/00016348409154654 (1984). [DOI] [PubMed] [Google Scholar]
- 36.Tu, X., You, B., Jing, M., Lin, C. & Zhang, R. Progestin-primed ovarian stimulation versus mild stimulation protocol in advanced age women with diminished ovarian reserve undergoing their first in vitro fertilization cycle: a retrospective cohort study. Front. Endocrinol.12, 801026. 10.3389/fendo.2021.801026 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng, Q. et al. Progestin-primed ovarian stimulation vs mild stimulation in women with advanced age above 40: a retrospective cohort study. Reprod. Biol. Endocrinol.17, 91. 10.1186/s12958-019-0518-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu, C. M. et al. Progestin-primed ovarian stimulation improves the outcomes of IVF/ICSI cycles in infertile women with diminished ovarian reserve. J. Chin. Med. Assoc.82, 845–848. 10.1097/JCMA.0000000000000177 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Turkgeldi, E. et al. Effectiveness of the flexible progestin primed ovarian stimulation protocol compared to the flexible gnrh antagonist protocol in women with decreased ovarian reserve. Hum. Fertil.25, 306–312. 10.1080/14647273.2020.1794060 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Atabekoglu, C. S. et al. A feasible option before cycle cancellation for poor responders; STOP-START protocol. Int. J. Fertil. Steril.15, 300–302. 10.22074/IJFS.2021.13462 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orvieto, R. Stop GnRH-agonist/GnRH-antagonist protocol: a different insight on ovarian stimulation for IVF. Reprod. Biol. Endocrinol.21, 13. 10.1186/s12958-023-01069-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones, H. J. et al. The importance of the follicular phase to success and failure in in vitro fertilization. Fertil. Steril.40, 317–321. 10.1016/s0015-0282(16)47293-6 (1983). [DOI] [PubMed] [Google Scholar]
- 43.Ben-Rafael, Z. et al. Follicular maturation parameters associated with the failure of oocyte retrieval, fertilization, and cleavage in vitro. Fertil. Steril.45, 51–57. 10.1016/s0015-0282(16)49096-5 (1986). [DOI] [PubMed] [Google Scholar]
- 44.Fisher, S., Grin, A., Paltoo, A. & Shapiro, H. M. Falling estradiol levels as a result of intentional reduction in gonadotrophin dose are not associated with poor IVF outcomes, whereas spontaneously falling estradiol levels result in low clinical pregnancy rates. Hum. Reprod.20, 84–88. 10.1093/humrep/deh543 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Grin, L. et al. Do spontaneously decreasing estradiol levels prior to triggering of ovulation adversely impact in vitro fertilization outcomes?. Clin. Exp. Reprod. Med.-Cerm.47, 213–220. 10.5653/cerm.2019.03419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bortoletto, P., Willson, S. F., Romanski, P. A., Davis, O. K. & Rosenwaks, Z. Outcomes in, and characteristics of, patients who undergo intrauterine insemination immediately after failed oocyte retrieval. F S Rep.1, 239–242. 10.1016/j.xfre.2020.10.002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du, L., Song, J., Fan, W., Ye, T. & Kong, H. Safety profiles of offspring born from early-follicular long-acting GnRH agonist protocol and daily mid-luteal GnRH agonist protocol: a retrospective study. Bmc Pregnancy Childbirth.24, 393. 10.1186/s12884-024-06589-7 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized and analyzed in the current study is accessible from the corresponding author upon reasonable request.