Abstract
The management and creation of Marine Protected Areas (MPAs) is currently under great focus, with international organisations aiming to protect 30% of our oceans by 2030. The success of MPAs depends on a nuanced understanding of local ecological dynamics and threats, which can significantly influence ecosystem balance. Herbivory can be a stressor for foundation species, namely kelp forests, contributing to their decline in several regions of the globe. However, the dynamics inherent to herbivory and MPA’s implementation are still poorly understood. Here, the impact of protection status, depth, kelp species, and grazer type on herbivory (occurrence, rate, and grazer frequency) was assessed through a comprehensive experimental approach involving tethering experiments and faunal characterisation of macro-herbivores. The research was conducted in habitats off the central coast of Portugal: Peniche (PEN) and the MPA Berlengas Archipelago (MPA-BER). Our findings revealed that herbivory occurrence and rate are higher within the MPA, especially at greater depths. Instead of urchins, fish are the significant contributors to kelp consumption, showing a preference for the kelp S. polyschides. Results provide the first experimental evidence in the Atlantic region identifying fish as the dominant herbivores driving increased kelp biomass loss, a relationship potentially magnified by MPA implementation. Hence, protection status may not benefit all ecosystem components, enhancing the need for robust MPA management to balance trophic interactions and support biodiversity and ecosystem resilience.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82557-7.
Keywords: MPAs, Herbivory, Kelp, Herbivorous fish, Trophic interactions
Subject terms: Biodiversity, Conservation biology, Ecosystem ecology, Marine biology
Introduction
The dynamic balance of marine ecosystems is maintained through intricate interactions among their biological and physical components. Within this context, Marine Protected Areas (MPAs) have emerged as a cornerstone of marine conservation efforts. The effectiveness of MPAs in conserving marine biodiversity and safeguarding critical habitats has been increasingly recognised1–3. This prompted a global effort to expand MPA coverage as part of broader strategies to protect marine ecosystems and ensure the sustainability of ocean resources4, and international organisations have set the goal of protecting 30% of the ocean area by 20305. In Europe, a recently approved Restoration law also targets the protection of 20% of land/ocean by 2030 and all ecosystems in need of restoration by 20506.
MPAs’ most general purpose is to act as an effective management tool to reverse and mitigate the impacts of overfishing and increase resilience to other stressors, such as climate change3,7–9. They not only facilitate the recovery of species directly affected by harvesting but can also indirectly promote broader ecosystem recovery and improved ecosystem health by re-establishing lost trophic interactions, such as trophic cascades9,10. However, a broad understanding of all ecosystem dynamics is required to achieve its full potential and benefits. Beyond fish protection, in several regions of the globe the conservation of marine forests, particularly kelp forests, has increasingly become a crucial point of MPA design and management11. Kelp forests are among the ocean’s most productive and biodiverse ecosystems, providing essential services such as habitat provision for commercially important species and nutrient cycling. However, they are currently degraded and jeopardised by several stressors12–14. Climate change, herbivory, and harvesting are the most common drivers of change affecting kelp forests negatively15, and the efficacy of MPAs is contingent upon the nuanced understanding of these threats7,16,17. Recent advances in marine conservation have highlighted the role of MPAs in mitigating some of the critical threats to kelp forests, including overfishing, herbivory and habitat degradation, and shown that well-designed MPAs, with effective enforcement and management plans, can lead to significant increases in biomass and biodiversity within kelp forests9,11. Nonetheless, climate change (heatwaves) and disturbance caused directly by abiotic factors are more difficult to mitigate with MPAs11, which is why it is rarely a central goal considered when planning and designing them.
Like in terrestrial forests18, herbivory is a key aspect to consider while perceiving the dynamics of kelp forests19–21. The grazing activities of macro-herbivores, such as sea urchins and certain fish species, can significantly influence the health and sustainability of kelp forests. In the absence of natural predators, herbivore populations can explode, namely sea urchins, leading to overgrazing and the potential devastation of kelps—a phenomenon known as “urchin barrens”13,20,22. Conversely, controlled levels of herbivory are necessary for the natural cycling and renewal of kelp forests, illustrating the complex nature of these interactions. Herbivory can also be potentiated by other biotic and abiotic factors, including species composition of herbivores, the availability of kelp species, and environmental conditions such as depth, temperature, and light availability23–25. These interactions have significant implications for the health and stability of kelp habitats, affecting their capacity to support diverse marine communities and provide other ecosystem services12,14,26. With the crescent need for MPA implementation, understanding how MPAs can affect herbivory is pivotal. Equally crucial is understanding how fish-oriented marine protected areas will affect or are currently affecting pre-existing foundation species. Removing fishing pressures may lead to changes in herbivore populations and impact the dynamics of these ecosystems, as seen in some Mediterranean seagrass and other brown algae forests where MPAs increased herbivorous fish populations, leading to a decrease in primary producers27,28.
Based on recent research and field observations, we know that this may be the case in kelp forests in the Northeast Atlantic. Kelp forests are degraded inside MPAs while healthy in the adjacent areas, which is paradoxical29. Here, the protection from fishing pressures within MPAs is hypothesised to increase the abundance of herbivorous fish29, which, in turn, can lead to changes in herbivory rates and potentially affect the structure and resilience of kelp forest ecosystems. Given the crucial importance of understanding these intricate dynamics, our study focuses on assessing the effects of protection status, depth, kelp species, and grazer type on herbivory rates and macro-herbivore abundance. This contributes to a growing body of evidence that emphasises the need for integrated management approaches that consider both the protection of kelp forests and the regulation of herbivore populations within MPAs. In light of these considerations, this work contributes to the ongoing debate on the ecological effects of MPAs, providing insights into the complex interactions between marine herbivores and kelp forest ecosystems. Through a comprehensive analysis of herbivory dynamics inside and outside an MPA, this research aims to enhance our understanding of marine ecosystem functioning and inform the development of effective conservation strategies that support marine biodiversity and ecosystem resilience.
Results
Herbivory
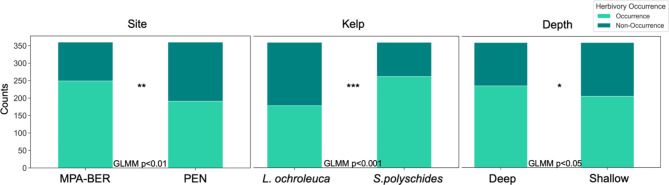
Site (protection status), Kelp species, and Depth had a significant effect on the occurrence of herbivory (frequency of grazed kelp pieces) (GLMM p < 0.01; p < 0.05; p < 0.001, respectively; dispersion parameter of 1; Supplementary Table S1.a). The protected area MPA-BER registered 249 herbivory occurrences, while the non-protected area of Peniche 191. A higher number of occurrences also happened in the kelp species S. polyschides (262 counts) and in deeper zones (235 counts) (Fig. 1).
Fig. 1.
Occurrence of herbivory across the factors Site (protection status); Kelp species and Depth.
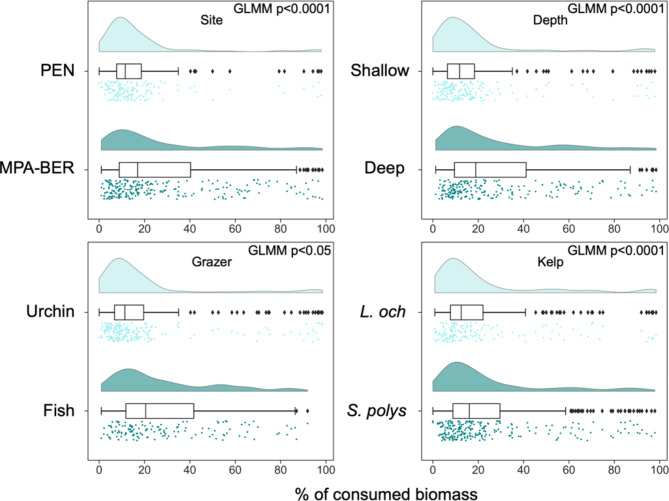
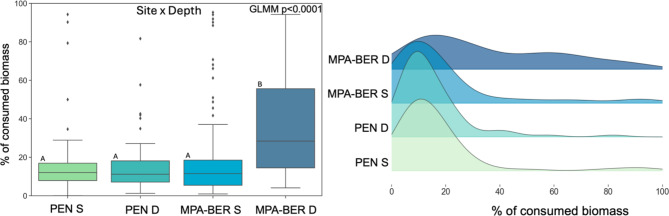
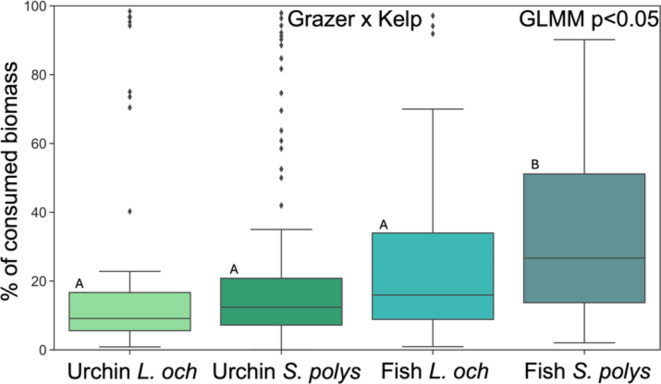
In cases where herbivory indeed occurred, the percentage of consumed biomass was significantly influenced by the factors: Site (protection status) (GLMM p < 0.0001, Supplementary Table S1), Depth (GLMM p < 0.0001, Supplementary Table S1), Kelp species (GLMM p < 0.0001, Supplementary Table S1) and grazer type (GLMM p < 0.05, Supplementary Table S1) (Fig. 2) and ultimately by the interaction of the factors Site&Depth (GLMM p < 0.0001, Supplementary Table S1) (Fig. 3) and Grazer&Kelp (GLMM p < 0.05, Supplementary Table S1) (dispersion range 0.5-2) (Fig. 4). The overall mean percentage of consumed biomass was 23.67% ± 24.02 SD. The protected area MPA-BER registered higher values of consumed biomass (28.08% ± 25.96 SD) than PEN (17.03% ± 18.77 SD) (Fig. 2). Deeper reefs showed more consumed biomass (28.73% ± 25.29 SD) when compared with shallow (18.34% ± 21.30 SD) Fig. 2). The kelp species S. ployschides also registered a higher mean of consumed biomass (25.22% ± 23.95 SD) than L. ochroleuca (21.66% ± 24.04 SD) (Fig. 2). The herbivore responsible for the higher rates of herbivory was fish (29.59% ± 23.67 SD) in relation to sea urchins (19.35% ± 23.39 SD) (Fig. 2). However, what influenced herbivory rates the most was the interaction of the factors Site (protection status) and Depth. Herbivory rates are reported to be higher especially inside MPA-BER and at a higher depth (35.62% ± 25.22 SD) (Fig. 3). The interaction of the factors Grazer type and Kelp species also revealed to be relevant in the degree of herbivory rate, with fish being the responsible for higher biomass consumed, especially in the species S. polyschides (33.01% ± 23.96 SD) (Fig. 4).
Fig. 2.
Percentage of consumed biomass (mean ± SD) in both sites (MPA-BER and PEN), both depths (Shallow and Deep), between grazer types (Fish and Urchin), and between the two kelp species L. ochroleuca and S. polyschides.
Fig. 3.
Percentage of consumed biomass (mean ± SD) in both sites (MPA-BER and PEN) at both depths (S-Shallow and D-Deep).
Fig. 4.
Percentage of biomass (mean ± SD) consumed by different grazers in both kelp species (L. ochroleuca and S. polyschides).
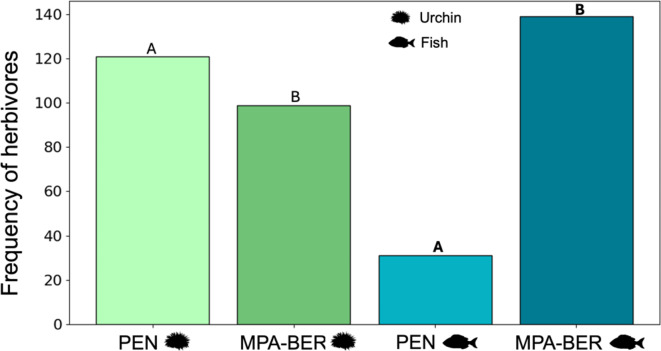
The frequency of different grazer bitemarks was also significantly different in both sites (GLMM p < 0.0001; dispersion range 1–5; Supplementary Table S2). MPA-BER registered 139 kelp pieces with fish bitemarks, while PEN registered only 31. As for sea urchin bitemarks, these were significantly higher in PEN (121) than in MPA-BER (99) (GLMM p < 0.05, dispersion range 1–5 Supplementary Table S2 (Fig. 5). The total N for the frequency of bitemarks is lower than the total number of samples because it was not always possible to identify bitemarks properly.
Fig. 5.
Bitemarks’ frequency at each site (MPA-BER and PEN).
Macro-herbivore abundance
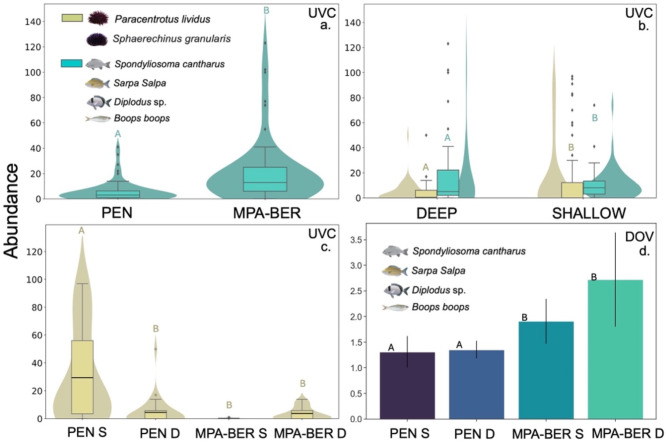
Both Site (protection status) (GLM p < 0.0001, Supplementary Table S3) and Depth (GLM p < 0.05, dispersion range 1–5; Supplementary Table S3) had a significant impact on the abundance of herbivorous fish registered in the Underwater Visual Census (UVC). As for sea urchins, Depth significantly influenced abundance (GLM p < 0.0001; Supplementary Table S3) and the interaction of Depth and Site (protection status) (GLM p < 0.0001; dispersion range 1–5; Supplementary Table S3). A higher herbivorous fish abundance was recorded inside the MPA (21.38 ± 25.56 SD) especially at MPA-BER deep (26.00 ± 33.10 SD) and lower outside the MPA (5.85 ± 8.18 SD), with PEN shallow having the lowest values (4.73 ± 4.73 SD) (Fig. 6). Concerning sea urchins, the highest abundance happened in PEN shallow (29.47 ± 33.53 SD) and the lowest at MPA-BER shallow (0.13 ± 0.35 SD) (Fig. 6, Supplementary Table S4). The abundance of herbivorous fish obtained using Diver Operated Videos (DOV) was also significantly impacted by Site (protection status) (GLM p < 0.0001; dispersion range 1–5; Supplementary Table S3). The highest DOV fish abundance happened in MPA-BER deep (2.72 ± 0.92 SD) and the lowest at PEN shallow (1.31 ± 0.31 SD) (Fig. 6, Supplementary Table S5).
Fig. 6.
a Abundance (number of individuals (mean ± SD)) of herbivorous fish assessed by Underwater Visual Census (UVC) in MPA-BER and PEN. b Abundance (number of individuals (mean ± SD)) of sea urchins and herbivorous fish assessed by Underwater Visual Census (UVC) in both depths (S-Shallow; D-Deep). c Abundance (number of individuals (mean ± SD)) of sea urchins assessed by Underwater Visual Census (UVC) in both sites (MPA-BER and PEN) and both depths (S-Shallow; D-Deep). d Abundance (number of individuals (mean ± SD)) of herbivorous fish assessed by Diver Operated Videos (DOV) in MPA-BER and PEN at the two considered depths (S-Shallow; D-Deep).
Discussion
Our study provides significant insights into the dynamics of herbivory in marine ecosystems, particularly within and outside Marine Protected Areas (MPAs). By examining the influence of protection status, depth, kelp species, and grazer type on herbivory occurrence, herbivory rates, and macro-herbivore abundance in the surroundings, we have uncovered patterns that could have broad implications for the management and conservation of these crucial habitats. Importantly, this research offers the first empirical evidence from the Atlantic region identifying fish as the predominant grazers in kelp forests and that this grazing can be intensified by MPA implementation.
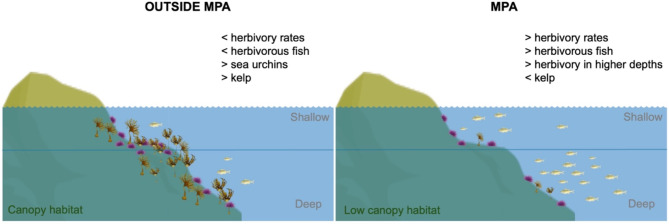
As initially hypothesised, most herbivory occurred inside the Marine Protected Area (MPA) and was performed by fish. In addition, it was found that a higher herbivory rate happened in depth and especially in the kelp species S. polyschides. Importantly, protection status also significantly affected grazer marks’ frequency. Fish bites were significantly more abundant inside the MPA-BER, and urchin ones were more abundant in PEN (Fig. 7). The enhanced abundance of fish in deeper areas within the MPA can be explained by the increased protection from any fishing and recreational activities that happen at the surface, allowing for a safer habitat for these species30. Alternatively, the higher urchin abundance in shallower areas outside the MPA highlights potential differences in habitat quality or predation pressures. Fish preference for the kelp species S. polyschides can be due to the higher abundance of this species in the surroundings12, possibly creating a consumption habit.
Fig. 7.
Graphical representation of the higher herbivory rates and fish grazers found inside the MPA – BER (Berlengas), especially at higher depths (image partially created with BioRender.com).
Our analysis of grazer marks frequency and herbivory rates is further supported by the higher abundance of fish found through UVC and DOV surveys inside the MPA-BER, particularly at greater depths. In contrast, urchin abundance was more pronounced in PEN, especially in shallower waters.
Results showed that MPAs with fisheries restrictions alongside depth gradients significantly influence the distribution and abundance of different herbivore groups. This underscores the importance of protection status and grazers’ identity in shaping the dynamics of kelp forests. It suggests that herbivorous fish might play a more dominant role in these ecosystems than previously thought, despite potential limitations in the conclusions due to using tethered kelp to assess herbivory rates. While this method provides a standardised measure of grazing pressure across different sites and depths, it may introduce an element of artificial food availability. However, it is important to note that within the MPA, there is already an abundance of various algal species, including smaller kelps like Phyllariopsis spp. Despite this natural diversity, herbivorous fish actively consumed the tethered Laminaria ochroleuca and Saccorhiza polyschides, indicating a selective feeding choice rather than a forced consumption due to the absence of alternatives. In addition, this method is widely used in various studies19,31–33.
Outside the MPA, the algal community also includes a mix of kelp species and other algae34, providing herbivores with the same natural food options. This reduces the likelihood that the observed herbivory is solely an artifact of tethering. Furthermore, sea urchins in both areas are known to be opportunistic and non-selective feeders35, preying on a wide range of algal species when available. Thus, while the possibility of an artifact effect from tethering exists, its impact is likely minimal, given the broader context of algal availability and feeding behaviour observed in both protected and unprotected sites.
These findings suggest that the increased grazing rates inside the MPA are a reflection of actual preferences and ecological interactions rather than an experimental artifact. Nonetheless, future studies could include direct observations of natural feeding behaviour or additional experiments using untethered kelp to further corroborate these results.
Although here, kelp forests are degraded inside the MPA, several studies report the opposite, namely ones representing habitats where sea urchins are the biggest threat to kelp. For ecosystems where sea urchins pose as the primary menace to kelp forests in terms of grazing, the implementation of MPAs with reduced/none fishing policies can more easily have a positive effect on lowering grazing and protecting the habitat and associated fauna, including fish and overall facilitating the recovery of kelp9,17. However, for ecosystems where herbivorous fish have a more impactful role in kelp grazing, the implementation of MPAs has more complex dynamics associated and can have a critical impact on these habitats. It creates an increase in fish populations, including, inevitably, herbivorous fish. This can lead to a significant downfall of kelp forests21,29,36.
The collapse of kelp forests due to herbivorous fish increment in MPAs can be even more accentuated in climatic transition zones, like our case, especially when under climate change effects. In situations like this, species distributions are often very sensitive and prone to shifts occurring due to changes in seawater temperature26. It is well known that tropicalisation can lead to increased consumer pressure by herbivorous fish in primary producers like kelp37–39.
This phenomenon is quite known in the Mediterranean Sea, where the protection of specific areas, especially seagrass meadows and other brown canopy-forming seaweeds, promoted the ascension of herbivorous fish populations, compromising the existing primary producers28,40,41. Despite being a relatively common phenomenon in the Mediterranean Sea, it is relatively unknown in the Atlantic. The fact that it is happening, namely in kelp habitats, can indicate a degree of tropicalisation and a changing habitat. A part of this problem in the Mediterranean comes from the rise of the herbivorous fish species Sarpa salpa42. In our case, we suggest S. salpa is also the most predominant herbivorous fish, although we could not identify precisely the amount of biomass it consumed. This species is known to be increasing its numbers and distribution across continental Portugal as a consequence of tropicalisation43. However, its impacts on grazing and ecosystem dynamics are still quite unknown and should be further investigated.
Our results are based on a specific region, but they are supported by other studies’ outcomes, suggesting that they can accurately reflect the reality of ecosystems in transitional temperate zones. Similar examples in the Mediterranean, as mentioned above, as well as other temperate regions, show increased herbivorous fish populations due to MPA implementation. For instance, in New Zealand’s oldest marine reserve, a growing number of herbivorous fish was registered over time inside the reserve44. In Australia, studies evaluating the effects of marine reserves on herbivorous fish populations and their grazing activities on temperate reefs also revealed higher numbers of several herbivorous fish and grazing rates inside the reserves as opposed to outside45. Perhaps the most relevant example here is the one from the coast of Galicia, Spain, where the kelp forests inside the MPA reached a degraded state due to overgrazing by herbivorous fish inside the reserve, despite the protection status21,29. Although there are few studies with conditions directly comparable to ours, this does not undermine the validity of our findings. As demonstrated by the studies cited, the impacts of MPAs on herbivorous fish populations and their grazing pressure are context-dependent and well-documented in various regions.
While our tethering experiments provided valuable insights into herbivory rates within and outside the MPA, we acknowledge that this approach does not fully capture long-term ecological dynamics or the broader impacts of increased herbivory on kelp forests. The observed increase in herbivorous fish populations within the MPA and their selective grazing on kelp species indicate a potential shift in trophic interactions. However, to comprehensively understand the implications of these interactions, long-term studies are necessary. Future research should focus on monitoring changes in kelp cover, shifts in fish populations, and the overall ecosystem structure over extended periods. This will help to clarify whether increased grazing pressure leads to sustained reductions in kelp abundance or triggers shifts in the ecosystem over time.
MPA implementation can facilitate the establishment of herbivorous fish more rapidly46, contributing to a more accelerated phase shift and potential collapse of kelp populations. It is essential to be aware that different ecosystem types require different MPA implementation strategies and that inferring implementation success can be misleading and should be made focusing on key groups10,47, not solely on fish populations.
Macroalgae, such as kelp, can be indeed a vital indicator species when assessing MPA implementation success since they are sessile, react very strongly to biotic and abiotic factors, and can increase or reduce biomass and biodiversity very quickly47–49. In addition, the prevalence and resilience of macroalgae forests, namely kelp, is a very crucial issue nowadays, given their unarguable value in terms of oxygen production50, potential carbon sequestration51, and habitat for other species. Associated with kelp loss, a myriad of ecosystem services are also lost13,14.
Given this, and since MPAs may not guarantee that every component of the ecosystem will see a benefit16,29,52, there is a clear need for further efforts to a better understanding of ecosystem interactions, namely the interactions of kelp within the food web and abiotic factors. Interactions within the top-down cascades47 are a critical research point that needs to be addressed. Changes in trophic relationships resulting from the implementation of no-take/ controlled fisheries’ MPAs and the importance of these processes in habitat restoration need to be well comprehended since differing habitats are expected to exhibit differing interactions between primary producers and herbivores according to benthic habitat type and desired habitat goals47,53,54. This highlights the need for MPA-specific management for different habitats and possible outcomes if fisheries are reduced or totally restricted. Moreover, to fully understand the dynamics of the ecosystems that are targeted for restoring an increase in resilience and stability, it is mandatory to have consistent and complete long-term studies and information about these habitats to understand the time frames and successional pathways involved7,9,55. Besides the study of biological interactions and trophic relations, it is also important to address the interaction with abiotic parameters such as depth and seawater temperature, among others, in a way that climate change adaptation can be incorporated into MPA design56.
In cases where the balanced status is already disturbed, some measures can be taken to mitigate the rise of herbivorous fish and increase the number of primary producers. These measures are quite complex and need to be adapted for each scenario.
Methods like fish-deterring devices can protect seaweeds from fish grazers; however, they are expensive and difficult to scale up20,28,57. Transplantations or other reforestation methods, such as green gravel58, can also work, but they need to be applied together with other population measures so that new recruitments can thrive and surpass the shift threshold20,57.
Herbivore population control is, to date, the best-suited option to control herbivore impact. This has been widely applied in temperate reefs where sea urchins have decimated kelp forests22,59,60. However, for herbivorous fish, particularly inside an MPA, this strategy is not as efficient and is much more complicated to set in practice. For this case, a good approach could be to promote the commercial value and applications of herbivorous fish, namely S. salpa, within the local population so it could gain value in fish markets57. This would increment fisheries of S. salpa instead of only being fished by bycatch and most likely thrown away. In addition, applying fisheries’ restrictions to key herbivore predators like seabass could also be an option to consider. However, this requires a multidisciplinary approach and a proper assessment done beforehand.
Here, the observed variations in herbivory rates and herbivore abundance across different sites and depths underline the need for tailored conservation strategies that consider the specific ecological dynamics of each marine habitat. The observed higher numbers of fish populations, mainly herbivorous species, within the MPA boundaries underscores the efficacy of fisheries protection in conserving aquatic life. However, this conservation success story harbours a nuanced ecological dilemma. While MPAs are a very important tool that aims to bolster fish populations by offering refuge from fishing pressures, the resultant surge in herbivorous fish could lead to heightened herbivory, potentially destabilising kelp forest ecosystems. As such, the design and management of MPAs should be informed by understanding these complex interactions to ensure the effective conservation of marine biodiversity and the sustainability of kelp forest ecosystems.
Materials and methods
Study area
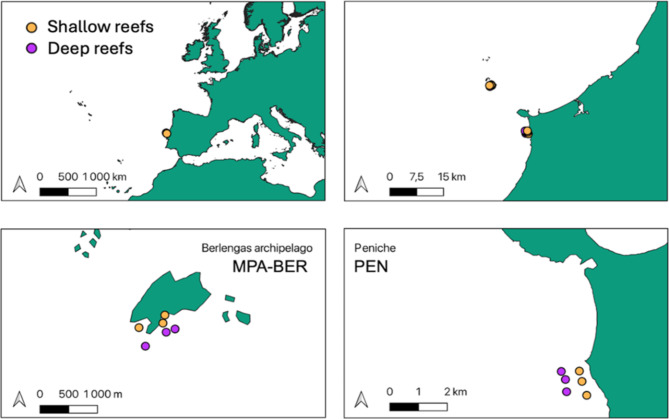
The study was conducted in the region of central Portugal both in the coast of Peniche (PEN) (39.34°N, – 9.36°W) and in Berlengas’ archipelago (MPA-BER) (39.45°N, – 9.53°W) (Fig. 8). Peniche is situated in a coastal upwelling area and is characterised by sheltered rocky shores and open reefs that support dense forests of the kelp species Laminaria ochroleuca and Saccorhiza polyschides19. This kelp presence is consistent with past records26,34. This site has no fisheries protection status. The Berlengas Archipelago, located about 10 km off the coast of Peniche, became a Marine Protected Area (MPA) in 1981 with restricted fisheries. It consists of several rocky islands and reefs with very scarce kelp (especially Phyllariopsis spp.) and more prevalence of foliose/turf-forming seaweeds such as Asparagopsis armata52,61–63. There are minimal differences in abiotic factors between the MPA and non-MPA sites64. Although the MPA is located around an archipelago and the comparison site is on the mainland, the two areas share similar physical and oceanographic characteristics. Both regions experience the same coastal upwelling events, which drive nutrient-rich waters into the area, and their rocky bottoms have comparable compositions and substrate types64. High wave dynamics are consistent in both locations, contributing similarly to nutrient mixing and habitat structuring64. Additionally, seawater temperatures across the sites remain within the optimal range for Laminaria ochroleuca, and Saccorhiza polyschides23, supporting kelp populations historically in both areas.
Fig. 8.
Study sites (Berlengas archipelago MPA-BER and Peniche PEN) and reefs where the experiment occurred. Outward dots represent deep reefs, while inward dots represent shallow reefs.
Although nowadays, kelps inside MPA-BER are scarce, and mainly the genus Phyllariopsis spp. this was not always the case and records of the species Laminaria ochroleuca and Saccorhiza polyschides exist and indicate the presence of kelp forests in the past61,65–67.
Underwater tethering experiment
During the summer (August) and autumn (September) of 2022, fieldwork was conducted at the two selected sites, PEN and MPA-BER. All experimental procedures were replicated for both periods.
In each season, before deployment, seaweed fronds were collected from 180 L. ochroleuca and S. polyschides individuals, totalling 360 samples (n = 360). Upon collection, fronds were immersed in aerated seawater tanks at 15 °C overnight to minimise potential seaweed degradation. Each blade was cut into a ~ 25 cm x 10 cm sample in the laboratory, and its initial weight was determined. Subsequently, using standard pegs, 10 samples were fixed to each of 36 stainless steel chains (Supplementary Fig. 1). Standard pegs were secured to the chain using plastic cable ties, with every chain accommodating 5 samples from each kelp species, placed alternated. Samples were enclosed in seawater-damp fabric bags within a cooling box for transportation.
For deployment, two depths were selected at each site: shallow (5–10 m) and deep (15–25 m) (Fig. 8). Within each depth, three reefs were randomly chosen (n = 12), and in each of these reefs, three stainless steel chains (n = 36) containing previously weighted fragments of both kelp species attached were deployed on the substrate (approx. 3 m distance from each other) during 72 h. After this period, chains were retrieved and placed inside fabric bags in a cooling box until processed at the lab. The final weight and the presence/type of grazer bite marks were assessed for every sample.
Once at the laboratory, bite marks in the samples can be distinguished between sea urchins and fish (Supplementary Fig. 2). Every sample was placed on a white surface, and the bite pattern was analysed following Franco et al.19 to assess one of the two grazing categories: ‘urchin’ or ‘fish.’
The measured parameters allowed the estimation of ‘Herbivory Occurrence’ by comparing the number of kelp with and without grazer marks; ‘Herbivory Rate’ by assessing the percentage of consumed biomass in each kelp; and ‘Frequency of Grazer’ by counting the frequency of kelp with different grazer marks.
Faunal characterisation of the area—macro-herbivore abundance
A faunal characterisation of each reef was performed using Underwater Visual Census (UVC) and Diver-Operated Videos (DOV). UVC was done to assess the abundance and diversity of fish and macroinvertebrates and consisted of 5 × 25 m radial transects, 4 m wide for fish and 2 m wide for sea urchins12. A total of 12 × 25 m transects were performed each season, ~ 2400 m2 of transects for fish and ~ 1200 m2 of transects for urchins. For DOV surveys and video analysis, a swimming stereo camera system adapted for two GoPros from SeaGIS company (www.seagis.com.au) was used as well as SeaGIS EventMeasure software for the assessment of species abundance and diversity through counts and species identification68,69. Video recording and analysis can also be done with a standard camera and video software. Videos were recorded through 5 × 25 m radial transects in each reef. Due to logistic constraints, the DOV surveys were only performed in autumn, totalling ~ 1200 m2 of transects.
For faunal characterisation, the sea urchin species Paracentrotus lividus and Sphaerechinus granularis were the target invertebrate species since it is the species present in this region. Regarding fish, both strictly herbivorous and omnivorous were considered: Boops boops, Diplodus spp., Sarpa salpa, and Spondyliosoma cantharus. Sarpa salpa is the only species regarded as strictly herbivorous. The other species are considered omnivorous and are also known to consume seaweeds. For example, Diplodus spp. predominantly feeds on seaweed during its juvenile stage19,21,70. All the mentioned species are commonly fished outside the MPA and occasionally within it, by recreational anglers. However, Sarpa salpa is mainly caught incidentally, as it holds little commercial value compared to the other species71.
Statistical analysis
All analyses of herbivory occurrence, herbivory rate, frequency of grazer, and herbivore abundances were done with generalised linear mixed models (GLMM) and generalised linear models (GLM) and appropriate data distributions using the R package glmmTmB72.
Model diagnostics, essential for validating the model’s assumptions, were conducted using visual inspection and the ‘DHARMa’ package for residual analysis.
We evaluated the occurrence of herbivory by comparing cases where consumed biomass was zero against cases where it registered any positive value. Here, fixed factors were Site (protection status), Depth, and Kelp species; random factors were Reef and Season and a binomial distribution was used. As for herbivory rate and frequency of grazer, the percentage of consumed biomass and frequency of different types of bitemarks were considered, respectively. Here, the considered fixed factors were Site (protection status), Depth, Kelp species, and Grazer. Random factors remained the same.
For the herbivore characterisation of the study areas, abundance of herbivorous fish and sea urchins was compared between the factors Site (protection status) and Depth.
A gamma distribution was chosen for the herbivory rate and a negative binomial for the remaining ones.
To ascertain the robustness of our findings, we explored various configurations of GLMMs and GLMs, assessing different combinations of factors. This model selection process utilised the ‘MuMIn’ package73, allowing the estimation of variable importance through model averaging. This method integrates results from multiple models, identifying the most parsimonious model based on the Akaike Information Criterion (AIC) and its importance weights. The best-fitting model was tested for model assumptions using visual inspection and the ‘DHARMa’ package. In addition, post hoc tests to identify specific group differences for the factors in the model were performed using the R ‘emmeans’ (Estimated Marginal Means) package.
All plots and figures were created using Python and the packages ‘pandas’, ‘seaborn’, and ‘matplotlib’.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Pieter van der Linden and Jonas de Azevedo for helping in the fieldwork and Rodrigo Correia for helping with video analysis. This research was supported by the strategic funding UIDB/04423/2020 (https://doi.org/10.54499/UIDB/04423/2020) and UIDP/04423/2020 (https://doi.org/10.54499/UIDP/04423/2020) and LA/P/0101/2020 (https://doi.org/10.54499/LA/P/0101/2020) through national funds provided by FCT – Fundação para a Ciência e a Tecnologia. This work was also supported by the project BLUEFORESTING- Climate Resilient Marine Forests for a Sustainable Future (PT-INNOVATION-0077) from Iceland, Liechtenstein and Norway through the EEA Grants-Blue Growth Program. Additional support from the European Union’s Horizon 2020 project FutureMARES (grant 869300) and by the project ACTNOW funded by European Union’s Horizon Europe Research and Innovation Programme under grant agreement No 101060072. Bianca Reis PhD fellowship (2020.08068.BD) was granted by Fundação para a Ciência e Tecnologia (FCT, Portugal). We are grateful to the editors and to the anonymous reviewers for the constructive comments on an earlier version of the manuscript.
Author contributions
BR, JNF and FA contributed to the study’s conception and design. Funding was acquired by ISP, FA and JNF. Material preparation, assembling and data collection were performed by BR, JNF and ÁSG. Fieldwork and sample processing were done by BR, JNF, ÁSG and AFSM. Data processing and analysis were performed by BR. The first draft of the manuscript was written by BR, and all authors commented and participated in the manuscript. All authors read and approved the final manuscript.
Data availability
A part of data is provided within supplementary information, the rest of the dataset generated during and/or analysed during the current study is available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
We confirm that all experimental research and field studies involving plants in this study, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation. No species at risk of extinction were involved in this research, and all ethical and legal standards were followed.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Horta e Costa, B et al. A regulation-based classification system for Marine protected areas (MPAs). Mar. Policy. 72, 192–198. 10.1016/j.marpol.2016.06.021 (2016). [Google Scholar]
- 2.Cabral, R. B. et al. A global network of marine protected areas for food. Natl. Geographic Soc.96744, 28134–28139. 10.1073/pnas.2000174117/-/DCSupplemental (2021). [Google Scholar]
- 3.Benedetti-Cecchi, L. et al. Marine protected areas promote stability of reef fish communities under climate warming. Nat. Commun.15, 18–22 10.1038/s41467-024-44976-y (2024). [DOI] [PMC free article] [PubMed]
- 4.Zhao, Q. et al. Where Marine protected areas would best represent 30% of ocean biodiversity. Biol. Conserv.244, 108536 10.1016/j.biocon.2020.108536 (2020).
- 5.Convention on Biological Diversity. Decision adopted by the conference of the parties to the Convention on Biological Diversity 15/4. Kunming-Montreal Global Biodiversity Framework. (2022).
- 6.European Commission. Regulation of the European Parliament and of the Council on Nature Restoration and Amending Regulation (EU) 2022/869. Brussels: European Parliament and the Council. PE-CONS 74/23 (European Parliament and the Council, 2024).
- 7.Bates, A. E. et al. Climate resilience in marine protected areas and the ‘Protection Paradox’. Biol. Conserv.236, 305–314. 10.1016/j.biocon.2019.05.005 (2019). [Google Scholar]
- 8.Duarte, C. M. et al. Rebuilding Marine Life. Nature 580, 13–15, https://doi.org/10.1038/s41586-020-2146-7 (2020) [DOI] [PubMed]
- 9.Peleg, O., Blain, C. O. & Shears, N. T. Long-term marine protection enhances kelp forest ecosystem stability. Ecol. Appl.33, 2895 10.1002/eap.2895 (2023). [DOI] [PubMed]
- 10.Babcock, R. C. et al. Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Natl. Acad. Sci. U.S.A.107, 18256–18261. 10.1073/pnas.0908012107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filbee-Dexter, K. et al. Marine protected areas can be useful but are not a silver bullet for kelp conservation. J. Phycol.60, 203–213. 10.1111/jpy.13446 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Franco, J. N. Kelps across Iberia: from patterns of abundance and distribution to top-down and bottom-up regulatory processes. PhD diss., Universidade do Porto (Portugal), (2017)
- 13.Wernberg, T., Krumhansl, K., Filbee-Dexter, K. & Pedersen, M. in Chapter 3. Status and trends for the world’s kelp forests, World Seas: an Environmental Evaluation, Academic Press: London, UK 2nd edition, 57–78, (2019).
- 14.Eger, A. M. et al. Playing to the positives: using synergies to Enhance Kelp Forest Restoration. Front. Mar. Sci.7, 544. 10.3389/fmars.2020.00544 (2020).
- 15.Cavanaugh, K. et al. in Chapter 3 Status, trends and future projections, Into the Blue - Securing a Sustainable Future for Kelp Forests. United Nations Environment Programme (2023).
- 16.Malakhoff, K. D. & Miller, R. J. After 15 years, no evidence for trophic cascades in marine protected areas. Proc. Royal Soc. B: Biol. Sci.288, 20203061. 10.1098/rspb.2020.3061 (2021). [DOI] [PMC free article] [PubMed]
- 17.Hopf, J. K., Caselle, J. E. & White, J. W. No-take marine protected areas enhance the benefits of kelp-forest restoration for fish but not fisheries. Ecol. Lett.25, 1665–1675. 10.1111/ele.14023 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe, D. B. et al. Herbivory makes major contributions to ecosystem carbon and nutrient cycling in tropical forests. Ecol. Lett.17, 324–332. 10.1111/ele.12233 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Franco, J. N. et al. Herbivory drives kelp recruits into ‘hiding’ in a warm ocean climate. Mar. Ecol. Prog. Ser.536, 1–9. 10.3354/meps11445 (2015). [Google Scholar]
- 20.Zarco-Perello, S., Bosch, N. E., Bennett, S., Vanderklift, M. A. & Wernberg, T. Persistence of tropical herbivores in temperate reefs constrains kelp resilience to cryptic habitats. J. Ecol.109, 2081–2094. 10.1111/1365-2745.13621 (2021). [Google Scholar]
- 21.Barrientos, S., Piñeiro-Corbeira, C. & Barreiro, R. Temperate Kelp Forest Collapse by Fish Herbivory: a detailed demographic study. Front. Mar. Sci.9, 817021. 10.3389/fmars.2022.817021 (2022).
- 22.Miller, K. I. et al. Large-scale one-off sea urchin removal promotes rapid kelp recovery in urchin barrens. Restor. Ecol.10.1111/rec.14060 (2023). [Google Scholar]
- 23.Franco, J. N. et al. The ‘golden kelp’ Laminaria ochroleuca under global change: integrating multiple eco-physiological responses with species distribution models. J. Ecol.106, 47–58. 10.1111/1365-2745.12810 (2018). [Google Scholar]
- 24.Sánchez-Barredo, M. et al. Effects of Heat waves and light deprivation on Giant Kelp juveniles (Macrocystis pyrifera, Laminariales, Phaeophyceae). J. Phycol.56, 880–894. 10.1111/jpy.13000 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Starko, S. et al. Microclimate predicts kelp forest extinction in the face of direct and indirect marine heatwave effects. Ecol. Appl.32, 2673. 10.1002/eap.2673 (2022). [DOI] [PubMed]
- 26.Azevedo, D. J., Franco, N. J., Vale, G. C., Lemos, L. F. M. & Arenas, F. Rapid tropicalization evidence of subtidal seaweed assemblages along a coastal transitional zone. Sci. Rep.13, 11720. 10.1038/s41598-023-38514-x (2023). [DOI] [PMC free article] [PubMed]
- 27.Gianni, F. et al. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine protected areas. Adv. Oceanogr. Limnol.4, 83–101. 10.1080/19475721.2013.845604 (2013). [Google Scholar]
- 28.Gianni, F. et al. Threats to large brown algal forests in temperate seas: the overlooked role of native herbivorous fish. Sci. Rep. 1–13. 10.1038/s41598-017-06394-7 (2017). [DOI] [PMC free article] [PubMed]
- 29.Barrientos, S., Barreiro, R. & Piñeiro-Corbeira, C. Paradoxical failure of Laminaria ochroleuca (Laminariales, Phaeophyceae) to consolidate a kelp forest inside a Marine National Park. Eur. J. Phycol.58, 72–82. 10.1080/09670262.2022.2065365 (2023). [Google Scholar]
- 30.O’Leary, B. C. & Roberts, C. M. Ecological connectivity across ocean depths: implications for protected area design. Global Ecol. Conserv.15, 00431. 10.1016/j.gecco.2018.e00431 (2018).
- 31.Curley, G. B., Kingsford, J. M. & Gillanders, M. B. Spatial and habitat-related patterns of temperate reef fish assemblages: implications for the design of Marine protected areas. Mar. Freshw. Res.53, 1197. 10.1071/MF01199 (2002). [Google Scholar]
- 32.Steneck, S. R., Leland, A., Mcnaught, C. D. & Vavrinec, J. Ecosystem flips, locks, and Feedbacks: the lasting effects of fisheries on Maine’s Kelp Forest Ecosystem. Bull. Mar. Sci.89, 31–55. 10.5343/bms.2011.1148 (2013). [Google Scholar]
- 33.Rhoades, K. O., Patrick, J. C. & Ogburn, B. M. Reviewing theory, design, and analysis of tethering experiments to enhance our understanding of predation. Mar. Biol.171, 1–14. 10.1007/s00227-024-04503-5 (2024).
- 34.Tuya, F. et al. Patterns of landscape and assemblage structure along a latitudinal gradient in ocean climate. Mar. Ecol. Prog. Ser.466, 9–19. 10.3354/meps09941 (2012). [Google Scholar]
- 35.Cardoso, A. C., Arenas, F., Sousa-Pinto, I., Barreiro, A. & Franco, J. N. Sea urchin grazing preferences on native and non-native macroalgae. Ecol. Ind.111, 106046. 10.1016/j.ecolind.2019.106046 (2020).
- 36.Marta, D. I., Vergés, A., Powell, S., Smith, S. & Poore, A. Marine protected areas are linked to higher predation rates by fish in shallow urbanised reefs, but only in no-take reserves. Mar. Ecol. Prog. Ser.721, 135–150. 10.3354/meps14421 (2023). [Google Scholar]
- 37.Vergés, A., Doropoulos, C., Malcolm, H. A. & Skye, M. & Garcia-pizá, M. Long-Term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. (2016). 10.1073/pnas.1610725113 [DOI] [PMC free article] [PubMed]
- 38.Zarco-perello, S., Wernberg, T., Langlois, T. J. & Vanderklift, M. A. Tropicalization strengthens consumer pressure on habitat- forming seaweeds. Sci. Rep.7(1), 1–8. 10.1038/s41598-017-00991-2 (2017). [DOI] [PMC free article] [PubMed]
- 39.Longo, O. G., Ferreira, L. E. C. & Floeter, R. S. Herbivory drives large-scale spatial variation in reef fish trophic interactions. Ecol. Evol.4, 4553–4566. 10.1002/ece3.1310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prado, P., Farina, S., Tomas, F., Romero, J. & Alcoverro, T. Marine protection and meadow size alter fish herbivory in seagrass ecosystems. Mar. Ecol. Prog. Ser.371, 11–21. 10.3354/meps07662 (2008). [Google Scholar]
- 41.Planes, S., Raventos, N., Ferrari, B. & Alcoverro, T. Fish herbivory leads to shifts in seagrass posidonia oceanica investments in sexual reproduction. Mar. Ecol. Prog. Ser.431, 205–213. 10.3354/meps09089 (2011). [Google Scholar]
- 42.Raventos, N., Ferrari, B. & Planes, S. Differences in population parameters and behaviour of the herbivorous fish Sarpa salpa between protected and unprotected seagrass meadows in the north-western Mediterranean. J. Mar. Biol. Assoc. U.K.89, 1153–1159. 10.1017/S0025315409000423 (2009). [Google Scholar]
- 43.Bañón, R. et al. Tropicalization of fish fauna of galician coastal waters, in the NW Iberian upwelling system. Reg. Stud. Mar. Sci.70, 103369. 10.1016/j.rsma.2024.103369 (2024).
- 44.Allard, H., Ayling, A. M. & Shears, N. T. Long-term changes in reef fish assemblages after 40 years of no-take marine reserve protection. Biol. Conserv.265, 109405. 10.1016/j.biocon.2021.109405 (2022). [Google Scholar]
- 45.Ferguson, M. A., Harvey, S. E. & Knott, A. N. Herbivore abundance, site fidelity and grazing rates on temperate reefs inside and outside marine reserves. J. Exp. Mar. Biol. Ecol.478, 96–105. 10.1016/j.jembe.2016.02.008 (2016). [Google Scholar]
- 46.Dimitriadis, C. et al. Evaluating the long term effectiveness of a Mediterranean Marine protected area to tackle the effects of invasive and range expanding herbivorous fish on rocky reefs. Mar. Environ. Res.193, 106293. 10.1016/j.marenvres.2023.106293 (2024). [DOI] [PubMed]
- 47.Gilby, B. L. & Stevens, T. Meta-analysis indicates habitat-specific alterations to primary producer and herbivore communities in marine protected areas. Global Ecol. Conserv.2, 289–299. 10.1016/j.gecco.2014.10.005 (2014). [Google Scholar]
- 48.Araújo, R. M. et al. Status, trends and drivers of kelp forests in Europe: an expert assessment. Biodiversity and Conservation25, 1319–1348 (2016).
- 49.Reed, D. et al. Extreme warming challenges sentinel status of kelp forests as indicators of climate change. Nat. Commun.7, 13757. 10.1038/ncomms13757 (2016). [DOI] [PMC free article] [PubMed]
- 50.Reis, B. et al. Benthic incubation Chamber (BIC) for in-situ assessment of primary productivity in different canopy-forming communities. Mar. Biol.171, 1–11 (2024). [Google Scholar]
- 51.Filbee-Dexter, K. et al. Carbon export is facilitated by sea urchins transforming kelp detritus. Oecologia192, 213–225. 10.1007/s00442-019-04571-1 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Blanco, A., Neto, J. M., Troncoso, J., Lemos, M. F. L. & Olabarria, C. Effectiveness of two western Iberian Peninsula Marine protected areas in reducing the risk of macroalgae invasion. Ecol. Ind.108, 105705. 10.1016/j.ecolind.2019.105705 (2020).
- 53.Edgar, G. J. et al. Global conservation outcomes depend on marine protected areas with five key features. Nature506, 216–220. 10.1038/nature13022 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Coleman, M. A. et al. Functional traits reveal early responses in marine reserves following protection from fishing. Divers. Distrib.21, 876–887. 10.1111/ddi.12309 (2015). [Google Scholar]
- 55.Marta, I. R. D., Vergés, A., Powell, S., Smith, S. M. & Poore, A. G. B. Marine protected areas are linked to higher predation rates by fish in shallow urbanised reefs, but only in no-take reserves. Mar. Ecol. Prog. Ser.721, 135–150. 10.3354/meps14421 (2023). [Google Scholar]
- 56.Wilson, K. L., Tittensor, D. P., Worm, B. & Lotze, H. K. Incorporating climate change adaptation into marine protected area planning. Glob. Change Biol.26, 3251–3267. 10.1111/gcb.15094 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Kuwahara, H. The evaluation of current status for grazing control techniques of herbivorous fish. Fisheries Eng.51, 253–257 (2015). [Google Scholar]
- 58.Fredriksen, S. et al. Green gravel: a novel restoration tool to combat kelp forest decline. Sci. Rep.1010.1038/s41598-020-60553-x (2020). [DOI] [PMC free article] [PubMed]
- 59.Ling, S. D., Ibbott, S. & Sanderson, J. C. Recovery of canopy-forming macroalgae following removal of the enigmatic grazing sea urchin Heliocidaris Erythrogramma. J. Exp. Mar. Biol. Ecol.395, 135–146 (2010). [Google Scholar]
- 60.Filbee-Dexter, K. & Scheibling, R. E. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser.495, 1–25. 10.3354/meps10573 (2014). [Google Scholar]
- 61.Gaspar, R. Contribuição para o conhecimento das espécies de macroalgas da reserva natural da Berlenga. Relatório do projecto: Contribuição para o conhecimento das espécies de macroalgas da reserva natural da Berlenga e da área de protecção especial dos Farilhões. (1993).
- 62.Jacinto, D., Cruz, T., Silva, T. & Castro, J. J. Gestión de la explotación de percebe (Pollicipes pollicipes) en la Reserva Natural De Berlengas (Portugal): Evaluación Del tope de capturas y talla mínima. Scientia Mar.75, 439–445. 10.3989/scimar.2011.75n3439 (2011). [Google Scholar]
- 63.Franco, J. N., Lemos, M. F. L. & Neto, J. M. Macroalgas das Berlengas e Costa de Prata: Do conhecimento à aplicação. Instituto Politécnico de Leiria1, 1–38. https://doi.org/10.25766/rwp2-8k68 (2022).
- 64.Marques, A. F. S. et al. Assessing Atlantic Kelp Forest Restoration Efforts in Southern Europe. Sustainability16(21), 9176 (2024).
- 65.Assis, J. et al. Finding kelp, a GIS-Based Community Participation Project to assess Portuguese Kelp Conservation Status. J. Coastal. Res. Special Issue 1, 1469–1473 (2009).
- 66.Queiroga, H., Leão, F. & Coutinho, M. Candidatura das Berlengas a Reserva da Biosfera da UNESCO (2008).
- 67.Amado, A., Preto, A. & Bártolo, P. Versão Final do Relatório do Plano De Ordenamento Da RNB. Equipa Técnica COORDENAÇÃO: COLABORAÇÃO INTERNA NO ICNB (2007).
- 68.López-Macías, J. et al. Comparison of two Stereo-Video Software for the Assessment of Marine resources. Thalassas: Int. J. Mar. Sci.39, 395–404. 10.1007/s41208-022-00507-4 (2023). [Google Scholar]
- 69.SeaGIS (2024). https://www.seagis.com.au/index.html#about
- 70.Froese, R. & Pauly, D. Fish Base, (2024). https://www.fishbase.se/search.php
- 71.Paiva, R. Salema, Sarpa salpa (Linnaeus 1758): stock structure in the eastern Atlantic and biological characterization off the Portuguese coast PhD thesis, University of Lisbon, (2015).
- 72.Brooks, M. et al. & Package ‘glmmtmb’. R Packag Vers 1.1 (2023).
- 73.Barton, K. MuMIn: multi-model inference. R Package Cran-R. 1, 289–290 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A part of data is provided within supplementary information, the rest of the dataset generated during and/or analysed during the current study is available from the corresponding author on reasonable request.