Abstract
Colorectal cancer (CRC) is one of the most common malignant tumors worldwide, with a high incidence rate and mortality. The analysis of serum biomarkers for colorectal cancer diagnosis has attracted more and more attention because of its low cost, repeatability, and quantification. This study was aimed to evaluate the diagnostic performance of serum Ephrin-A1 in patients with CRC. We retrospectively analyzed CRC cases in a test cohort (121 patients and 108 controls) and validated them in a validation cohort (119 patients and 118 controls). The concentration of Ephrin-A1 in serum was detected by Enzyme-linked immunosorbent assay (ELISA) and the diagnostic performance of serum Ephrin-A1 was evaluated by receiver operating characteristic (ROC) analysis. In the test cohort, serum Ephrin-A1 levels in patients with all-stage CRC and early-stage CRC were significantly higher than those in healthy controls. The area under the ROC curve (AUC), sensitivity and specificity of all-stage CRC and early-stage CRC were 0.709 (95% CI 0.644–0.775) and 0.660 (95% CI 0.530–0.790), 48.76% and 45.00%, 81.48% and 81.48%, respectively. Similar results were observed in the validation cohort. Serum Ephrin-A1 might be served as a potential biomarker in the diagnosis of CRC.
Keywords: Ephrin-A1, Colorectal cancer, Serum biomarker, Diagnosis
Subject terms: Biological techniques, Cancer, Molecular medicine
Introduction
As a common malignant tumor of the digestive system, colorectal cancer (CRC) was responsible for over 1.9 million new cases and 904,000 deaths in 2022, making it the third most frequently diagnosed cancer and the second leading cause of cancer death worldwide1. In China, CRC ranks second in the most commonly diagnosed cancer types and fourth in the most common causes of cancer-related deaths2. The 5-year relative survival rate for CRC ranges from 90% in patients diagnosed with localized disease to 14% for those diagnosed with distant metastatic disease3. Therefore, early diagnosis is very important for the treatment and prognosis of patients with colorectal cancer.
Colonoscopy serves as the gold standard for colorectal cancer screening. Among all screening methods, colonoscopy is the most sensitive and is the only one that involves the resection of precancerous lesions and/or the biopsy of neoplastic lesions4. However, invasive, unpleasant bowel preparation, risk of intestinal perforation, or bleeding limit it to being a regular screening method5. Noninvasive screenings, such as fecal occult blood tests and stool DNA analyses, are valued for being straightforward and nonintrusive. However, their limited sensitivity and specificity can lead to misdiagnoses or undetected conditions6. In recent years, the use of protein markers in serum has been explored as strategies for tumor diagnosis. Serum-based tumor markers are considered to be simple, relatively cheap, reproducible, quantitative, and objective diagnostic tools. Carcinoembryonic antigen (CEA) is the only marker recommended by American Society of Clinical Oncology (ASCO) in 2006 for the management of CRC patients but has insufficient sensitivity and specificity7,8. Therefore, it is urgent to explore new, high sensitivity and specific molecular markers for the early diagnosis of colorectal cancer.
Ephrins (EFNs) are ligands for erythropoietin-producing hepatoma (Eph) receptors, which together comprise the largest subfamily of receptor protein-tyrosine kinases. Ephrin-A1, a member of the ephrins family, is upregulated in a variety of tumors, such as esophageal squamous cell carcinoma9,10, cervical cancer11, gastric cancer12, renal cancer13, and colorectal cancer14. Ephrin-A1 is widely involved in tumorigenesis by influencing tumor neovascularization, invasion, and metastasis15–17, and it is closely related to the prognosis of many tumors18,19.
Salem, E. et al. have suggested that the rs12904 A > G polymorphism located in the 3′-untranslated region (3′-UTR) of the Ephrin-A1 gene is pivotal in the pathogenesis and metastatic progression of CRC. This polymorphism may be utilized as a biomarker for the molecular diagnosis and prognostication of metastatic potential in CRC20. Furthermore, previous studies have shown that EFNA1 mRNA expression is associated with poor prognosis of CRC patients, and is considered to be an independent prognostic factor of CRC14. However, little is known about the concentration of serum Ephrin-A1 in CRC patients. Here, we compared the serum levels of Ephrin-A1 between CRC patients and healthy persons and investigated the value of the serum Ephrin-A1 as a potential biomarker for CRC diagnosis.
Materials and methods
Study samples
Totally, 466 serum samples were selected in this study. In the test cohort, 121 serum samples from patients with CRC and 108 serum samples from normal controls were collected from the First Affiliated Hospital of Shantou University Medical College, from June 2017 to August 2018. In the validation cohort, 119 serum samples from patients with CRC and 118 serum samples from normal controls were collected from the Cancer Hospital of Shantou University Medical College, from January 2019 to January 2021. All participants in the cancer group were newly diagnosed CRC patients without any anticancer treatment before blood collection, while participants in the normal controls group were qualified blood donors and all of them have no evidence of cancer. The peripheral blood of the patients and the normal group were centrifuged at 2500 g for 10 min. The supernatant was collected as serum samples and stored at -80℃ until analysis.
We classified tumor stage aligned with the eighth edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual21 and defined I-IIA AJCC TNM stage as early-stage CRC. All participants provided informed consent and the study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College and the Ethics Committee of the Cancer Hospital of Shantou University Medical College, which was undertaken according to the principles of the Helsinki Declaration of 1975 (as revised 2008).
Enzyme-linked immunosorbent assay (ELISA)
The concentration of Ephrin-A1 in serum was detected by ELISA kit (CSB-EL007460HU, Cusabio, Wuhan, Hubei, China) according to the user manual. Briefly, the Ephrin-A1 protein standard was diluted to 10, 5, 2.5, 1.25, 0.625, 0.315, 0.156, and 0 ng/ml to make the standard curve, while the serum samples were diluted with sample diluent at the ratio of 1:1. The 96-well microplates were pre-coated with an antibody specific for Ephrin-A1. 100 µl of diluted standard and sample were pipetted into the wells and incubated at 37 ℃ for 2 h. After removing any unbound substrates, biotin-conjugated Ephrin-A1 specific antibody was added to the wells and incubated at 37 ℃ for one hour. After three washes with the automatic plate washer (5165000, Thermo Fisher Scientific), avidin conjugated Horseradish Peroxidase (HRP) was added to the wells and incubated at 37 ℃ for one hour. Following five washes to remove any unbound avidin-enzyme reagent, a substrate solution was added to the wells and color develops in proportion to the amount of bound Ephrin-A1. The color development was stopped after 20 min. The intensity of the color was measured within 5 min, using a microplate reader (ELX800, BioTek® Instruments, Winooski, VT, USA) set to 450 nm and 630 nm. The optimum cut-off values were obtained from the Youden’s indexes of the ROC curves, which yield maximum values of sensitivity plus (100%-specificity).
Statistical analysis
Statistical analyses were performed using SigmaPlot 10.0 (Systat Software Inc., San Jose, California, USA), SPSS Statistics 21.0 (SPSS Inc., Chicago, IL, USA), and GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA). Readings at 630 nm were subtracted from the readings at 450 nm to correct for optical imperfections in the plat. The corrected optical density value was changed to concentration according to the standard curve. The differences of Ephrin-A1 level between the normal control group and CRC group or early-stage CRC were tested by performing Mann–Whitney U test. Receiver operating characteristic (ROC) analysis was performed to identify the diagnostic value of Ephrin-A1, which was determined by calculating the area under the ROC curve (AUC) with the 95% confidence interval (95% CI), sensitivity, specificity, and cut-off value. Correlation between Ephrin-A1 and clinical data in CRC patients was evaluated by the chi-square test. P < 0.05 was considered statistically significant. Serum CEA was quantified at the First Affiliated Hospital of Shantou University Medical College and Shantou University Medical College Cancer Hospital using the UniCel DXi 800 and Roche E601, respectively. According to the manufacturer’s instructions, the cutoff value for normal CEA was less than 9.7 and 3.8 ng/ml, respectively.
Results
The level of serum Ephrin-A1 in normal controls and CRC patient
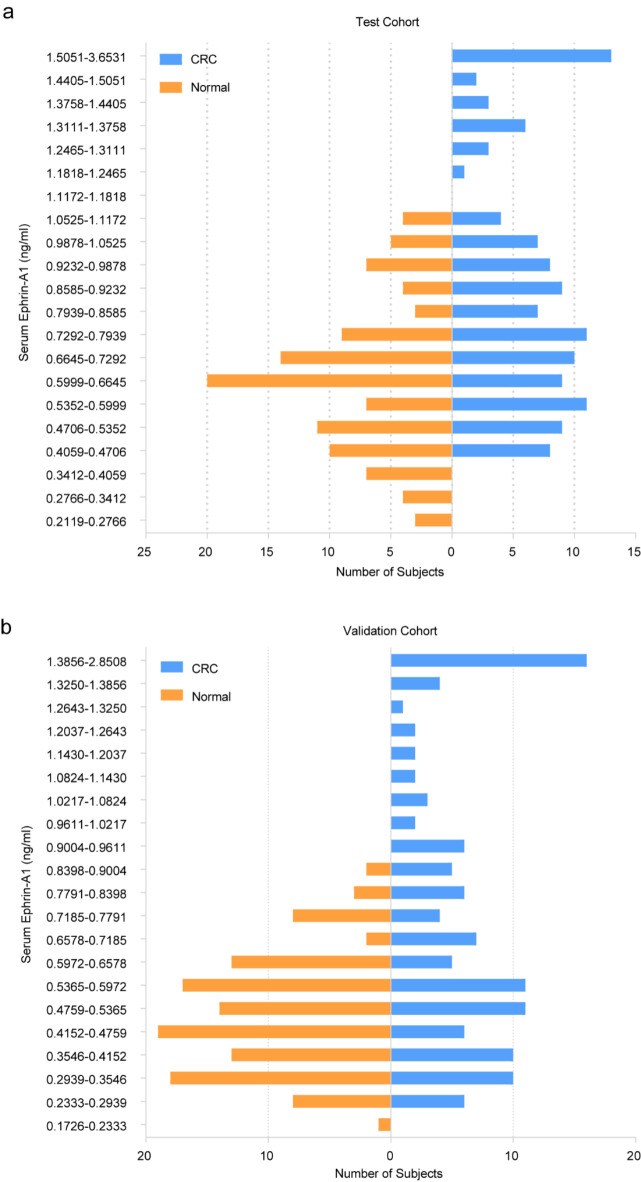
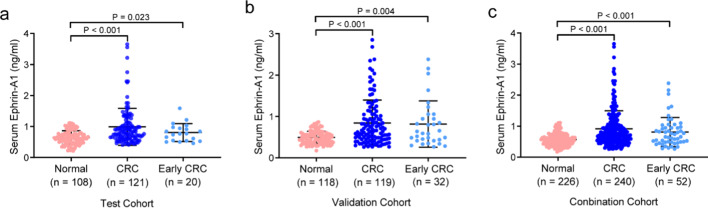
The pathological data of patients in the test cohort (121 patients and 108 controls) and the validation cohort (119 patients and 118 controls) were summarized in Table 1. In the test cohort, the mean age of CRC patients and normal controls was 64 years (range 26–85 years) and 50 years (range 25–83 years), respectively. The subjects in the validation cohort had a similar mean and range of age. Focused on the Ephrin-A1 concentration distribution of CRC and controls, we found that the CRC group accounted for more histogram volume at high concentration, while the normal group accounted for more at low concentration (Fig. 1a). This result was confirmed in the validation cohort (Fig. 1b). In the test cohort, the mean concentration of serum Ephrin-A1 (mean ± SD) in the normal group, CRC group and early-stage CRC group was 0.646 ± 0.217 ng/ml, 0.989 ± 0.598 ng/ml and 0.803 ± 0.287 ng/ml (Table 2). These values in the validation cohort were 0.494 ± 0.150 ng/ml, 0.841 ± 0.554 ng/ml and 0.816 ± 0.560 ng/ml, respectively (Table 2). When the two cohorts of data were combined, the serum concentration of Ephrin-A1 in the control group, CRC group, and early-stage CRC group were 0.567 ± 0.200 ng/ml, 0.916 ± 0.580 ng/ml, and 0.811 ± 0.471 ng/ml, respectively. Serum Ephrin-A1 in patients with CRC and early-stage CRC was significantly higher than that in normal controls (Fig. 2a). This result was verified in the validation cohort and the combination cohort (Fig. 2b and c).
Table 1.
Patient demographics and clinical characteristics.
| Variable | Test cohort | Validation cohort | ||
|---|---|---|---|---|
| CRC patients (n = 121) | Normal control (n = 108) | CRC patients (n = 119) | Normal control (n = 118) | |
| Age (years) | ||||
| Mean ± SD | 64 ± 12 | 50 ± 15 | 65 ± 11 | 51 ± 11 |
| Range | 26–85 | 25–83 | 33–88 | 36–82 |
| Gender | ||||
| Male | 69 (57.02%) | 70 (64.81%) | 72 (60.50%) | 77 (65.25%) |
| Female | 52 (42.98%) | 38 (35.19%) | 47 (39.50%) | 41 (34.75%) |
| Location | ||||
| Colon | 83 (68.60%) | 65 (54.62%) | ||
| Rectum | 38 (31.40%) | 54 (45.38%) | ||
| Tumor size | ||||
| <=5 cm | 64 (52.89%) | 55 (46.22%) | ||
| > 5 cm | 51 (42.15%) | 28 (23.53%) | ||
| Unknown | 6 (4.96%) | 36 (30.25%) | ||
| Grade | ||||
| Low | 0 (0%) | 11 (9.24%) | ||
| Moderate | 98 (80.99%) | 59 (49.58%) | ||
| High | 2 (1.65%) | 3 (2.52%) | ||
| Unknown | 21 (17.36%) | 46 (38.66%) | ||
| Invasion depth | ||||
| Tis | 0 (0%) | 1 (0.84%) | ||
| T1 | 2 (1.65%) | 5 (4.2%) | ||
| T3 | 12 (9.92%) | 10 (8.40%) | ||
| T3 | 10 (8.26%) | 28 (23.53%) | ||
| T4 | 93 (76.86%) | 62 (52.10%) | ||
| Unknown | 4 (3.31%) | 13 (10.92%) | ||
| Lymphatic metastasis | ||||
| N0 | 61 (50.41%) | 49 (41.18%) | ||
| N1 | 36 (29.75%) | 31 (26.05%) | ||
| N2 | 16 (13.22%) | 15 (12.61%) | ||
| N3 | 2 (1.65%) | 1 (0.845%) | ||
| Unknown | 6 (4.96%) | 21 (17.65%) | ||
| Distant metastasis | ||||
| M0 | 102 (84.30%) | 77 (64.71%) | ||
| M1 | 14 (11.57%) | 20 (16.81%) | ||
| Unknown | 5 (4.13%) | 22 (18.49%) | ||
| TNM stage | ||||
| I-IIA | 20 (16.53%) | 32 (26.89%) | ||
| IIB-IV | 97 (80.17%) | 72 (60.50%) | ||
| Unknown | 4 (3.31%) | 15 (12.61%) | ||
CRC, colorectal cancer; SD, standard deviation.
Fig. 1.
A distribution bar charts of the predicted probability of serum Ephrin-A1 in CRC patients and normal control. (a) The lowest concentration was 0.2119 ng/ml in normal control and the highest one was 3.6531 ng/ml in normal control in the test cohort. The concentration between 0.2119 ng/ml and 1.5051 ng/ml was divided equally for 20 sections, and the concentration higher than 1.5051 ng/ml was classified as one section. (b) The lowest concentration was 0.1726 ng/ml in normal control and the highest one was 2.8508 ng/ml in normal control in the validation cohort. The concentration between 0.1726 ng/ml and 1.3856 ng/ml was divided equally for 20 sections, and the concentration higher than 1.3856 ng/ml was classified as one section. CRC, colorectal cancer.
Table 2.
Comparison between groups.
| No. | Mean ± SD | P value* | |
|---|---|---|---|
| Test cohort | |||
| Normal control | 108 | 0.646 ± 0.217 | – |
| CRC | 121 | 0.989 ± 0.598 | < 0.001 |
| Early-stage CRC | 20 | 0.803 ± 0.287 | 0.023 |
| Validation cohort | |||
| Normal control | 118 | 0.494 ± 0.150 | – |
| CRC | 119 | 0.841 ± 0.554 | < 0.001 |
| Early-stage CRC | 32 | 0.816 ± 0.560 | 0.003 |
| Combination cohort | |||
| Normal control | 226 | 0.567 ± 0.200 | – |
| CRC | 240 | 0.916 ± 0.580 | < 0.001 |
| Early-stage CRC | 52 | 0.811 ± 0.471 | < 0.001 |
CRC, colorectal cancer; SD standard deviation.
* Compared with the matched normal control group.
Fig. 2.
The concentration of serum Ephrin-A1 in CRC patients, early-stage CRC patients, and normal control. (a) The concentration of serum Ephrin-A1 in the test cohort. (b) The concentration of serum Ephrin-A1 in the validation cohort. (c) The concentration of serum Ephrin-A1 in combination cohort. The lines in the scatter plot represented mean and SD. P < 0.05 was considered statistically significant. CRC, colorectal cancer.
Diagnostic value of Ephrin-A1 in CRC
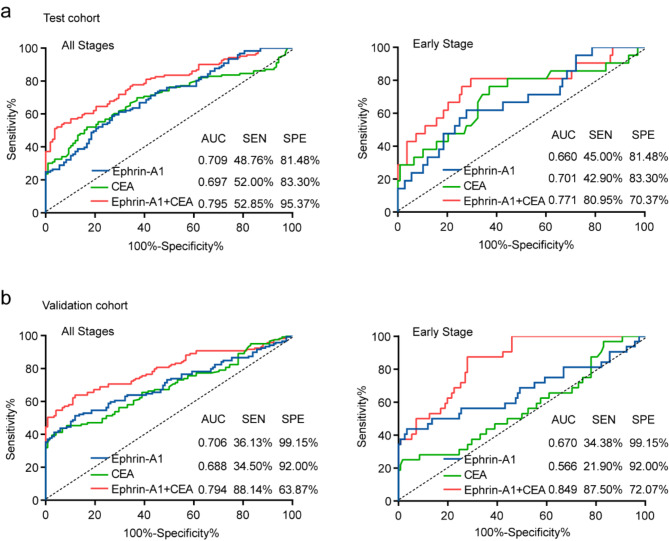
ROC curve was established to assess the diagnostic value of Ephrin-A1 in CRC. CEA is the most common diagnostic marker for CRC. In order to visually compare the differences between Ephrin-A1 and CEA, we integrated clinical data and conducted a joint analysis of both. The cutoff value for distinguishing between CRC and normal controls using Ephrin-A1 was determined to be 0.836 ng/ml, and this same cutoff value was applied in the validation cohort. For CEA, the cutoff value in the test cohort was 9.7 ng/ml, while in the validation cohort, it was adjusted to 3.8 ng/ml. The combined diagnostic efficiency of serum Ephrin-A1 and CEA (AUC 0.795, sensitivity 52.85%, specificity 95.37%) for CRC patients outperformed the individual tests (Ephrin-A1 (AUC 0.709, sensitivity 48.76%, specificity 81.48%), CEA (AUC 0.697, sensitivity 52.00%, specificity 83.30%)) (Fig. 3a). Notably, the diagnostic performance of Ephrin-A1 was comparable to that of CEA. In patients with early-stage CRC, the diagnostic ability of serum Ephrin-A1 combined with CEA to distinguish cancer patients from normal controls (AUC 0.771, sensitivity 80.95%, specificity 70.37%) (Fig. 3a). Similar results were obtained in the comparison between CRC patients, early-stage CRC patients, and normal controls in the validation cohort (Fig. 3b). To better explain the clinical value of Ephrin-A1, more relevant indicators such as false-positive rate (FPR), false-negative rate (FNR), positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) had been calculated and shown in Table 3. These data suggest that the combination of Ephrin-A1 with CEA can improve the clinical diagnostic performance for early-stage CRC patients. Furthermore, Ephrin-A1 may serve as a potential clinical biomarker for the early diagnosis of CRC.
Fig. 3.
ROC curve analysis of the diagnostic performance of Ephrin-A1 for CRC. (a) The ROC curves for serum Ephrin-A1, CEA and combination of Ephrin-A1 and CEA for patients with CRC and early-stage CRC in the test cohort. (b) The ROC curves for serum Ephrin-A1, CEA and combination of Ephrin-A1 and CEA for patients with CRC and early-stage CRC in the validation cohort. The area under the black line is 0.5, for reference. ROC curve, receiver operating characteristic curve; CRC, colorectal cancer.
Table 3.
Results for measurement of Ephrin-A1 in the diagnosis of CRC.
| AUC (95%CI) | Sensitivity (%) | Specificity (%) | FPR (%) | FNR (%) | PPV (%) | NPV (%) | PLR | NLR | |
|---|---|---|---|---|---|---|---|---|---|
| CRC vs. NC | |||||||||
| Test cohort | 0.709 (0.644–0.775) | 48.76 | 81.48 | 25.32 | 41.33 | 74.68 | 58.67 | 2.63 | 0.63 |
| Validation cohort | 0.706 (0.638–0.773) | 36.13 | 99.15 | 2.27 | 39.38 | 97.73 | 60.62 | 42.64 | 0.64 |
| Early-stage CRC vs. NC | |||||||||
| Test cohort | 0.660 (0.530–0.790) | 45.00 | 81.48 | 68.97 | 11.11 | 31.03 | 88.89 | 2.43 | 0.68 |
| Validation cohort | 0.670 (0.543–0.796) | 34.38 | 99.15 | 8.33 | 15.22 | 84.78 | 85.40 | 40.56 | 0.66 |
AUC, area under the curve; 95% CI, 95% confidence interval; FPR, false positive rate; FNR, false negative rate; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; CRC, colorectal cancer; NC, normal controls.
Correlation between serum concentration of Ephrin-A1 and clinical data
The correlation analysis of pathological data and serum Ephrin-A1 in patients with CRC was shown in Table 4. There was no significant correlation between Ephrin-A1 and gender, tumor size, degree of differentiation, lymphatic metastasis, TNM stage in both test and validation cohorts. In the test cohort, serum Ephrin-A1 was not related to age and invasion depth. However, the expression of Ephrin-A1 was affected by age and invasion depth in the validation cohort.
Table 4.
Correlation between Ephrin-A1 and clinical data in CRC patients.
| Variable | Test Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| Total | Positive | P | Total | Positive | P | |
| Age | ||||||
| < 64 | 54 | 23 (42.59%) | 0.223 | 51 | 13 (25.49%) | 0.036 |
| >=64 | 67 | 36 (53.73%) | 68 | 30 (44.12%) | ||
| Gender | ||||||
| Male | 69 | 36 (52.17%) | 0.387 | 72 | 26 (36.11%) | 0.995 |
| Female | 52 | 23 (44.23%) | 47 | 17 (36.17%) | ||
| Location | ||||||
| Colon | 83 | 43 (51.81%) | 0.322 | 65 | 27 (41.54%) | 0.178 |
| Rectum | 38 | 16 (42.11%) | 54 | 16 (29.63%) | ||
| Tumor size | ||||||
| <=5 cm | 64 | 26 (40.63%) | 0.069 | 55 | 18 (32.73%) | 0.098 |
| > 5 cm | 51 | 28 (54.90%) | 28 | 7 (25.00%) | ||
| Unknown | 6 | 5 (83.33%) | 36 | 18 (50.00%) | ||
| Grade | ||||||
| Low | 0 | 0 (0.00%) | 0.365 | 11 | 4 (36.36%) | 0.366 |
| Moderate | 98 | 48 (48.98%) | 59 | 19 (32.20%) | ||
| High | 2 | 0 (0.00%) | 3 | 0 (0.00%) | ||
| Unknown | 21 | 11 (52.38%) | 46 | 20 (43.48%) | ||
| Invasion depth | ||||||
| Tis + T1 + T2 + T3 | 24 | 9 (37.50%) | 0.296 | 44 | 11 (25.00%) | 0.046 |
| T4 | 93 | 47 (50.54%) | 62 | 24 (38.71%) | ||
| Unknown | 4 | 3 (75.00%) | 13 | 8 (61.54%) | ||
| Lymphatic metastasis | ||||||
| N0 | 61 | 31 (50.82%) | 0.104 | 51 | 18 (35.29%) | 0.663 |
| N1 | 36 | 18 (50.00%) | 31 | 10 (32.26%) | ||
| N2 + N3 | 18 | 5 (27.78%) | 16 | 5 (31.25%) | ||
| Unknown | 6 | 5 (83.33%) | 21 | 10 (47.62%) | ||
| Distant metastasis | ||||||
| M0 | 102 | 46 (45.10%) | 0.146 | 77 | 27 (35.06%) | 0.639 |
| M1 | 14 | 9 (64.29%) | 20 | 9 (45.00%) | ||
| Unknown | 5 | 4 (80.00%) | 22 | 7 (31.82%) | ||
| TNM stage | ||||||
| I-IIA | 20 | 9 (45.00%) | 0.544 | 32 | 11 (34.38%) | 0.662 |
| IIB-IV | 97 | 47(48.45%) | 72 | 25 (34.72%) | ||
| Unknown | 4 | 3 (75.00%) | 15 | 7 (46.67%) | ||
CRC, colorectal cancer.
Discussion
CRC is one of the leading causes of cancer-related death worldwide. More than 80% of the CRC patients were over 60 years old when they were diagnosed with colorectal cancer, and over 20% of the patients had distant metastasis with a five-year survival rate of less than 15%3,22. Early screening of colorectal cancer is very important for the treatment and survival rate of patients. Fecal immunochemical test (FIT), sigmoidoscopy, and colonoscopy (offered if FIT or sigmoidoscopy positive) are recommended for CRC screening in asymptomatic subjects 50 years or older23,24.
Ephrin-A1 was originally separated from human umbilical vein endothelial cells as a secretory protein induced by tumor necrosis factor (TNF)25. Ephrin-A1 and its most common receptor, Eph receptor 2, were associated with various types of cancer, including breast cancer and gastric cancer26,27. Although Ephrin-A1 is upregulated in a variety of cancers, its expression pattern may differ across different tumor types. Studies have shown that Ephrin-A1 expression is higher in the advanced stages of gastric tissues28 and renal cell carcinoma tissues compared29 to the early stages. In contrast, in colorectal cancer tissues exhibit higher Ephrin-A1 expression in the early stages, suggesting that Ephrin-A1 may have specificity for the early detection of colorectal cancer28. However, the role of Ephrin-A1 as a biomarker for cancer diagnosis is rarely studied.
The development of liquid biopsy-based biomarker detection screening methods has emerged as an alternative to traditional screening approaches30. Although there is not sufficient evidence to suggest the analysis of biomarkers such as DNA, RNA, or protein in the blood, with the rapid development of molecular biological technology tools, the improvement of sensitivity, and the reduction of cost, these tools will be gradually applied to clinical practice, and will probably be developed on a large scale31. In recent decades, more and more studies are looking for ideal blood biomarkers for CRC with the characteristics of easy quantification, high specificity and sensitivity, reliability, and good repeatability. Previous studies have shown that circulating cell-free DNA32–34, microRNAs (e.g., miR-21, miR-24)35–37, long noncoding RNAs (e.g., HIF1A-AS1, CCAT1, HOTAIR)38,39 and protein (e.g., IGFBP2, L1CAM, KT1)40–42 can be considered as potential biomarkers for the diagnosis of CRC.
The mSEPT9 assay is the first blood test approved by the FDA for colorectal cancer screening43. However, studies have found that it excels in late-stage diagnosis but lacks sensitivity in early-stage detection44,45. Kemal, Y. et al. found that HE4 can be used as a diagnostic marker for CRC, but its detection efficacy also mainly targets patients with advanced stages46. The currently used clinical serum diagnostic markers such as CEA and CA19-9, have limitations in the early diagnosis of CRC, and their combined analysis does not increase the sensitivity of CEA, which is commonly employed for prognostic analysis in CRC patients47,48.
In our study, serum Ephrin-A1 was significantly elevated in CRC patients versus that in healthy controls and showed a good diagnostic ability for CRC. Considering that serum biomarker test is a kind of screening requiring high sensitivity to reduce false negatives, the sensitivity of the Ephrin-A1 test might limit the clinical application in screening purposes in general or high-risk individuals. The combination of multiple biomarkers has been proved to improve the specificity and sensitivity of diagnosis42,49. Our combined analysis of CEA and Ephrin-A1 showed an AUC of 0.849, with a sensitivity of 87.50% and a specificity of 72.07%, demonstrating excellent early diagnostic capability. Nevertheless, the sample size in our study was limited, necessitating an expansion of the sample size for subsequent validation. This indicates that Ephrin-A1 may be a potential diagnostic biomarker for CRC, and its combined analysis with CEA has shown good early diagnostic efficacy. In future studies, we will seek biomarkers of high sensitivity and specificity for further optimization with the test of serum Ephrin-A1.
Conclusion
We confirmed that the serum level of Ephrin-A1 protein in patients with CRC was significantly higher than that in normal subjects, and was the first to evaluate serum Ephrin-A1 as a potential biomarker for the diagnosis of CRC.
Acknowledgements
We thank the patients and negative volunteers for their participation. We thank the reviewers for their critical comments.
Author contributions
Qi-Xin Su, Ze-Jun Zheng, and Ying-Hua Xie searched the literature and wrote the manuscript. Ling-Yu Chu and Yin-Qiao Liu performed the experiments, analyzed and interpreted the data. Yi-Wei Lin and Xin-Xin Li collected the serum samples and clinical data.Yu-Hui Peng designed the study and revised the paper. Yi-Wei Xu and Jian-Jun Xie conceptualized and designed the study, supervised the project, and revised the paper. All authors read and approved the final manuscript.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi-Xin Su, Ze-Jun Zheng and Ying-Hua Xie contributed equally to this work.
Contributor Information
Yi-Wei Xu, Email: yiwei512@126.com.
Jian-Jun Xie, Email: xiejj0816@foxmail.com.
References
- 1.Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229–263 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Han, B. et al. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent.4, 47–53 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel, R. L. et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin.70, 145–164 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Gimeno-Garcia, A. Z. & Quintero, E. Role of colonoscopy in colorectal cancer screening: Available evidence. Best Pract. Res. Clin. Gastroenterol.66, 101838 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Porcaro, F., Voccola, S., Cardinale, G., Porcaro, P. & Vito, P. DNA methylation biomarkers in stool samples: Enhancing colorectal cancer screening strategies. Oncol. Rev.18, 1408529 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, J., Li, Z. P., Ruan, W. J. & Wang, W. Colorectal cancer screening: the value of early detection and modern challenges. World J. Gastroenterol.30, 2726–2730 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKeown, E. et al. Current approaches and challenges for monitoring treatment response in colon and rectal cancer. J. Cancer. 5, 31–43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locker, G. Y. et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol.24, 5313–5327 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Jiang, H. et al. Targeting EFNA1 suppresses tumor progression via the cMYC-modulated cell cycle and autophagy in esophageal squamous cell carcinoma. Discov Oncol.14, 64 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu, L. Y. et al. Secreted proteins encoded by super enhancer-driven genes could be promising biomarkers for early detection of esophageal squamous cell carcinoma. Biomed. J.47, 100662 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen, X. et al. An integrated analysis of single-cell and bulk transcriptomics reveals EFNA1 as a novel prognostic biomarker for cervical cancer. Hum. Cell.35, 705–720 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Cui, Y. et al. Ephrin A1 stimulates CCL2 secretion to facilitate pre-metastatic niche formation and fromote gastric cancer liver metastasis. Cancer Res., (2024). [DOI] [PubMed]
- 13.Toma, M. I. et al. Lack of ephrin receptor A1 is a favorable independent prognostic factor in clear cell renal cell carcinoma. PLoS One. 9, e102262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto, H. et al. Ephrin-A1 mRNA is associated with poor prognosis of colorectal cancer. Int. J. Oncol.42, 549–555 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Hao, Y. & Li, G. Role of EFNA1 in tumorigenesis and prospects for cancer therapy. Biomed. Pharmacother. 130, 110567 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Ma, T. T. et al. Hypoxia-induced cleavage of soluble ephrinA1 from cancer cells is mediated by MMP-2 and associates with angiogenesis in oral squamous cell carcinoma. Onco Targets Ther.12, 8491–8499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiuan, E. et al. Host deficiency in ephrin-A1 inhibits breast cancer metastasis. F1000Res9, 217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, F. F., Zhang, S. R., Peng, H., Chen, Y. Z. & Cui, X. B. Integrative genomics analysis of hub genes and their relationship with prognosis and signaling pathways in esophageal squamous cell carcinoma. Mol. Med. Rep.20, 3649–3660 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao, M., Huang, W., Zou, S., Shen, Q. & Zhu, X. A five-genes-based prognostic signature for cervical cancer overall survival prediction. Int. J. Genomics. 8347639, (2020). (2020). [DOI] [PMC free article] [PubMed]
- 20.Salem, E. et al. Role of EFNA1 SNP (rs12904) in tumorigenesis and metastasis of colorectal cancer: a bioinformatic analysis and HRM SNP genotyping verification. Asian Pac. J. Cancer Prev.23, 3523–3531 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amin, M. B. et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more personalized approach to cancer staging. CA Cancer J. Clin.67, 93–99 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin.71, 7–33 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Helsingen, L. M. et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a clinical practice guideline. BMJ367, l5515 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Force, U. S. P. S. T. et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA325, 1965–1977 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Holzman, L. B., Marks, R. M. & Dixit, V. M. A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol. Cell. Biol.10, 5830–5838 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura, R. et al. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci.96, 42–47 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youngblood, V. M. et al. The Ephrin-A1/EPHA2 signaling axis regulates glutamine metabolism in HER2-positive breast cancer. Cancer Res.76, 1825–1836 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki, K. et al. EphA4 is a prognostic factor in gastric cancer. BMC Clin. Pathol.13, 19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, J. S. et al. Expression of EphA2 and EphrinA1 in human renal cell carcinoma and its relationship with angiogenesis. Zhonghua Zhong Liu Za Zhi. 31, 438–441 (2009). [PubMed] [Google Scholar]
- 30.Loomans-Kropp, H. A. The utility of liquid biopsy-based methylation biomarkers for colorectal cancer detection. Front. Oncol.14, 1351514 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh, H. H. & Joo, Y. E. Novel biomarkers for the diagnosis and prognosis of colorectal cancer. Intest Res.18, 168–183 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao, T. B. et al. Circulating cell-free DNA in serum as a biomarker for diagnosis and prognostic prediction of colorectal cancer. Br. J. Cancer. 111, 1482–1489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Gayar, D., El-Abd, N., Hassan, N. & Ali, R. Increased free circulating DNA integrity index as a serum biomarker in patients with colorectal carcinoma. Asian Pac. J. Cancer Prev.17, 939–944 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Morris, V. K. & Strickler, J. H. Use of circulating cell-free DNA to guide precision medicine in patients with colorectal cancer. Annu. Rev. Med.72, 399–413 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Kanaan, Z. et al. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann. Surg.256, 544–551 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Fang, Z. et al. Plasma levels of microRNA-24, microRNA-320a, and microRNA-423-5p are potential biomarkers for colorectal carcinoma. J. Exp. Clin. Cancer Res.34, 86, (2015). [DOI] [PMC free article] [PubMed]
- 37.Jung, G., Hernandez-Illan, E., Moreira, L., Balaguer, F. & Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol.17, 111–130 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong, W., Tian, M., Qiu, H. & Yang, Z. Elevated serum level of lncRNA-HIF1A-AS1 as a novel diagnostic predictor for worse prognosis in colorectal carcinoma. Cancer Biomark.20, 417–424 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Zhao, W., Song, M., Zhang, J., Kuerban, M. & Wang, H. Combined identification of long non-coding RNA CCAT1 and HOTAIR in serum as an effective screening for colorectal carcinoma. Int. J. Clin. Exp. Pathol.8, 14131–14140 (2015). [PMC free article] [PubMed] [Google Scholar]
- 40.Chu, L. Y. et al. The diagnostic value of serum L1CAM in patients with colorectal cancer. Technol. Cancer Res. Treat.19, 1533033820920971 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liou, J. M. et al. Plasma insulin-like growth factor-binding protein-2 levels as diagnostic and prognostic biomarker of colorectal cancer. J. Clin. Endocrinol. Metab.95, 1717–1725 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Singh, S., Kumar, R., Kumar, U. & Kumari, R. Clinical significance and role of TK1, CEA, CA19-9 and CA72-4 levels in diagnosis of colorectal cancers. Asian Pac. J. Cancer Prev.21, 3133–3136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song, L., Jia, J., Peng, X., Xiao, W. & Li, Y. The performance of the SEPT9 gene methylation assay and a comparison with other CRC screening tests: A meta-analysis. Sci. Rep.7, 3032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Church, T. R. et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut63, 317–325 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potter, N. T. et al. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin. Chem.60, 1183–1191 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Kemal, Y. N. et al. Serum human epididymis protein 4 levels in colorectal cancer patients. Mol. Clin. Oncol.7, 481–485 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lakemeyer, L. et al. Diagnostic and prognostic value of CEA and CA19-9 in colorectal cancer. Diseases9, (2021). [DOI] [PMC free article] [PubMed]
- 48.Dai, X. et al. Development and validation of nomograms based on pre-/post-operative CEA and CA19-9 for survival predicting in stage I–III colorectal cancer patients after radical resection. Front. Oncol.14, 1402847, (2024). [DOI] [PMC free article] [PubMed]
- 49.Li, X. et al. Potential diagnostic value of combining inflammatory cell ratios with carcinoembryonic antigen for colorectal cancer. Cancer Manag Res.11, 9631–9640 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.