Abstract
Liver function affects the prognosis of patients with hepatocellular carcinoma (HCC). This study aimed to investigate the prognostic impact of the functional liver imaging score (FLIS), assessed using gadoxetic acid-enhanced magnetic resonance imaging, on long-term outcomes following hepatectomy for HCC. The FLIS was assessed in 235 patients who underwent initial hepatectomy for HCC. The relationship between FLIS and prognosis was retrospectively analyzed. The FLIS was 6 in 185, and 2–5 in 50, patients. The 5-year recurrence-free and overall survival rates were 43.6% and 76.4% in patients with an FLIS of 6, and 23.0% and 42.4% in patients with an FLIS of 2–5, respectively; both recurrence-free and overall survival were significantly better in patients with an FLIS of 6 (P = 0.012 and 0.001, respectively). Multivariable analyses revealed that microvascular invasion (hazard ratio: 3.611; P = 0.002) and an FLIS of 2–5 (hazard ratio: 2.558; P = 0.027) were independently associated with shorter overall survival. After propensity-score matching, overall survival was significantly better in patients with an FLIS of 6. A low FLIS was significantly associated with poor prognosis following initial hepatectomy for HCC, suggesting that surgical indications must be carefully considered in patients with a low FLIS.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82741-9.
Keywords: Enhanced MRI, Gadoxetic acid, Hepatectomy, Hepatocellular carcinoma, Liver function
Subject terms: Oncology, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the leading cause of mortality worldwide1. Despite recent advances in systemic chemotherapy, including the development of molecular targeted agents and immune checkpoint inhibitors, hepatectomy remains a strong option to achieve cure2. Several prognostic factors following hepatectomy for HCC have been reported, including tumor number, size, differentiation, and microvascular invasion3,4. These factors are associated with the biological behavior of each tumor. Apart from these factors, liver function is important factor affecting the prognoses in patients with HCC5,6. Several indicators of liver function are currently used in clinical practice, such as the Child–Pugh, Model for End-Stage Liver Disease, Fibrosis-4, and Albumin–Bilirubin (ALBI) scores7–10.
The functional liver imaging score (FLIS) is a recently developed score reflecting liver function11. This score is calculated using three parameters derived from the hepatobiliary-phase features of gadoxetic acid-enhanced magnetic resonance imaging (MRI) scans. The FLIS and existing liver function indices such as the ALBI and Child–Pugh scores are strongly correlated12 and a significant association between the FLIS and orthotopic liver transplant graft survival has also been reported11. However, no study has assessed the predictive value of the FLIS on prognosis following hepatectomy in patients with HCC.
This study aimed to investigate the association between the FLIS and long-term outcomes following hepatectomy for HCC.
Patients and methods
Patients
Two hundred and sixty-two patients underwent initial hepatectomy for HCC at Chiba University Hospital between January 2013 and December 2019; of these, 236 patients underwent preoperative gadoxetic acid-enhanced MRI within 1 month before hepatectomy. These 236 patients were consecutively included in the present study, and their medical records retrospectively reviewed. One patient died of post-hepatectomy liver failure within 90 days after surgery, and this patient was excluded from the study. As a result, the present study analyzed the data of 235 patients (Supplementary Fig. 1). The study protocol was approved by the Institutional Ethics Committee of the College of Medicine at Chiba University (approval number: M10101). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and the 1964 Declaration of Helsinki. Because of the retrospective nature of the study, informed consent was waived by the Institutional Ethics Committee of the College of Medicine, Chiba University. As an alternative, an opt-out consent was approved by the committee and obtained via our websites, where permission was requested for the use of the participants’ personal information in this study.
Follow-up assessments
Postoperative follow-up consisted of physical examination and measurement of the levels of tumor markers, such as alpha fetoprotein, alpha fetoprotein-L3, and/or protein induced by vitamin K absence. Abdominal ultrasonography, computed tomography of the abdomen and chest, and gadoxetic acid-enhanced MRI of the liver were performed every 3–6 months.
MRI protocol
MRI was performed using a 1.5-T magnetic resonance scanner (Signa HDxt; GE Healthcare, Milwaukee, WI, USA) with a 12-channel phased-array body coil or a 3.0-T magnetic resonance scanner (Discovery MR750; GE Healthcare) with a 32-channel phased-array body coil. For enhanced MRI, gadoxetic acid (0.025 mmol/kg; Primovist/Eovist; Bayer Healthcare) was injected intravenously at a standard dose of 1.0 mL/s, followed by a 20-mL normal saline flush. Twenty minutes after gadoxetic acid injection, a hepatobiliary-phase enhanced sequence with fat-suppressed 3D spoiled gradient-echo T1-weighted imaging examination was performed. MRI was performed within 1 month before surgery. The MRI protocol is provided in supplementary Tables 1, 2.
Imaging analysis
The FLIS was calculated as described previously11,13,14. Briefly, three types of scores were determined using axial and coronal hepatobiliary-phase enhanced sequence scans: enhancement quality score, excretion quality score, and portal vein sign quality score. The enhancement quality score was determined by comparing the uptake of gadoxetic acid by the liver and right kidney. A score of 0, 1, or 2 was assigned if the right kidney was hypo-, iso-, or hyperintense, respectively, in comparison with the liver. The excretion quality score was determined based on the extent of gadoxetic acid excretion from the biliary tract. A score of 0, 1, or 2, respectively, was assigned if no excretion in the biliary tract; excretion only in the intrahepatic bile ducts; or excretion into the common hepatic duct, common bile duct, or duodenum was observed. The portal vein sign quality score was determined based on the signal intensity of the portal vein relative to that of the liver parenchyma. A score of 0, 1, or 2 was assigned if the signal intensity of the portal vein was high, iso, or low relative to that of the liver parenchyma, respectively. The FLIS was calculated as the sum of the enhancement, excretion, and portal vein sign quality scores and ranged from 0 to 6.
The FLIS was evaluated by two surgeons with more than 10 years of experience as hepatobiliary pancreatic surgeons. Both surgeons were blinded to clinical and laboratory data. Evaluation was performed after obtaining an interobserver consensus using sample images obtained from recent cases (not included in the present study due to the short observation period following hepatectomy).
Propensity-score analysis
A propensity score (PS) analysis was performed to eliminate bias in patient characteristics between the low- and high-FLIS groups. PSs were calculated using logistic regression analysis and matching was performed on a 1 to 1 basis with the closest estimated PS and within 0.2 of a standard deviation. The variables used in the PS analysis were tumor size and microvascular invasion, because these variables are associated with tumor biology and significant difference were observed between low- and high-FLIS groups. Liver function was not adjusted in the PS analysis.
Statistical analysis
All data were collected retrospectively, and differences were considered statistically significant at P-values of < 0.05. The relationships between categorical variables were assessed using either the chi-square test or Fisher’s exact test, as appropriate. Survival outcomes after hepatectomy for HCC were evaluated using the Kaplan–Meier method and log-rank test. Survival data were evaluated using univariable and multivariable Cox proportional hazard regression analyses. All statistical analyses were performed using JMP Pro software (version 13; SAS Institute Japan, Tokyo, Japan).
Results
Patient characteristics and FLIS assessed using gadoxetic acid-enhanced MRI
The patients’ demographic and clinicopathological characteristics are shown in Table 1. The median and mean follow-up durations were 47 and 50 months, respectively. The median and mean FLIS values were 6 and 5.7, respectively. The FLIS was 6 in 185 (78.7%), 5 in 28 (11.9%), 4 in 19 (8.1%), 3 in 1 (0.4%), and 2 in 2 (0.9%) patients.
Table 1.
Patient characteristics.
| All patients (N = 235) | |
|---|---|
| Sex, male/female, n (%) | 176/59 (74.9/25.1) |
| Median age (years) | 72 |
| HBV infection, yes/no, n (%) | 50/185 (21.3/78.7) |
| HCV infection, yes/no, n (%) | 71/164 (30.2/69.8) |
| Number of tumors, median | 1 (1–6) |
| Solitary/multiple, n (%) | 178/57 (75.7/24.3) |
| Largest tumor size (cm), median | 3.6 |
| Serum AFP level (ng/mL), median | 8.9 (1–37780) |
| Child–Pugh grade, A/B, n (%) | 227/8 (96.6/3.4) |
| ALBI grade, I/II or III, n (%) | 231/4 (98.3/1.7) |
| FLIS 6/2–5, n (%) | 185/50 (78.7/21.3) |
| Microscopic portal vein invasion, +/-, n (%) | 43/192 (18.3/81.7) |
| Microscopic hepatic vein invasion, +/-, n (%) | 12/223 (5.1/94.9) |
| Microvascular invasion, +/-, n (%) | 55/180 (23.4/76.6) |
| Surgical procedure, minor resection/major resection, n (%) | 202/33 (86.0/14.0) |
| Anatomic resection/non-anatomic resection, n (%) | 174/61 (74.0/26.0) |
HBV Hepatitis B virus, HCV Hepatitis C virus, AFP alpha-fetoprotein, ALBI grade albumin–bilirubin grade, FLIS Functional liver imaging score.
Association between FLIS and existing liver function indices (ALBI and child–pugh scores)
The FLIS was significantly associated with ALBI grade (Supplementary Fig. 2a). Similarly, the FLIS was significantly associated with the Child-Pugh score (Supplementary Fig. 2b).
FLIS and long-term outcomes following initial hepatectomy
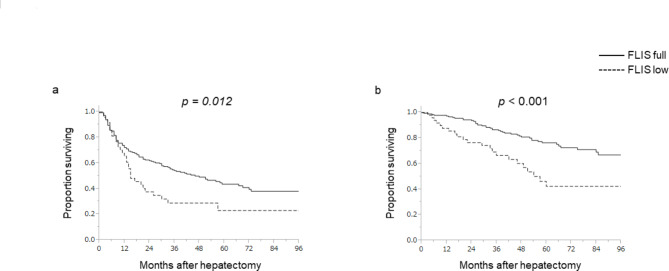
The 5-year recurrence-free survival (RFS) and 5-year overall survival (OS) rates in the total cohort were 40.5% and 74.4%, respectively. As a significant difference in long-term survival between ALBI grade I and II in patients with HCC has been previously reported15, , a receiver operating characteristic analysis in accordance with ALBI grade (I/ II or III) was conducted. Based on the results of the analysis (cut-off value = 5; area under the curve = 0.814), the 235 patients were divided into two groups: low FLIS (FLIS = 2–5; Fig. 1a,c) or high FLIS (FLIS = 6; Fig. 1d,f). In patients with a high FLIS, the median RFS was 46 months, and the 5-year RFS rate was 43.6%. In patients with a low FLIS, the median RFS was 15 months, and the 5-year RFS rate was 23.0% (Fig. 2a). Further, in patients with a high FLIS, the median OS was not reached, and the 5-year OS rate was 76.4%. In patients with a low FLIS, the median OS was 57 months, and the 5-year OS rate was 42.4% (Fig. 2b). Both RFS and OS were significantly better in patients with a high FLIS than in those with a low FLIS (P = 0.012 and P < 0.001, respectively).
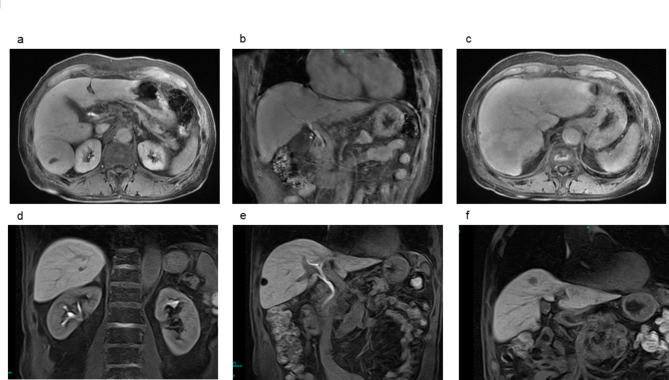
Fig. 1.
Magnetic resonance images of a representative case of a low (a–c) and high (d–f) functional liver imaging score. A 73-year-old male with a 4-cm HCC in segment 6 and FLIS of 4 (a–c). The signal intensity of the liver parenchyma was similar to that of the right kidney (enhancement quality score: 1). (a) Gadoxetic acid excretion was detected in the common bile duct (excretion quality score: 2). (b) The signal intensity of the portal vein was similar to that of the liver parenchyma (portal vein sign quality score: 1). (c) The patient developed hepatic recurrence 3 months after hepatectomy and died of HCC 30 months after hepatectomy. A 73-year-old male with a 1-cm HCC in segment 8 and FLIS of 6 (d–f). The signal intensity of the liver parenchyma was higher than that of the right kidney (enhancement quality score: 2). (d) Gadoxetic acid excretion was detected in the common bile duct (excretion quality score: 2). (e) The signal intensity of the portal vein was lower than that of the liver parenchyma (portal vein sign quality score: 2). (f) The patient is alive, with no recurrence for 117 months. HCC hepatocellular carcinoma, FLIS functional liver imaging score.
Fig. 2.
Recurrence-free survival (a) and overall survival (b) in relation to the functional liver imaging score. FLIS functional liver imaging score.
Furthermore, univariable analyses revealed that age > 72 years (P = 0.023), multiple tumors (P = 0.006), largest tumor size > 3 cm (P = 0.008), microvascular invasion (P < 0.001), and a low FLIS (P < 0.001) were significantly associated with a shorter OS (Table 2). In the multivariable analyses, the following variables that showed a significant association with OS in the univariable analysis were used: age, number of tumors (solitary/ multiple), largest tumor size (< 3 cm/ >3 cm), microvascular invasion, and FLIS (6/ 2–5). The multivariable analyses demonstrated that microvascular invasion (hazard ratio: 3.611; P = 0.002) and low FLIS (hazard ratio: 2.558; P = 0.027) were independently associated with shorter OS (Table 3). The ALBI score was not significantly associated with RFS or OS.
Table 2.
Factors predicting overall survival.
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| HR | p-value | HR | 95% CI | p-value | |
| Sex, male | 1.027 | 0.926 | |||
| Age, > 72 years | 1.724 | 0.023 | 1.272 | 0.760–2.148 | 0.361 |
| HBV infection, yes | 0.801 | 0.462 | |||
| HCV infection, yes | 0.856 | 0.556 | |||
| Number of tumors | 1.500 | 0.006 | |||
| Number of tumors, multiple | 2.079 | 0.006 | 1.611 | 0.904–2.791 | 0.104 |
| Largest tumor size, > 3 cm | 1.939 | 0.008 | 1.547 | 0.890–2.777 | 0.123 |
| Serum AFP level (ng/mL) | 1.575 | 0.808 | |||
| Child–Pugh grade, B | 1.463 | 0.485 | |||
| ALBI grade, II or III | 2.330 | 0.206 | |||
| Microscopic portal vein invasion, +/-, + | 3.325 | < 0.001 | |||
| Microscopic hepatic vein invasion, +/- | 2.323 | 0.079 | |||
| Microvascular invasion, + | 3.611 | < 0.001 | 2.564 | 1.433–4.499 | 0.002 |
| Surgical procedure, major resection | 1.300 | 0.421 | |||
| Anatomic resection | 1.124 | 0.669 | |||
| FLIS, 2–5 | 2.558 | < 0.001 | 1.189 | 1.077–3.226 | 0.027 |
HR hazard ratio, CI confidence interval, HBV Hepatitis B virus, HCV Hepatitis C virus, AFP alpha-fetoprotein, ALBI grade albumin–bilirubin grade, FLIS Functional liver imaging score.
Table 3.
Factors predicting recurrence-free survival.
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| HR | p-value | HR | 95% CI | p-value | |
| Sex, male | 0.835 | 0.350 | |||
| Age, > 72 years | 1.261 | 0.163 | |||
| HBV infection, yes | 0.861 | 0.459 | |||
| HCV infection, yes | 0.943 | 0.745 | |||
| Number of tumors | 1.473 | < 0.001 | |||
| Number of tumors, multiple | 2.231 | < 0.001 | 2.103 | 1.414–3.078 | < 0.001 |
| Largest tumor size, > 3 cm | 1.579 | 0.007 | 1.463 | 1.014–2.131 | 0.042 |
| Serum AFP level (ng/mL) | 1.000 | 0.244 | |||
| Child–Pugh grade, B | 1.728 | 0.166 | |||
| ALBI grade, II or III | 1.667 | 0.352 | |||
| Microscopic portal vein invasion, +/-, + | 2.496 | < 0.001 | |||
| Microscopic hepatic vein invasion, +/- | 2.240 | 0.022 | |||
| Microvascular invasion, + | 2.526 | < 0.001 | 1.720 | 1.107–2.616 | 0.017 |
| Surgical procedure, major resection | 1.188 | 0.457 | |||
| Anatomic resection | 0.919 | 0.655 | |||
| FLIS, 2–5 | 1.665 | 0.019 | 1.487 | 0.963–2.237 | 0.073 |
HR hazard ratio, CI confidence interval, HBV Hepatitis B virus, HCV Hepatitis C virus, AFP alpha-fetoprotein, ALBI grade albumin–bilirubin grade, FLIS Functional liver imaging score.
Patient characteristics after PS matching
The low- and high-FLIS groups showed differences in tumor size and incidence of microvascular invasion (Table 4); thus, PS matching was performed to minimize these differences. Tumor size and the presence of microvascular invasion were used as variables for PS matching. After PS matching, the patient characteristics showed no significant differences in relation to the FLIS (Table 5).
Table 4.
Patient characteristics in relation to the FLIS.
| FLIS = 6 (n = 185) | FLIS < 6 (n = 50) | P | |
|---|---|---|---|
| Sex, male, n (%) | 142 (76.8) | 34 (68.0) | 0.205 |
| Median age (years) | 72 | 74 | 0.332 |
| HBV infection, yes, n (%) | 43 (23.2) | 7 (14.0) | 0.177 |
| HCV infection, yes, n (%) | 52 (28.1) | 19 (38.0) | 0.224 |
| Number of tumors, median | 1 | 1 | 0.764 |
| Solitary, n (%) | 141 (76.2) | 37 (74.0) | 0.715 |
| Largest tumor size (cm), median | 3.4 | 4.1 | 0.032 |
| Largest tumor size, > 3 cm, n (%) | 100 (54.1) | 31 (62.0) | 0.340 |
| Serum AFP level (ng/mL), median | 8.3 | 11.7 | 0.507 |
| Child–Pugh grade, A, n (%) | 183 (98.9) | 44 (88.0) | 0.001 |
| ALBI grade, I, n (%) | 184 (99.5) | 47 (94.0) | 0.031 |
| Microscopic portal vein invasion, +, n (%) | 28 (15.1) | 15 (30.0) | 0.023 |
| Microscopic hepatic vein invasion, +, n (%) | 9 (4.9) | 3 (6.0) | 0.722 |
| Microvascular invasion, +, n (%) | 37 (20.0) | 18 (36.0) | 0.024 |
| Surgical procedure, minor resection, n (%) | 163 (88.1) | 39 (78.0) | 0.105 |
| Anatomic resection, n (%) | 142 (76.8) | 32 (64.0) | 0.072 |
Values represent the number of patients or mean or median values, as indicated.
FLIS Functional liver imaging score, HBV Hepatitis B virus, HCV Hepatitis C virus, AFP alpha-fetoprotein, ALBI albumin–bilirubin grade.
Table 5.
Patient characteristics in relation to the FLIS after PS matching.
| FLIS = 6 (n = 49) | FLIS < 6 (n = 49) | P | |
|---|---|---|---|
| Sex, male, n (%) | 39 (79.6) | 33 (67.3) | 0.252 |
| Median age (years) | 71 | 74 | 0.266 |
| HBV infection, yes, n (%) | 12 (24.5) | 7 (14.3) | 0.307 |
| HCV infection, yes, n (%) | 16 (32.7) | 18 (36.7) | 0.832 |
| Number of tumors, median | 1 | 1 | 0.778 |
| Solitary, n (%) | 40 (81.6) | 36 (73.5) | 0.468 |
| Largest tumor size (cm), median | 40 | 40 | 0.623 |
| Largest tumor size, > 3 cm, n (%) | 29 (59.2) | 30 (61.2) | 1.000 |
| Serum AFP level (ng/mL), median | 9.5 | 11.8 | 0.183 |
| Child–Pugh grade, A, n (%) | 49 (100) | 43 (87.8) | 0.027 |
| ALBI grade, I, n (%) | 49 (100) | 46 (93.9) | 0.242 |
| Microscopic portal vein invasion, +, n (%) | 16 (32.7) | 14 (28.6) | 0.827 |
| Microscopic hepatic vein invasion, +, n (%) | 3 (6.1) | 3 (6.1) | 1.000 |
| Microvascular invasion, +, n (%) | 16 (32.7) | 15 (30.6) | 1.000 |
| Surgical procedure, minor resection, n (%) | 40 (81.6) | 39 (79.6) | 1.000 |
| Anatomic resection, n (%) | 38 (77.6) | 31 (63.3) | 0.184 |
Values represent the number of patients or mean or median values, as indicated.
FLIS, Functional liver imaging score; HBV, Hepatitis B virus; HCV, Hepatitis C virus; AFP, alpha-fetoprotein; ALBI, albumin–bilirubin grade.
Oncological outcomes after PS matching
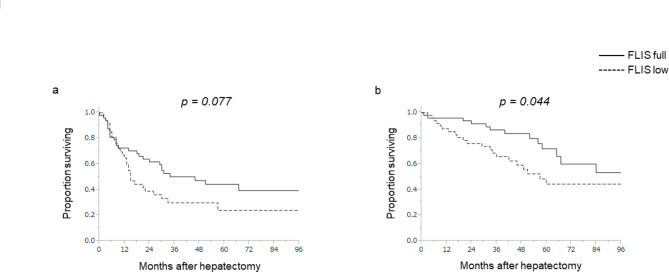
RFS and OS were reanalyzed using this cohort after PS matching. In patients with a high FLIS, the median RFS was 46 months and the 5-year RFS rate was 44.2%. In patients with a low FLIS, the median RFS was 15 months and the 5-year RFS rate was 23.7% (P = 0.077; Fig. 3a). Moreover, in patients with a high FLIS, the median OS was not reached, and the 5-year OS rate was 71.9%. In patients with a low FLIS, the median OS was 57 months, and the 5-year OS rate was 44.3%. OS, but not RFS, was significantly better in patients with a high FLIS, as observed in the original cohort (P = 0.044; Fig. 3b). A subgroup analysis comparing patients with or without microvascular invasion demonstrated that RFS was better in patients with a high FLIS without microvascular invasion compared to those with microvascular invasion (the median RFS 52 vs. 21 months); however this difference was not significant. In the subgroup with microvascular invasion, median RFS was comparable between patients with a high and low FLIS (the median RFS 9 vs. 8 months) (Supplementary Fig. 3).
Fig. 3.
Recurrence-free survival (a) and overall survival (b) in relation to the functional liver imaging score after propensity-score matching. FLIS functional liver imaging score.
Additional analyses
To confirm robustness of the statistical analyses in the present study, sensitivity analyses were conducted. Multivariable analyses, excluding age, number of tumors, largest tumor size, and microvascular invasion, were performed. These analyses similarly identified FLIS as an independent prognostic factor for OS (Supplementary Tables 3–6).
Discussion
The present study demonstrated that the FLIS assessed using gadoxetic acid-enhanced MRI was significantly associated with OS and RFS after initial hepatectomy in patients with HCC. Multivariable analyses demonstrated that a low FLIS was an independent prognostic factor for shorter OS. Moreover, even after minimizing the bias related to tumor-related factors by PS matching, OS was significantly worse in patients with a low FLIS. To the best of our knowledge, this is the first study to demonstrate that the FLIS is significantly associated with long-term outcomes after hepatectomy for HCC.
Previous studies have revealed several prognostic factors for HCC, including tumor number, tumor size, macro- and microvascular invasion, and tumor marker16–18. In addition to factors related to tumor biology, liver function is another important factor affecting clinical outcomes in patients with HCC19,20. When considering surgical indications for HCC, in addition to clarifying the prognostic impact on long-term outcomes, preoperative evaluation of liver function is critical to avoid post-hepatectomy liver failure21,22. Several indicators are used in current clinical practice to precisely assess liver function, including the Child–Pugh, Model for End-Stage Liver Disease, Fibrosis-4, and ALBI scores7–10. The ALBI score is a recently developed scoring system that reflects liver function15. This scoring system was originally developed to evaluate liver function, which is significantly associated with survival outcomes in patients with HCC. In addition to HCC, recent studies have demonstrated that the ALBI grade is associated with clinical outcomes in various types of cancer and other diseases23–27.
The FLIS is a recently developed semi-quantitative score used to assess liver function; it is derived from three types of evaluations based on gadoxetic acid-enhanced MRI13. The usefulness of the FLIS was first demonstrated by Bastati et al. in 2016. The authors demonstrated that the FLIS could predict 1- to 3-year graft survival in patients who underwent orthotopic liver transplantation11. Subsequently, some studies demonstrated that the FLIS is significantly associated with the outcomes of patients with chronic liver disease and HCC12,14,28,29. Our data are the first to demonstrate that patients with HCC and a low FLIS had a shorter RFS and OS following hepatectomy. Multivariable analysis demonstrated that the FLIS was an independent risk factor for poor OS but not for RFS. The difference between OS and RFS in these data may be explained as follows: namely, OS reflects not only tumor biology, but also the patient’s general condition and/or the effects of treatments for recurrence. In contrast, because adjuvant therapy is not common after hepatectomy for HCC, RFS may be more likely to reflect tumor biology.
In contrast to the association between FLIS and long-term survival, Child–Pugh and ALBI grades were not associated with the OS or RFS after hepatectomy for HCC in the present study. One possible explanation for this result is a selection bias in the patients included in the present study. Because all patients underwent hepatectomy, the possibility of a substantial bias in terms of liver function cannot be ruled out. The Child–Pugh grade was A in 227 of 235 (96.6%) patients, and the ALBI grade was I or II in 231 of 235 (98.3%) patients. Therefore, the sensitivity of the Child–Pugh and ALBI grades may have been insufficient to discriminate differences in liver function that could affect long-term outcomes following hepatectomy for HCC. In contrast, the FLIS was 6 in 185 of 235 (78.7%) patients and 2–5 in 50 of 235 (21.3%) patients. Although the majority of patients had an FLIS of 6, the distribution of the FLIS was more varied than that of the Child–Pugh and ALBI scores; therefore, the FLIS might be able to better discriminate differences in liver function compared to the Child–Pugh and ALBI scores, even in a biased population of patients who underwent hepatectomy.
Another advantage of the FLIS is that it is simple and easy to evaluate. The FLIS does not require specific MRI protocols. Moreover, assessment of the FLIS using the actual images of each patient does not require any image analysis software or measurement-specific indices. The FLIS is calculated by the simple addition of each factor (0–2), with a maximum score of just 6. In addition, the FLIS can be evaluated with very high inter-reader reliability and is independent of MRI-related factors such as vendor, scanner type, and magnetic field strength, indicating that inter-operator variability is negligible13,30.
A potential disadvantage of FLIS is that the evaluation of FLIS requires an MRI to be performed before hepatectomy. While MRI is becoming more common as a preoperative assessment, it is not necessarily a routine examination at all institutions. Additionally, it may be difficult to evaluate FLIS in patients with obstructive jaundice, as it requires to assess EOB excretion into the biliary tracts (i.e. the excretion quality score), although this is similarly the case for the ALBI score.
Our data do not indicate that the FLIS can completely replace existing liver function indices, but rather support the FLIS as a simple and easily calculable score based on preoperative MRI imaging. As demonstrated in the present study, the FLIS provides better risk assessment for, at least, a specific, biased patient group undergoing hepatectomy; in contrast, the Child-Pugh and ALBI scores did not stratify patients well and were not significantly associated with long-term outcome after hepatectomy for HCC. Consistent with our data, Luo et al. demonstrated that FLIS may be a better predictor of post hepatectomy liver failure than the MELD score, ALBI score, and ICG-R15 clearance28.
The present study had some limitations. All data were retrospectively collected from the database of a single institution. All patients included in the present study underwent hepatectomy, indicating the presence of a certain selection bias related to liver function and the risk factors for recurrence and survival. Moreover, the association between the FLIS and long-term outcomes following nonsurgical treatment was not evaluated and should be explored in future studies. Due to potential biases related to patient selection, surgical technique, and post-recurrence treatment strategies, our findings need to be externally validated through larger studies involving multiple institution.
In conclusion, FLIS assessed using gadoxetic acid-enhanced MRI was significantly associated with RFS and OS following initial hepatectomy for HCC. A low FLIS is a poor prognostic factor for long-term outcomes; therefore, surgical indications must be carefully considered for patients with a low FLIS. Careful follow-up and multidisciplinary treatment strategies are warranted for these patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
N.S. designed the study and acquired data. N. S., T. T., S. T., and D. S. analyzed and interpreted the data. N.S. drafted the manuscript. N.S. and M. O. critically revised the manuscript. All authors have reviewed and approved the manuscript for publication.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kelley, R. K. et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet401, 1853–1865. 10.1016/S0140-6736(23)00727-4 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Taketomi, A. Hepatic resection for hepatocellular carcinoma in the era of molecular-targeted agents and Immune checkpoint inhibitors in Japan. JMA J.4, 241–245. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han, J. et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford)21, 962–971. 10.1016/j.hpb.2018.11.005 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Masuda, Y. et al. Factors affecting overall survival and disease-free survival after surgery for hepatocellular carcinoma: a nomogram-based prognostic model-a western European multicenter study. Updates Surg.76, 57–69. 10.1007/s13304-023-01656-8 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Ito, T. & Nguyen, M. H. Perspectives on the underlying etiology of HCC and its effects on treatment outcomes. J. Hepatocell. Carcinoma10, 413–428. 10.2147/jhc.s347959 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki, K. et al. Effect of background liver cirrhosis on outcomes of hepatectomy for hepatocellular carcinoma. JAMA Surg.152, e165059. 10.1001/jamasurg.2016.5059 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Zhou, P. et al. Comparison of FIB-4 index and child-pugh score in predicting the outcome of hepatic resection for hepatocellular carcinoma. J. Gastrointest. Surg.24, 823–831. 10.1007/s11605-019-04123-1 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Na, S. K. et al. ALBI versus child-pugh grading systems for liver function in patients with hepatocellular carcinoma. J. Surg. Oncol.117, 912–921. 10.1002/jso.24992 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka, A. et al. Albumin-bilirubin (ALBI) Grade as part of the evidence-based clinical practice guideline for HCC of the Japan society of hepatology: a comparison with the liver damage and child-pugh classifications. Liver Cancer6, 204–215. 10.1159/000452846 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng, Y. et al. Child-Pugh, MELD, and ALBI scores for predicting the in-hospital mortality in cirrhotic patients with acute-on-chronic liver failure. Expert Rev. Gastroenterol. Hepatol.10, 971–980. 10.1080/17474124.2016.1177788 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Bastati, N. et al. Assessment of orthotopic liver transplant graft survival on gadoxetic acid-enhanced magnetic resonance imaging using qualitative and quantitative parameters. Invest. Radiol.51, 728–734. 10.1097/RLI.0000000000000286 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Lee, H. J. et al. Validation of functional liver imaging scores (FLIS) derived from gadoxetic acid-enhanced MRI in patients with chronic liver disease and liver cirrhosis: the relationship between child-pugh score and FLIS. Eur. Radiol.31, 8606–8614. 10.1007/s00330-021-07955-1 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Bastati, N. et al. Does the functional liver imaging score derived from gadoxetic acid-enhanced MRI predict outcomes in chronic liver disease? Radiology294, 98–107. 10.1148/radiol.2019190734 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Bastati, N. et al. Gadoxetic acid-enhanced MRI-derived functional liver imaging score (FLIS) and spleen diameter predict outcomes in ACLD. J. Hepatol.77, 1005–1013. 10.1016/j.jhep.2022.04.032 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Johnson, P. J. et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J. Clin. Oncol.33, 550–558. 10.1200/JCO.2014.57.9151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tung-Ping Poon, R., Fan, S. T. & Wong, J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann. Surg.232, 10–24. 10.1097/00000658-200007000-00003 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoda, H. et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J. Hepatol.49, 223–232. 10.1016/j.jhep.2008.04.013 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Ivanics, T. et al. Dynamic risk profiling of HCC recurrence after curative intent liver resection. Hepatology76, 1291–1301. 10.1002/hep.32411 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Toyoda, H. et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br. J. Cancer114, 744–750. 10.1038/bjc.2016.33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki, A. et al. Remnant liver function is associated with long-term survival in patients with hepatocellular carcinoma undergoing hepatectomy. Sci. Rep.13, 15637. 10.1038/s41598-023-42929-x (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soreide, J. A. & Deshpande, R. Post hepatectomy liver failure (PHLF) - recent advances in prevention and clinical management. Eur. J. Surg. Oncol.47, 216–224. 10.1016/j.ejso.2020.09.001 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Chin, K. M. et al. Early prediction of post-hepatectomy Liver failure in patients undergoing major hepatectomy using a PHLF prognostic nomogram. World J. Surg.44, 4197–4206. 10.1007/s00268-020-05713-w (2020). [DOI] [PubMed] [Google Scholar]
- 23.Matsukane, R. et al. Prognostic significance of pre-treatment ALBI grade in advanced non-small cell lung cancer receiving immune checkpoint therapy. Sci. Rep.11, 15057. 10.1038/s41598-021-94336-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto, Y. et al. Association of the modified ALBI grade with endoscopic findings of gastroesophageal varices. Vivo35, 1163–1168. 10.21873/invivo.12364 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao, P. et al. Application of the ALBI scoring system for mortality outcome prediction in patients with hypertrophic cardiomyopathy. Glob Heart17, 73. 10.5334/gh.1163 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evlice, M. et al. The relationship between echocardiographic parameters and albumin bilirubin (ALBI) score in patients with isolated secundum type atrial septal defect. Echocardiography40, 350–358. 10.1111/echo.15556 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Zhu, Q. et al. Baseline ALBI grade predicts benefits after splenectomy for cirrhotic patients with hypersplenism. J. Gastrointest. Surg.27, 1130–1140. 10.1007/s11605-023-05610-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo, N. et al. A functional liver imaging score for preoperative prediction of liver failure after hepatocellular carcinoma resection. Eur. Radiol.32, 5623–5632. 10.1007/s00330-022-08656-z (2022). [DOI] [PubMed] [Google Scholar]
- 29.Aslan, S., Eryuruk, U., Tasdemir, M. N. & Cakir, I. M. Determining the efficacy of functional liver imaging score (FLIS) obtained from gadoxetic acid-enhanced MRI in patients with chronic liver disease and liver cirrhosis: the relationship between Albumin-Bilirubin (ALBI) grade and FLIS. Abdom. Radiol. (NY)47, 2325–2334. 10.1007/s00261-022-03557-7 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Kim, N. H. & Kang, J. H. Inter-reader reliability of functional liver imaging score derived from gadoxetic acid-enhanced MRI: a meta-analysis. Abdom. Radiol. (NY)48, 886–894. 10.1007/s00261-022-03785-x (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.