Abstract
Rectal cancer is a prevalent global malignancy. Recurrence and metastasis significantly impact patient survival over the long term. This study aims to identify independent risk factors associated with distant metastases in rectal cancer (RC) patients and develop a prognostic columnar-line diagram. This retrospective analysis encompasses data from 1,118 RC patients treated at the Department of Anorectal Surgery, Chifeng Municipal Hospital, between December 2015 and October 2023. These patients were diagnosed with stage I-IV RC. Univariate and multivariate Cox proportional hazard regression models identified risk factors for distant metastases development. The median follow-up duration was 61.3 months (range 2.24–96.33 months). The identified factors linked to distant metastases in RC included hemoglobin levels, body mass index (BMI), leukocyte neutrophil percentage, tumour diameter, pathology type, differentiation degree, number of detected lymph nodes, and T and N stages. These factors are significant risk indicators for distant metastases in RC patients. Incorporating these identified risk factors into a columnar-line diagram effectively predicts the likelihood of distant metastasis in RC patients. This approach aids in devising precise treatment strategies during the initial patient consultation.
Keywords: Rectal cancer, Distant, Hemoglobin, BMI, Nomogram
Subject terms: Cancer, Gastrointestinal diseases
Introduction
RC is among the most common cancers in China and globally, occupying the fourth highest position in both incidence and mortality worldwide1,2. Despite RC presenting a relatively better prognosis compared to other gastrointestinal tumors, the overall prognosis has not seen significant enhancement. The issues of recurrence and metastasis continue to be critical factors adversely impacting the long-term survival of patients3. Furthermore, although there have been advancements in radiotherapy and chemotherapy for RC treatment, there is still a deficiency in effective prognostic tools4. Individualized prediction models, which have proven more accurate than clinical staging in various malignant tumors such as breast, lung, and gastric cancers5–7, hold potential. In this context, this study has conducted a statistical evaluation of a large patient cohort with RC at our hospital. The objective is to establish a model that correlates specific clinicopathological features of RC with the likelihood of distant metastasis, aiming to identify high-risk factors predictive of distant metastasis in RC patients. This approach is intended to facilitate early intervention and thereby improve patient prognosis.
Materials and methods
Study population
A retrospective analysis was conducted on 1118 patients with RC, who underwent treatment at the Department of Anorectal Surgery, Chifeng Municipal Hospital, from December 2015 to October 2023. The inclusion criteria were: patients having undergone radical surgery for systemic RC and a postoperative pathologically confirmed diagnosis of RC. The exclusion criteria included: patients with incomplete test results, missing follow-up data, a history of other malignant tumors, or multiple primary tumors. Of these 1118 RC patients8, 703 were male and 415 were female; the average age was 61.91 ± 10.68 years. All patients in the cohort underwent laparoscopic surgeries for rectal cancers. This study received approval from the Ethics Committee of Chifeng Municipal hospital. This study was a retrospective analysis, so the Ethics Committee of the Chifeng Municipal hospital waived informed consent. We confirm that all methods are carried out in accordance with the relevant guidelines and regulations.
Extraction of patients’ clinical data
The study encompassed various parameters including age, sex, length of hospital stay, BMI(Most of the epidemiologic data on obesity are based on BMI (in kg/m2) and use the ranges of 18.5–24.9 for normality, 25-29.9 for overweight, and ≥ 30 for obesity9), levels of hemoglobin, white blood cells, neutrophil percentage, carcinoembryonic antigen (CEA), and carbohydrate antigen 19 − 9 (CA19-9) It also took into consideration factors like preoperative radiotherapy, diameter of the tumor, the pathological category (adenocarcinoma, mucinous carcinoma, imprinted cell carcinoma, adenocarcinoma with mucus secretion), degree of differentiation, pathological stages T and N, location of tumor (several previous literatures described that tumor location has some impact on the prognosis in rectal cancer8), number of lymph nodes detected, and cases of distant metastases. A comprehensive data collection process was performed and subsequently revised by two independent data entry personnel.
Establishment of predictive model for nomogram
Comprehensive clinical data was collected throughout the study based on postoperative pathological reports of the patients. These data include age, sex, duration of hospitalization, BMI, hemoglobin levels, leukocytes count, neutrophils percentage, CEA, CA19-9, preoperative radiotherapy status, tumor diameter, tumor category, degree of differentiation, T and N pathological stages, number of lymph nodes detected, and presence of distant metastasis. R software was used for nomogram construction and univariate and multivariate Cox regression analyses, allowing visual observation. The prognostic scores were easy to calculate directly, as well as the distance metastasis probability in single cases. The accuracy of survival prediction was evaluated using Harrell’s concordance index (C-index), and the correlation between the predicted probability of distant metastasis from the column plot and the actual probability on the standard curve was assessed. Statistical significance was established at a P-value of less than 0.05.
Prognostic follow-up
In this study, the primary endpoint, disease-free survival (DFS), was defined as the interval from the date of surgery to either the first detection of distant metastases or the follow-up cut-off in October 2023. Follow-up procedures were meticulously conducted by dedicated staff through telephone calls, letters, and clinic visits, enabling the accurate determination of the timing of distant metastasis occurrence in patients.
Statistical analysis
For data analysis, the R software (Version 4.0.5) was employed. Variables were processed using R-Studio (Version 1.2.5033) as an auxiliary tool, and the ‘rms’ and ‘survival’ packages were utilized for constructing a nomogram. A P-value of less than 0.05 was deemed to indicate statistical significance.
Results
Screening results
Between December 2015 and October 2023, a total of 1,201 RC patients treated at the Department of Anorectal Surgery, Chifeng Municipal Hospital, were identified from the pathology system. Out of these, 1118 patients met the inclusion criteria of having RC as the primary tumor. However, exclusions were made as follows: 47 patients were excluded due to the absence of TNM staging, 3 patients due to not undergoing radical surgery, and 33 patients due to incomplete test, examination, and pathological information.
Clinical characteristics of RC patients
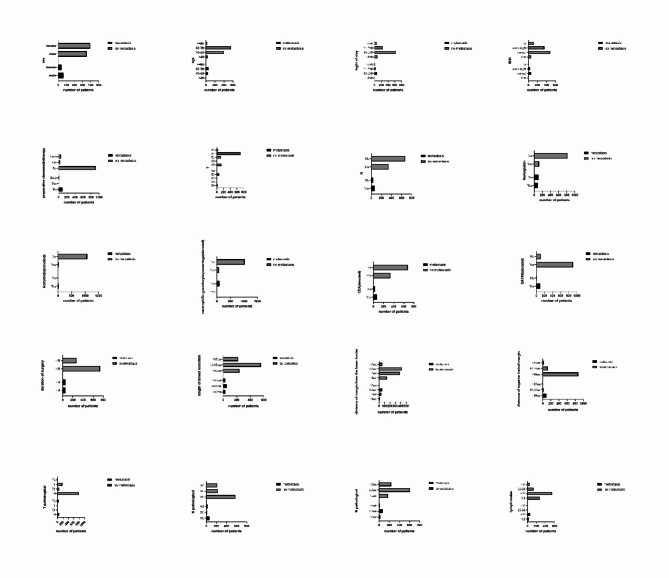
In this study, a final cohort of 1,118 patients was included. Of these, 703 patients (62.8%) were male, and 415 patients (37.12%) were female. The majority of the patients were aged between 60 and 79 years (54.47%) and 40–59 years (39.09%). Detailed case information and clinicopathologic feature of these patients are presented in Table 1. Then we made the bar graph (Fig. 1).
Table 1.
Number of clinicopathologic feature in patients.
| Clinicopathologic feature | Total | Distant metastasis | ||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| 1118 | 106 | 9.48% | 1012 | 90.52% | ||
| Sex | ||||||
| Male | 415 | 37.12% | 64 | 15.42% | 351 | 84.58% |
| Female | 437 | 39.09% | 42 | 9.61% | 395 | 90.39% |
| Age | ||||||
| < 40 | 38 | 3.40% | 7 | 18.42% | 31 | 81.58% |
| 40–59 | 437 | 39.09% | 39 | 8.92% | 398 | 91.08% |
| 60–79 | 609 | 54.47% | 54 | 8.87% | 555 | 91.13% |
| >=80 | 34 | 3.04% | 6 | 17.65% | 28 | 82.35% |
| Length of stay | ||||||
| < 15d | 91 | 8.14% | 5 | 5.49% | 86 | 94.51% |
| 16-25d | 692 | 61.90% | 69 | 9.97% | 623 | 90.03% |
| 26-35d | 269 | 24.06% | 25 | 9.29% | 244 | 90.71% |
| > 35d | 66 | 5.90% | 7 | 10.61% | 59 | 89.39% |
| BMI | ||||||
| Slim | 66 | 5.90% | 6 | 9.09% | 60 | 90.91% |
| Normal | 538 | 48.12% | 54 | 10.04% | 484 | 89.96% |
| Overweight | 389 | 34.79% | 37 | 9.51% | 352 | 90.49% |
| Fat | 125 | 11.18% | 9 | 7.20% | 116 | 92.80% |
| Preoperative chemoradiotherapy | ||||||
| No | 1018 | 91.06% | 94 | 9.23% | 924 | 90.77% |
| Yes | 39 | 3.49% | 5 | 12.82% | 34 | 87.18% |
| Both | 61 | 5.46% | 7 | 11.48% | 54 | 88.52% |
| T | ||||||
| T0 | 151 | 13.51% | 17 | 11.26% | 134 | 88.74% |
| T1 | 9 | 0.81% | 1 | 11.11% | 8 | 88.89% |
| T2 | 135 | 12.08% | 8 | 5.93% | 127 | 94.07% |
| T3 | 774 | 69.23% | 72 | 9.30% | 702 | 90.70% |
| T4 | 49 | 0.0438 | 8 | 0.1633 | 41 | 0.8367 |
| N | ||||||
| Yes | 409 | 0.3658 | 68 | 0.1663 | 341 | 0.8337 |
| No | 709 | 0.6342 | 38 | 0.0536 | 671 | 0.9464 |
| Location | ||||||
| High | 383 | 0.3426 | 37 | 0.0966 | 346 | 0.9034 |
| Medium | 468 | 0.4183 | 37 | 0.0791 | 431 | 0.9209 |
| Low | 267 | 0.2388 | 32 | 0.1199 | 235 | 0.8802 |
| Hemoglobin (reduce) | ||||||
| Yes | 202 | 18.07% | 86 | 42.57% | 116 | 57.43% |
| No | 916 | 81.93% | 103 | 11.24% | 813 | 88.76% |
| Neutrophilic granulocyte percentage(elevated) | ||||||
| Yes | 83 | 7.42% | 6 | 7.23% | 77 | 92.77% |
| No | 1035 | 92.58% | 100 | 9.66% | 1029 | 99.42% |
| CEA | ||||||
| Yes | 402 | 35.96% | 69 | 17.16% | 333 | 82.84% |
| No | 716 | 64.04% | 37 | 5.17% | 679 | 94.83% |
| CA199 | ||||||
| Yes | 999 | 89.36% | 97 | 9.71% | 902 | 90.29% |
| No | 119 | 10.64% | 9 | 7.56% | 110 | 92.44% |
| Duration of surgery | ||||||
| > 3 h | 789 | 70.57% | 55 | 6.97% | 734 | 93.03% |
| < 3 h | 320 | 28.62% | 51 | 15.94% | 269 | 84.06% |
| Length of bowel resection | ||||||
| < 10 cm | 263 | 23.52% | 26 | 9.89% | 237 | 90.11% |
| 10–15 cm | 607 | 54.29% | 51 | 8.40% | 556 | 91.60% |
| > 15 cm | 248 | 22.18% | 29 | 11.69% | 219 | 88.31% |
| Distance of margin from the lower border | ||||||
| < 1 cm | 153 | 13.69% | 10 | 6.54% | 143 | 93.46% |
| 1–2 cm | 425 | 38.01% | 37 | 8.71% | 388 | 91.29% |
| 2–5 cm | 477 | 42.67% | 55 | 11.53% | 422 | 88.47% |
| > 5 cm | 63 | 5.64% | 4 | 6.35% | 59 | 93.65% |
| Distance of superior incisal margin | ||||||
| < 10 cm | 959 | 85.78% | 88 | 9.18% | 871 | 90.82% |
| 10–15 cm | 135 | 12.08% | 17 | 12.59% | 118 | 87.41% |
| > 15 cm | 24 | 2.15% | 1 | 4.17% | 23 | 95.83% |
| T pathological | ||||||
| T0 | 839 | 75.04% | 60 | 7.15% | 779 | 92.85% |
| T1 | 64 | 5.72% | 5 | 7.81% | 59 | 92.19% |
| T1 | 180 | 16.10% | 6 | 3.33% | 174 | 96.67% |
| T3 | 35 | 3.13% | 35 | 100.00% | 0 | 0.00% |
| N pathological | ||||||
| N0 | 638 | 57.07% | 66 | 10.34% | 572 | 89.66% |
| N1 | 243 | 21.74% | 17 | 7.00% | 226 | 93.00% |
| N2 | 237 | 21.20% | 23 | 9.70% | 214 | 90.30% |
| Diameter of tumor | ||||||
| < 2 cm | 195 | 17.44% | 25 | 12.82% | 170 | 87.18% |
| 2–5 cm | 676 | 60.47% | 69 | 10.21% | 607 | 89.79% |
| > 5 cm | 247 | 22.09% | 12 | 4.86% | 235 | 95.14% |
| Lymph node | ||||||
| < 12 | 309 | 27.64% | 33 | 10.68% | 276 | 89.32% |
| 13–22 | 608 | 54.38% | 60 | 9.87% | 548 | 90.13% |
| 23–32 | 149 | 13.33% | 9 | 6.04% | 140 | 93.96% |
| > 32 | 52 | 4.65% | 4 | 7.69% | 48 | 92.31% |
Fig. 1.
The bar graph of clinicpathological feature.
Selection of RC distant metastasis factors
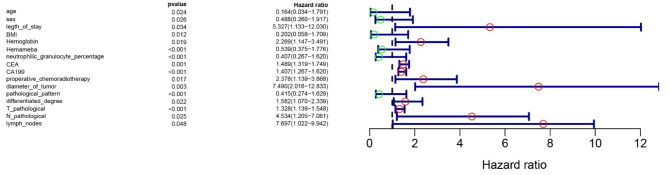
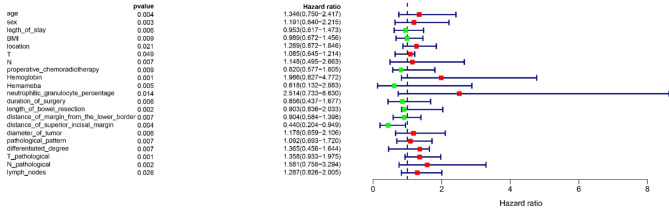
The study utilized a basic dataset to analyze unifactorial and multifactorial Cox proportional hazard regression models, with patient prognosis (distant metastases: Y = 1, no distant metastases: Y = 2) as the dependent variable and clinical data as independent variables (X). The variable screening was conducted using a forward selection method (likelihood ratio), with an inclusion criterion of 0.05 and an exclusion criterion of Univariate analysis revealed that factors such as age, sex, length of hospital stay, Body Mass Index (BMI), hemoglobin levels, leukocyte count, percentage of neutrophils, CEACA19-9, preoperative radiotherapy, tumor diameter, tumor type, degree of differentiation, T and N stages, and the number of lymph nodes detected were associated with distant metastasis in patients, as detailed in Table 2. Subsequently, significant risk factors were visualized and a forest plot was constructed using R-studio (Fig. 2). Multifactorial analysis results, demonstrating clinicopathological features associated with distant metastases in RC patients, are presented in Table 3. These were also visualized using R-studio to construct a forest plot (Fig. 3).
Table 2.
Unicox.
| Clinicopathologic feature | HR | L95CI | H95CI | p value |
|---|---|---|---|---|
| Age | 0.164 | 0.034 | 1.7909 | 0.02423 |
| Sex | 0.4883 | 0.2602 | 1.9167 | 0.0257 |
| Length of stay | 5.327 | 1.133 | 12.03 | 0.0341 |
| BMI | 0.2024 | 0.05779 | 1.7089 | 0.0125 |
| Hemoglobin | 2.2692 | 1.147 | 3.491 | 0.0186 |
| Neutrophilic granulocyte percentage | 0.406573 | 0.266588 | 1.620063 | 0.0000292 |
| CEA | 1.488599 | 1.31863 | 1.749236 | 0.001025128 |
| CA199 | 1.406573 | 1.266588 | 1.620063 | 0.0000292 |
| Preoperative chemoradiotherapy | 2.378 | 1.139 | 3.868 | 0.0174 |
| Diameter of tumor | 7.4901 | 2.0156 | 12.833 | 0.00264 |
| Pathological pattern | 0.414723 | 0.273568 | 1.62871 | 0.0000338 |
| Differentiated degree | 1.582085 | 1.069977 | 2.339297 | 0.021509636 |
| T pathological | 1.328052 | 1.139075 | 1.548381 | 0.00029157 |
| N pathological | 4.5342 | 1.2051 | 7.061 | 0.0254 |
| lymph nodes | 7.696608 | 1.022363 | 9.942044 | 0.047542275 |
Fig. 2.
The forest graph of unicox.
Table 3.
Mulcox.
| Clinicopathologic feature | HR | L95CI | H95CIH | p value |
|---|---|---|---|---|
| Age | 1.345952 | 0.749517 | 2.417005 | 0.0035 |
| Sex | 1.190825 | 0.640148 | 2.215213 | 0.0032 |
| Length of stay | 0.95315 | 0.61684 | 1.472821 | 0.0058 |
| BMI | 0.989429 | 0.672197 | 1.456373 | 0.0089 |
| Location | 1.268772 | 0.871984 | 1.846113 | 0.0213 |
| T | 1.085012 | 0.645297 | 1.213777 | 0.049 |
| N | 1.14778 | 0.494769 | 2.662654 | 0.007 |
| Preoperative_chemoradiotherapy | 0.820211 | 0.576692 | 1.80483 | 0.009452 |
| Hemoglobin | 1.98613 | 0.82657 | 4.772388 | 0.00125 |
| Neutrophilic granulocyte percentage | 2.514268 | 0.732549 | 8.629516 | 0.0143 |
| Duration of surgery | 0.856363 | 0.437186 | 1.67745 | 0.0061 |
| Length of bowel resection | 0.903462 | 0.835627 | 2.033218 | 0.00243 |
| Distance of margin from the lower border | 0.903871 | 0.584475 | 1.397806 | 0.0066 |
| Distance of superior incisal margin | 0.439825 | 0.203847 | 0.948977 | 0.003631 |
| Diameter of tumor | 1.178372 | 0.659197 | 2.106442 | 0.005797 |
| Pathological pattern | 1.091619 | 0.692676 | 1.720331 | 0.007056 |
| Differentiated degree | 1.365466 | 0.455567 | 1.644175 | 0.00659 |
| T pathological | 1.357694 | 0.933229 | 1.975222 | 0.001099 |
| N pathological | 1.580683 | 0.758443 | 3.294326 | 0.002217 |
| Lymph nodes | 1.286915 | 0.826041 | 2.004926 | 0.02648 |
Fig. 3.
The forest graph of mulcox.
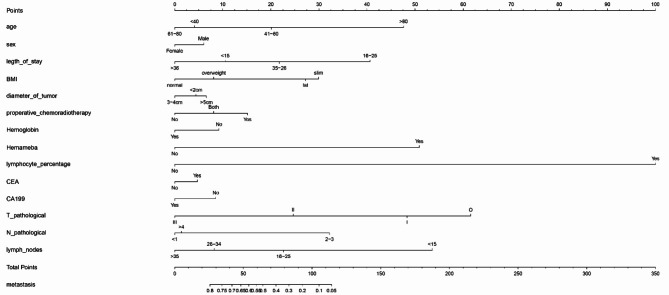
Construction of prognostic model by drawing nomogram
This study incorporated a range of independent prognostic factors to construct a Nomogram, predicting the likelihood of distant metastases in patients (Fig. 4). The column-line graph indicates that male patients have a higher risk of distant metastases compared to females. Patients with reduced hemoglobin levels are at an elevated risk for metastasis. An increase in the percentage of leukocytes and neutrophils is associated with a decreased risk of metastasis. Higher T-stage and N-stage classifications correlate with a reduced probability of metastasis. CA19-9 are indicative of an increased likelihood of distant metastasis. Age-wise, the highest metastasis probability is observed in the 61–80 years age group, followed by those < 40 years; the risk is comparatively lower in patients aged 41–60 years and is the lowest in patients > 80 years. Regarding BMI categories, the likelihood of metastasis descends in the order of normal, overweight, obese, and underweight. The highest metastasis risk is associated with tumor diameters ranging from 2 to 5 cm, followed by those > 5 cm. For each patient, a vertical line is drawn corresponding to each variable to calculate specific points. These points are then summed, and the decimal at the intersection of the vertical line and the survival axis on the total point line represents the patient’s probability of experiencing metastasis.
Fig. 4.
The nomogram of the risk factors in RC patients.
Validation of the column line plot prediction model
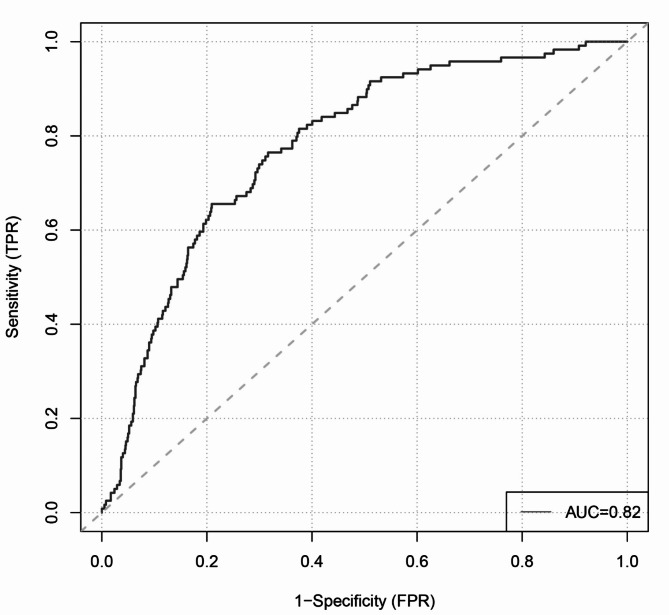
The calculation of the C-index for the column-line diagram model yielded a C- index of 0.808, with a P-value of 0.007. The ROC curve analysis yielded an Area Under the Curve (AUC) of 0.82 (Fig. 5) for the prognostic prediction model designed for estimation of the probability of distant metastases for the validated cohort of RC patients. These results showed that the model had a high degree of reliability and reproducibility in prognostic prediction. Therefore, this prophetic chart predictive model is able to predict when RC patients remote metastasize from the original focus reasonably.
Fig. 5.
The ROC of the model.
Discussion
The tumor lymph node metastasis (TNM) staging system, as outlined by the International American Joint Committee on Cancer10, serves as the primary assessment criterion in our clinic. Research has consistently demonstrated that higher TNM stages are associated with worse prognoses, a trend also evident in our findings. However, the prognostic efficacy of TNM staging is limited, offering insufficient detail for comprehensive patient assessment. Column line graphs, depicting complex statistical models in a simplified, visual format, show promise for widespread application in clinical practice and research. We developed a prognostic prediction model using extensive case-control studies, analyzing the clinical characteristics of RC patients and their risk of developing distant metastases. This model facilitates the inclusion of various indicators such as clinical tests and pathology, enhancing the specificity of clinical evaluations. CEA and CA19-9, known to correlate with RC prognosis in numerous publications11–13, were also found in our study to impact the development of distant metastases in RC patients. Data from 128 RC patients, compiled by Dr. Elif Tuğba Tuncel at Manisa State Hospital, Turkey, indicated that non-anemic patients with a hemoglobin/nutritional prognostic index above the threshold had significantly better survival and prognosis compared to anemic patients14. This aligns with the statistical data from 1118 RC patients at our center. In 26 colorectal cancer (CRC) clinical trials involving 22,674 patients at the University of Sydney Clinical Trials Centre, Australia, total white blood cell counts were associated with progression-free survival in CRC, but not with overall survival15.Martin R.Weiser, MD MD et al. analysed data from 1400 patients with rectal cancer at Memorial Sloan Kettering Cancer Center and constructed a nomogram of recurrence-free survival and overall survival. Unlike us, they incorporated AJCC, positive lymph nodes, DTAV, venous invasion, PNI and other metrics into the nomogram, which was used to predict risk factors for rectal cancer16. However, our findings suggest that elevated leukocyte and neutrophil counts are protective factors against the development of distant metastases in RC patients.
The precision in determining the number of lymph nodes excised intraoperatively and the length of the resected bowel segment has varied across studies. Data from Steven S. Shen at the Department of Pathology, Weill Hospital, Cornell University, revealed that factors such as patient age, tumor location, and the length of the resected bowel segment are correlated with the number of lymph nodes in surgical resections for CRC. They identified tumor location and the length of the resected bowel segment as independent predictors for the removal of 12 or more lymph nodes in resection specimens17. Research conducted by Xishan Wang’s team at Union Hospital, involving 7694 Chinese patients with RC across six medical centers, indicated that 15 lymph nodes represent the optimal threshold for quality assessment in lymph node examinations18. Many clinical studies, including imaging analyses, use tumor location as an observable indicator of rectal cancer11,19,20. However, a multicenter retrospective cohort study shows that primary tumor location did not affect survival in metastatic rectal cancer21, This is the same trend as our study.
In a multifactorial analysis, factors like preoperative radiotherapy, the length of resected bowel, and the tumor’s upper and lower margins showed correlation with the occurrence of metastases in patients. Our current study found that preoperative radiotherapy, intraoperative length of resected bowel, and the tumor’s margins served as protective factors against metastasis in RC patients. Notably, the probability of distant metastases in RC patients was lower in the subgroup with fewer than 15 detected lymph nodes.
It is noteworthy that not all RC patients who developed distant metastases exhibited an association with overall survival (OS). Particularly, within the subgroup of patients with distant metastases, the presence of lung metastases did not correlate with overall survival15. This observation offers valuable insights for our future research endeavors.
Conclusions
The presented results suggest a prediction model of good predictive capability. Clinicians may well predict the metastasis probability in RC patients with good chances of accuracy at the initial consultation using the column chart model. This helps them effectively communicate the prognosis to the family of the patient and together plan an optimized treatment, promoting patient’s compliance and better prognostic outcomes. In patients identified with high risk for distant metastases, timely intervention is important. Aggressive measures in this regard would include surgery, lymph node dissection, and preoperative radiotherapy to guard against chances of distant metastasis. Currently, while our model holds significant relevance, its predictive accuracy is confined to patients under similar conditions at this center. The applicability of the model to varied patient populations in different centers is still subject to validation through larger-scale studies. In conclusion, this model offers enhanced prediction of DFS in RC patients. Integrating these clinical indicators into the treatment evaluation framework as key reference points to refine clinical management and prognosis assessment, and thereby reducing metastatic rates, remains a critical challenge for clinicians. An user-friendly CUP nomogram which made by MD Anderson Cancer Center integrating commonly available baseline factors provides robust personalized prognostication which can aid clinical decision making and selection/stratification for clinical trials21. Our article has a little more patient cases and a little more study metrics relative to this study. They selected 281 patients with rectal cancer who developed distant metastases for analysis, whereas we selected 1118 patients and divided them into two groups based on whether or not distant metastases occurred. However, our study still has some limitations, such as the fact that although our sample size is large, we only have the results from a single center, and we need to subsequently obtain samples from more research centers for analysis and comparison. Subsequent studies will establish links with other centers in order to validate our results.
Acknowledgements
The authors thank all patients and institutions involved in this study, especially the ability to have open access to the R software.
Author contributions
Conceptualization, Mingyue Guo; methodology, Jia.Qiong.and Guoli Li.; software, Jia.Qiong.; validation, Jia.Qiong.; formal analysis, Mingyue Guo.; investigation, Mingyue Guo.; resources, Jia.Qiong and Zhangmin; data curation, Mingyue Guo, Zhang Min and Qiong Jia.; writing—original draft preparation, Jia.Qiong.; writing—review and editing, Mingyue Guo.; visualization, Jia.Qiong; supervision, Jia.Qiong.and Guoli Li; project administration, Jia.Qiong and Guoli Li. All authors have read and agreed to the published version of the manuscript.”
Data availability
All data generated or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao, W., Chen, H. D., Yu, Y. W., Li, N. & Chen, W. Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin. Med. J. (Engl)134(7), 783–791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu, S. et al. Total neoadjuvant therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer: a systematic review and meta-analysis. Oncologist26(9), e1555–e1566 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciardiello, F. et al. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J. Clin.72(4), 372–401 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Kuipers, E. J. et al. Colorectal cancer. Nat. Rev. Dis. Primers1, 15065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanghani, M. et al. Validation of a web-based predictive nomogram for ipsilateral breast tumor recurrence after breast conserving therapy. J. Clin. Oncol.28(5), 718–722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong, C., Miao, Q., Zheng, J. & Wu, J. A novel nomogram for predicting the decision to delayed extubation after thoracoscopic lung cancer surgery. Ann. Med.55(1), 800–807 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong, D. et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann. Oncol.30(3), 431–438 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, L-J. et al. Distinct prognosis of high Versus Mid/Low rectal Cancer: a Propensity score–matched cohort study. J. Gastrointest. Surg.23(7), 1474–1484 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Caballero, B. Humans against obesity: who Will Win? Adv. Nutr.10, S4–S9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol.17(6), 1471–1474 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Hao, C. et al. The clinical value of the combined detection of enhanced CT, MRI, CEA, and CA199 in the diagnosis of rectal Cancer. J. Oncol.2021, 1–8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh, S., Kumar, R., Kumar, U. & Kumari, R. Clinical significance and role of TK1, CEA, CA 19 – 9 and CA 72 – 4 levels in diagnosis of colorectal cancers. Asian Pac. J. Cancer Prev.21(11), 3133–3136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao, J., Zhao, H., Jia, T., Yang, S. & Wang, X. Combination of changes in CEA and CA199 concentration after neoadjuvant chemoradiotherapy could predict the prognosis of stage II/III rectal cancer patients receiving neoadjuvant chemoradiotherapy followed by total mesorectal excision. Cancer Manag. Res.14, 2933–2944 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuncel, E. T., Parvizi, M., Kut, E., Aydin, M. & Kasap, E. Prognostic significance of Hemoglobin/Prognostic Nutritional Index and Hemoglobin/Red blood cell distribution in rectal Cancer. Turk. J. Gastroenterol.34(2), 128–134 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjoquist, K. M. et al. Personalizing survival predictions in advanced colorectal cancer: the ARCAD Nomogram Project. J. Natl. Cancer Inst.110(6), 638–648 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiser, M. R. et al. Development and Assessment of a clinical calculator for estimating the likelihood of recurrence and survival among patients with locally advanced rectal Cancer treated with chemotherapy, Radiotherapy, and surgery. JAMA Netw. Open4(11) (2021). [DOI] [PMC free article] [PubMed]
- 17.Steven, S. et al. R. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch. Pathol. Lab. Med.133(5), 781–786 (2016). [DOI] [PubMed]
- 18.Guan, X. et al. Optimal examined lymph node number for accurate staging and long-term survival in rectal cancer: a population-based study. Int. J. Surg.109(8), 2241–2248 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao, Z. et al. Clinical atlas of rectal cancer highlights the barriers and insufficient interventions underlying the unfavorable outcomes in older patients. Heliyon9(5) (2023). [DOI] [PMC free article] [PubMed]
- 20.Ciardiello, F. et al. Clinical management of metastatic colorectal cancer in the era of precision medicine. Cancer J. Clin.72(4), 372–401 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Unal, O. U. et al. Survival outcomes according to the tumor location and prognostic factor in metastatic rectal cancer: a multicenter retrospective cohort study. Front. Oncol. 14 (2024). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during the current study available from the corresponding author on reasonable request.