Abstract
This study explored the causal relationships among primary sclerosing cholangitis (PSC), ulcerative colitis (UC), and hepatobiliary cancer (HBC) by using bidirectional two-sample, two-step Mendelian randomization (MR) analysis. Genetic variants associated with PSC and UC from the FinnGen research database were used for instrumental variable-based analyses. Mediation analyses were conducted to examine the role of PSC and UC in HBC risk. The findings revealed a causal effect of genetic predisposition to UC on PSC risk (inverse-variance-weighted [IVW] analysis odds ratio [OR] 1.145, p < 0.001), whereas no reverse causality was observed. Although UC showed no direct causal effect on HBC risk, genetic susceptibility to PSC significantly increased the risk of HBC (IVW analysis OR = 1.855, p < 0.001). Mediation analysis further identified PSC as a significant mediator amplifying the causal effect of UC on HBC risk (effect size = 0.083). These results established a causal link between genetic susceptibility to UC and increased risk of PSC, and highlighted the critical role of PSC in mediating the impact of UC on HBC risk.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83085-0.
Keywords: causal relationship, hepatobiliary cancer, Mendelian randomization, mediator, primary sclerosing cholangitis, ulcerative colitis
Subject terms: Cancer epidemiology, Cancer prevention, Cancer screening, Cancer
Introduction
Hepatobiliary cancer (HBC) includes primary liver cancers, such as hepatocellular carcinoma (HCC), cholangiocarcinoma (CC), and gallbladder cancer (GBC). HBC is the sixth most common malignancy and the third leading cause of cancer-related mortality. Given the marked increase in susceptibility to HCC as compared to the general population, primary sclerosing cholangitis (PSC) has garnered attention as a potential predisposing factor for hepatobiliary cancer1. PSC is a rare, chronic, immune-mediated cholangiopathy characterized by inflammation and progressive fibrotic narrowing of the bile ducts2,3. Existing literature demonstrates a recent increase in its prevalence, with reported rates ranging from 0.78 to 31.7 per 100,000 individuals4. Although the prevailing consensus attributes PSC to a combination of environmental and genetic factors, the precise etiology of this condition remains elusive3. The natural progression of PSC culminates in the emergence of biliary cirrhosis and eventually in liver failure, with the potential for further advancement to HBC5. However, the underlying causes of PSC have not been definitively characterized6.
Furthermore, the presence of inflammatory bowel disease (IBD), a persistent inflammatory condition affecting the gastrointestinal tract, particularly ulcerative colitis (UC), has been observed in approximately 70–80% of individuals diagnosed with PSC7, and significantly increases the risk of colorectal cancer to greater than that associated with UC alone8,9. Despite the well-established and extensively researched link between PSC and UC, the precise nature of this relationship, in terms of chronological precedence and severity, remains unclear10.
Hence, establishing a definitive causal connection between UC and PSC is of paramount importance for disease management. Such determination serves as a pivotal foundation for developing efficacious treatment strategies. Furthermore, a pertinent question is whether UC contributes to the pathogenesis of HBC.
Specific genetic variants that exhibit clear associations with potential risk factors can function as unbiased proxies for these risk factors. This approach facilitates the establishment of causality and is known as Mendelian randomization (MR), a genetic methodology that bolsters the precision of inferences pertaining to the causal link between an exposure and its corresponding outcome. Notably, MR mitigates susceptibility to confounding factors and effectively eliminates concerns related to reverse causality, both of which are prevalent limitations of conventional observational investigations11. Compared with conventional multivariate regression methods, MR analysis offers enhanced robustness against measurement errors, confounding factors, and reverse causation12.
In this study, we used summary statistics extracted from a genome-wide association study (GWAS) that encompassed UC, PSC, and HBC. We endeavored to investigate the genetic underpinnings of these traits. To this end, we comprehensively evaluated genome-wide genetic correlations. Furthermore, we conducted a two-sample, bidirectional MR analysis, which enabled exploration of the potential causal relationship between UC and PSC. Additionally, we investigated the intricate interplay among UC, PSC, and the risk of HBC using a meticulous two-step MR analysis.
Methods
Study design and data sources
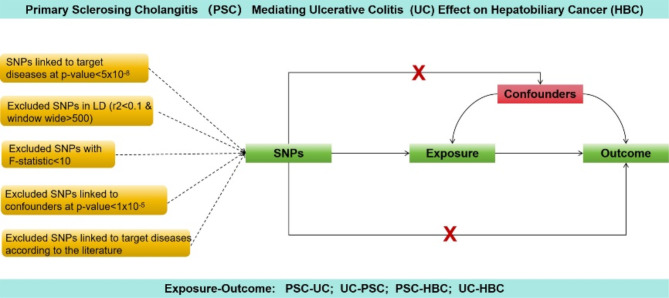
To derive causal insights encompassing PSC, UC, and HBC, we employed a bidirectional two-sample, two-step MR design, following the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) statement13. Figure 1 shows a schematic diagram summarizing the MR investigation.
Fig. 1.
This Figure illustrates a diagram of the MR study. The study’s flowchart is structured upon three core assumptions. (I) Relevance to Exposure: The instrumental variables (IVs) must exhibit a statistically significant connection to the exposure under investigation (II) Independence from Confounding: The IVs should remain disconnected from any recognized confounding factors that could potentially modify the relationship between an exposure and its associated outcome. (III) Exclusion Restriction: The IVs should bear no direct influence on the outcomes; their impact is solely mediated by their effects on the exposure of interest.
The MR study was based on three fundamental assumptions. First, genetic variants meticulously chosen as instrumental variables (IVs) exhibited robust associations with their respective exposures. Second, the selected IVs were devoid of connections to potential confounding factors. Third, IVs exerted a direct influence on the outcomes solely through exposure pathways, rather than via alternative routes.
Our study obtained summary statistics from a GWAS conducted as part of the FinnGen Research Project. The sample sizes were as follows: PSC cases, 1,715; PSC controls, 330,903; UC cases, 5,034; UC controls, 371,530; HBC cases, 304; and HBC controls, 174,006. The FinnGen initiative facilitates investigation of the intricate interplay between disease patterns and genetic variations in isolated populations. Supplementary Table 1 provides comprehensive details of all GWAS included in our study.
Genetic instrument selection
Genome-wide significant single nucleotide polymorphisms (SNPs) (p-value < 5.0E − 08) were extracted from the dataset14. Subsequently, SNP selection and filtering were performed to ensure data quality. SNPs exhibiting a low linkage disequilibrium (r2 < 0.1) within a region of 500 kb15 were excluded, adhering to the principle of diminishing confounding due to genetic correlations. To assess the strength of each SNP, F-statistics were computed using the formula F = R2 [(N − 2) / (1 - R2)], where R2 represents the proportion of exposure factor variance elucidated by the IVs, and N signifies the overall sample size16,17. SNPs with weak instrumental characteristics were discarded using a threshold of F = 10 as the cut-off value.
Furthermore, SNPs linked to potential confounders (Supplementary Tables S2–S5) were removed from the analysis. SNPs demonstrating horizontal pleiotropic effects were pruned using the PhenoScanner database18. This meticulous curation process was designed to uphold two pivotal assumptions in Mendelian randomization: the independence of the IVs from potential confounding factors and the exclusion restriction.
MR analysis
A bidirectional two-sample MR analysis was performed to investigate the potential association between PSC and UC. Subsequently, two-step MR analysis was conducted to delve deeper into the intricate relationships among PSC, UC, and HBC. This comprehensive approach aimed to ascertain not only pairwise connections, but also meticulously assess conceivable mediating pathways within the causal framework.
In our primary analyses, we employed the inverse variance-weighted (IVW) method, assuming the validity of all genetic variants as IVs19,20. To investigate the third hypothesis of MR, addressing potential violations of the IV assumption stemming from directional-level pleiotropy, we employed the MR-Egger method21. Additionally, we employed the weighted median approach, which computes the median of weighted estimates and can provide reliable effect estimates, even when pleiotropy affects up to 50% of the IVs22,23.
Three supplementary analytical methods were also used. These encompass weighted pattern estimation, simple pattern estimation, and IVW analysis for random-effects models. The inclusion of weighted and simple pattern estimates facilitates the relaxation of the assumptions intrinsic to IV analysis, consequently augmenting the dependability and consistency of causal effect detection. Notably, IVW analysis for the random-effects models exhibited robustness, even in the presence of heterogeneity.
The outcome results were presented as the odds ratio (OR) of the respective outcome variable, accompanied by its corresponding 95% confidence interval, signifying a unit standard deviation change in the OR exposure factor. This comprehensive process was predominantly executed using the TwoSample MR package24.
The mediation proportions were computed utilizing the following formula: β × β2 / [(β1 × β2) + β], where β represents the total effect derived from the primary analysis, β1 represents the effect of exposure on the mediator, and β2 represents the effect of the mediator on HBC (Fig. S1)25.
Sensitivity analysis
Heterogeneity testing was performed for both the IVW analysis and MR-Egger regression. Cochran’s Q test was employed to evaluate IV heterogeneity, with a p-value of less than 0.05 indicating significant heterogeneity26. When a genetic variant utilized as an IV is associated with risk factors beyond the intended exposure of interest and exerts a causal effect on the outcome, a bias due to horizontal pleiotropy may occur, thus violating MR assumptions 2 and 3. To gauge and mitigate such bias stemming from horizontal pleiotropy, an MR-Egger regression was employed. The absence of horizontal pleiotropy was indicated by an intercept close to zero (p > 0.05).
In addition, a leave-one-out approach was integrated into the analyses. This entailed the sequential removal of each SNP and subsequent calculation of the pooled effect of the remaining SNPs. This step aimed to identify potential SNPs that might influence the causal effect.
Statistical analysis
All statistical analyses conducted in this study were executed utilizing the “Two-sample MR” package within R version 4.0.1 (https://cran.r-project.org/bin/windows/base/old/). Statistical significance was defined as a two-sided p-value of less than 0.05.
Results
Quality control for genetic instruments
The MR results are reported in accordance with the STROBE-MR guidelines (Supplementary Material 2).
Supplementary Tables S2–S5 give the details of the included SNPs. All PSC SNPs had an F-statistic > 199, which was above the threshold of 10, suggesting sufficient instrumental strength27,28. Horizontal pleiotropy was not detected for individual SNPs associated with PSC in the pleiotropy test (Supplementary Table S6). Furthermore, no indications of inter-SNP heterogeneity were observed in either IVW or MR-Egger regression analyses of UC (Supplementary Table S6, Figs. S2, S3). In the context of PSC, although some heterogeneity in SNP selection may emerge when UC is the outcome, it did not exert any influence on IVW outcomes within the random-effects model, confirming the reliability of our conclusions (Supplementary Table S6, Fig. S2).
Causal estimates between PSC and UC
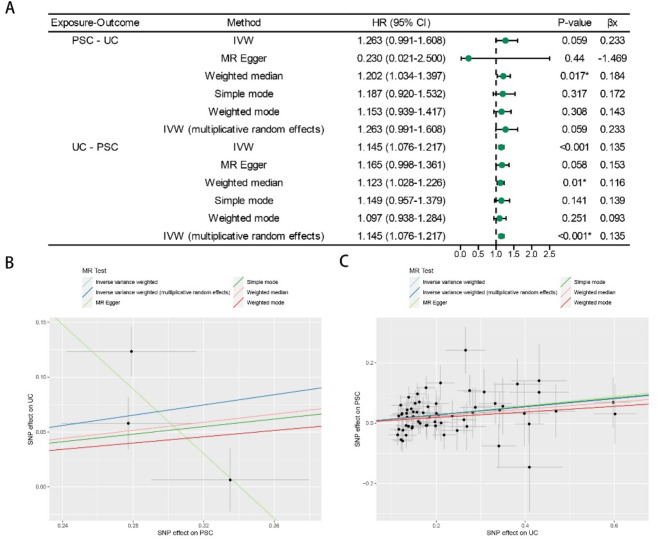
Our IVW analysis findings (p = 0.059, OR = 1.263) indicated that PSC did not exert a discernible influence on the risk of UC. These outcomes demonstrated remarkable consistency with the results derived from alternative MR techniques: MR-Egger (p-value = 0.440, OR = 0.230), simple mode (p-value = 0.317, OR = 1.187), weighted mode (p-value = 0.308, OR = 1.153), and weighted median (p-value = 0.017, OR = 1.202) (Fig. 2A).
Fig. 2.
MR estimates derived from the fixed-effect IVW method, MR-Egger regression, weighted median method, weighted-mode method, simple-mode and random-effect IVW method to assess the causal effect between PSC and UC. (A) Results of forward MR and reverse MR analysis between PSC and UC. (B) Scatter plots showing the MR effect of each exposure on PSC. (C) Scatter plots showing the MR effect of each exposure on UC. PSC primary sclerosing cholangitis, UC ulcerative colitis.
In contrast, the outcomes of the reverse MR analyses consistently suggested a conspicuous causal connection between UC and the risk of PSC: IVW p < 0.001, OR = 1.145; weighted median p = 0.010, OR = 1.123; MR-Egger p = 0.058, OR = 1.165; simple model p = 0.141, OR = 1.150; and weighted model p = 0.251, OR = 1.097 (Fig. 2A).
Notwithstanding the varying significance of the different methods, the findings indicated a substantial level of concurrence in the direction of the estimated causal effect (Fig. 2B, C). The reported estimates signified the effects of genetic susceptibility to PSC (or UC) on the risk of UC (or PSC). Furthermore, the outcomes of the leave-one-out sensitivity assessments confirmed that the non-causal associations were not influenced by individual genetic variants in either the MR or reverse MR analyses (Supplementary Fig. S4).
Causal estimates between PSC and HBC or UC and HBC
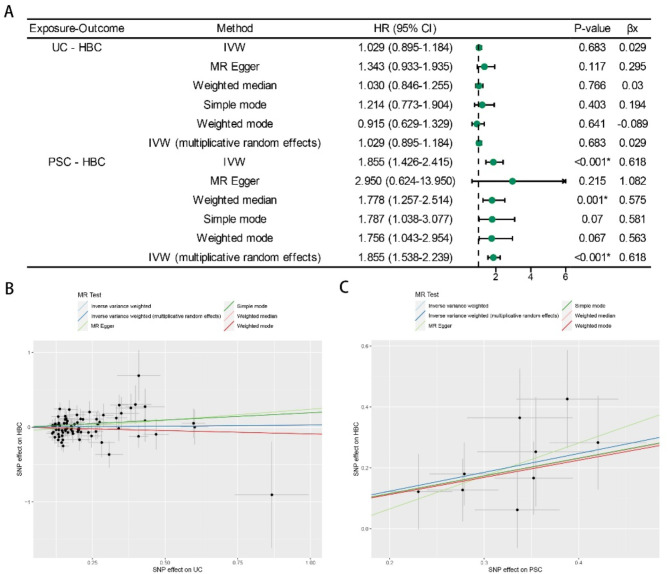
The IVW analysis yielded compelling evidence of a positive association between PSC and HBC (p < 0.001, OR = 1.855), with consistent results found using the weighted median method (p = 0.001, OR = 1.778) (Fig. 3A). Notably, the outcomes from the MR-Egger test (p = 0.215, OR = 2.950), simple mode (p = 0.070, OR = 1.787), and weighted mode (p = 0.067, OR = 1.756) did not reveal any association between PSC and HBC. Nevertheless, these results did not influence our principal assessment based on IVW analysis.
Fig. 3.
MR estimates derived from the fixed-effect IVW method, MR-Egger regression, weighted median method, weighted-mode method, simple-mode and random-effect IVW method to assess the causal effect of UC on HBC and PSC on HBC. (A) Results of MR analysis between liability to UC or PSC and the HBC risk. (B) Scatter plots showing the MR effect of each exposure on PSC. (C) Scatter plots showing the MR effect of each exposure on UC. UC ulcerative colitis, PSC primary sclerosing cholangitis, HBC hepatobiliary cancer.
Conversely, MR analyses investigating the association between UC and an increased risk of HBC showed no significant association: IVW p = 0.683, OR = 1.029; weighted median p = 0.766, OR = 1.030; MR-Egger p = 0.117, OR = 1.343; simple mode p = 0.403, OR = 1.214; or weighted mode p = 0.641, OR = 0.915 (Fig. 3A).
Notwithstanding the varying significance of the different methods, the findings indicated a substantial level of concurrence in the direction of the estimated causal effect (Fig. 3B, C). This finding suggested a noteworthy causal relationship between genetic susceptibility to PSC and HBC risk, whereas UC has no direct causal relationship with HBC risk. Moreover, leave-one-out sensitivity tests indicated that non-causal associations were unaffected by individual genetic variants in both the MR and reverse MR analyses (Supplementary Fig. S5).
PSC as mediator in UC progression to HBC
These findings indicate a causal link between the predisposition to UC and an elevated risk of PSC (IVW: p < 0.001). Similarly, a causal relationship between PSC susceptibility and an increased risk of HBC was evident (IVW: p < 0.001). Importantly, the results did not support a direct causal relationship between UC and HBC. These results imply that PSC potentially functions as a comprehensive mediator, wherein susceptibility to UC precipitates an augmented risk of HBC, largely via PSC.
Regarding the causal impact of UC on HBC, according to the IVW method, the mediating effect of PSC was 0.083, representing 73.8% of the total mediating effect. Moreover, the direct and overall effects of UC on HBC were calculated as 0.029 and 0.113, respectively (Figs. 2 and 3).
Discussion
To the best of our knowledge, this study represents a pioneering endeavor to scrutinize the causal associations among UC, PSC, and HBC by applying a bidirectional two-sample, two-step MR analysis. Our comprehensive findings underscore the lack of substantial evidence supporting a causal relationship between genetic susceptibility to PSC and the risk of UC. However, a distinct causal association emerged between the genetic predisposition to UC and the heightened risk of PSC, suggesting a potential convergence of shared genetic mechanisms between these two conditions. This conclusion was supported by a recent study on the relationship between IBD and PSC29.
UC, as a long-term exposure factor, evidently increases the risk of HBC. A study by Huang et al. provides further support for our findings30. Although direct causality between PSC and HBC has not been established, our results unmistakably confirmed the causal role of PSC in an increased risk of HBC. This inference lends credence to the notion that PSC operates as an essential mediator of the induction of HBC development from UC. The insights gleaned from our study provide a compelling rationale for the early implementation of preventive measures and therapeutic interventions targeting both PSC and HBC.
Cancers affecting the hepatobiliary tract represent a particularly pernicious group of malignancies, and previous studies have also investigated the relationship between PSC and the risk of HBC31. Following the diagnosis of PSC, the annual incidence of CC ranges from 1 to 2%, with a cumulative risk of 5–10% over a patient’s lifetime32. Furthermore, individuals with PSC display heightened vulnerability to GBC. This is evidenced by the fact that 14% of patients with PSC undergoing cholecystectomy present with a gallbladder mass, of which 57% involves malignancy33,34. While HCC is relatively less prevalent in the context of PSC, a concern remains that the risk of HCC could be similar to that observed for other forms of cirrhosis once cirrhosis manifests5. Despite the perceived association between UC and HBC, existing evidence remains limited and scholarly discourse on this topic is scarce35,36. Considering the frequent coexistence of UC and PSC37,38, we hypothesized that UC might exacerbate HBC risk by inducing PSC. Our findings lend credence to this hypothesis, indicating that PSC may act as a comprehensive mediator of HBC induced by UC. The collective impact of UC on HBC was estimated at 0.1125, with PSC accounting for 73.8% of this effect through indirect mediation. Consequently, prevention and management of PSC are of paramount significance in the broader context of disease control and intervention.
PSC is a relatively uncommon yet increasingly more prevalent cholestatic liver disorder. Despite its increasing incidence, the underlying etiological factors remain unclear. Moreover, established medical interventions currently do not confer a verified benefit in curtailing disease progression39. Previous epidemiological studies have consistently underscored the high prevalence of IBD in approximately 70–80% of patients with PSC, with the majority of these cases (approximately 80%) having UC7. In contrast, only a small subset of patients with IBD, ranging from 2 to 8%, ultimately develop PSC during the course of their ailment40,41. The nuanced clinical presentation of PSC, which often manifests with few symptoms, and its frequent incidental diagnosis may inadvertently lead to underdiagnosis of PSC in individuals with UC42. However, asymptomatic individuals do not equal the absence of risk. Research has shown that approximately 22% of patients diagnosed with asymptomatic PSC exhibit clinical symptoms within 5 years of diagnosis43. The median survival period for symptomatic patients prior to succumbing or undergoing liver transplantation is estimated to be approximately 9 years. Additionally, the coexistence of PSC and UC engenders a parallel risk, leading to more extensive colitis and a four-fold increased susceptibility to colorectal tumors compared to isolated UC42,44. The outcomes of our study unequivocally pointed to a causal link between susceptibility to UC and PSC risk, although insufficient evidence exists to establish a causal effect of PSC on UC risk. This underscores the importance of diligently monitoring liver function in individuals with UC and actively participating in regular PSC screenings. Notably, the effective management and treatment of UC are pivotal for facilitating the early prevention and diagnosis of PSC.
At present, only liver transplant has demonstrated the ability to alter the natural trajectory of PSC. Nevertheless, a substantial proportion of patients (approximately 20–25%) experience PSC relapse within a decade of undergoing liver transplant43. Research has highlighted that total colectomy performed either before or during liver transplant can mitigate the risk of PSC recurrence by up to 50%45. Certain studies have investigated the plausible association between UC and PSC from the point of view of the “leaky gut” hypothesis46–48. The intricate interplay between the intestines and liver is upheld via the biliary tract, portal vein, and systemic circulation. According to this hypothesis, the breach of the intestinal epithelial barrier could result in the migration of intestinal flora into the portal vein circulation, facilitated by the “enterohepatic axis”48. This sequence of events can induce an immune response and foster chronic inflammation within the hepatic biliary system46,48,49. Concomitantly, interventions targeting the intestinal microbiota may offer potential efficacy in managing UC–PSC interfaces. These findings align with our evidence for a causal relationship between UC and PSC.
Our study had several strengths. First, we harnessed the potency of bidirectional, two-sample MR analysis, a strategy adept at uncovering causal relationships between UC and PSC, while attenuating the impacts of reverse causality and confounding variables. Second, our employment of MR revealed a fresh vantage point, highlighting the pivotal role of PSC in driving the emergence of HBC triggered by UC. Furthermore, our stringent selection criteria for SNPs ensured their significant association with the exposure variable, without direct links to the outcome variable. The F-statistics for each SNP surpassed the customary threshold of 10 (F > 199), reaffirming the robustness and trustworthiness of the statistical findings.
Despite these strengths, some limitations of the study should also be acknowledged. Given that all GWAS summary statistics incorporated in our analysis originated from samples of European descent, the generalizability of the observed estimates across other racial backgrounds remains uncertain. Subgroup analyses based on variables, such as sex, age, and comorbidities were constrained, prompting a cautious interpretation of the individual conclusions. Therefore, validation of our outcomes in future studies encompassing larger, diverse ethnic cohorts is imperative. In addition, MR studies have inherent limitations. Exposure factors may influence genetic risk through multiple pathways, which we attempted to address using the MR-Egger regression and weighted median methods. However, these approaches may not fully account for all the complexities. Genetic pleiotropy, in which a genetic variant affects multiple traits, remains a challenge despite our adjustments. In addition, our analysis did not consider epigenetic factors that could have affected our results. Future research should incorporate these elements to enhance the robustness of the causal inferences.
In summary, our study underscores the causal role of susceptibility to UC in increasing the risk of PSC, although no evidence for bidirectionality was found. Moreover, our findings indicate that heightened susceptibility to PSC substantially amplifies the likelihood of developing HBC while concurrently operating as a pivotal mediator in augmenting UC’s influence of UC on HBC. The implications of our findings underscore the importance of vigilant early surveillance and ongoing management of both UC and PSC, which are pivotal for preventing PSC recurrence and for reducing the risk of HBC.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
Fangming W: Conceptualization, Formal analysis, Methodology, Writing-original draft, Writing-review & editing; Junhui X: Investigation, Validation, Visualization, Writing-original draft.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fangming Wang and Junhui Xiao contributed equally to this work.
References
- 1.Yang, Y. et al. Emerging roles of long noncoding RNAs in cholangiocarcinoma: Advances and challenges. Cancer Commun.40 (2020). [DOI] [PMC free article] [PubMed]
- 2.van Munster, K. N., Dijkgraaf, M. G. W., Elferink, O., Beuers, R. P. J., Ponsioen, C. Y. & U. & Symptom patterns in the daily life of PSC patients. Liver Int.42, 1562–1570. 10.1111/liv.15271 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasztelan-Szczerbinska, B., Rycyk-Bojarzynska, A., Szczerbinska, A. & Cichoz-Lach, H. Selected aspects of the intricate background of immune-related cholangiopathies: A critical overview. Nutrients10.3390/nu15030760 (2023). [DOI] [PMC free article] [PubMed]
- 4.Trivedi, P. J. & Hirschfield, G. M. Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut70, 1989–2003. 10.1136/gutjnl-2020-322362 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Bowlus, C. L., Lim, J. K. & Lindor, K. D. AGA clinical practice update on surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis: Expert review. Clin. Gastroenterol. Hepatol.17, 2416–2422. 10.1016/j.cgh.2019.07.011 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Prokopi, M. & Beuers, U. Management of primary sclerosing cholangitis and its complications: an algorithmic approach. Hep. Intl.15, 6–20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Der Have, M. & Oldenburg, B. Is ulcerative colitis associated with primary sclerosing cholangitis an undertreated condition? Inflamm. Bowel Dis.26, 780–781. 10.1093/ibd/izz211 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Adamowicz, M., Stukan, I. & Milkiewicz, M. K. P. Agnieszka. Modulation of mismatch repair and the SOCS1/p53 axis by microRNA-155 in the colon of patients with primary sclerosing cholangitis. Int. J. Mol. Sci.23 (2022). [DOI] [PMC free article] [PubMed]
- 9.Shah, S. C. et al. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease. Clin. Gastroenterol. Hepatol.1610.1016/j.cgh.2018.01.023 (2018). [DOI] [PubMed]
- 10.Barberio, B. et al. Prevalence of primary sclerosing cholangitis in patients with inflammatory bowel disease: A systematic review and meta-analysis. Gastroenterology161, 1865–1877. 10.1053/j.gastro.2021.08.032 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Smith, G. D. & Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol.32 (2003). [DOI] [PubMed]
- 12.Richmond, R. C. & Davey Smith, G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb Perspect. Med.10.1101/cshperspect.a040501 (2022). [DOI] [PMC free article] [PubMed]
- 13.Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. JAMA326, 1614–1621. 10.1001/jama.2021.18236 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Xu, J. X., Zhu, Q. L., Bi, Y. M. & Peng, Y. C. New evidence: Metformin unsuitable as routine adjuvant for breast cancer: a drug-target mendelian randomization analysis. BMC Cancer24, 691. 10.1186/s12885-024-12453-w (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun, L. et al. Association between human blood metabolome and the risk of Alzheimer’s disease. Ann. Neurol.92, 756–767. 10.1002/ana.26464 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Park, S. et al. Nonlinear causal effects of estimated glomerular filtration rate on myocardial infarction risks: Mendelian randomization study. BMC Med.20, 1–12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadimitriou, N. et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat. Commun.11, 597. 10.1038/s41467-020-14389-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamat, M. A. et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics35, 4851–4853. 10.1093/bioinformatics/btz469 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, L. et al. Causal associations of obesity with achilles tendinopathy: A two-sample Mendelian randomization study. Front. Endocrinol. (Lausanne). 13, 902142. 10.3389/fendo.2022.902142 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, S. et al. Investigating causal relations between genetic-related intermediate endophenotype and risk of chronic prostatitis: Mendelian randomization study. Oxid. Med. Cell. Longev.2022, 4560609. 10.1155/2022/4560609 (2022). [DOI] [PMC free article] [PubMed]
- 21.Yavorska, O. O. & Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol.46, 1734–1739. 10.1093/ije/dyx034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alipour, P. et al. Investigation of the causal relationship between ALS and autoimmune disorders: a Mendelian randomization study. BMC Med.20, 1–7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, H. et al. Mendelian randomization highlights significant difference and genetic heterogeneity in clinically diagnosed Alzheimer’s disease GWAS and self-report proxy phenotype GWAX. Alzheimers Res. Ther.14, 17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu, J. X., Chen, Y. Y., Qi, L. N. & Peng, Y. C. Investigation of the causal relationship between breast cancer and thyroid cancer: a set of two-sample bidirectional Mendelian randomization study. Endocrine10.1007/s12020-024-03976-0 (2024). [DOI] [PubMed] [Google Scholar]
- 25.Thompson, J. R., Minelli, C. & Del Greco, M. Mendelian randomization using public data from genetic consortia. Int. J. Biostat.10.1515/ijb-2015-0074 (2016). [DOI] [PubMed]
- 26.Greco, M., Minelli, F. D., Sheehan, C., Thompson, J. R. & N. A. & Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med.34, 2926–2940. 10.1002/sim.6522 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open. Res.4, 186. 10.12688/wellcomeopenres.15555.3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden, J., Hemani, G. & Davey Smith, G. Invited commentary: Detecting individual and global horizontal pleiotropy in Mendelian Randomization-A job for the humble heterogeneity statistic? Am. J. Epidemiol.187, 2681–2685. 10.1093/aje/kwy185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao, J., Li, K., Liao, X. & Zhao, Q. Causal associations between inflammatory bowel disease and primary biliary cholangitis: a two-sample bidirectional Mendelian randomization study. Sci. Rep.13, 10950. 10.1038/s41598-023-35785-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, J. et al. Inflammatory bowel disease increases the risk of hepatobiliary pancreatic cancer: A two-sample Mendelian randomization analysis of European and East Asian populations. Cancer Med.12, 13599–13609. 10.1002/cam4.6057 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyson, J. K., Beuers, U., Jones, D. E. J., Lohse, A. W. & Hudson, M. Primary sclerosing cholangitis. Lancet391, 2547–2559. 10.1016/S0140-6736(18)30300-3 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Schrumpf, E. & Boberg, K. M. Hepatic and extrahepatic malignancies and primary sclerosing cholangitis. Gut52, 165 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vázquez-Elizondo, G., Muciño-Bermejo, J. & Méndez-Sánchez, N. Gallbladder disease in patients with primary sclerosing cholangitis. Ann. Hepatol.7, 182–183 (2008). [PubMed] [Google Scholar]
- 34.Buckles, D. C., Lindor, K. D., Larusso, N. F., Petrovic, L. M. & Gores, G. J. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am. J. Gastroenterol.97, 1138–1142 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Castro, F. A. et al. Increased risk of hepatobiliary cancers after hospitalization for autoimmune disease. Clin. Gastroenterol. Hepatol.10.1016/j.cgh.2013.11.007 (2014). [DOI] [PubMed]
- 36.Erichsen, R. et al. Hepatobiliary cancer risk in patients with inflammatory bowel disease: A Scandinavian population-based cohort study. Cancer Epidemiol. Biomark. Prev.30, 886–894. 10.1158/1055-9965.EPI-20-1241 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Tyson, G. L. & El-Serag, H. B. Risk factors for cholangiocarcinoma. Hepatology (Baltimore Md)54, 173–184. 10.1002/hep.24351 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trivedi, P. J. et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology159, 915–928. 10.1053/j.gastro.2020.05.049 (2020). [DOI] [PubMed] [Google Scholar]
- 39.de Krijger, M. et al. Epigenetic signatures discriminate patients with primary sclerosing cholangitis and ulcerative colitis from patients with ulcerative colitis. Front. Immunol.13, 840935. 10.3389/fimmu.2022.840935 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Vries, A. B., Janse, M., Blokzijl, H. & Weersma, R. K. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J. Gastroenterol.21, 1956–1971. 10.3748/wjg.v21.i6.1956 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmela, C., Peerani, F., Castaneda, D., Torres, J. & Itzkowitz, S. H. Inflammatory bowel disease and primary sclerosing cholangitis: A review of the phenotype and associated specific features. Gut Liver12, 17–29. 10.5009/gnl16510 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Da Cunha, T., Vaziri, H. & Wu, G. Y. Primary sclerosing cholangitis and inflammatory bowel disease: A review. J. Clin. Transl Hepatol.10, 531–542. 10.14218/JCTH.2021.00344 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eaton, J. E., Talwalkar, J. A., Lazaridis, K. N., Gores, G. J. & Lindor, K. D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology145, 521–536. 10.1053/j.gastro.2013.06.052 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soetikno, R. M., Lin, O. S., Heidenreich, P. A., Young, H. S. & Blackstone, M. O. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest. Endosc.56, 48–54 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Lindström, L. et al. Risk factors and prognosis for recurrent primary sclerosing cholangitis after liver transplantation: a Nordic Multicentre Study. Scand. J. Gastroenterol.53, 297–304. 10.1080/00365521.2017.1421705 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Tripathi, A. et al. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol.15, 397–411. 10.1038/s41575-018-0011-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamoto, N. et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol.4, 492–503. 10.1038/s41564-018-0333-1 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Özdirik, B., Müller, T., Wree, A., Tacke, F. & Sigal, M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci.10.3390/ijms22136975 (2021). [DOI] [PMC free article] [PubMed]
- 49.Seki, E. & Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J. Physiol.590, 447–458. 10.1113/jphysiol.2011.219691 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the corresponding author.