Abstract
Background and Objectives:
The most common cause of severe foodborne salmonellosis is S. Typhimurium. Its interaction with intestinal epithelial cells is little known. Lactic acid bacteria (LAB) were recognized as a prominent probiotic gastrointestinal microbiota of humans and animals that confer health-promoting and protective effects. This study aims to determine the anti-invasion and antibacterial effects of heat-killed LAB (HK-LAB) isolates against S. Typhimurium towards human intestinal cells.
Materials and Methods:
12 HK-LAB isolates from 3 sources of origin (stingless bee, plant, and food) were tested to determine the adhesion of HK-LAB to Caco-2 cells, anti-invasion and antibacterial activities against S. Typhimurium, the adhesion and invasion pattern of S. Typhimurium on intestinal epithelial cells (Caco-2) and assessing the effect of LAB on the S. Typhimurium-host cell interaction.
Results:
Tairu isolates from food have the highest adhesion rate with 19 ± 1.32/10 Caco-2 cells followed by HK-LAB R-isolate from plant 17 ± 0.70/10 Caco-2 cells, which is similar to the control (Lactobacillus casei). In the anti-invasion assay, the two HK-LAB isolates that had the strongest adherence to Caco-2 cells, Tairu-isolate inhibited at 78.1 ± 3.06% and R-isolate inhibited at 64.76 ± 9.02% compared to the positive control (63.81 ± 1.15%), which led to increased suppression of S. Typhimurium accordingly. Tairu and R isolates were tested for their antibacterial ability against S. Typhimurium. Both R and Tairu isolates displayed strong inhibition zones (27 ± 0.06 mm, 23 ± 0.06 mm) respectively.
Conclusion:
These findings suggest that the anti-invasion activities of HK-LAB R and Tairu may correlate to their bactericidal effects that serve to protect the host from infection.
Keywords: Salmonella Typhimurium, Lactic acid bacteria, Caco-2 cells, Anti-invasion
INTRODUCTION
Salmonella enterica is a Gram-negative bacterium and is known as one of the major foodborne pathogens which causes human foodborne infection each year affecting millions of people worldwide (1). The high incidence of Salmonella outbreak in Malaysia has been associated with the country’s environment, which is optimum for the growth of most bacteria (2). Ministry of Health Malaysia (MOH) has reported the occurrence of this outbreak in several states of Malaysia; infection among children in Sarawak from 2011 to 2016 (3), food poisoning affecting 43 students in Perak (4), 13 patients and 1 food handler in night market (5), and 21 schools (6), in Terengganu, as well as two deaths along with 83 infections in Kedah, Perak, and Selangor (7). Recently, Salmonella contamination has caused the recall of various imported food items, including eggs from Malaysia (8), and Jif peanut butter (9). Salmonella is considered as the most common foodborne organism in imported foods from Africa to the European Union. A large proportion (72.4%) of the foodborne salmonellosis outbreaks were caused by Salmonella enterica serovar Enteritidis (10). In the European Union/European Economic Area (EU/EEA) and the United Kingdom (UK), a cross-border outbreak of Salmonella Mbandaka ST413 has been ongoing for over two years since September 2021. By 30 November 2022, 196 cases had been recorded and published in a joint European Centre for Disease Prevention and Control (ECDC) and European Food Safety Authority (EFSA) Rapid Outbreak Assessment. It increased to 300 cases (an increase of 104 cases) by 15 March 2024, according to the European case definition (11). CDC estimates Salmonella bacteria cause about 1.35 million infections, 26,500 hospitalizations, and 420 deaths in the United States every year (12).
The pathogenicity of Salmonella is governed by its ability to adhere to and invade intestinal cells (13, 14). Studies on Salmonella adhesion and invasion have been extensively reported on intestinal Caco-2 cell lines (15). Various anti-adhesion and anti-invasion studies have been reported as a therapeutic strategy to prevent Salmonella adhesion/invasion to intestinal cells at the early stages of infection (16). For example, Salmonella enterica serovar Typhimurium (S. Typhimurium) is shown to be inhibited from adhering to the Caco-2 cell line by bovine milk-derived Muc1 (17–21).
In recent years, lactic acid bacteria (LAB) have received extensive attention due to their various beneficial effects, including their capacity to maintain intestinal permeability, improve the physical mucosal layers, and boost the immune system to protect the host from pathogens (22). LAB are Gram-positive, rodor cocci-shaped bacteria that belong to several genera; including Lactobacillus, Pediococcus, Lactococcus, Streptococcus, Bifidobacterium, Carnobacterium, Sporolactobacillus, and Leuconostoc (23), and have been discovered in an array of sources; including food, plants, animals, and insects (24). LAB are commonly known as probiotic microorganisms (25), determined by several characteristics such as acid tolerance, bile tolerance, high antimicrobial resistance against foodborne pathogens, and capability to adhere to intestinal cells (26). The ability of LAB to protect their hosts by secreting antimicrobials and triggering the host immune response has been documented in both humans and animals (27). A good probiotic LAB produces a variety of antimicrobial chemicals, including lactic acid, acetic acid, propanoic acid, alcohol, and diacetyl, demonstrating outstanding anti-bacterial, anti-fungal, and anti-viral characteristics (26). These antimicrobial chemicals produced by LAB can inhibit and protect against dangerous microorganisms by bringing the pH down to a point where the protein membrane is denatured resulting in dysfunctional membrane permeability. In addition, LAB also produces bacteriocins that can aid in the inhibition of pathogen growth (26). LAB is effective against a variety of foodborne pathogens via a wide range of antimicrobial mechanisms (28).
Many in vivo and in vitro studies have shown the protective role of LAB strains in preventing various intestinal infections caused by pathogenic bacteria (29). For example, probiotics Bacillus coagulans have shown protective effects on Caco-2 cells against S. Typhimurium infection by inhibiting their adhesion and invasion (28). Aside from that, the adherence of S. Typhimurium to Caco-2 cells is reduced by 7-fold by the pre-incubation with LAB Lactobacillus paracasei (29). Although there may be advantages, live probiotics are not frequently used in clinical settings because of the risk of septicemia in immunocompromised patients and the shorter shelf life since probiotic bioactivity tends to decline with time (30). Additionally, safety concerns regarding the use of live probiotic strains have emerged in certain patient populations, including neonates and vulnerable patients, particularly due to the translocation of bacteria from the gut to the systemic circulation, which has increased interest in the use of non-viable heat-killed probiotics (20). Moreover, heat-killed probiotics are stable and usable in a wide pH range and heat-processed foods, therefore offering a potentially safe alternative for manufacturers and vulnerable individuals (22). Thus, the use of non-viable microorganisms or microbial cell extracts as probiotics, particularly heat-killed lactic acid bacteria (HK-LAB) in place of live LAB because they improved natural defense mechanisms against pathogenic infection, is becoming more and more popular as a way to minimize these hazards (20).
Studies have shown that HK-LAB has a variety of positive effects. In vitro and in vivo competition between HK-LAB and intestinal pathogens such as Salmonella (22), Staphylococcus aureus (31), Campylobacter jejuni (32), Escherichia coli (33) and Helicobacter pylori (34), for adhesion sites at the gastrointestinal level and anti-invasion capabilities have been documented. Contrary to popular belief, HK-LAB strains are more effective than live LAB strains at reducing pathogenic bacterial adhesion (31). Therefore, this study aimed to evaluate the adhesion properties of heat-killed (HK)-LAB from various sources (stingless bees, plants, and food origin) on intestinal Caco-2 cells and to determine their anti-invasion activities against S. Typhimurium.
MATERIALS AND METHODS
Source and isolation of lactic acid bacteria.
For isolation of lactic acid bacteria, 10 grams of each (stingless bee, plant, and food) samples were weighed and homogenized with sterilized peptone physiological saline solution PPSA (1% peptone (Oxoid, England), 0.9% NaCl) for about 1–4 minutes aseptically. Proper serial dilutions (10−1 to 10−6) were prepared for each sample using 1 ml of homogenate. A volume of 0.1 ml of each dilution was spread-plated on MRS (Oxoid, England) agar media. Then the plates were incubated for 48 hours in an anaerobic jar at 32°C. Typical LAB characteristics colonies were randomly picked up and purified by streaking 2 or 3 times on freshly prepared MRS agar plates followed by morphological and microscopic examinations. Based on colony and cell morphological properties, the colonies showing the lactic acid bacteria’s general characteristics were chosen from each plate for physiological and biochemical tests, Simple characterizations were done such as Gram staining and catalase test (35).
A total of 12 LAB isolates obtained from various sources (stingless bees, plants, and food) were used in this study (Table 1). A total of 4 isolates were obtained from the stingless bee gut (1 from Heterotrigona itama, and 3 from Tetragonula laeviceps), 2 isolates from two plants (pomegranate and grass), and 6 isolates from different food sources namely fermented food (tempoyak) (6), cow’s milk (2), cheese (2) and Kimchi (6). The isolates from stingless bee gut and Tempoyak were received in glycerol stock while others were from MRS broth.
Table 1.
LAB isolates from different sources
| No. | LAB isolates | Sources |
|---|---|---|
| Origin: Stingless Bee | ||
| 1. | I5 | Heterotrigona itama |
| 2. | L1 | |
| 3. | L3 | Tetragonula laeviceps |
| 4. | L6 | |
| Origin: Plant | ||
| 5. | Dp | Pomegranate |
| 6. | R | Grass |
| Origin: Food | ||
| 7. | LAB 2 | Fermented food (tempoyak) |
| 8. | SL | Cow’s milk |
| 9. | Tairu | |
| 10. | Cheddar | Cheese |
| 11. | BC | |
| 12 | Kimchi | Kimchi |
Bacterial strains and growth conditions.
ATCC strains of S. Typhimurium (ATCC 14028) provided by the Faculty of Pharmacy, UiTM Puncak Alam were maintained in Luria-Bertani (LB) broth (Miller’s LB agar, Condalab, Spain), while LAB isolates were grown in de Mann, Rogosa and Sharpe (MRS) broth (Oxoid, England).
Before use in experiments, cultures were sub-cultured onto LB agar (S. Typhimurium) or MRS agar (LAB isolates), and individual colonies were grown in LB broth and incubated at 37°C for 16 h with shaking at 150 rpm (19) or in MRS broth in anaerobic condition at 37°C. Morphological evaluation of LAB isolates was done by simple characterizations using Gram staining and catalase test.
For the Salmonella adhesion and invasion experiment, overnight culture of S. Typhimurium was diluted in LB broth to achieve approximately (108 CFU/mL) at a wavelength of 600 nm (OD600 = 0.2) (21).
Caco-2 cell culture.
Caco-2 cells were routinely maintained in a 25 cm2 cell culture flask with Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA), 1% antibiotics (penicillin/ streptomycin) (Sigma-Aldrich, USA), 1% non-essential amino acids (NEAA) (Sigma-Aldrich, USA) and 1% L-glutamine (Sigma-Aldrich, USA) (36). The cultures were incubated at 37°C in a humidified incubator with 5% CO2. Cultural mediums were replaced every other day. Before the adhesion and invasion assay, the Caco-2 cells (confluency between 80–90%) were harvested from flasks using trypsin-EDTA (Sigma Aldrich, USA) and subsequently seeded into a cell culture plate. Several 2 × 104 cells/mL were seeded on 24-well plates (37) and were used as differentiated cells after 15 ± 1 days in culture with medium change every other day (17, 37). After the Caco-2 cells had reached full differentiation, the medium was exchanged for an antibiotics-free medium for 24 h before adhesion and invasion studies (19).
Salmonella adhesion assay.
The adhesion study of S. Typhimurium towards Caco-2 cells was performed as described previously (18, 19) with slight modifications. Overnight cultures of S. Typhimurium were recovered by centrifugation at 10,000 rpm for 5 min. Then, the bacteria were washed twice with PBS and re-suspended in an antibiotic-free medium. A volume of 500 μL/well of antibiotic-free medium containing 108 CFU/mL S. Typhimurium strains was added to 15 ± 1 days Caco-2 cells. The infected cells were incubated at 37°C for interval time of 1 h and 2 h. After the incubation period, the medium was removed, and cells were washed three times with PBS to remove non-adhered Salmonella. Cells with adhered Salmonella were treated with 250 μL of trypsin-EDTA per well for 10 min at 37°C followed by the addition of 250 μL culture medium containing FBS to stop the trypsin reaction. Then, serial dilutions of the cell suspension were plated on LB agar and incubated at 37°C for 24 h to enumerate adhering bacteria (CFU/mL).
Salmonella invasion assay.
The invasion study was determined using gentamicin-protective assay, as described previously by Mechesso et al. (19), Birhanu et al. (18), Burkholder et al. (38), Dostal et al. (39) and Gagnon et al. (40), with slight modifications (18, 19). Briefly, a bacterial suspension (500 μL/well) that was prepared as described for the adhesion assay was applied on Caco-2 cells and incubated at 37°C for an interval time of 1 h and 2 h. The infected cells were washed three times with PBS before the addition of 250 μL DMEM containing 300 μg/mL gentamicin per well and were incubated at 37°C for an additional 60 min to kill extracellular bacteria that had not invaded the cells. After a further washing step with PBS, 250 μL of trypsin-EDTA was added followed by another incubation step for 10 min at 37°C. The Caco-2 cells were then disrupted by adding 250 μL of 1% (v/v) Triton X-100 per well. After 10 min incubation at 37°C, samples were collected for the determination of bacterial counts as described in the cell adhesion study. The adhesion and invasion patterns of S. Typhimurium were expressed as the relative percentage of the number of adhered and invaded bacteria to the total number of bacteria used. Both assays were performed in triplicate and conducted in three independent experiments.
Preparation of Heat-Killed (HK) LAB.
The LAB cultures (1 × 108 CFU/mL) were harvested by centrifugation at 14,000 × g for 5 min at 4°C after incubation at 37°C for 18 to 24 h and the supernatant was discarded. The LAB pellets were washed three times with PBS and then resuspended in 10 mL PBS. The heat-killed (HK) treatment was done by heating the bacteria suspension at 80°C for 30 min (14, 41).
Adhesion assay of HK-LAB to Caco-2 cells.
The adhesion assay of HK-LAB was performed according to the method previously described (42). Caco-2 cells were seeded into 24 well-plates at 2 × 104 cells/mL and incubated at 37°C in a 5% CO2 until 15 days. After 15 days, 250 μL (approximately 5 × 107 CFU/mL) HK-LAB strains were added into the well and incubated at 37°C for 2 h in a 5% CO2 atmosphere. After incubation, cells in each well were washed three times with PBS followed by fixation with 10% of formalin for 30 min. After that, the cells were stained with 0.5% crystal violet followed by three times washing with PBS. The cells were visually counted by using an inverted microscope. The number of adhered HK-LAB on the Caco-2 cell lines was enumerated in ten random Caco-2 cells. This adhesion assay was done in three independent replicates and the mean ± standard deviation of adhered HK cells was calculated (10.28).
Anti-invasion assay of HK-LAB strains against S. Typhimurium.
The anti-invasion assay of HK-LAB strains was done according to the previously described protocol with some modifications (42). An overnight culture of S. Typhimurium was prepared in 10 mL LB broth at 37°C. The OD reading of S. Typhimurium was adjusted to OD600 = 0.25 (approximately 2 × 108 CFU/mL) followed by centrifugation at 10,000 rpm, for 5 min and the pellet was resuspended in antibiotic-free DMEM. The Caco-2 cells were seeded into 24 well-plates at 2 × 104 cells/mL and incubatedat 37°C in a 5% CO2 atmosphere until day 15. A volume of 250 μL HK-LAB strains (approximately 1 × 108 CFU/mL) was seeded in the well and incubated at 37°C for 2 h in a 5% CO2 . After incubation, the cells were washed three times with PBS followed by treating 250 μL overnight culture of S. Typhimurium (2 × 108 CFU/mL) and incubated for another 2 h. The cells were washed again with PBS three times. Next, a volume of 500 μL of gentamicin (300 μg/mL) was added to each well and incubated for 1 h. The cells were washed three times again with PBS followed by the addition of 500 μL 1% Triton X-100 for lysis of cells. The invaded S. Typhimurium supernatant upon lysis was serially diluted and plated on LB agar followed by incubating at 37°C for 24 h. This assay was done in triplicate and the number of invaded S. Typhimurium was enumerated and the percentage was calculated (43).
Where A1 and A2 indicate the absence and the presence of LAB strains, respectively.
Antibacterial activity against S. Typhimurium.
The antibacterial assay against S. Typhimurium was done according to the method by Reuben et al. (44). S. Typhimurium was incubated for 18 h at 37°C in nutrient broth (Oxoid, England). The overnight culture of the tested pathogen was adjusted to 0.5 Mc-Farland standard (approximately 1 × 108 CFU/mL). S. Typhimurium was then spread onto the surface of Muller Hinton Agar (MHA) plates. The wells were punctured using cork-borer and 100 μL of HK-LAB cell lysate was added into the well. The plate was incubated at 37°C for 48 h. This well diffusion assay was repeated in triplicate and the inhibition zone was measured. The positive control used was L. casei strain Shirota provided by the Faculty of Pharmacy, UiTM Puncak Alam.
Statistical analysis.
All assays were done in triplicate. The results were expressed as mean ± standard deviation (SD). Analysis of variance (ANOVA) post-hoc Tukey was conducted to find significant differences (p< 0.05).
RESULTS
Isolation of LAB from different sources.
A total of 12 LAB was isolated in this study from three different sources: stingless bees, plants, and food (Table 1).
Adhesion and invasion of S. Typhimurium on Caco-2 Cells.
Results for adhesion and invasion rate of S. Typhimurium on Caco-2 cells are shown in Table 2.
Table 2.
Percentage of S. Typhimurium Adhesion and Invasion on Caco-2 Cells
| Incubation time | Relative % to control | |
|---|---|---|
|
| ||
| Adhesion | Invasion | |
| 1 h | 10.9 ± 0.76 | 0.1 ± 0.03 |
| 2 h | 16.2 ± 0.66 | 0.6 ± 0.06 |
Results are expressed as mean ± standard deviation of three independent experiment
The adhesion rate of S. Typhimurium increased from 10.9 ± 0.76% to 16.2 ± 0.66%, during 2 h incubation time. A similar pattern was observed in the invasion study, where the percentage of invasion rose from 0.1 ± 0.03% to 0.6 ± 0.06% at 2 h incubation. Thus, to examine the potential of LAB isolates to prevent the invasion of S. Typhimurium on the Caco-2 cells, we used a longer incubation time (2 h) for the anti-invasion studies.
Adhesion of HK-LAB isolates on Caco-2 cells.
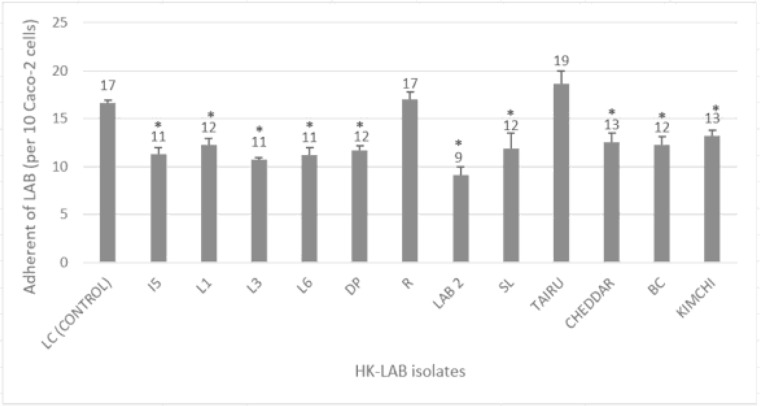
All 12 strains of HK-LAB isolates were evaluated for their capacity to adhere to intestinal Caco-2 cells. The counting of adhered cells was performed in 10 random cells for three independent experiments. The number of HK-LAB isolates that adhered to Caco-2 cells is shown in Fig. 1.
Fig. 1.
Adhesion of HK-LAB Isolates on Caco-2 Cells. The data shows the means of triplicate experiments and error bars indicate standard deviations. LC serves as a positive control. Values with an asterisk (*) are significantly different (p < 0.05) from the control group.
Anti-invasion activities of HK-LAB isolates against S. Typhimurium.
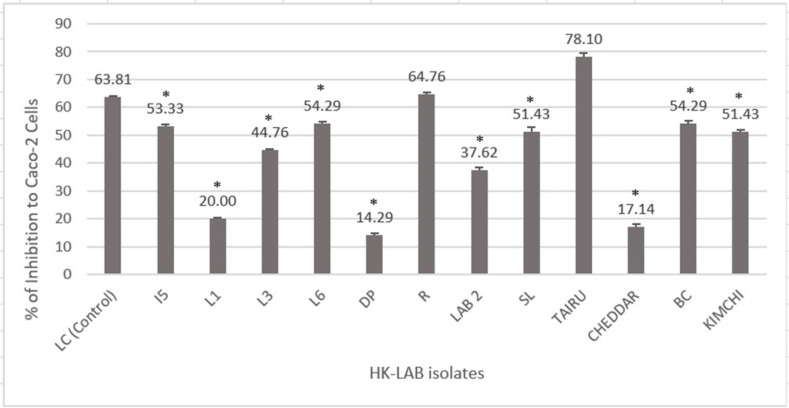
In this work, 12 different HK-LAB isolates were pre-incubated with Caco-2 cells before a 2 h infection with S. Typhimurium to test their ability to inhibit S. Typhimurium from invading Caco-2 cells (Fig. 2). The highest percentage of inhibition was demonstrated by Tairu isolate (78.1 ± 3.06%). Followed by R (grass) isolates (64.76 ± 9.02%) and the positive control group (63.81 ± 1.15%). There was no difference between the percentage of inhibition. While the lowest percentage was found for the DP isolate (14.29).
Fig. 2.
Percentage Inhibition of LAB Isolates on Caco-2 Cells against Invasion of S. Typhimurium. The data shows the means of triplicate experiments and error bars indicate standard deviations. LC serves as a positive control. Bars with an asterisk (*) are significantly different (p < 0.05) from the control group.
Antibacterial activities against S. Typhimurium.
Based on the excellent anti-invasion activities, HK-LAB isolates of R and Tairu were chosen and tested for their antibacterial potential against S. Typhimurium (Table 3). Both isolates showed a very high inhibition zone against S. Typhimurium, the highest was for R (27 ± 0.06 mm) followed by (23 ± 0.06 mm) for Tairu and the positive control LC (2424 ± 0.10). Statistically, both R and Tairu isolates significantly differed from the positive control LC (p>0.05).
Table 3.
Antimicrobial Activity of Promising HK-LAB Isolates against S. Typhimurium
| HK-LAB isolate | Zone of inhibition (mm) ± SD |
|---|---|
| R | 27 ± 0.06bcdef |
| Tairu | 23 ± 0.06bcdef |
| LC (positive control) | 24 ± 0.10b |
Values are expressed in mean ± standard deviation of three independent experiments. The (−) indicates no zone of inhibition; weak (6–9 mm); medium (9–12 mm); strong (12–15 mm); very strong (>15 mm). Means with different letters indicating significant differences (p < 0.05).
DISCUSSION
Adhesion of HK-LAB isolates on Caco-2 cells.
Adhesion to intestinal cells is important as one of the probiotic characteristics. Ahmad et al. (2018) (45), stated that Lactobacilli adhesion has been recognized as necessary for exerting positive probiotic effects in the large intestine (45). The Shirota’s strain L. casei (LC) was selected as a positive control since this strain has proven to protect against E. coli and Salmonella sp. infection (46). From the result, all 12 tested isolates could adhere to Caco-2 cells with different adhesion activities. In comparison to control (LC), most HK-LAB isolates generally showed much lower adherence to Caco-2 cells. It is interesting to note that one of the isolates (R-grass) displayed a pattern that was identical to the control group, with an adhesion of 17 ± 0.70 per 10 Caco-2 cells. Another isolate (Tairu) demonstrated marginally stronger adherence to Caco-2 cells (19 ± 1.32 per 10 Caco-2 cells) as compared to the control group (17 ± 0.25 per 10 Caco-2 cells).
The adhesion result in this study is consistent with the study by Lin et al. (2007) in which the heat-killed Lactobacillus acidophilus was able to adhere to the Caco-2 cells with >20 per 100 Caco-2 cells [~ >2 per 10 Caco-2 cells] (42). Similarly, a study by Chen et al. (2013) (47), showed that all heat-killed L. acidophilus, Enterococcus faecium, Lactobacillus fermentum, and Lactobacillus plantarum reported a high adherent capability at 104 ± 2.82, 131 ± 2.11, 87 ± 1.89 and 125 ± 2.71 per 100 Caco-2 cells, respectively [~ (10.4), (13.1), (8.7) and (12.5) per 10 Caco-2 cells] (47). Both researchers (42, 47), also claimed that the exopolysaccharides and lipoteichoic acid, two non-protein cell-wall components, play a vital role in regulating the adherence of heat-killed LAB cells to intestinal cells and the activation of the host immune system.
Anti-invasion activities of HK-LAB isolates against S. Typhimurium.
The colonization of pathogenic microorganisms can be inhibited by the adhesion of HK probiotic bacteria to the intestinal epithelium which effectively modulates the human immune system (26).
Based on previous studies, pre-incubating Caco-2 cells with heat-killed L. casei and L. paracasei (29) before exposing them to S. enterica dramatically reduces their infection. Pre-incubation with heat-killed probiotics resulted in an 8-fold reduction in Salmonella adherence when compared to the co-incubation trial (4-fold) (29).
According to our findings, the data on HK-LAB adhesion to Caco-2 cells is consistent with the percentage of inhibition against S. Typhimurium invasion. There was no difference between the percentage of inhibition of R (grass) isolates (64.76 ± 9.02%) and the positive control group (63.81 ± 1.15%). Similarly, the highest percentage of inhibition was demonstrated by Tairu isolate (78.1 ± 3.06%). These results imply that the adherent characteristics of the R and Tairu HK-LAB isolates allowed them to prevent S. Typhimurium from invading intestinal Caco-2 cells. This result agrees with the findings by Feng et al. (2016) (48), in which, the highest adhesion abilities of LAB significantly inhibited the adhesion and invasion of Salmonella enteritidis to Caco-2 cells with a percentage inhibition of more than 80% compared to Lactobacillus rhamnosus and L. casei (48).
Findings from this study suggest that non-viable HK-LAB has significant roles in avoiding S. Typhimurium invasion on intestinal cells. Several mechanisms have been reported on how HK-LAB could have protective effects against pathogenic infection. According to Lin et al. (2007), the immunomodulatory function of activated macrophages may be responsible for HK-LAB’s prevention of Salmonella invasion in mouse organs (42). On the other hand, Tian et al. (2023) speculated that certain substances on the cell surface of heat-killed L. casei could effectively reduce the rate of S. Typhimurium internalization into HT-29 cells as well as prevent Salmonella colonization and translocation in mice (22). HK-LAB strains have also been demonstrated to inhibit pathogen adhesion/invasion by preventing colonization through competitive exclusion with cell receptors (29). However, it is also possible that the inhibition of adhesion and invasion is related to the antimicrobial substances produced by HK-LAB (29).
Growing evidence has shown that HK-LAB can reduce the colonization of pathogenic bacteria by improving the integrity of the epithelial barrier. A recent study by Tian et al. (2023) (22) found that the permeability of HT-29 cells increased after S. Typhimurium challenge, while HK-LAB could recover epithelium permeability by up-regulating the level of tight junction’s protein (ZO-1 and Claudin-1) resulted in decreased invasion of S. Typhimurium (22). Pre-treatment with HK-LAB significantly improved the host’s intestinal barrier due to their adherence properties to the host intestinal epithelium and led to the suppression of their adhesion and invasion, as well as prevented intestinal injury induced by the pathogenic bacteria including Salmonella sp. and E. coli (42, 48). Additionally, the combination of the HK-LAB strains in yogurt (L. acidophilus, Streptococcus thermophilus, and Lactobacillus bulgaricus) was also reported to prevent epithelial barrier dysfunction (49).
Antibacterial activities against S. Typhimurium.
S. Typhimurium was listed as “the most threatening to public health” by the Centers for Disease Control and Prevention due to its frequent adulteration of beef and poultry food products and its association with multidrug resistance. Therefore, developing new strategies to inhibit this bacterium is urgent (50). The antibacterial activity of HK-LAB isolates of R and Tairu against S. Typhimurium indicated that both isolates showed a very strong inhibition zone against S. Typhimurium and significantly differed from the positive control LC. These results indicate that anti-invasion activities of HK-LAB R and Tairu can potentially be caused by their ability to kill S. Typhimurium thereby protecting the host from their infection. This activity can be attributed to the bacteriocins and the exopolysaccharides produced by Lactobacillus species which have a good potential in the biocontrol of pathogens (51, 52).
CONCLUSION
This study demonstrated the utilization of HK-LAB isolates in inhibiting S. Typhimurium infections by suppressing their invasion towards the intestinal cell lines (Caco-2). The use of LAB from various sources in this study provides an exceptional understanding of the adhesion and anti-invasion properties against S. Typhimurium. From the data obtained, two predominant HK-LAB isolates with good adhesion on Caco-2 cells could reduce the invasion of S. Typhimurium at a similar rate (R-isolate) and slightly higher (Tairu-isolate) than the positive control (L. casei). A very substantial inhibition zone in antibacterial activity against S. Typhimurium was also present in both isolates. These two isolates (R and Tairu) could potentially be good probiotic candidates. The results of this study may make a significant contribution to the biopharmaceutical industry, particularly in the development of probiotics as biotherapeutics for food-borne diseases that modulate many beneficial functions to the host.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the Ministry of Higher Education of Malaysia and Universiti Teknologi MARA for the financial and research funding support under the FRGS-RACER Grant (RACER/1/2019/STG05/UITM//1).
REFERENCES
- 1.Jajere SM. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet World 2019; 12: 504–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdul-Mutalib NA, Syafinaz AN, Sakai K, Shirai Y. An overview of foodborne illness and food safety in Malaysia. Int Food Res J 2015; 22: 896–901. [Google Scholar]
- 3.Mohan A, Munusamy C, Tan Y-C, Muthuvelu S, Hashim R, Chien S-L, et al. Invasive Salmonella infections among children in Bintulu, Sarawak, Malaysian Borneo: A 6-year retrospective review. BMC Infect Dis 2019; 19: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamaludin MH. (2016). Canteen operators, food suppliers to have contracts terminated if linked to food poisoning cases. from: http://www.nst.com.my/news/2016/08/167226/canteen-operators-food-suppliers-have-contracts-terminated-if-linked-food
- 5.Karim BA, Latip AL, Shukor ASA, Rashid NA, Mohd WMW, Kamaludin F. A large common source outbreak of Salmonella Typhimurium Linked to Kuala Terengganu Night Markets, Malaysia, 2014. OSIR 2017; 10: 1–7. 10.59096/osir.v10i2.263136 [DOI] [Google Scholar]
- 6.Abdullah NBA, Ismail AA. Food poisoning outbreaks among school children in Terengganu and their associated factors. Sains Malays 2021; 50: 1027–1036. [Google Scholar]
- 7.Bernama (2018). Laksa poisoning is caused by Salmonella. New Straits Times. https://www.nst.com.my/news/nation/2018/10/423469/laksa-poisoning-caused-salmonella
- 8.Bernama (2021). Singapore recalls eggs from yet another Malaysian farm. New Straits Times. https://www.nst.com.my/news/nation/2021/03/675438/singapore-recalls-eggs-yet-another-malaysia-farm
- 9.Yusof TA. (2022). Jif peanut butter recalled over Salmonella fears. New Straits Times. https://www.nst.com.my/news/nation/2022/05/800716/jif-peanut-butter-recalled-over-salmonella-fears
- 10.Somorin YM, Odeyemi OA, Ateba CN. Salmonella is the most common foodborne pathogen in African food exports to the European Union: Analysis of the rapid alert system for food and feed (1999–2019). Food Control 2021; 123: 107849. [Google Scholar]
- 11.European Centre for Disease Prevention and Control (2024). Salmonellosis outbreaks. https://www.ecdc.europa.eu/en/infectious-diseases-and-public-health/salmonellosis/threats-and-outbreaks
- 12.Centers for Disease Control and Prevention CDC (2024). Salmonella Infection https://www.cdc.gov/salmonella/index.html
- 13.Krausova G, Hynstova I, Svejsti R, Mrvikova I, Kadlec R. Identification of synbiotics conducive to probiotics adherence to intestinal mucosa using an in vitro Caco-2 and HT29-MTX cell model. Processes 2021; 9: 569. [Google Scholar]
- 14.Sharma S, Kanwar SS. Adherence potential of indigenous lactic acid bacterial isolates obtained from fermented foods of Western Himalayas to intestinal epithelial Caco-2 and HT-29 cell lines. J Food Sci Technol 2017; 54: 3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasbullah NI, Aminah S, Syed Mohamad SA, Iberahim R, Hasan N, Ahmad N, et al. A review on in vitro cell culture model for bacterial adhesion and invasion: From simple monoculture to co-culture human intestinal epithelium model. J Pharm Res Int 2021; 33: 97–106. [Google Scholar]
- 16.Asadi A, Razavi S, Talebi M, Gholami M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019; 47: 13–23. [DOI] [PubMed] [Google Scholar]
- 17.Paula Menezes Barbosa P, Roggia Ruviaro A, Mateus Martins I, Alves Macedo J, LaPointe G, Alves Macedo G. Effect of enzymatic treatment of citrus by-products on bacterial growth, adhesion and cytokine production by Caco-2 cells. Food Funct 2020; 11: 8996–9009. [DOI] [PubMed] [Google Scholar]
- 18.Birhanu BT, Lee EB, Lee SJ, Park SC. Targeting Salmonella Typhimurium invasion and intracellular survival using pyrogallol. Front Microbiol 2021; 12: 631426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mechesso AF, Quah Y, Park S-C. Ginsenoside Rg3 reduces the adhesion, invasion, and intracellular survival of Salmonella enterica serovar Typhimurium. J Ginseng Res 2021; 45: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker P, Sando L, Pearson R, Kongsuwan K, Tellam RL, Smith S. Bovine Muc1 inhibits the binding of enteric bacteria to Caco-2 cells. Glycoconj J 2010; 27: 89–97. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Li J, Yin Y, Guo D, Jin T, Guan N, et al. Antibiofilm activity of coenzyme Q0 against Salmonella Typhimurium and its effect on adhesion-invasion and survival-replication. Appl Microbiol Biotechnol 2019; 103: 8545–8557. [DOI] [PubMed] [Google Scholar]
- 22.Tian L, Zhong C, He Y, Lu Q, Wang Y, Zhao X, et al. Preventive of Lacticcaseibacillus casei WLCA02 against Salmonella Typhimurium infection via strengthening the intestinal barrier and activating the macrophages. J Funct Foods 2023; 104: 105507. [Google Scholar]
- 23.Ngalimat MS, Abd Rahman RNZR, Yusof MT, Amir Hamzah AS, Zawawi N, Sabri S. A review on the association of bacteria with stingless bees. Sains Malays 2020; 49: 1853–1863. [Google Scholar]
- 24.Jang HJ, Song MW, Lee NK, Paik HD. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum ln1 isolated from kimchi. J Food Sci Technol 2018; 55: 3174–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somashekaraiah R, Shruthi B, Deepthi BV, Sreenivasa MY. Probiotic properties of lactic acid bacteria isolated from neera: A naturally fermenting coconut palm nectar. Front Microbiol 2019; 10: 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta R, Jeevaratnam K, Fatima A. Lactic acid bacteria: Probiotic characteristic, selection criteria, and its role in human health (a review). J Emerg Technol Innov Res 2018; 5: 411–424. [Google Scholar]
- 27.Kathiresan K, Thiruneelakandan G. Prospects of lactic acid bacteria of marine origin. Indian J Biotechnol 2008; 7: 170–177. [Google Scholar]
- 28.Gao Z, Daliri EB, Wang J, Liu D, Chen S, Ye X, et al. Inhibitory effect of lactic acid bacteria on foodborne pathogens: A review. J Food Prot 2019; 82: 441–453. [DOI] [PubMed] [Google Scholar]
- 29.Jankowska A, Laubitz D, Antushevich H, Zabielski R, Grzesiuk E. Competition of Lactobacillus paracasei with Salmonella enterica for adhesion to Caco-2 cells. J Biomed Biotechnol 2008; 2008: 357964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katkowska M, Garbacz K, Kusiak A. Probiotics: Should all patients take them? Microorganisms 2021; 9: 2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karbowiak M, Gałek M, Szydłowska A, Zielińska D. The influence of the degree of thermal inactivation of probiotic lactic acid bacteria and their postbiotics on aggregation and adhesion inhibition of selected pathogens. Pathogens 2022; 11: 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tareb R, Bernardeau M, Gueguen M, Vernoux JP. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J Med Microbiol 2013; 62: 637–649. [DOI] [PubMed] [Google Scholar]
- 33.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 2004; 28: 405–440. [DOI] [PubMed] [Google Scholar]
- 34.Aiba Y, Ishikawa H, Tokunaga M, Komatsu Y. Anti-Helicobacter pylori activity of non-living, heat-killed form of Lactobacilli including Lactobacillus johnsonii No.1088. FEMS Microbiol Lett 2017; 364: 10.1093/femsle/fnx102. [DOI] [PubMed] [Google Scholar]
- 35.Wassie M, Wassie T. Isolation and Identification of Lactic Acid Bacteria from Raw Cow Milk. Int J Adv Res Biol Sci 2016; 3: 44–49. [Google Scholar]
- 36.Antunes F, Andrade F, Araújo F, Ferreira D, Sarmento B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur J Pharm Biopharm 2013; 83: 427–435. [DOI] [PubMed] [Google Scholar]
- 37.Campana R, Merli A, Verboni M, Biondo F, Favi G, Duranti A, et al. Synthesis and evaluation of saccharide-based aliphatic and aromatic esters as antimicrobial and antibiofilm agents. Pharmaceuticals (Basel) 2019; 12: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burkholder KM, Fletcher DH, Gileau L, Kandolo A. Lactic acid bacteria decrease Salmonella enterica Javiana virulence and modulate host inflammation during infection of an intestinal epithelial cell line. Pathog Dis 2019; 77: ftz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dostal A, Gagnon M, Chassard C, Zimmermann MB, O’Mahony L, Lacroix C. Salmonella Adhesion, invasion and cellular immune responses are differentially affected by iron concentrations in a combined in vitro gut Fermentation-Cell model. PLoS One 2014; 9(3): e93549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagnon M, Zihler Berner A, Chervet N, Chassard C, Lacroix C. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J Microbiol Methods 2013; 94: 274–279. [DOI] [PubMed] [Google Scholar]
- 41.Chen W-H, Luo G-F, Lei Q, Hong S, Qiu W-X, Liu L-H, et al. Overcoming the heat endurance of tumour cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy. ACS Nano 2017; 11: 1419–1431. [DOI] [PubMed] [Google Scholar]
- 42.Lin W-H, Y-u B, Lin C-K, Hwang W-Z, Tsen H-Y. Immune effect of heat-killed multistrain of Lactobacillus acidophilus against Salmonella Typhimurium invasion to mice. J Appl Microbiol 2007; 102: 22–31. [DOI] [PubMed] [Google Scholar]
- 43.Vasiee A, Falah F, Behbahani BA, Tabatabaee-Yazdi F. Probiotic characterization of Pediococcus strains isolated from Iranian cereal-dairy fermented product: Interaction with pathogenic bacteria and the enteric cell line Caco-2. J Biosci Bioeng 2020; 130: 471–479. [DOI] [PubMed] [Google Scholar]
- 44.Reuben RC, Roy PC, Sarkar SL, Alam RU, Jahid IK. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol 2019; 19: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmad A, Yap WB, Kofli NT, Ghazali AR. Probiotic potentials of Lactobacillus plantarum isolated from fermented durian (Tempoyak), a Malaysian traditional condiment. Food Sci Nutr 2018; 6: 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mokoena MP, Omatola CA, Olaniran AO. Applications of lactic acid bacteria and their bacteriocins against food spoilage microorganisms and foodborne pathogens. Molecules 2021; 26: 7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C-Y, Tsen H-Y, Lin C-L, Lin C-K, Chuang L-T, Chen C-S, et al. Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. J Med Microbiol 2013; 62: 1657–1664. [DOI] [PubMed] [Google Scholar]
- 48.Feng J, Wang L, Zhou L, Yang X, Zhao X. Using in vitro immunomodulatory properties of lactic acid bacteria for selection of probiotics against Salmonella infection in broiler chicks. PLoS One 2016; 11(1): e0147630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng J, Jiang J, Zhu W, Chu Y. Heat-killed yogurt-containing lactic acid bacteria prevent cytokine-induced barrier disruption in human intestinal Caco-2 cells. Ann Microbiol 2016; 66: 171–178. [Google Scholar]
- 50.Tan W, Tian Y, Zhang Q, Miao S, Wu W, Miao X, et al. Antioxidant and antibacterial activity of Apis laboriosa honey against Salmonella enterica serovar Typhimurium. Front Nutr 2023; 10: 1181492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atta H, Gimba A, Bamgbose T. Antimicrobial activity of lactic acid bacteria isolated from garri on Escherichia coli strains isolated from clinical and environmental samples. Agric Sci Technol 2020; 12: 357–365. [Google Scholar]
- 52.Abdalla AK, Ayyash MM, Olaimat AN, Osaili TM, Al-Nabulsi AA, Shah NP, et al. Exopolysaccharides as antimicrobial agents: Mechanism and spectrum of activity. Front Microbiol 2021; 12: 664395. [DOI] [PMC free article] [PubMed] [Google Scholar]