Abstract
By virtue of applying small tidal volumes, high-frequency ventilation is advocated as a method of minimizing ventilator-induced lung injury. Lung protective benefits are established in infants, but not in other patient cohorts. Efforts to improve and extend the lung protection potential should consider how fundamental modes of gas transport can be exploited to minimize harmful tidal volumes while maintaining or improving ventilation.
This research investigates different models of gas transport during high-frequency ventilation and discusses the extent to which the gas transport mechanisms are considered in each. The research focuses on the rationale for current ventilation protocols, how they were informed by these models, and investigates alternative protocols that may improve gas transport and lung protection. A review of high-frequency ventilation physiology and fluid mechanics literature was performed, and dimensional analyses were conducted showing the relationship between clinical data and the model outputs. We show that contemporary protocols have been informed by resistor-inductor-capacitor, or network, models of the airway-lung system that are formulated around a ventilation pressure cost framework. This framework leads to clinical protocol selection that ventilates patients at frequencies that excite a resonance in the lung. We extend on these models by considering frequencies that are much higher than resonance which further optimize gas transport in the airway via alternative gas transport mechanisms to bulk advection that operate for very low tidal volumes. Our findings suggest it is unlikely that gas transport is optimally exploited during current approaches to high-frequency ventilation and protocols that differ significantly from those currently in use could achieve ventilation while using very low tidal volumes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-03049-w.
Keywords: High-frequency ventilation, High-frequency oscillatory ventilation, Mechanical ventilation, Nonlinear mean streaming
Background
The focus of this manuscript relates to the gas transport mechanisms that are likely available during clinical high-frequency ventilation (HFV). Of course, gas transport is not the only consideration for successfully administering HFV. Regardless, this manuscript aims to highlight, via a review of the literature and then applying the concepts identified while considering patient and airway dimensions, that current HFV protocols may not optimally exploit the gas transport mechanisms that operate in the airway.
During conventional ventilation, gas transport can occur via bulk advection, as the lungs are at least partially emptied and refilled each inflation cycle [1]. This relies on the tidal volume, , being larger than the airway dead space volume, . High-frequency oscillatory ventilation, in particular, purports to use an alternative set of gas transport mechanisms that operate even when the tidal volume is less than the dead space (see the online supplementary Table 1 for a summary of these mechanisms: https://github.com/leontini/gas_transport_supplement.git). These include nonlinear mean streaming (asymmetric velocity profiles), turbulent diffusion, Taylor dispersion, Pendelluft, molecular diffusion [2] and even cardiogenic mixing [1]. The ability to support gas exchange with low tidal volumes is the rationale behind HFV being potentially lung protective, although above have been reported during HFV [3]. The use of high frequencies and associated low tidal volumes aims to avoid high volume and pressure excursions that may lead to volutrauma. When coupled with a high and constant mean airway pressure it may also avoid atelectrauma [4].
While the alternative gas transport mechanisms outlined by Chang [2] are well-accepted, the exploitation of these mechanisms is not explicitly considered in the selection of current ventilator protocols. HFV has been successfully applied in the treatment of infants [5] with primary respiratory failure for decades, but it is less successful in large trials in the treatment of adults, suggesting a change in the efficacy of gas transport that is dependent on the size of the patient. A meta-analysis by Maitra et al. [6] including 1,759 patients from seven randomized controlled trials suggested variable outcomes using HFV in adults and highlighted a lack of a standardized strategy for setting ventilator parameters including the oscillatory frequency [6]. HFV did not reduce mortality compared to conventional ventilation [6]. Interpretation of these results is complicated and suggests that the optimal approach to delivering HFV currently eludes clinicians.
HFV frequency is generally set based upon the underlying lung mechanics and patient size. Higher frequencies are used for smaller patients and less compliant lungs, with lower frequencies in more resistive states and larger patients [7]. For example, frequencies of 12–15 Hz are often used in preterm infants with surfactant deficient respiratory distress syndrome (RDS), and frequencies of 3–6 Hz in adults with acute respiratory distress syndrome. This range of frequencies typically align with what is predicted as optimal from network, or resistor-inductor-capacitor (RLC-type) models, as proposed in the work of Venegas and Fredberg [8]. These empirical models implicitly consider the contribution of the alternative mechanisms to ventilation as a global sum and model the fluid mechanics down to a scale of the airway diameter. I.e., they do not capture the complex flows through the airway. More recent advanced versions of RLC-type models have attempted to include, explicitly, the contributions of some of these alternative mechanisms [9–11] with some success. For example, the onset of turbulence and the subsequent effects of diffusion [9] have been included empirically.
We consider the important transition between the conventional and high-frequency ventilation domains identified in the work detailed in [8] and [12] and show how this modelling leads to a choice of frequency that targets the resonance, or corner, frequency of the lungs. We extend on this work, by consideration of patient and airway dimensions, and show that lung resonance or corner frequencies are unlikely to be optimal for gas transport in the airway using the alternative mechanisms proposed by Chang [2]. We suggest that a second optimum exists at higher frequencies than currently employed and point to recent computational modelling efforts that demonstrate ways in which these alternative mechanisms may be targeted to exploit this second optimum.
Methods
This research commenced with a review of contemporary HFV protocols to understand the modelling that informs current clinical practice. The review was initiated using the search terms:
“High-frequency oscillatory ventilation `OR' high-frequency ventilation `AND' ventilator protocol `OR' ventilator settings”
where ten, predominantly review-style, papers were selected as the starting point. Where ventilation protocols were detailed, and referenced, the listed study was obtained repeating the process until the origin of the protocol or some rationale was reached. Based on the review findings, we investigated the modelling approach that informs current clinical practice including the parameters used to construct them and their outputs, in particular the frequency of ventilation. We compared the model outputs with the protocols used in randomized controlled trials, including both neonatal and adult cohorts, to show alignment between modelling and clinical practice. We then performed dimensional analyses of the airway-lung system to show there are at least two characteristic frequencies to consider. The first is associated with the resonance or corner frequency of the lungs and the second is associated with the gas transport in the airway which influences the alternative mechanisms detailed by Chang [2]. Finally, we review recent numerical and experimental models that investigate the alternative gas transport mechanisms that suggest higher ventilation frequencies are required to exploit them effectively and consider these higher frequencies in the framework that currently informs clinical practice.
Results
Outcomes from RLC-type models
RLC-type models of the airway-lung system have been used extensively to understand the dynamics during HFV. Early results were reviewed by Drazen et al. [13], with a large contribution to the contemporary understanding stemming from the work of Venegas et al. [12] and Venegas and Fredberg [8]. The seminal work of Venegas and Fredberg [8] analyzed the problem of “achieving adequate alveolar ventilation at minimal pressure cost” [8] by formulating two simpler problems to “assess the total pressure cost of high-frequency ventilation” [8]. Their work proposed that the total pressure cost of ventilation is the product of the flow cost of achieving ventilation, and the pressure cost of achieving flow [8].
The non-dimensional flow cost of achieving ventilation was expressed as
| 1 |
where f is the frequency of ventilation and is the rate of alveolar ventilation, and related to a single nondimensional frequency parameter
| 2 |
In the conventional ventilation domain, the functional relationship between these two nondimensional parameters was expressed as
| 3 |
which is derived from the expression for conventional alveolar ventilation
| 4 |
where we recall that is the tidal volume and is the dead space volume. In the HFV domain, the non-dimensional flow cost of achieving ventilation was established from an empirical relationship, fitted to data from various species, expressed as
| 5 |
For the case of the high-frequency flow cost of achieving ventilation, (Q), the expression for alveolar ventilation, detailed in Eq. (4) is not applicable due to the requirement of the tidal volume to be larger than the dead space volume for ventilation to occur. Although an expression is not explicitly defined in [8] it is described as a ratio of output to alveolar concentration in the steady state [12].
The pressure cost of achieving flow is equal to the airway-lung system impedance, which is the pressure gradient required to generate a given tidal volume. The RLC-type model of the airway-lung system in [8] is constructed from electrical hardware components to predict the pressure distending the airway. Resistive elements of the model capture the viscous pressure losses; inductors model the impacts associated with inertial loads; and capacitors model the impacts due to the elastic loads. The pressure and oscillatory flows in the airway are analogous, in this model, to the voltage and alternating current respectively [8]. The pressure cost of achieving flow is minimized when the frequency of ventilation is equal to the natural frequency of the airway-lung system, i.e., the system is resonating. However, this is only the case for underdamped airway-lung systems and in many scenarios the system is overdamped in which case the corner frequency becomes the point in the system up to which the largest reductions in the pressure cost are observed [8]. For the overdamped system the corner frequency is less than the natural frequency.
Some important points arise from this modelling. A transition point is identified between the conventional and the high-frequency domains where a reduction in the rate of increase of the flow cost of achieving ventilation with increasing frequency is apparent. In the conventional ventilation domain, the flow cost of achieving ventilation (Eq. 3) scales linearly with the non-dimensional frequency, . After the transition, the flow cost of achieving ventilation in the high-frequency ventilation domain (Eq. 5) scales with the non-dimensional frequency raised to the power of suggesting an increase in ventilation efficiency (compared to what would be expected if the trend from conventional ventilation continued). It should be noted, however, the flow cost of achieving ventilation is predicted by this model to continually increase with increasing frequency, and therefore there is little apparent benefit to the overall cost from manipulating the frequency. The pressure cost of achieving flow, for high-frequency gas exchange, is optimized when the frequency of ventilation is set at, or near, the natural or corner frequency of the airway-lung system and it is the reduction in the pressure cost of ventilation that is the focus when the overall pressure cost of flow is considered.
Alignment of currently used frequencies with those suggested by RLC-type models
An output of this modelling, i.e., the suggestion to exploit resonance, aligns with the current selection of frequencies used during HFV for the treatment of both infants and adults. For infants, resonance frequencies are predicted in the range of by Venegas and Fredberg [8], in line with the range of reported by Weber et al. [14] and reported by Lee et al. [15]. The online supplementary Table 2 (https://github.com/leontini/gas_transport_supplement.git) summarizes ventilation protocols used in trials comparing first-intention HFV to conventional ventilation for term (reproduced from [16]) and preterm infants (reproduced from [17]) with severe pulmonary dysfunction. All the studies listed use frequencies in the range of , with the exception of one study [18] where frequencies were as high as , and all were within the reported range of resonance frequencies. It is notable that a number of the studies set the pressure amplitude via visible movement, jiggle, or bouncing of the chest wall, a crude approach that may encourage inadvertent high tidal volume. Several other studies recommend, or report, frequencies in the resonance range [4, 5, 19, 20], and recent work discuss or support the concept of exploiting resonance or corner frequencies [21–26].
A similar situation is apparent when considering HFV in adults, where reported resonance frequencies are as low as [11], or in the range [27]. Online supplementary Table 3 (https://github.com/leontini/gas_transport_supplement.git) reports the ventilation protocols used in trials of HFV for the treatment of adults with acute respiratory distress syndrome (ARDS), reproduced from [6]. All except one study use a frequency in the range of . Similar to the studies involving infants, the frequencies used in the treatment of adults align with the reported resonance frequency.
In both infant and adult cohorts, there is an effort to use frequencies that target an assumed resonance frequency. Despite this apparent consistency in approach, there is a difference in clinical benefit. While the difference in benefit across infant and adult cohorts is likely due to various factors, we propose that one important aspect for consideration is the impact of the ventilation frequency on the efficacy of alternative gas transport mechanisms in the airway. In the following section, we show that currently selected frequencies do not target these alternative mechanisms of gas transport, and therefore gas transport through the airway is likely to be sub-optimal in all patient cohorts.
Dimensional analysis suggests airway details should be accounted for
Scaling to incorporate the flow cost of ventilation and the pressure cost of flow into a single dimensionless relationship
Equation (7) provides a way to scale parameters from one patient (or patient cohort) to another. If the ratios on the right-hand side are kept the same between two cases, then the scaled ventilation on the left-hand side is also expected to be the same. If the flows in the two cases transport gas in the same way, the values of the groups on the right-hand side of Eq. (7) that result in optimal ventilation in one case should also lead to optimal ventilation in the second case.
Dimensional analysis is a method for systematically finding the minimum set of dimensionless variables that need to be considered in a given dynamic system [28, 29]. For the airway-lung system, this is achieved by considering the dimensional variables of the system, and characteristic mass, length, and time scales. For the current RLC-type models, the simplest dimensional relationship between the rate of alveolar ventilation, patient physiology, and ventilator protocol can be written as
| 6 |
where is a function to be determined, is the natural, or resonance frequency, is a length characterizing the airway-lung system, and is a mass characterizing the inertia of the system. This can be reduced to the dimensionless relationship
| 7 |
which suggests ventilation is a function of only two dimensionless groups (a dimensionless group being a ratio of parameters that have the same dimensions or units). The first group on the right-hand side of this relationship is the ratio of the frequency of ventilation to the natural, or resonance frequency, and the second group is the ratio of the applied pressure amplitude to the elastic stress in the lungs. This nondimensionalization uses the inverse of the natural frequency as a consistent time scale in all parameters, and as the length scale. Doing so ensures that the ventilation frequency appears in only one group. Details of the dimensional analyses are provided in the online supplement (https://github.com/leontini/gas_transport_supplement.git).
The expression in Eq. (7) naturally incorporates the impact of frequency and pressure and can be related to the concept of the pressure cost of achieving flow proposed in [8]. Equation (7) can equally be rearranged to find an expression for the required pressure as
| 8 |
If the dead space volume is used to define the representative length scale, , then the quotient of the two parameters on the right-hand side recovers the nondimensional frequency, of [8] from Eq. (2). I.e.,
| 9 |
Combining the two parameters suggests that the pressure amplitude is only a function of the parameter , i.e.,
| 10 |
Equations (3) and (5) (from [12]) define the dimensionless flow cost of ventilation parameter, , as a function of the same dimensionless frequency, . Therefore, it could be taken that the relationship between pressure and is an implicit one moderated by , i.e.,
| 11 |
Written this way, the flow cost of achieving ventilation and the pressure cost of achieving flow are incorporated into a single non-dimensional relationship. It makes it clear that for a given flow cost of ventilation, , the only parameter to minimize is , and this is likely to occur when using a frequency near the resonance frequency (i.e., ). However, in order to arrive at this relationship, the two independent dimensionless parameters on the right-hand side of Eq. (8) need to be combined.
The section titled: Alignment of currently used frequencies with those suggested by RLC-type models outlines that in most studies across infant and adult cohorts. There is an effort to maintain this dimensional similitude, suggesting parameters are scaled between the two cohorts in the way suggested by Eq. (11).
However, we highlight two points:
this final relationship for a non-dimensional pressure cost of achieving flow is built by combining two, potentially independent, parameters into a single parameter, ; and,
the relationship outlined in Eq. (8) contains no parameters related to the airway resistance or the fluid properties of air, that will have a significant impact on the pressure loss and gas transport properties.
Scaling to explicitly consider the impact of the flow variation in an airway vessel and the properties of air
Adding consideration of an additional parameter, the kinematic viscosity , to address the lack of airway flow resistance leads to the dimensional relationship
| 12 |
which, when reduced, produces the dimensionless relationship
| 13 |
noting that a third group appears which represents the ratio of the fluid inertia to the viscous force (the Reynolds number) and is directly proportional to the oft-used Womersley parameter [30] (noting that here this parameter is a characteristic of the entire airway-lung system – a Womersley parameter can be defined locally for each airway vessel). To arrive at this group, the relationship has been nondimensionalized, again, using the inverse of the natural frequency as the consistent time scale, and then the product of two parameters multiplied, producing the square of the Womersley parameter, .
When the square of the Womersley parameter is large the flow inertia is high and so the effects of viscosity are relatively small. Viscosity tends to “smear out” small-scale flow features such as eddies and vortices (here, “small-scale” means smaller than the diameter of an airway vessel). Turbulent flows, by nature, consist of small-scale features. Therefore, without the smearing effect of viscosity, turbulence is most likely in flows where the Womersley parameter is large.
Conversely, when the square of the Womersley parameter is small the flow inertia is low and so the effects of viscosity are relatively large. In this case, the flow is laminar (free of small-scale features), and turbulence cannot occur. The square of the Womersley parameter, therefore, relates to the “shape” of the flow at a scale below the size of the airway diameter. The shape of the flow influences the rate of gas transport by alternative mechanisms including nonlinear mean streaming and turbulent diffusion. So, explicit consideration of the square of the Womersley parameter is necessary if gas transport by alternative mechanisms is to be targeted.
Importantly, the square of the Womersley parameter cannot be controlled while maintaining across patient cohorts, due to its dependence on the frequency of ventilation. There are at least three dimensionless groups and only two can be matched by controlling the ventilator settings of and .
This highlights there are at least two characteristic time scales to consider:
the inverse of the natural or resonance frequency which is primarily associated with the inertia and elasticity of the lungs; and,
a viscous time scale , which is primarily associated with details of the gas flow in the airway.
Current practice targets the resonance frequency at the expense of the viscous time scale. However, in doing so, this neglects explicit consideration of the alternative transport mechanisms that depend on the viscous time scale and details of the flow in the airway.
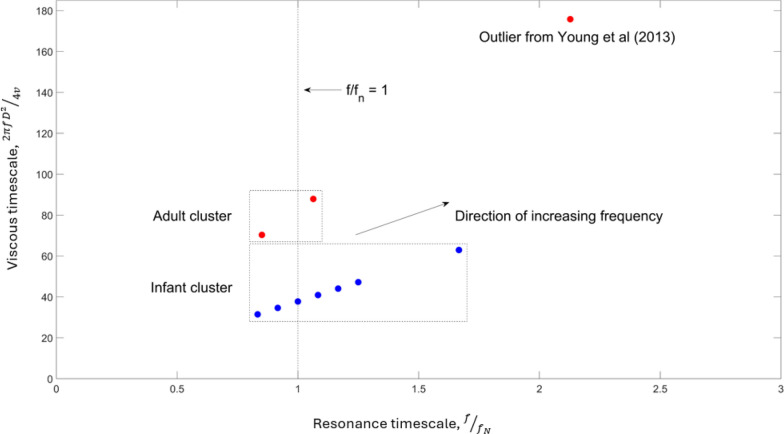
The current frequency parameters used in clinical practice for the treatment of infant and adult patients, detailed in the online supplementary Tables 2 and 3, are represented in terms of the dimensionless groups introduced in Eq. (13) and are plotted in Fig. 1. The parameters, in both the infant and adult cohorts, cluster around (with the exception of the outlier from the study in [31]) as outlined in the section titled: Alignment of currently used frequencies with those suggested by RLC-type models. Importantly, the plot illustrates that this similarity between infant and adult cohorts is not maintained with respect to the square of the Womersley parameter involving the viscous time scale . The trachea diameter has been used as the characteristic length with values taken from the data reported in [32]; for infants a value of and for adults a value of [32]. The values used for the resonance frequency of the infant and adult lungs are [14] and [11] respectively. For the data plotted on the viscous timescale in Fig. 1, we have applied a factor of to maintain similarity with the definition of the square of the Womersley parameter used in other studies that are reviewed in this manuscript.
Fig. 1.
Current infant and adult frequencies reported in clinical trials plotted with respect to the viscous and resonance time scale parameters. Infant data is represented as blue markers. Adult data is represented as red markers. For the viscous time scale a trachea diameter of for infants and for adults was used. For the resonance time scale a resonance frequency of for infants and for adults was used. Oscillation frequencies for infants and adults are from the studies detailed in the online supplementary Tables 2 and 3 respectively
Consideration of ventilation efficiency in terms of the viscous timescale
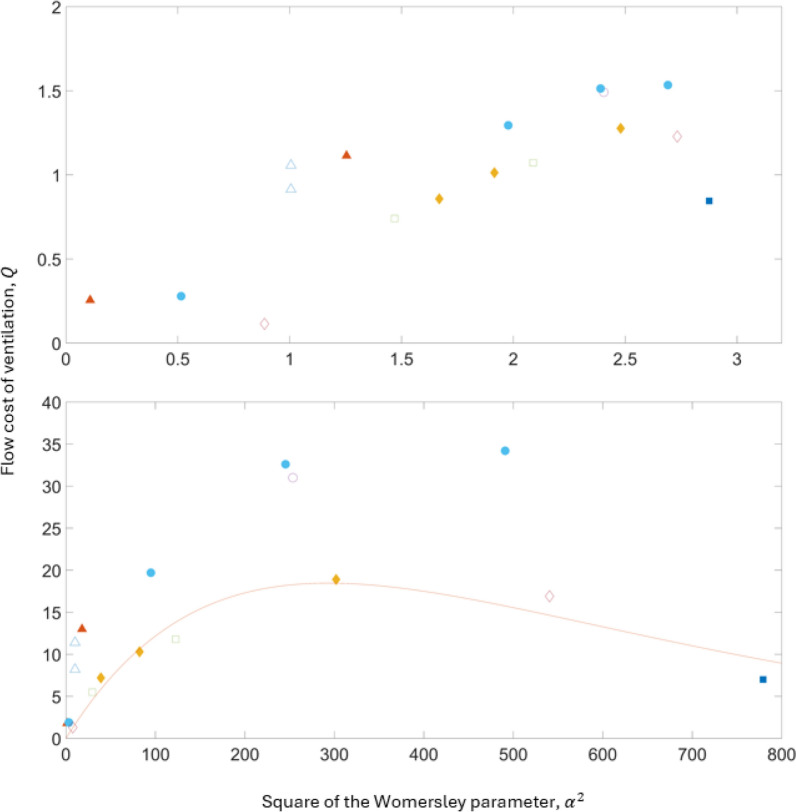
In Fig. 2 we re-plot the original data pertaining to the flow cost of achieving ventilation, , from [8] and [12] as a function of the square of the Womersley parameter (α2), rather than the non-dimensional frequency and we use the trachea diameter as the length scale, , sourced from the literature for the various species in place of . The plots in Fig. 2 (log scale at the top and linear scale at the bottom) show the flow cost of achieving ventilation starting to flatten before turning over and eventually reducing as the square of the Womersley parameter increases suggesting an increase in ventilation efficiency at much higher Womersley parameters than those typically used in clinical practice. In the linear plot, we have fitted a curve to the “lower branch” using a least squares method expressing the flow cost of ventilation, , as a function of the square of the Womersley parameter, . I.e.,
| 14 |
Fig. 2.
The flow cost of achieving ventilation, , plotted as a function of the square of the Womersley parameter, . The flow cost of achieving ventilation has been reproduced from studies [8] and [12]. The plot on the top is log scale and the plot on the bottom is linear scale. The marker shapes representing each species in both plots are the same shape as the original data. Triangles: rats, filled triangles: rabbits, filled diamonds: monkeys, circles: dogs, squares: dogs, filled circles: dogs, diamonds: humans, and filled squares: horses. We have used different colors here for visibility. The length scale for the Womersley parameter is based on the trachea diameter for each species sourced from the literature which are detailed in the online supplementary Table 4 (https://github.com/leontini/gas_transport_supplement.git). The square of the Womersley parameter has been calculated from the expression,
From the expression for in Eq. 14, if the kinematic viscosity, tracheal diameter and required rate of ventilation is known, a tidal volume can be established from the expression. For a specified the required frequency can be determined. After establishing from Eq. 14, a tidal volume can be calculated from the expression for in Eq. 1. This illustrates, for a specific clinical scenario, how the tidal volume is likely to scale with increasing the frequency (and subsequently ). This process is detailed in the online supplement.
It is these much higher frequencies displayed in Fig. 2 relating to large Womersley parameters , which pertain to the viscous time scale, that are required to exploit the alternative gas transport mechanisms reported by Chang [2]. At present, the Womersley parameter (α2) is not prioritized. If these are to be exploited, it is this parameter that needs to be considered, and this can only be done by considering frequencies other than the resonance or corner frequency.
Using dimensional analysis to scale between patient cohorts
Using the technique detailed in the section titled: Dimensional analysis suggests airway details should be accounted for to scale the frequency of ventilation from infant to adult patients based on the resonance time scale requires matching the first group from Eq. (13). I.e.,
where subscripts and represent the adult and infant cases respectively, is the frequency of ventilation and is the natural frequency. Rearranging to solve for gives
and substituting approximately the median value from current infant frequency protocol, , and values for adult and infant resonance frequencies, and , gives a dynamically similar adult ventilation frequency of , which aligns with current adult protocol detailed in Table 3 (https://github.com/leontini/gas_transport_supplement.git) and Fig. 1. However, scaling from infant to adult patients using the viscous time scale requires matching the third group from Eq. (13) which is the ratio of the fluid inertia to the viscous force. I.e.,
where is the trachea diameter and is the kinematic viscosity of air. Rearranging to solve for gives
and substituting the median value from current infant frequency protocol, , trachea diameters of and for the infant and adult respectively, noting that the kinematic viscosity cancels, gives a dynamically similar adult frequency of .
When matching the frequencies of ventilation between infants and adults using the viscous time scale using current infant protocols, the dynamically similar adult frequency is actually lower than current adult protocol. However, considering the findings reported in this manuscript, current infant frequency protocol is likely too low to leverage the alternative gas transport mechanisms available to the viscous time scale. If an optimized frequency for an infant is used as the starting point for scaling, both the infant and adult frequencies will be considerably higher than current clinical practice.
Discussion
Does a focus on resonance miss an opportunity for lung protection?
Previous sections have outlined that current ventilator protocols are guided by an effort to ventilate at the resonant or corner frequency of the airway-lung system, in order to minimize the pressure cost of achieving flow, which, implicitly leads to increasing tidal volume to improve or maintain ventilation. Large tidal volumes however increase the risk of both volutrauma and atelectrauma and a number of studies report during HFV [5, 33] suggesting that the alternative transport mechanisms listed by Chang [2] are important. Therefore, a strategy targeting resonance may effectively ventilate, but it may not be the strategy that adequately ventilates and optimally reduces the risk of ventilator-induced lung injury. The dimensional analyses detailed in the section titled: Dimensional analysis suggests airway details should be accounted for shows that alternative transport mechanisms are not currently targeted, and if they are to be, other ventilation frequencies are required. Here, we review recent studies that suggest what these frequencies might be.
Recent modelling suggests alternative transport mechanisms are optimized at higher frequencies
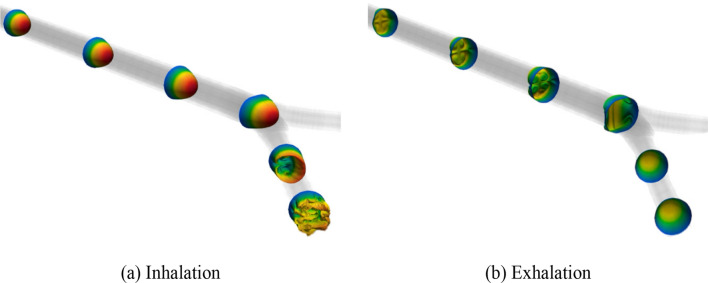
The alternative mechanisms detailed in the section titled: Background rely on details of the flow of gas inside the airway, and so their characterization requires high-resolution computation or experimental measurements. Figure 3 shows the complex flow structure and turbulence that is generated through a simple bifurcation during inhalation and exhalation at conditions modelling the flow during HFV at the trachea of a full-term infant. These images were generated using a high-resolution computational fluid dynamics (CFD) model [34] that ran on high-performance computing facilities.
Fig. 3.
Turbulence during the inhalation and exhalation phases of high-frequency ventilation as gas moves through a single airway bifurcation from the study in [34] which current models are unable to predict
A CFD study in [35] has shown that all the identified alternative mechanisms can operate during HFV in different regions of the airway. Other CFD studies [34, 36] suggest that for current ventilator settings used for infants, nonlinear mean streaming alone can provide a recirculating flow rate adequate to provide oxygen in at least the first five generations of the airway [34, 36]. The operation of these mechanisms during HFV is also supported by recent experimental work [37–39] in phantom models which allow high-resolution measurements of the entire three-dimensional flow field in airway sections.
The studies detailed in [11, 40] investigated higher frequencies up to 19 Hz and 40 Hz, whereas the study of Wanigasekara and co-workers [41], although the upper limit is not currently clinically practical, reported frequencies up to sixteen times greater than those currently used in clinical practice. The studies [40, 41] report that gas transport can be maintained with increased frequencies, and proportionally smaller tidal volumes, and therefore subsequent reduction in the fluctuation of cyclic tissue strain.
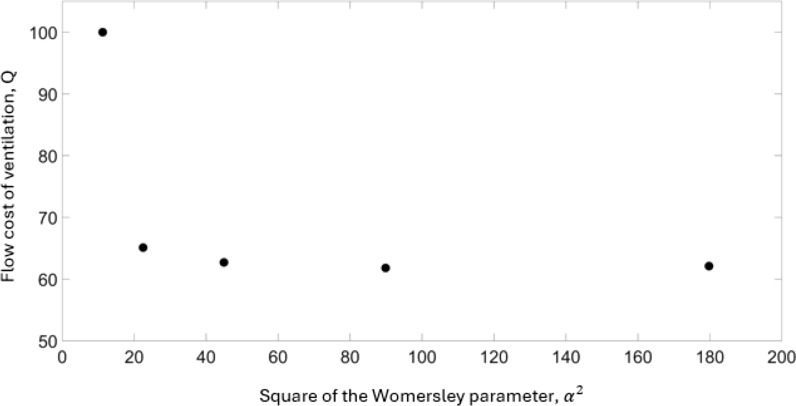
In Fig. 4 we show the results from [41] in the same parameters used for the physiological measurement data displayed in Fig. 2. Again, the flow cost of achieving ventilation is plotted as a function of the square of the Womersley parameter in the very high frequency domain. Here we consider ventilation as the strength of the nonlinear mean streaming. An important finding from the work of Venegas et al. [12] and Venegas and Fredberg [8] was the observation of a transition point, discussed here in the section titled: Outcomes from RLC-type models. This transition point between the conventional and high-frequency domains indicated that the flow cost of achieving ventilation in the high-frequency domain scaled differently to that of the conventional domain, with respect to the frequency, suggesting a change in the mechanisms of gas transport. The work of [41] explored significantly higher frequency protocols than that of contemporary modelling and clinical practice and Figs. 2 and 4 suggest that there is a second transition at much higher Womersley parameters.
Fig. 4.
The flow cost of achieving ventilation, , plotted as a function of the square of the Womersley parameter, , from the data in the study from Wanigasekara and co-workers [41]. The length scale for the Womersley parameter is based on a trachea diameter of 3 mm
The modelling detailed in [8] doesn’t explicitly consider the contributions from individual gas transport mechanisms, but rather considers a global contribution to the overall gas transport. Figure 4, reproduced from the work in [41], considers explicitly one alternative gas transport mechanism operating in the airway, namely nonlinear mean streaming. The first data point () in Fig. 4 corresponds to values close to the current clinical protocol for the treatment of infants (). Figure 4 shows that when the square of the Womersley parameter is doubled () there is a significant increase in the efficiency of ventilation. These results from [41] displayed using the flow cost of achieving ventilation framework proposed in [8] support the observation, detailed in Fig. 2, that there is potentially another optimum that is significantly removed from the current implementation of clinical HFV when the viscous time scale is targeted.
Although it is not clear from these studies exactly what the optimal frequency of ventilation is, it is significantly higher, and likely at least two to four times greater than that of current practice in the treatment of infants (that is or greater). In adults this would suggest that frequencies may be optimal. In the framework proposed in [8], the pressure cost of achieving flow for very high frequency modes would be large due to the high impedance when oscillating the airway-lung system far from resonance. However, this implies a rapid pressure drop with progression through the airway, so that a large pressure cost of flow does not necessarily suggest an increase in intra-lung pressure precluding the use of these modes of ventilation. The study detailed in [42] investigated the effects of frequency selection on the pressure cost of ventilation during high-frequency oscillatory ventilation in preterm infants. With the carbon dioxide diffusion coefficient held constant, they reported higher frequencies resulted in lower pressure amplitudes at the alveolar compartment [42]. Under these conditions, the study in [42] found the lowest pressure cost of ventilation did not coincide with the resonant frequency. The study detailed in [43] measured the pressure transmission through an artificial airway model for increasing ventilation frequencies. They found the pressure measured at the distal lung compartments reduced with frequency over the range tested [43]. Furthermore, the study detailed in [44] based on a computational canine airway network suggested that a frequency bandwidth may exist pertaining to optimal conditions for high-frequency oscillatory ventilation. The range, potentially, occurring between the resonant and anti-resonant frequencies, defined as “according to the local minimum and maximum impedance magnitudes” [44, 45].
Other considerations for ventilation at very high frequencies
The high-fidelity computational and experimental models discussed here relate to the optimal frequencies for gas transport through the airways. Our study reports that in order to fully leverage these alternative gas transport mechanisms, much higher frequencies than current clinical protocols may be useful. Here, we consider other factors that will need to be considered for these higher frequencies to be used in the clinic.
Ventilating at higher frequencies implies an increase in pressure amplitude () at the ventilator is required which could expose the proximal airways to higher pressure swings, in comparison to what occurs under current clinical settings. However, the study in [41] reports the pressure drop between successive airway generations (for e.g., between generation 1 and generation 2, between generation 2 and generation 3 etc.) increases with increasing the frequency. Therefore, even though a higher driving pressure at the trachea is required for ventilating at higher frequencies, similar to the findings in [42, 43], this does not necessarily translate to higher intra-lung pressures. The findings reported in these numerical [41, 42] and artificial [43] models used to understand how pressure distends the airways are likely to be good approximations of the human respiratory system; however, these models are idealized and do not consider all complex dynamics of the respiratory system and need to be applied with care.
Other considerations on how gas transport in distal regions of injured, and heterogenous, lungs might be affected when ventilating at very high frequencies, discussed in [9], should also be taken into account.
Furthermore, the optimal frequencies required for gas transport discussed in this study are currently beyond the limits of contemporary ventilator technology. However, the experimental and numerical studies reviewed in this manuscript indicate that significant improvements in gas transport due to alternative mechanisms are still available that are within the operating ranges of currently available technology.
Despite the high-fidelity respiratory gas transport models and dimensional analyses discussed here, it is important to note that these are not a complete physiological representation of the respiratory system and cannot capture all the physics and physiological variation. There are patient- and cohort-specific features such as airway branching angles and relative size, parenchymal tissue properties and heterogeneity, the addition of surfactant, air leak, etc., that will affect the details of the flow in the airway which will impact on gas transport. However, the dimensional analyses presented in this study, besides the parameters which are encapsulated in the Womersley parameter (a single measure of size , the frequency , and the kinematic viscosity ), consider other independent dimensionless groups and which incorporate the pressure fluctuation, the inertia of the airway-lung system, and the lung elasticity via the natural frequency. With the rescaling presented in Fig. 2, and the consistency of its suggestion of using higher frequencies with the simulation data and recent clinical and physiological studies, it suggests that the dimensional analysis has captured the most important variables relating to clinical high-frequency ventilation.
Importantly, the clinically set frequency needs to carefully consider all aspects of the respiratory system, including effective alveolar gas exchange, lung protection, cardio-pulmonary interactions and the imposed work of breathing, as well as technological limitations. Some of these aspects may require a lower frequency than what is optimal for gas transport and a balance will need to be considered when ventilating at very high frequencies of > in infants and > in adults.
Conclusion
This review has highlighted that across infant and adult cohorts, high-frequency ventilation protocols use frequencies of ventilation that target resonance that implicitly increase tidal volumes. While the concurrence of ventilation and resonance frequency is maintained, we have shown that frequencies relevant to the gas transport via alternative mechanisms to bulk advection are not specifically targeted. Recent models have shown that these mechanisms may be available at higher frequencies, and subsequently lower tidal volumes, than those currently used. This suggests that there may be ventilation strategies which better optimize for ventilation and lung protection that are yet to be fully explored. While the proposed optimal frequencies may not be fully achievable with currently available ventilators, this review provides some insight for consideration into the limitations of the strategy in its current implementation. Furthermore, this study recommends investigating higher frequencies that are within current ventilator operating ranges and considering the findings of this work as a guide in the development of future ventilator technology and subsequent ventilation strategies.
Supplementary Information
Acknowledgements
TJAS acknowledges the Australian Government and Swinburne University of Technology for the provision of a Research Training Program Scholarship. JSL and CJ acknowledge the support of resources and services from the National Computational Infrastructure (NCI) via competitive grant IZ4 which is supported by the Australian Government and the OzSTAR national facility at Swinburne University of Technology which is funded by Swinburne University of Technology. Two images in this manuscript were obtained from work that was performed on the OzSTAR national facility at Swinburne University of Technology. The OzSTAR program receives funding in part from the Astronomy National Collaborative Research Infrastructure Strategy (NCRIS) allocation provided by the Australian Government, and from the Victorian Higher Education State Investment Fund (VHESIF) provided by the Victorian Government. DGT is supported by a National Health and Medical Research Council Leadership Level 1 Investigator Grant (Grant ID 2008212) and the Victorian Government Operational Infrastructure Support Program (Melbourne, Australia).
Author contributions
TJAS, JSL and DGT developed the concept. TJAS and JSL wrote the first draft. DGT provided clinician feedback on the draft manuscript. CJ provided images in the section “Recent modelling that suggests alternative transport mechanisms are optimized at higher frequencies”. All authors contributed to the review and editing of the manuscript.
Funding
DGT is supported by a National Health and Medical Research Council Leadership Level 1 Investigator Grant (Grant ID 2008212) and the Victorian Government Operational Infrastructure Support Program (Melbourne, Australia).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
DGT has received support from Getinge Healthcare (Sweden) for DSMB membership, and development of educational presentations unrelated to this work from Getinge Healthcare and SLE UK Ltd. The authors have no other competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Standiford TJ, Morganroth ML. High-frequency ventilation. Chest. 1989;96:1380–9. [DOI] [PubMed] [Google Scholar]
- 2.Chang HK. Mechanisms of gas transport during ventilation by high-frequency oscillation. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:553–63. [DOI] [PubMed] [Google Scholar]
- 3.Tingay DG, Mills JF, Morley CJ, Pellicano A, Dargaville PA. Indicators of optimal lung volume during high-frequency oscillatory ventilation in infants. Crit Care Med. 2013;41:237–44. [DOI] [PubMed] [Google Scholar]
- 4.Facchin F, Fan E. Airway pressure release ventilation and high-frequency oscillatory ventilation: potential strategies to treat severe hypoxemia and prevent ventilator-induced lung injury. Respir Care. 2015;60:1509–21. [DOI] [PubMed] [Google Scholar]
- 5.Meyers M, Rodrigues N, Ari A. High-frequency oscillatory ventilation: a narrative review. Can J Respir Ther. 2019;55:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maitra S, Bhattacharjee S, Khanna P, Baidya DK. High-frequency ventilation does not provide mortality benefit in comparison with conventional lung-protective ventilation in acute respiratory distress syndrome: a meta-analysis of the randomized controlled trials. Anesthesiology. 2015;122:841–51. [DOI] [PubMed] [Google Scholar]
- 7.Hibberd J, Leontini J, Scott T, Pillow JJ, Miedema M, Rimensberger PC, et al. Neonatal high-frequency oscillatory ventilation: where are we now? Arch Dis Child Fetal Neonatal Ed. 2024;109:467–74. [DOI] [PubMed] [Google Scholar]
- 8.Venegas JG, Fredberg JJ. Understanding the pressure cost of ventilation: why does high-frequency ventilation work? Crit Care Med. 1994;22:S49-57. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann J, Tawhai MH, Kaczka DW. Regional gas transport in the heterogeneous lung during oscillatory ventilation. J Appl Physiol. 2016;121:1306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann J, Tawhai MH, Kaczka DW. Parenchymal strain heterogeneity during oscillatory ventilation: why two frequencies are better than one. J Appl Physiol. 2018;124:653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann J, Lilitwat W, Tawhai MH, Kaczka DW. High-frequency oscillatory ventilation and ventilator-induced lung injury: size does matter. Crit Care Med. 2020;48:e66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venegas JG, Hales CA, Strieder DJ. A general dimensionless equation of gas transport by high-frequency ventilation. J Appl Physiol. 1986;60:1025–30. [DOI] [PubMed] [Google Scholar]
- 13.Drazen JM, Kamm RD, Slutsky AS. High-frequency ventilation. Physiol Rev. 1984;64:505–43. [DOI] [PubMed] [Google Scholar]
- 14.Weber K, Courtney SE, Pyon KH, Chang GY, Pandit PB, Habib RH. Detecting lung overdistention in newborns treated with high-frequency oscillatory ventilation. J Appl Physiol. 2000;89:364–72. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Alexander J, Blowes R, Ingram D, Milner AD. Determination of resonance frequency of the respiratory system in respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 1999;80:F198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson-Smart DJ, De Paoli AG, Clark RH, Bhuta T. High frequency oscillatory ventilation versus conventional ventilation for infants with severe pulmonary dysfunction born at or near term. Cochrane Database Syst Rev. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015;2015:CD000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rettwitz-Volk W, Veldman A, Roth B, Vierzig A, Kachel W, Varnholt V, et al. A prospective, randomized, multicenter trial of high-frequency oscillatory ventilation compared with conventional ventilation in preterm infants with respiratory distress syndrome receiving surfactant. J Pediatr. 1998;132:249–54. [DOI] [PubMed] [Google Scholar]
- 19.Miller AG, Tan HL, Smith BJ, Rotta AT, Lee JH. The physiological basis of high-frequency oscillatory ventilation and current evidence in adults and children: a narrative review. Front Physiol. 2022;13:813478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchut J-C, Godard J, Claris O, Weiskopf RB. High-frequency oscillatory ventilation. J Am Soc Anesthesiol. 2004;100:1007–12. [DOI] [PubMed] [Google Scholar]
- 21.Bunnell JB. High-frequency ventilation: general concepts. In: Donn SM, Mammel MC, Kaam AHLC, editors. Manual of neonatal respiratory care. Springer; 2022. p. 371–86. [Google Scholar]
- 22.Ackermann BW, Klotz D, Hentschel R, Thome UH, van Kaam AH. High-frequency ventilation in preterm infants and neonates. Pediatr Res. 2023;93:1810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollisch-Singule M, Ramcharran H, Satalin J, Blair S, Gatto LA, Andrews PL, et al. Mechanical ventilation in pediatric and neonatal patients. Front Physiol. 2022;12:805620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos-Navarro C, González-Pacheco N, de Rodríguez-Sánchez AB, Sánchez-Luna M. Effect of a new respiratory care bundle on bronchopulmonary dysplasia in preterm neonates. Eur J Pediatr. 2020;179:1833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jager P, Kamp T, Dijkstra SK, Burgerhof JGM, Markhorst DG, Curley MAQ, et al. Feasibility of an alternative, physiologic, individualized open-lung approach to high-frequency oscillatory ventilation in children. Ann Intensive Care. 2019;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer K, Nof E, Sznitman J. Revisiting high-frequency oscillatory ventilation in vitro and in silico in neonatal conductive airways. Clin Biomech (Bristol, Avon). 2019;66:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desiraju K, Agrawal A. Impulse oscillometry: the state-of-art for lung function testing. Lung India. 2016;33:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckingham E. On physically similar systems: illustrations of the use of dimensional equations. Phys Rev. 1914;4:345–76. [Google Scholar]
- 29.Bolster D, Hershberger R, Donnelly R. Dynamic similarity, the dimensionless science. Phys Today. 2011;64:42–7. [Google Scholar]
- 30.Womersley JR. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J Physiol. 1955;127:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, OSCAR Study Group, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–13. [DOI] [PubMed] [Google Scholar]
- 32.Griscom NT. Computed tomographic determination of tracheal dimensions in children and adolescents. Radiology. 1982;145:361–4. [DOI] [PubMed] [Google Scholar]
- 33.Hupp SR, Turner DA, Rehder KJ. Is there still a role for high-frequency oscillatory ventilation in neonates, children and adults? Expert Rev Respir Med. 2015;9:603–18. [DOI] [PubMed] [Google Scholar]
- 34.Jacob C, Tingay DG, Leontini JS. The impact of steady streaming and conditional turbulence on gas transport during high-frequency ventilation. Theor Comput Fluid Dyn. 2021;35:265–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth CJ, Förster KM, Hilgendorff A, Ertl-Wagner B, Wall WA, Flemmer AW. Gas exchange mechanisms in preterm infants on HFOV - a computational approach. Sci Rep. 2018;8:13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob C, Tingay D, Leontini J. The role of mean streaming and turbulent mixing for gas transport during high-frequency ventilation. In: Australian Biomedical Engineering Conference 2019 (ABEC 2019): Technology & Research in Australian Medical Science: Technology & Research in Australian Medical Science 2019 Jan 1 (pp. 57–60). Melbourne: Engineers Australia.
- 37.Jalal S, Van de Moortele T, Nemes A, Amili O, Coletti F. Three- dimensional steady and oscillatory flow in a double bifurcation airway model. Phys Rev Fluids. 2018;3:103101. [Google Scholar]
- 38.Jalal S, Van de Moortele T, Amili O, Coletti F. Steady and oscillatory flow in the human bronchial tree. Phys Rev Fluids. 2020;5:063101. [Google Scholar]
- 39.Nof E, Heller-Algazi M, Coletti F, Waisman D, Sznitman J. Ventilation-induced jet suggests biotrauma in reconstructed airways of the intubated neonate. J R Soc Interface. 2020;17:20190516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Pacheco N, Sánchez-Luna M, Ramos-Navarro C, Navarro-Patiño N, de la Blanca AR. Using very high frequencies with very low lung volumes during high-frequency oscillatory ventilation to protect the immature lung. A pilot study. J Perinatol. 2016;36:306–10. [DOI] [PubMed] [Google Scholar]
- 41.Wanigasekera D, Jacob C, Manasseh R, Leontini J. Mean streaming in reciprocating flow in a double bifurcation. Fluid Dyn Res. 2024. 10.1088/1873-7005/ad6289. [Google Scholar]
- 42.Zannin E, Dellaca RL, Dognini G, Marconi L, Perego M, Pillow JJ, et al. Effect of frequency on pressure cost of ventilation and gas exchange in newborns receiving high-frequency oscillatory ventilation. Pediatr Res. 2017;82:994–9. [DOI] [PubMed] [Google Scholar]
- 43.Mukerji A, Belik J, Sanchez-Luna M. Bringing back the old: time to reevaluate the high-frequency ventilation strategy. J Perinatol. 2014;34:464–7. [DOI] [PubMed] [Google Scholar]
- 44.Amini R, Kaczka DW. Impact of ventilation frequency and parenchymal stiffness on flow and pressure distribution in a canine lung model. Ann Biomed Eng. 2013;41:2699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson AC, Lutchen KR. Physiological basis for resonant frequencies in respiratory system impedances in dogs. J Appl Physiol. 1991;70:1051–8. [DOI] [PubMed] [Google Scholar]
- 46.Clark RH, Yoder BA, Sell MS. Prospective, randomized comparison of high-frequency oscillation and conventional ventilation in candidates for extracorporeal membrane oxygenation. J Pediatr. 1994;124:447–54. [DOI] [PubMed] [Google Scholar]
- 47.Rojas MA, Lozano JM, Rojas MX, Bose CL, Rondón MA, Ruiz G, Columbian Neonatal Research Network, et al. Randomized, multicenter trial of conventional ventilation versus high-frequency oscillatory ventilation for the early management of respiratory failure in term or near-term infants in Colombia. J Perinatol. 2005;25:720–4. [DOI] [PubMed] [Google Scholar]
- 48.Clark RH, Gerstmann DR, Null DM Jr, deLemos RA. Prospective randomized comparison of high-frequency oscillatory and conventional ventilation in respiratory distress syndrome. Pediatrics. 1992;89:5–12. [PubMed] [Google Scholar]
- 49.Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT, Neonatal Ventilation Study Group. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N Engl J Med. 2002;347:643–52. [DOI] [PubMed] [Google Scholar]
- 50.Craft AP, Bhandari V, Finer NN. The sy-fi study: a randomized prospective trial of synchronized intermittent mandatory ventilation versus a high-frequency flow interrupter in infants less than 1000 g. J Perinatol. 2003;23:14–9. [DOI] [PubMed] [Google Scholar]
- 51.Dani C, Bertini G, Pezzati M, Filippi L, Pratesi S, Caviglioli C, et al. Effects of pressure support ventilation plus volume guarantee vs. high-frequency oscillatory ventilation on lung inflammation in preterm infants. Pediatr Pulmonol. 2006;41:242–9. [DOI] [PubMed] [Google Scholar]
- 52.Durand DJ, Asselin JM, Hudak ML, Aschner JL, McArtor RD, Cleary JP, et al. Early high-frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation in very low birth weight infants: a pilot study of two ventilation protocols. J Perinatol. 2001;21:221–9. [DOI] [PubMed] [Google Scholar]
- 53.Gerstmann DR, Minton SD, Stoddard RA, Meredith KS, Monaco F, Bertrand JM, et al. The Provo multicenter early high-frequency oscillatory ventilation trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics. 1996;98:1044–57. [PubMed] [Google Scholar]
- 54.HIFI Study Group. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants. N Engl J Med. 1989;320:88–93. [DOI] [PubMed] [Google Scholar]
- 55.Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, United Kingdom Oscillation Study Group, et al. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med. 2002;34:633–42. [DOI] [PubMed] [Google Scholar]
- 56.Lista G, Castoldi F, Bianchi S, Battaglioli M, Cavigioli F, Bosoni MA. Volume guarantee versus high-frequency ventilation: lung inflammation in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2008;93:F252–6. [DOI] [PubMed] [Google Scholar]
- 57.Moriette G, Paris-Llado J, Walti H, Escande B, Magny JF, Cambonie G, et al. Prospective randomized multicenter comparison of high-frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics. 2001;107:363–72. [DOI] [PubMed] [Google Scholar]
- 58.Ogawa Y, Miyasaka K, Kawano T, Imura S, Inukai K, Okuyama K, et al. A multicenter randomized trial of high frequency oscillatory ventilation as compared with conventional mechanical ventilation in preterm infants with respiratory failure. Early Hum Dev. 1993;32:1–10. [DOI] [PubMed] [Google Scholar]
- 59.Plavka R, Kopecký P, Sebron V, Svihovec P, Zlatohlávková B, Janus V. A prospective randomized comparison of conventional mechanical ventilation and very early high frequency oscillatory ventilation in extremely premature newborns with respiratory distress syndrome. Intensive Care Med. 1999;25:68–75. [DOI] [PubMed] [Google Scholar]
- 60.Salvo V, Zimmermann LJ, Gavilanes AW, Barberi I, Ricotti A, Abella R, et al. First intention high-frequency oscillatory and conventional mechanical ventilation in premature infants without antenatal glucocorticoid prophylaxis. Pediatr Crit Care Med. 2012;13:72–9. [DOI] [PubMed] [Google Scholar]
- 61.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–107. [DOI] [PubMed] [Google Scholar]
- 62.Sun H, Cheng R, Kang W, Xiong H, Zhou C, Zhang Y, et al. High-frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation plus pressure support in preterm infants with severe respiratory distress syndrome. Respir Care. 2014;59:159–69. [DOI] [PubMed] [Google Scholar]
- 63.Thome U, Kössel H, Lipowsky G, Porz F, Fürste HO, Genzel-Boroviczeny O, et al. Randomized comparison of high-frequency ventilation with high-rate intermittent positive pressure ventilation in preterm infants with respiratory failure. J Pediatr. 1999;135:39–46. [DOI] [PubMed] [Google Scholar]
- 64.Van Reempts P, Borstlap C, Laroche S, Van der Auwera JC. Early use of high frequency ventilation in the premature neonate. Eur J Pediatr. 2003;162:219–26. [DOI] [PubMed] [Google Scholar]
- 65.Vento G, Matassa PG, Ameglio F, Capoluongo E, Zecca E, Tortorolo L, et al. HFOV in premature neonates: effects on pulmonary mechanics and epithelial lining fluid cytokines. A randomized controlled trial. Intensive Care Med. 2005;31:463–70. [DOI] [PubMed] [Google Scholar]
- 66.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial (MOAT) Study Investigators, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–8. [DOI] [PubMed] [Google Scholar]
- 67.Bollen CW, van Well GT, Sherry T, Beale RJ, Shah S, Findlay G, et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care. 2005;9:R430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demory D, Michelet P, Arnal JM, Donati S, Forel JM, Gainnier M, et al. High-frequency oscillatory ventilation following prone positioning prevents a further impairment in oxygenation. Crit Care Med. 2007;35:106–11. [DOI] [PubMed] [Google Scholar]
- 69.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, OSCILLATE Trial Investigators; Canadian Critical Care Trials Group, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. [DOI] [PubMed] [Google Scholar]
- 70.Mentzelopoulos SD, Roussos C, Koutsoukou A, Sourlas S, Malachias S, Lachana A, et al. Acute effects of combined high-frequency oscillation and tracheal gas insufflation in severe acute respiratory distress syndrome. Crit Care Med. 2007;35:1500–8. [DOI] [PubMed] [Google Scholar]
- 71.Oakes JM, Scadeng M, Breen EC, Marsden AL, Darquenne C. Rat airway morphometry measured from in situ MRI-based geometric models. J Appl Physiol. 2012;112:1921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arendt TB, Loeber SJ, Schroeder CA, Lasarev MR, Ferreira TH. Computed tomographic laryngotracheal dimensions in adult domestic rabbits (Oryctolagus cuniculus) are positively associated with body weight and the laryngotracheal lumen is narrowest at the level of the thyroid cartilage. Am J Vet Res. 2023;84:8. [DOI] [PubMed] [Google Scholar]
- 73.Valverde CR, Christe KL. CHAPTER 22 - radiographic imaging of nonhuman primates. In: Wolfe-Coote S, editor. The laboratory primate. Academic Press; 2005. p. 371–86. [Google Scholar]
- 74.Mostafa AA, Berry CR. Radiographic vertical tracheal diameter assessment at different levels along the trachea as an alternative method for the evaluation of the tracheal diameter in non-brachycephalic small breed dogs. BMC Vet Res. 2022. 10.1186/s12917-022-03160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breatnach E, Abbott GC, Fraser RG. Dimensions of the normal human trachea. Am J Roentgenol. 1984;142:903–6. [DOI] [PubMed] [Google Scholar]
- 76.Carstens A, Kirberger RM, Grimbeek RJ, Donnellan CMB, Saulez MN. Radiographic quantification of tracheal dimensions of the normal thoroughbred horse. Vet Radiol Ultrasoun. 2009;50:492–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.