Abstract
Background
A precise observation is that the cervix’s solid tumors possess hypoxic regions where the oxygen concentration drops below 1.5%. Hypoxia negatively impacts the host’s immune system and significantly diminishes the effectiveness of several treatments, including radiotherapy and chemotherapy. Utilizing oncolytic spores of Clostridium novyi-NT to target the hypoxic regions of solid tumors has emerged as a noteworthy treatment strategy.
Methods
The transplantation procedure involved injecting TC-1 cells, capable of expressing HPV-16 E6/7 oncoproteins, into the subcutaneous layer of 6-8-week-old female C57/BL6 mice. The TC-1 cell line, was subcutaneously transplanted into 6-8-week-old female C57/BL6 mice. The tumor-bearing mice were randomly divided into 4 groups, and after selecting the control group, they were treated with different methods. Group 1- control without treatment (0.1 ml sterile PBS intratumor) Group 2- received cisplatin intraperitoneally (10 mg/kg) Group 3- received 107Clostridium novyi-NT spores systemically through the tail vein Group 4-tumor mice received 107Clostridium novyi-NT spores intratumorally. 20 days after the start of treatment, the mice were sacrificed and tumor tissues were isolated. In order to clarify the mechanism of the therapeutic effect with spores, the amount of ROS and ceramide was measured by ELISA technique, and the expression level of cytochrome c, cleaved caspase- 3, Bax, Bcl-2, HIF-1α, and VEGF proteins was measured by western blotting.
Results
Our results clearly showed that the injection of Clostridium novyi-NT spores (either intratumorally or intravenously) causes the regression of mouse cervical tumors. Spore germination induces internal apoptosis in cancer cells by inducing ROS production and increasing total cell ceramide, releasing cytochrome c and damaging mitochondria. Additionally, the results provided clear evidence of a significant decrease in the expression of HIF-1 alpha and VEGF proteins among the tumor groups that received spores, when compared to both the cisplatin-treated group and the control group.
Conclusions
The study’s outcomes demonstrated that the introduction of Clostridium novyi-NT spores triggered apoptosis in cervical cancer cells (derived from the TC-1 cell line) via the mitochondrial pathway, subsequently resulting in tumor regression in a mouse model.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-024-04742-5.
Keywords: Clostridium novyi-NT, HPV-positive cervical, Apoptosis, TC-1 cell line, Oncolytic bacteria
Background
Cervical cancer is the fourth most common cancer among women worldwide. In 2020, 604,127 new cases of this disease were registered [1]. Approximately 90% of deaths are related to less developed or developing countries. Subunits 16 and 18 of the papillomaviruses (HPV) are the most important causes of precancerous and cancerous lesions of the cervix. HPV, by affecting proto-oncogenes and tumor suppressor genes, causes changes in the shape of normal cells and leads them to become cancerous. It is well known that the E6 and E7 proteins in high-risk types of HPV play prominent roles in host cell carcinogenesis [2]. Oncoprotein E6 stimulates the degradation of the p53 protein (a critical transcription factor that stimulates the expression of genes involved in cell cycle arrest and apoptosis), and oncoprotein E7 exerts significant biological changes in cell growth by inactivating pRb and two proteins, P106 and P130, playing an essential role in genetic instability and normal cell growth. Despite the development of detection and prevention methods through the vaccination of adolescents against dangerous HPV subunits, this disease is still considered a vital treatment challenge in most poor or developing countries [3]. Today, chemotherapy, surgery, radiotherapy, immunotherapy, or combined methods based on these methods are considered routine treatments for this disease. Despite the high rate of local control, the overall survival of patients with this type of malignancy is less than 50%, mainly due to the distant recurrence of this disease [4]. As a result, to improve the overall survival index, it is necessary to personalize the treatments as much as possible, and by modifying the routine treatment methods, the probability of recurrence and progression of the disease will decrease [5]. One of the most unfavorable features of solid tumors is the presence of hypoxic areas where the oxygen concentration is less than 1.5%. Regardless of the type of treatment method, these conditions weaken the treatment result. Therefore, therapeutic strategies to address hypoxic areas of the tumor are essential, especially in advanced stages of the disease where radiotherapy is often combined with cisplatin derivatives as the primary treatment option [6].
The interaction between E6/E7 oncogenes and host cells under hypoxic conditions has not been fully clarified. However, research has shown that cervical cancer cells in the hypoxic areas of the tumor can become inactive by reducing the expression of E6/E7 oncogenes of human papillomavirus [7]. After entering a reversible proliferative stop, they become dormant and cause immune escape, leading to therapeutic resistance. Furthermore, with reoxygenation, these cells resume E6/E7 expression and act as a reservoir for proliferation and recurrence after treatment. Recent findings clearly state the necessity of developing combined treatment strategies against hypoxic areas of HPV-positive cervical tumors. Various proteins and pathways are involved in how cells respond to hypoxic conditions [8]. The complex interplay that enables tumor cells to escape senescence is orchestrated by the phosphoinositide 3-kinase/AKT/mammalian target of rapamycin (mTOR) signaling pathway in hypoxic environments, even in the presence of decreased E6/E7 oncoprotein expression. Hypoxia-inducible factor 1-alpha (HIF-1α) serves as a key player in cancer cell adaptation to hypoxia, influencing angiogenesis and cancer development. In cervical cancer, the stabilization of HIF-1α under hypoxic conditions triggers the activation of regulated in development and DNA damage responses 1, leading to mTORC1 inhibition. Consequently, hypoxic HPV-positive cancer cells can evade senescence despite AKT-mediated suppression of E6/E7 [9, 10]. In general, the family of hypoxia-inducing factors (HIFs) plays a significant role in the adaptation of tumor cells to hypoxic conditions through the modulation or expression of various genes [11]. Therefore, it can be safely said that overcoming cancer cells located in the hypoxic areas of the tumor is a critical challenge in the success of treatment, and this aspect of the therapy requires new and targeted research [9].
Over a century ago, Dr. William Coley observed the unexpected regression of an inoperable tumor in a patient inadvertently infected with Streptococcus pyogenes infection. After this report, many researchers began to investigate the potential of bacteria to attack tumors [12]. Among these bacteria, researchers used a family of anaerobic gram-positive bacteria called Clostridium for antitumor treatments. Preclinical studies on animals showed that these bacteria can grow in the necrotic/hypoxic areas of the tumor [13]. Among this family, Clostridium novyi showed the best performance. However, the problem arose when the researchers observed that the tested mice died due to excessive toxicity of germinated spores of this bacterium. After the investigation, researchers finally created a nontoxic Clostridium novyi-NT strain by removing the alpha toxin gene of this bacterium through thermal processes, and the research was focused on this strain [14].
This bacterium has shown promising results in animal studies, including companion dogs with naturally occurring tumors [15]. Intravenous administration of Clostridium novyi-NT has demonstrated better tolerability and higher objective response rates than intravenous administration [16]. When Clostridium novyi-NT spores are administered intratumorally, they selectively colonize and replicate within the hypoxic regions of tumors. This colonization leads to the lysis of tumor cells and the release of tumor antigens, triggering an immune response. The immune response includes the activation of tumor-specific T cells, enhancing the body’s ability to recognize and destroy cancer cells. The localized nature of Clostridium novyi-NT treatment helps minimize systemic toxicity and potential side effects [17]. Clinical trials have been conducted to evaluate the safety and efficacy of Clostridium novyi-NT in the treatment of solid tumors. One such study involved 24 patients with treatment-refractory solid tumors. A single intratumoral injection of Clostridium novyi-NT led to bacterial spore germination and tumor lysis in 10 patients. The maximum tolerated dose was 1 × 106 spores, with grade four sepsis and gas gangrene being the dose-limiting toxicities. Other treatment-related toxicities included pathologic fracture, limb abscess, soft tissue infection, respiratory insufficiency, and rash. Overall, 41% of patients experienced a decrease in tumor size, and 86% had stable disease as the best overall response. The use of Clostridium novyi-NT in the treatment of solid tumors shows excellent promise. However, there are still challenges to be addressed. One of the main challenges is optimizing the dosing and delivery of Clostridium novyi-NT to maximize its therapeutic potential while minimizing toxicity. The identification and management of potential side effects such as sepsis and gas gangrene are crucial for the safe administration of this treatment [18]. Furthermore, more research is needed to comprehensively comprehend the processes by which Clostridium novyi-NT triggers tumor destruction and elicits an immune response. The mechanisms involved in this positive antitumor function are not completely clear. It seems that the antitumor process of this type of bacteria is due to the induction of a robust immune response in the host and the secretion of several extracellular proteins with the power of cell lysis [19, 20].
In this study, for the first time, an attempt was made to clarify the cellular and molecular mechanisms involved in the regression of solid tumors caused by the TC-1 cell line in the treatment using Clostridium novyi-NT spores. Additionally, the effectiveness of Clostridium novyi-NT spores in the conditions of intratumoral (IT) or intravenous (IV) injection was compared with each other and the chemotherapy drug cisplatin, which is the most common drug used in treating cervical cancer. We hope that the results obtained from this research can help researchers understand the strengths and weaknesses of treating solid tumors using Clostridium novyi-NT spores.
Methods
Preparation of cell culture and oncolytic bacteria (clostridium novyi-NT) spores
In this study, to create an animal model reflecting cervical cancer induced by human papillomavirus (HPV), the TC-1 cell line (TC-1 is the tumor cell line obtained from primary lung epithelial cells of C57BL/6 mice. Using the amphotropic retroviral vector LXSN16E6E7, the cells were immortalized and later transfected with plasmid pVEJB, which contains the active human c-Ha-ras oncogene) was acquired from the National Cell Bank of Iran affiliated with the Pasteur Institute in Tehran, Iran. RPMI1640 (Sigma, USA) medium supplemented with 10% fetal bovine serum (FBS) (Gibco, USA), 1% penicillin‒streptomycin (Sigma, USA), 25 mM HEPES (Sigma, USA), and 1% glutamine (Sigma, USA) was used to suspend the TC-1 cell line. Subsequently, the cells were incubated at 37 °C in an atmosphere with a CO2 concentration of 5%. In a prior investigation, Dr. Asghar Abdoli and his research team successfully developed modified spores of Clostridium novyi type B 46, which were intentionally designed to be devoid of the lethal alpha toxin gene. For the present study, we were graciously supplied with these spores by Dr. Abdoli and his team [21]. Once processed accordingly, lyophilized tablets enclosing 10 million spores of Clostridium novi-NT were prepared and subsequently preserved at -20 °C to be employed in forthcoming research studies.
Animal study design and development of a mouse model that simulates HPV-associated cervical cancer
It should be emphasized at the beginning that this study was reviewed and approved in terms of research ethics and working with laboratory animals by the Research and Technology Vice-Chancellor of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1398.434). During this study, all the animal works performed were regularly observed and reviewed under the supervision of this committee. In this study, 24 C57/BL6 female mice, 6–8 weeks old, weighing 18–22 g, were obtained from Pasteur Institute (Karaj, Iran) and fed according to laboratory standards for seven days. Then, the animals were randomly divided into four groups with six mice in each group (groups of 4 and n = 6). Four other groups were selected to challenge the tumors. To induce HPV-related cervical cancer, one million TC-1 cells were suspended in 0.2 mL of PBS (Sigma, USA) and injected subcutaneously into the right flank of each mouse. During this period, the mice were monitored daily, and when palpable tumors appeared, the size of the tumors was measured using calipers (once a day). The volume of tumors was determined using the standard formula (longest tumor diameter) × (shortest diameter) 2 × 0.5.
The therapeutic approach for mice carrying tumors with the TC-1 cell line involves the utilization of clostridium novyi-NT spores
The purpose of this study was to investigate the therapeutic efficacy of Clostridium novyi-NT spores against tumors caused by the TC-1 cell line and to understand the cellular and molecular mechanisms involved in this process to compare the way the model animal receives spores systematically or intratumorally. Finally, the efficiency of this treatment was compared with routine chemotherapy treatment based on cisplatin derivatives. After the average volume of tumors in the animal models reached 300 to 500 mm3, the mice were randomly divided into four groups, and the treatments were carried out as follows. First, the negative control group that did not receive any treatment and only 0.1 ml of sterile PBS was given intratumorally, and 0.1 ml of sterile PBS was received systemically through the tail vein. The second group - as a positive control group, received 10 mg/kg cisplatin (CAS 15663-27-1), which was purchased from Sigma Aldrich (Sigma, USA) and dissolved in 0.1 ml of sterile PBS intraperitoneally. The third group - each animal in this group received 10 million spores suspended in 0.1 sterile PBS systemically through the tail vein. In the fourth group, the prescribed treatment protocol for every animal member involved administering 10 million spores of Clostridium novyi-NT. This was done by injecting 0.1 ml of sterile PBS directly into the tumor’s center through intratumoral injection, ensuring a slow and controlled delivery. The conclusion of our treatment was based on a previous study that determined the 20th day to be the optimal end date [22]. All animals were given 30 µl of anesthetic solution, which consisted of a combination of 10% ketamine (100 mg/ml; Medistar, Ascheberg, Germany) and 2% xylazine (20 mg/ml; Riemser, Greifswald, Germany) in a single insulin syringe with a ratio of 2 parts ketamine to 1-part xylazine. When the animals were wholly anesthetized, the blood sample was taken from the heart muscle and kept at a concentration of 10% with the help of EDTA (ethylenediaminetetraacetic acid), which acted as an anticoagulant. After that in the etiquette of moving the neck vertebrae, the animals were sacrificed accordingly. Tumor tissue was then carefully isolated and kept at -70 °C for gene analysis and protein expression and in 10% formalin solution for histopathological examination.
The process of extracting RNA and synthesizing cDNA
Each mouse’s cervical cancer tissue was isolated, and 100 mg was used to extract total RNA with the help of an RNA extraction kit (Thermo Fisher Scientific, USA) following the manufacturer’s instructions. The purification of total RNA was evaluated by a NanoDrop ND-1000 (NanoDrop, USA) spectrophotometer. To assess RNA integrity, the extracted product was evaluated on a 2% agarose gel. cDNA synthesis was carried out using 500 µg of extracted RNA and a reverse transcription kit (Biotech Rabbit, Germany) following the guidelines provided by the manufacturer.
Using real-time polymerase chain reaction (RT‒PCR) analysis for detecting vegetative forms of clostridium novyi-NT
By conducting RT‒PCR, the expression level of the phospholipase C (NT01CX0979) gene was established. For SYBR-Green 2x Master Mix (Amplicon, Denmark), 2 µl of syntony cDNA was subjected to a PCR cycle. PCR conditions included predenaturation at 95°C for 15 minutes, denaturation at 95°C for 30 seconds, and denaturation at 60°C for 45 seconds for 40 cycles. As a reaction internal reference, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was utilized in the experiment. All the samples were analyzed 3 times. The specific primers were as follows: PLC, forward 5’- GGAGCATCAAGTAAAGCGTA-3’ and reverse 5’- CATTCGGATCATAATCAGGA-3’; GAPDH, forward 5’-GCCAAAGGGTCATCATCTC3’ and reverse 5’-GTAGAGGCAGGGATGATGTT-3’. Oligo 7 software was used to design all primers, and Metabion Company (Germany) synthesized them. By employing the control sample, the target mRNA value was determined, which was then calculated using the (ΔΔCt) method.
The quantitative determination of total ceramide
The Mouse Ceramide ELISA Kit (MyBioSource-USA) with catalog number MBS7255958 was used to measure the amount of total ceramide in tumor tissues. The CER ELISA kit uses a competitive enzyme immunoassay method using a polyclonal anti-CER antibody and CER-HRP conjugate. To carry out this process, the instructions related to using the kit provided by the manufacturing company were followed. For this procedure, to completely remove excess, tissues were rinsed in ice-cold PBS (0.02 mol/L, pH 7.0-7.2) and weighed 300–500 mg before homogenization. Tissues were chopped into small pieces and homogenized in 500 L of PBS with a glass homogenizer on ice. The resulting suspension was exposed to ultrasound waves to break the cell membrane further. After that, the homogenates were centrifuged for 15 min at 1500 × g (or 5000 rpm). The supernatant was removed, and the samples were stored at -20 °C. Next, to measure the amount of total ceramide, the manufacturer’s instructions were followed.
Analysis of reactive oxygen species (ROS)
The sandwich ELISA method was employed using the ROS1/ROS ELISA kit (LifeSpan BioSciences, USA) to assess the concentrations of active species. After washing the tumor tissues in PBS to remove any surplus, their weight was determined before homogenization. Using a glass homogenizer on ice, the minced tissues were homogenized in 10 ml of PBS. Following the kit instructions, all reagents, working standards, and samples were prepared and left at room temperature for 20 min. Then, 100 µl of sample and standard were added to each well and incubated for 2 h at 37 °C. The plate was emptied, and 100 µL of detection reagent A was added and incubated for 1 h at 37 °C. The plate was opened and washed three times with 400 µl of washing solution in the plate kit. Then, 100 µL of Detection Reagent B was added and incubated for 30 min at 37 °C. The plate was drained and washed three times with 400 µl of washing solution in the plate kit. Ninety milliliters of substrate solution were added to each well and incubated for 20 min in the dark at 37 °C. Finally, 50 µl of Stop solution was added to each well. Using an ELISA device, the amount of light absorption was read at 450 nm, and based on the amount of light absorption, a standard curve was drawn. Based on the slope and width of the first line, the ROS concentration in each well was measured.
Western blot analysis
After weighing the tumor tissue, a 5 mg piece of tissue was taken, and 300 µL of ice-cold lysis buffer was quickly added to the tube. The tissue was homogenized with an electric homogenizer. The blade was washed twice with 2 × 300 µl of lysis buffer. After that, it was kept at 4 °C for 2 h with constant stirring. Lysates were removed by centrifugation at 14,000 rpm for 20 min at 4 °C. According to the manufacturer’s instructions, the BCA Protein Quantification Kit was used to determine the protein concentration of the exosome lysate. The exosome lysates were mixed with an equal volume of 2X Laemmli sample buffer. Lysates (15 µg) were subjected to SDS‒PAGE after boiling for 5 min and subsequently transferred to a 0.2 μm immune-Blot™ polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, CA, USA). The membranes were then blocked with 5% BSA (Cat No: A-7888; Sigma Aldrich, MO, USA) in 0.1% Tween 20 for 1 h. Then, the membranes were incubated with anti-HIF-1 alpha antibodies (Abcam), anti-VEGF (Abcam), anti-cleaved caspase-3 antibody (Abcam), anti-Bax antibody (Abcam), anti-Bcl-2 antibody (Abcam), and anti-cytochrome C antibody (Abcam) as previously described. The preparation of cytosolic and mitochondrial extracts for Cyt-C analysis was performed using the Abcam Mitochondria Isolation Kit for Tissue (ab110168) and anti-beta actin loading control antibodies (Abcam) for one hour at room temperature. The membranes were then washed three times with TBST and incubated with goat anti-rabbit IgG H&L (HRP) secondary antibodies (Abcam). The membranes were then incubated with enhanced chemiluminescence (ECL) for 1–2 min. Protein expression was normalized to that of β-actin. Densitometry of protein bands was performed using Gel Analyzer version 2010a software (NIH, USA) in such a way that the portion of the area under the curve of each band was divided by the percentage of the area under the curve of the corresponding actin band, and then the values were calculated. Comparisons between groups were performed as previously described.
Histopathological studies and analysis
To preserve the tumor tissue, it was first fixed in a solution of 10% formalin and then dehydrated using ethanol of varying concentrations (70%, 90%, 96%, and 100%). Subsequently, the tissues were embedded in paraffin, and then 5 μm sections were extracted from the tissue using a microtome. In adherence to routine laboratory protocols, hematoxylin and eosin dyes were used to stain the slides. Finally, for the histology of tumors, including the calculation of the relative necrosis area and mitotic count and (MC) number of mitoses per unit area, the slides stained with normal hematoxylin and eosin (H&E) were examined under a light microscope. The formula for calculating the relative level of necrosis (%) of tumor tissues: relative area of necrosis (%) = area of necrosis in the tumor section/total area of the tumor.
Statistical analysis
In presenting all the data for this experiment, the mean ± SD was utilized. GraphPad Prism 9 software was used for statistical analysis, including one-way analysis of variance (ANOVA) and t test, and 0.05 was considered statistically significant.
Results
Infiltration of clostridium novyi-NT into HPV-positive cervical cancer tumors and extensive necrosis in tumor tissue due to germination
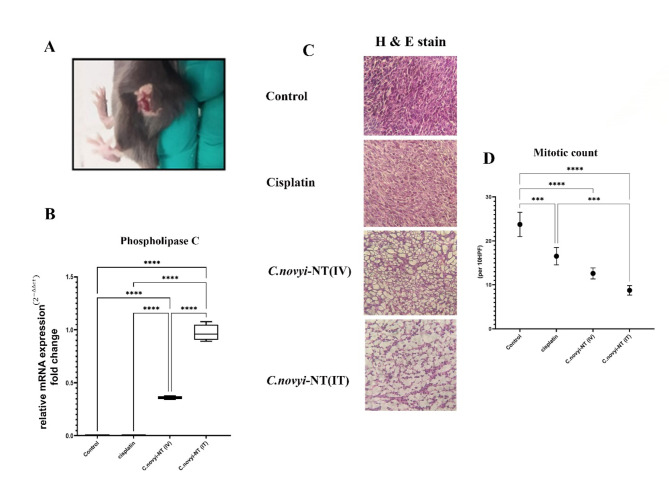
According to Fig. 1-A, after the intratumoral administration of 107Clostridium novyi-NT spores in less than 48 h, hemorrhagic necrosis is visible in the tumor tissue. On the other hand, to be sure of the germination of spores in the tumor tissue, using the RT‒PCR method, the native gene of phospholipase C (NT01CX0979), which is specific to Clostridium novyi-NT bacteria, was measured in the tumor tissue isolated from the mice of all four groups (Fig. 1-B). The results showed that the amount of this gene in the tumor tissues of the groups of mice receiving 107 spores intratumorally was significantly higher than that in the tumor tissues of the group receiving 107 spores systemically (**** P < 0.0001). As expected, the level of this gene was zero in the control and cisplatin-treated mouse groups (Fig. 1-B). The number of mitoses per unit area is a critical parameter often used to classify and grade some tumors. Additionally, this index can predict a good prognosis for both overall survival and response to treatment in most types of cancer. The results of the measurement of this parameter showed that the amount of this factor in the groups of mice receiving spores (either intratumorally or systemically) was significantly lower than that in the control group (**** P < 0.0001) (Fig. 1-D).
Fig. 1.
Clostridium novyi-NT spores, after systemic or intratumoral injection, penetrate and germinate in mouse tumor tissue created by the TC-1 cell line (HPV-positive cervical cancer model). They continue to destroy cancer cells and cause extensive necrosis in the tumor tissue. A: Less than 48 h after the intratumoral injection of 107Clostridium novyi-NT spores, hemorrhagic necrosis was noticeably observed in the tumor tissue. B: To ensure the rejuvenation of Clostridium novyi-NT spores in different areas of tumor tissue after injection, RT‒PCR was used to measure the native gene of phospholipase C (NT01CX0979) related to Clostridium novyi-NT bacteria. It was well established that the amount of this gene in tumor mice that received spores intratumorally is much higher than that in tumor mice that received spores systemically. Additionally, as expected, the amount of this gene was zero in tumor mice of the control and cisplatin receiving groups. C & D: The results of H&E staining and that the comparison of mitotic count of the tested groups shows that the amount of this factor is statistically significantly lower in the group receiving Clostridium novyi-NT spore’s intratumourally compared to the control group and the group receiving cisplatin. (* P < 0.05, **P < 0.005, *** P = 0.0001, **** P < 0.0001)
Clostridium novyi-NT spores cause regression and decrease the volume of HPV-positive cervical cancer tumors (derived from the TC-1 cell line) in a mouse model
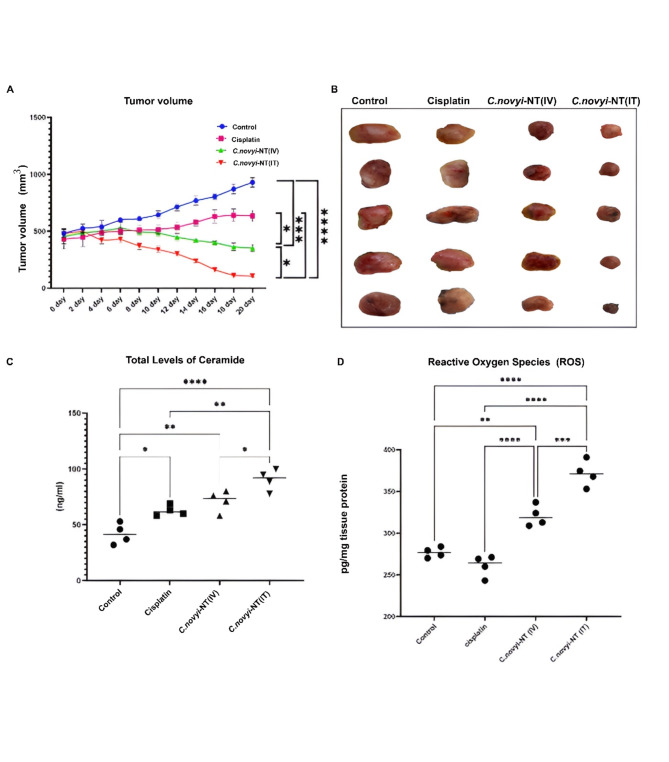
As the next goal, after ensuring the germination of spores in the groups of mice receiving spores, the volume of tumors was measured every two days using a digital caliper. The results showed that the volume of tumors in the group of mice receiving 107 spores (either intratumorally or systemically) started to decrease from the fourth day after the beginning of the treatment process. Finally, after separating the mouse tumors from the four mouse groups, it was found that the average volume of the tumors of the group receiving 107 spores intratumorally was significantly higher than the average volume of the tumors of the control group and the group receiving cisplatin, which had decreased (**** P < 0.0001). Additionally, the average tumor volume of the group receiving 107 spores systemically was significantly lower than that of the control group (*** P = 0.0001) and the group of rats receiving cisplatin (* P < 0.05). Finally, the volume of tumors in the group of mice receiving spores intratumorally was significantly lower than that in the group of tumor mice treated with spores systemically (* P < 0.05) (Fig. 2-A&B).
Fig. 2.
Tumor regression and a significant reduction in tumor volume were observed in HPV-positive cervical cancer mice treated with 107Clostridium novyi-NT spores (intratumoral and systemic spore reception) compared to mice in the control and recipient groups. Intraperitoneal cisplatin (10 mg/kg) was administered at the end of the 20-day treatment period. A and B: The comparison of the tumor volume of different treatment groups clearly shows that the final tumor volume of the mice that received 107 spores of Clostridium novyi-NT intratumorally (10 mg/kg), compared to the other three groups including the control group, the group receiving cisplatin intraperitoneally, and the group receiving 107Clostridium novyi-NT spores, has a statistically significantly decreased. C: For a clearer understanding of the mechanism involved in tumor regression caused by the treatment of HPV-positive cervical cancer mice, the amount of total ceramide was measured for each group using ELISA. The results showed that the level of total ceramide in the tumor tissue of the mice in the groups receiving 107 spores of Clostridium novyi-NT (both intratumoral and systemic) at the end of the 20-day treatment period was significantly higher than that in the control group and the group receiving cisplatin (10 mg/kg) in the peritoneal cavity. D: Comparison of intracellular levels of ROS in different treatment groups: The results showed that the number of reactive oxygen species (ROS) in tumor tissue cells of mice belonging to the group receiving 107 spores of Clostridium novyi-NT was statistically significantly higher than that in the control group and the group receiving cisplatin (10 mg/kg). (* P < 0.05, **P < 0.005, *** P = 0.0001, **** P < 0.0001)
Treatment of mice with HPV-positive cervical cancer tumors (derived from the TC-1 cell line) using clostridium novyi-NT spores increases the level of total ceramide in the tumor tissue
Ceramide functions as a second messenger, playing an essential role in activating the apoptotic cascade. Ceramide is utilized to signal apoptosis in response to various cytokine receptors and environmental stresses. Therefore, according to the heart plan from our previous studies, we used the ELISA kit, which is based on the competitive enzyme immunoassay method using anti-CER antibody and CER-HRP conjugate, to measure the ceramide of the tumors isolated from the mice of the four groups after the end of the treatment period. Our results showed that the amount of total ceramide in the group of tumor mice receiving 107 spores of Clostridium novyi-NT intratumorally was significantly higher than the amount of total ceramide in the tumor mice of the control group (**** P < 0.0001), tumor mice of the cisplatin receiving group (**P < 0.005), and tumor mice of the group receiving 107Clostridium novyi-NT spores systemically (* P < 0.05). The amount of this factor in the group receiving spores systemically was significantly higher than that in the control group (**P < 0.005) (Fig. 2-C).
Increasing the amount of reactive oxygen species (ROS) in mice with HPV-positive cervical cancer tumors (derived from TC-1 cell line) treated with Clostridium novyi-NT spores
Increased production of ROS, which is achieved by immune or therapeutic mechanisms, plays an important and fundamental role in killing tumor cells by inducing different types of cell death, including programmed cell death. As a result, in this research, we used the ROS1/ROS ELISA Kit (LifeSpan BioSciences, USA) to measure and compare the effect of Clostridium novyi-NT spores on the changes related to reactive oxygen species (ROS) among the four groups. The results showed that the amount of ROS in the group receiving 107 spores of Clostridium novyi-NT through tumor injection was significantly higher than that in the control and cisplatin-receiving tumor groups (**** P < 0.0001). Additionally, the amount of this factor in the spore-receiving group was also higher than that in the control group (**P < 0.005) and the cisplatin-receiving group (****P < 0.0001) (Fig. 2-D).
The ability of Clostridium novyi-NT spores to induce apoptosis via the mitochondrial pathway in mice with HPV-positive cervical cancer tumors (derived from the TC-1 cell line)
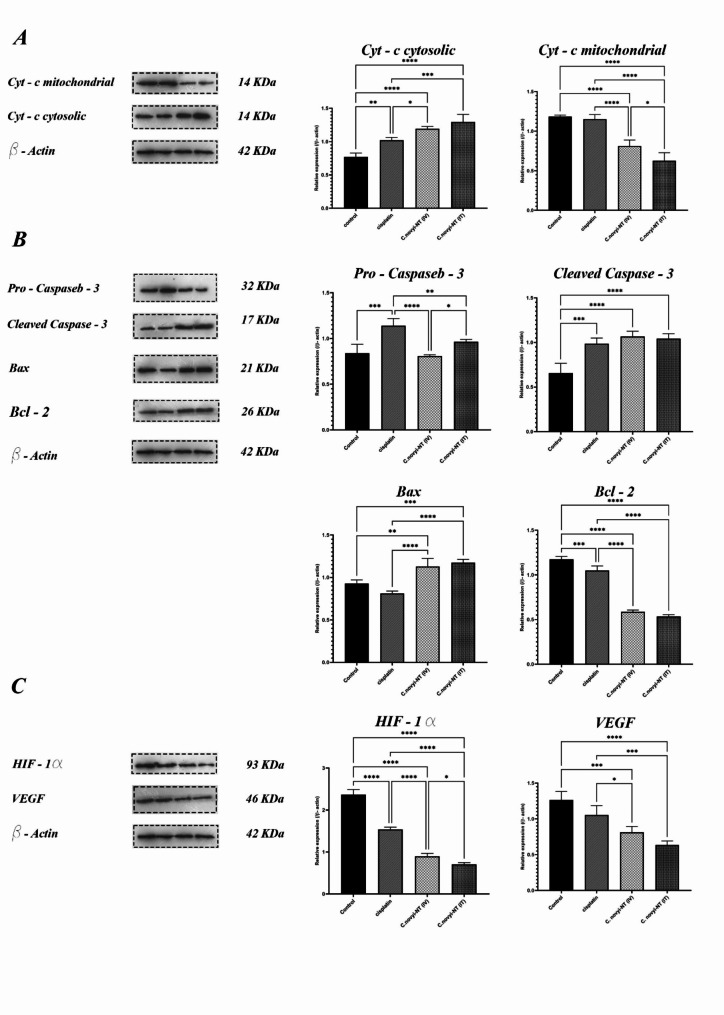
The process of apoptosis, or programmed cell death, is a protected method under the control of genes used to eliminate unwanted or unnecessary cells in living organisms. It interferes in many mechanisms of the immune system or diseases. The leading difference between this path and cell necrosis, which is the main path to remove unwanted cells, is that apoptosis does not cause inflammation, and its effect is limited to the target cells. Apoptosis plays a vital role in important biological processes such as natural development, tissue homeostasis, removal of damaged or virus-infected cells, and removal of activated immune cells against self-antigens [23]. This process is fundamental in regulating the rate of growth, proliferation of cells, development and health of the body. The occurrence of many autoimmune diseases, cancers, and viral infections is the result of poor performance or inhibition of the programmed cell death phenomenon. During apoptosis, cytochrome c is released from mitochondria through the activity of Bax and Bak proteins. The mechanism of this diffusion is unclear, but it appears to arise from the large number of Bax/Bak homo and heterodimers inserted into the outer membrane [24]. With this attitude, to clarify the possible cellular mechanism that causes tumor regression as a result of treatment with Clostridium novyi-NT spores, we measured the expression of cytochrome c protein present in the mitochondria. We released them into the cytosolic region of the cells related to the tumor tissue of the treated mice. To achieve this goal, we first isolated mitochondria from tumor tissue cells using the Abcam Mitochondria Isolation Kit for Tissue (ab110168). Then, we measured the amount of cytochrome c in both mitochondria and the cytosol using western blotting. We measured different treatments and compared them with each other. The results showed that the expression level of cytochrome c protein in the cytosolic area of the cells, related to the tumor tissue, was significantly higher in the groups treated with 107 spores of Clostridium novyi-NT (both intratumorally and systemically) than in the control group (***P < 0.0001) and the group receiving cisplatin (***P = 0.0001). It is interesting to note that there was no significant difference in the expression of cytochrome c between the two groups receiving spores intratumorally and the group receiving spores systemically (Fig. 3-A). In the following study, the theory of induction of programmed tumor cell death through the internal pathway by Clostridium novyi-NT spores was investigated. The expression levels of important proteins involved in this pathway, including Bax, Bcl-2, and cleaved caspase-3, were measured using Western blotting. The Bax molecule plays a significant role in the induction of programmed cell death. This protein resides in the cytoplasm and, in response to various stimuli, migrates to the mitochondria. It can cause the release of cytochrome C, thereby causing dimerization of APAF-1 and activation of the apoptosis cascade. Our results clearly showed that the expression level of this proapoptotic protein in the cancer cells of the tumor mouse group receiving 107Clostridium novyi-NT spores intratumorally was significantly higher than that in the tumor mice of the control group (*** P = 0.0001) and the group receiving cisplatin (**** P < 0.0001). The expression level of this factor in the group of tumor mice receiving spores systemically was also higher than that in the two groups of tumor mice receiving cisplatin (**** P < 0.0001) and the control (** P < 0.005). The Bcl-2 protein, as a proto-oncogene in germ cells, is involved in regulating the cell apoptosis process. In this way, the mentioned protein plays a role in inhibiting cell apoptosis. If the expression level of the Bcl-2 protein-encoding gene decreases, the process of programmed cell apoptosis starts due to the activation of other oncogenes, such as p53. Considering the role of caspases in different cells, studies have shown that Bcl-2 can inhibit the process of apoptosis by inhibiting the synthesis and production of caspases. More specifically, Bcl-2 proteins present in the walls of mitochondria prevent the release of cytochrome C or by binding to Apaf-1, preventing the formation of apopapoptosomes and triggering the caspase cascade. With this description, to understand the oncolytic functional nature of Clostridium novyi-NT spores, we measured the expression level of the antiapoptotic Bcl-2 protein in groups of tumor mice at the end of the treatment period using the western blot technique. Fortunately, the results showed that the amount of this anti-programmed cell protein in tumor mouse groups receiving 107Clostridium novyi-NT spores (both systemically and intratumorally) was significantly reduced compared to the control group) **** P < 0.0001). Caspases are critical mediators of programmed cell apoptosis. Among them, caspase-3 is a frequently activated protease that catalyzes the specific cleavage of many key cellular proteins. Caspase-3 specifically cleaves downstream apoptotic targets and caspase-6 and − 7, which in turn cleave their respective targets to induce cell death. In addition to being able to activate caspase-6 and − 7, caspase-3 also regulates the activity of caspase-9 and acts through a feedback loop. Caspase-3 is activated in apoptotic cells through extrinsic (ligand) and intrinsic (mitochondrial) pathways. Caspase-3 is cleaved at an aspartate residue to generate p12 and p17 subunits, forming the active enzyme caspase-3. Active caspase-3 cleaves several cellular proteins and is responsible for morphological changes and DNA fragmentation in cells during apoptosis. In this study, we measured the expression level of caspase 3 protein in both pro-caspase 3 and cleaved caspase states to understand whether our treatment activated the cell pathway through the caspase cascade. The results of western blot analysis showed that the amount of active or cleaved caspase 3 in the groups of mice receiving 107 spores of Clostridium novyi-NT was significantly higher than that of tumor mice in the control group (**** P < 0.0001) (Fig. 3-B).
Fig. 3.
Investigating the cellular mechanism involved in the regression of HPV-positive cervical cancer tumors originating from the TC-1 cell line after the 20-day treatment period using Clostridium novyi-NT spores by measuring the proteins involved in the apoptosis pathway and hypoxic conditions using the western blot method. A: One of the best methods to detect the induction of mitochondrial apoptosis by an antitumor agent is to measure the changes in cytochrome c protein expression of tumor cells inside mitochondria and cytosol. After separating the mitochondria of tumor cells from the four different groups, the western blot results showed that the amount of cytochrome c protein in the cytosolic space of tumor cells receiving 107 spores of Clostridium novyi-NT was significantly higher than that in the control and cisplatin (10 mg/kg)-receiving groups. This result can indicate damage to mitochondria in treatment groups receiving spores (both intratumoral and systemic). B: Next, to investigate the possible mechanism involved in the induction of tumor regression, the expression levels of proteins involved in internal apoptosis were measured and compared. The results of the western blot technique clearly indicated that despite slight changes in the expression of the pro-apoptotic caspase 3 protein in the four studied groups, the amount of active caspase 3 or cleaved caspase in the mouse tumor groups receiving 107 spores (either as intratumor and systemic) was significantly increased compared to that in the control group, which can indicate the induction of internal apoptosis as a result of this type of treatment. On the other hand, the western blot results showed that the expression level of the pro-apoptotic protein Bax in the tumor mouse groups receiving 107 spores was significantly higher than the expression level of this protein in the control group. It was also found that the expression level of the Bcl-2 antiapoptotic protein in the spore-receiving groups was statistically significantly reduced compared to that in the control and cisplatin-receiving groups. C: One of the significant obstacles in the treatment of solid tumors using routine methods is the presence of hypoxic areas inside the tumors. This negative characteristic can be measured by measuring the amount of HIF-1α and VEGF proteins. The western blot results showed that fortunately, the expression of both of these proteins in the group of mice receiving 107 spores intratumorally decreased significantly compared to the groups receiving cisplatin and the control. All experiments were performed in triplicate. (* P < 0.05, **P < 0.005, *** P = 0.0001, **** P < 0.0001). (The original full-length, uncut gel and blot are included in the supplemental files.)
Decreased expression levels of HIF-1α and VEGF proteins were observed in mice with HPV-positive cervical cancer tumors (derived from the TC-1 cell line) that were treated with Clostridium novyi-NT spores
Hypoxia-inducible factors (HIFs) have been identified in the hypoxic tumor microenvironment as essential transcription factors that regulate the expression of many genes related to angiogenesis, metastasis, cell proliferation, and resistance to chemotherapy and radiotherapy [25]. HIF-1α promotes cancer cell proliferation, and VEGF induces vascular endothelial cell division to promote tumor growth. High expression of HIF-1α and VEGF in cervical cancer tissues is associated with clinical stage, pathological grade, and lymph node metastasis [26]. To investigate the anti-hypoxic effect of administering Clostridium novyi-NT spores in the treatment of tumor mice, the expression levels of HIF-1α and VEGF proteins were analyzed by western blotting. Beta-actin was used as a loading control. The analysis of the results showed that there was a statistically significant difference in the levels of HIF-1α and VEGF protein expression in the quadruple mouse tumor groups. The expression levels of HIF-1α and VEGF proteins in the group of tumor mice receiving 107Clostridium novyi-NT spores intratumorally were significantly lower than those in the control group and the group of tumor mice receiving cisplatin (* ***P < 0.0001). Additionally, the amount of these two factors in the spore-receiving group decreased systemically compared to that in the control and cisplatin-receiving tumor mouse groups (*** P = 0.0001) (Fig. 3-C).
Discussion
Cervical cancer is one of the main causes of women of reproductive age in developing countries and the fourth most common malignant disease of women in the world [27]. 95% of cervical cancers are related to the HPV virus. With 85% of new cases and deaths directly linked to it, cervical cancer is a major concern in low- and middle-income countries [3]. Although various secondary prevention efforts have been successful in reducing incidence and mortality rates in developed nations, cervical cancer continues to be a primary cause of death related to cancer in women [28]. Improving the treatment and control process of cervical cancer requires more personalization of treatment, identification of factors that trigger relapse and progression, and finally improvement and correction of routine treatment methods [29]. As in other solid tumors, in cervical cancer, hypoxia is a well-known feature and an established therapeutic target. Low tumor oxygenation increases the risk of local invasion, metastasis, and treatment failure. During the development of cancer, hypoxia plays an essential role as a pathophysiological feature [4, 30]. Hypoxia-responsive molecules, known as HIFs, play a crucial role in the response to hypoxia. When facing hypoxic conditions, the combination of HIF-1α and the HIF-1β subunit occurs, leading to dimer formation, activation of gene expression in targeted areas, involvement in cell proliferation, angiogenesis, metabolic processes, immune response against cancer, and resistance to treatment. Vascular endothelial growth factor (VEGF) is a critical player in hypoxia-induced neo angiogenesis, and its promotion is facilitated by HIF-1α [31, 32]. The importance of treatment failure caused by hypoxic microenvironments in all types of solid tumors has led researchers to develop targeted treatment strategies against these areas [33, 34]. Meanwhile, the existence of physiological differences between the cells of the hypoxic and normoxic regions of the tumor is the main feature in the new treatment designs against solid tumors. This important feature has caused the revival of antitumor research based on the ability of obligate anaerobic bacteria to colonize and continue destroying the hypoxic areas of tumor tissue since the beginning of the 21st century [35, 36].
The anaerobic bacterium Clostridium novyi-NT possesses a unique ability to target and thrive in regions of absolute hypoxia and necrosis within solid tumors [13, 19]. The spores of this particular bacterium exhibit selective migration and germination in areas of hypoxic necrosis within tumors, resulting in the regression of the tumor [37]. The toxicological properties of Clostridium novyi-NT are favorable, exhibiting strong oncolytic effects, minimal tumor resistance, and robust targeting abilities. One of the most attractive applications of this bacterium is its potential use as a gene carrier for targeted gene therapy [38]. Clostridium novyi-NT, devoid of the primary toxin (toxin-A) responsible for toxicity, has become a nontoxic strain after its removal. Many studies have shown that combination therapy, such as the combination of Clostridium novyi-NT with traditional chemotherapy [14, 39] or radiation therapy [40, 41], has been effective in improving the results of treatment of various types of cancer. For example, it has been reported that Clostridium novyi-NT in combination with specific microtubule-interacting drugs showed dramatic effects on experimental tumors and caused hemorrhagic necrosis and tumor regression. Therapeutic responses vary depending on the mechanism of action of the microtubule-interacting agent. Thus, microtubule-stabilizing drugs lead to slow tumor regression, while microtubule destabilizers reduce blood flow to tumors and cause rapid hemorrhagic necrosis [39]. The injection of Clostridium novyi-NT inside the tumor led to spore germination and subsequent destruction of injected tumor masses in 10 out of the 24 patients involved in the trial, representing a success rate of 42% [16]. Even with extensive research conducted, there remains an incomplete understanding of the therapeutic mechanisms employed by this bacterium, although it is evident that the bacterium effectively triggers a robust immune response in the host [17]. Alternatively, after germinating in host tumor tissue, the spores of this bacterium destroy the lipid structure of the cell membrane and cause tumor regression by secreting several extracellular proteins, including phospholipase C (PLC) (NT01CX0979) [42]. A research project involving dogs with normal neoplasia discovered that the administration of Clostridium novyi-NT spores via intratumoral injection led to increased phagocytosis and the development of NK cell-like functionality. Administration of Clostridium novyi-NT spores through intravenous injection induces heightened TNF-α production triggered by LPS, elevated IL-10 production caused by LTA, and the development of NK cell-like functionality posttreatment. There is insufficient research to elucidate the cellular and molecular mechanisms implicated in the destruction of tumor cells using this bacterium’s spores [15]. With the aim of improving combined treatment methods based on this spore, our group focused for the first time on the mechanisms of cell lysis caused by the activity of this bacterium within the tumor tissue. As mentioned, the stimulation of host immunity against the tumor during the injection of Clostridium novyi-NT spores has been discussed. Based on our previous study, we predicted that the enzyme phospholipase C (PLC) (NT01CX0979) secreted during the germination of Clostridium novyi-NT spores at the tumor site could play a key role in inducing cancer cells. Independent studies on the antitumor effect of this enzyme related to the Clostridium novyi-NT strain have not been reported thus far. Fortunately, it has been found that this protein has considerable structural similarity with α-toxin related to Clostridium perfringens, about which there is good information. Extensive destruction of the plasma membrane and induction of necrosis have been demonstrated with lytic concentrations of clostridial phospholipase C (PLC) [43]. The results of our research also showed that the amount of necrotic surface in the tumor tissue was related to the tumor mouse groups that received 107 spores of Clostridium novyi-NT either intratumorally or systemically, compared to the control group and the group receiving intraperitoneal cisplatin, which increased significantly (Fig. 1). In addition, research conducted by L Monturiol-Gross et al. revealed a correlation between sublytic phospholipase C (PLC) levels and the activation of the MEK/ERK pathway and the production of reactive oxygen species (ROS) [44]. The results of our research also showed that the amount of reactive oxygen species (ROS) produced in cancer cells treated with Clostridium novyi-NT spores in mice with cervical cancer was significantly higher than that in the control mice and the cisplatin-receiving group (Fig. 2-D). Dysregulated production of bioactive lipids, ultimately leading to cellular malfunction, is observed when subjects are administered low doses of phospholipase C (PLC), according to a report by Marco M. Manni and colleagues. They reported that sphingomyelin (SM) metabolism induced by low-dose phospholipase C (PLC) produces pro-apoptotic mediators such as ceramides (CER), N-acylethanolamine, and saturated fatty acids [45, 46]. The role of CER as a second messenger involved in apoptosis has already been elaborately discussed [47]. Ceramide can induce the release of mitochondrial cytochrome C, thereby activating caspase-3 and inducing programmed cell death [48]. The results that we obtained are very much in line with this research. The results of total ceramide measurement showed that the amount produced in the group of mice that received Clostridium novyi-NT spores as treatment was significantly higher than the amount of this factor in the control and cisplatin-receiving groups (Fig. 2-C). On the other hand, the analysis related to the release of cytochrome c protein from the mitochondria to the cytosolic space showed that the amount of this important quantity in inducing intrinsic cell death in the groups receiving 107Clostridium novyi-NT spores (either intratumorally or intravenously) at the end of the treatment period increased much more than the rats in the control group and receiving cisplatin (10 mg/kg) (Fig. 3-A). Bcl-2 family proteins, tumor necrosis factors, and cell cycle enzymes are crucial in regulating cell proliferation and apoptosis. The interaction between mitochondria and certain Bcl-2 family proteins triggers the liberation of cytochrome c and other apoptosis-inducing factors. Approximately 20 proteins make up the Bcl-2 family, primarily serving as mediators for either promoting or inhibiting apoptosis [49]. The extensive study of the relationship between Bcl-2 family proteins and ceramides stems from their direct involvement in apoptosis. In vitro, the activity of ceramide synthase (CerS) was heightened when pro-apoptotic recombinant Bak was externally added, while anti-apoptotic Bcl-2 protein inhibition triggered C16-ceramide synthesis, which is impressive. As a result, it can be inferred that alternative methods exist for controlling the induction of programmed cell death through ceramide synthesis by the Bcl-2 group [50]. Following this argument, the results of the western blot technique to measure the amount of Bax pro-apoptotic protein showed that the amount of this factor increased significantly in the groups receiving Clostridium novyi-NT spores. At the same time, there was a notable reduction in the levels of Bcl-2 apoptosis-limiting protein in these spore-receiving groups when compared to both the control group and the cisplatin-receiving group. Based on our results, as expected, it was found that the cleaved amount of caspase-3 apoptosis-inducing protein was also significantly increased by treating cervical cancer mice with Clostridium novyi-NT spores. In this study, it was discovered that administering Clostridium novyi-NT spores directly into the tumors of mice resulted in a significant decrease in the levels of HIF-1α and VEGF proteins, which was not observed in the control group that did not receive the sporesFor the treatment of different forms of cancers, including cervical cancer, cisplatin is a commonly prescribed medicine. Oxygen-rich tumors show better response rates, while those lacking oxygen exhibit lower efficacy. The discovery by researchers indicates that cisplatin can trigger tumor cell death by upregulating a specific protein known as p53. However, when tumors lack sufficient oxygen, another protein called HIF-1α causes a significant decrease in p53 levels, resulting in decreased treatment efficacy [51]. Xu et al. conducted a comprehensive analysis of 3352 differentially expressed genes in 306 cervical cancer samples, leading them to propose that the HIF-1 signaling pathway, specifically related to TFRC, may have a crucial role in the development of cervical cancer [52]. In contrast, Liu et al. presented evidence suggesting that HIF-1α and VEGF could serve as valuable indicators for assessing the progression, metastasis, and prognosis of HPV-related cancers. Numerous investigations have demonstrated the significant involvement of VEGF in both angiogenesis and the development of cancer. Conversely, the abnormal expression of GLUT 1, a downstream gene of HIF-1α, in HPV-positive head and neck cancer tumors has been found to enhance the likelihood of invasion and metastasis. Consequently, a reduction in the expression of this gene can be regarded as a positive indication of the efficacy of diverse therapeutic approaches [53]. In summary, Clostridium novyi-NT spores can penetrate, colonize, and germinate after intratumoral or intravenous injection into HPV-positive cervical cancer mice. After germination, these spores begin to induce tumor regression in less than 48 h after injection.
Conclusion
This study has confirmed that the spores of Clostridium novyi-NT, whether administered systemically or intratumorally to mice with TC-1 cell line-derived tumors, returned to the tumor site and initiated germination. Our findings suggest that tumor regression in these conditions may be attributed to the induction of apoptosis through the mitochondrial pathway. Furthermore, we propose that the potential involvement of phospholipase C (PLC) enzyme in the regression of tumors treated with oncolytic Clostridium novyi-NT bacteria should be taken into account in understanding the anticancer effects of spores. According to the evidence presented, it seems that this enzyme could have direct effects on the cell wall, leading to cell lysis, and also trigger the release of mitochondrial cytochrome, further promoting intrinsic apoptosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We feel it necessary to thank the efforts of all the professors and staff of the Department of Pharmaceutical Biotechnology located in the Faculty of Pharmacy of Tabriz University of Medical Sciences, who helped us in doing this research.
Abbreviations
- HPV
Human Papillomavirus
- HIF-1α
Hypoxia-inducible factor 1-alpha
- GLUT-1
Glucose transporter 1
- ROS
Reactive Oxygen Species
- PLC
Phospholipase C
- FBS
Fetal Bovine Serum
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- RT-PCR
Reverse Transcriptase Polymerase Chain Reaction
- H&E
Hematoxylin and Eosin
- VEGF
Vascular Endothelial Growth Factor
- PCR
Polymerase Chain Reaction
- DNA
Deoxyribonucleic acid
- cDNA
Complementary DNA
- SMases
Sphingomyelinases
- CPA
C. perfringens alpha toxin
- IT
Intratumoral
Author contributions
D.A., E.B: Study conception and design. A.A., J.P., E.B., : Acquisition of data. E.B., D.A., A.A., J.P: Analysis and interpretation of data. E.B: Drafting of the manuscript.
Funding
This project (Ph.D. thesis) was financially supported by Tabriz University of Medical Sciences, Tabriz, Iran (Grant Number: 64371).
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Research and Technology Vice-Chancellor of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1398.434). This vice-chancellor has an ethics committee that fully supervises all the ethical aspects of the conducted research (including ethical discussions of working with laboratory animals and ethics in publishing research) with the help of a grant from Tabriz University of Medical Sciences. Additionally, all animal work was performed under the standards of the Iran National Committee for Ethics in Biomedical. All animal experiments comply with the ARRIVE guidelines and carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Global Health. 2023;11(2):e197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haręża DA, Wilczyński JR, Paradowska E. Human papillomaviruses as infectious agents in gynecological cancers. Oncogenic properties of viral proteins. Int J Mol Sci. 2022;23(3):1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull R, Mbele M, Makhafola T, Hicks C, Wang SM, Reis RM, et al. Cervical cancer in low and middle–income countries. Oncol Lett. 2020;20(3):2058–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datta A, West C, O’Connor JP, Choudhury A, Hoskin P. Impact of hypoxia on cervical cancer outcomes. Int J Gynecologic Cancer. 2021;31(11). [DOI] [PubMed]
- 5.Cohen AC, Roane BM, Leath CA. Novel therapeutics for recurrent cervical cancer: moving towards personalized therapy. Drugs. 2020;80:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narva SI, Seppänen MP, Raiko JR, Forsback SJ, Orte KJ, Virtanen JM, et al. Imaging of tumor hypoxia with 18F-EF5 PET/MRI in cervical cancer. Clin Nucl Med. 2021;46(12):952–7. [DOI] [PubMed] [Google Scholar]
- 7.Bossler F, Kuhn BJ, Günther T, Kraemer SJ, Khalkar P, Adrian S, et al. Repression of human papillomavirus oncogene expression under hypoxia is mediated by PI3K/mTORC2/AKT signaling. MBio. 2019;10(1):02323–18. 10.1128/mbio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoppe-Seyler K, Mändl J, Adrian S, Kuhn BJ, Hoppe-Seyler F. Virus/host cell crosstalk in hypoxic HPV-positive cancer cells. Viruses. 2017;9(7):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoppe-Seyler K, Bossler F, Lohrey C, Bulkescher J, Rösl F, Jansen L, et al. Induction of dormancy in hypoxic human papillomavirus-positive cancer cells. Proc Natl Acad Sci. 2017;114(6):E990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bossler F, Kuhn B, Günther T, Kraemer S, Khalkar P, Adrian S, et al. Repression of human papillomavirus oncogene expression under hypoxia is mediated by PI3K/mTORC2/AKT signaling. mBio. 2019;10:e02323–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozal K, Krześlak A. The role of hypoxia-inducible factor isoforms in breast cancer and perspectives on their inhibition in therapy. Cancers. 2022;14(18):4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoption Cann S, Van Netten J, Van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79(938):672–80. [PMC free article] [PubMed] [Google Scholar]
- 13.Sharafabad BE, Abdoli A, Abdolmohammadi Khiav L, Meskini M, Jamur P, Dilmaghani A. Therapeutic potential of Clostridium novyi-NT in Cancer: current knowledge and future perspectives. Curr Cancer Drug Targets. 2023;23(9):682–96. [DOI] [PubMed] [Google Scholar]
- 14.Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci. 2001;98(26):15155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krick EL, Sorenmo KU, Rankin SC, Cheong I, Kobrin B, Thornton K, et al. Evaluation of Clostridium novyi–NT spores in dogs with naturally occurring tumors. Am J Vet Res. 2012;73(1):112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts NJ, Zhang L, Janku F, Collins A, Bai R-Y, Staedtke V, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014;6(249):ra249111–249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeClue AE, Axiak-Bechtel SM, Zhang Y, Saha S, Zhang L, Tung D, et al. Immune response to C. novyi-NT immunotherapy. Vet Res. 2018;49:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janku F, Zhang HH, Pezeshki A, Goel S, Murthy R, Wang-Gillam A, et al. Intratumoral injection of Clostridium novyi-NT spores in patients with treatment-refractory advanced solid tumors. Clin Cancer Res. 2021;27(1):96–106. [DOI] [PubMed] [Google Scholar]
- 19.Feng X, He P, Zeng C, Li Y-H, Das SK, Li B, et al. Novel insights into the role of Clostridium novyi–NT related combination bacteriolytic therapy in solid tumors. Oncol Lett. 2021;21(2):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staedtke V, Roberts NJ, Bai R-Y, Zhou S. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016;3(2):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jafari FA, Abdoli A, Pilehchian R, Soleimani N, Hosseini SM. The oncolytic activity of Clostridium novyi nontoxic spores in breast cancer. BioImpacts: BI. 2022;12(5):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebadi Sharafabad B, Abdoli A, Panahi M, Abdolmohammadi Khiav L, Jamur P, Abedi Jafari F, et al. Anti-tumor effects of Cisplatin Synergist in Combined Treatment with Clostridium novyi-NT spores against hypoxic microenvironments in a mouse model of Cervical Cancer caused by TC-1 cell line. Adv Pharm Bull. 2023;13(4):817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang A, Wu Y, Lai HW, Yew D. Apoptosis–a brief review. Neuroembryology Aging. 2005;3(1):47–59. [Google Scholar]
- 24.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim et Biophys Acta (BBA)-Bioenergetics. 2006;1757(5–6):639–47. [DOI] [PubMed] [Google Scholar]
- 25.Hajizadeh F, Okoye I, Esmaily M, Chaleshtari MG, Masjedi A, Azizi G, et al. Hypoxia inducible factors in the tumor microenvironment as therapeutic targets of cancer stem cells. Life Sci. 2019;237:116952. [DOI] [PubMed] [Google Scholar]
- 26.Huang R, Zhou P-K. HIF-1 signaling: a key orchestrator of cancer radioresistance. Radiation Med Prot. 2020;1(1):7–14. [Google Scholar]
- 27.Zhang S, Xu H, Zhang L, Qiao Y. Cervical cancer: Epidemiology, risk factors and screening. Chin J Cancer Res. 2020;32(6):720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. 2020;112(2):229–32. [DOI] [PubMed] [Google Scholar]
- 29.Adiga D, Eswaran S, Pandey D, Sharan K, Kabekkodu SP. Molecular landscape of recurrent cervical cancer. Crit Rev Oncol/Hematol. 2021;157:103178. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Gao X, Huang Y, Zhu X, Chen Y, Xue L, et al. Tumor microenvironment promotes lymphatic metastasis of cervical cancer: its mechanisms and clinical implications. Front Oncol. 2023;13:1114042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietrobon V, Marincola FM. Hypoxia and the phenomenon of immune exclusion. J Translational Med. 2021;19(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abd GM, Laird MC, Ku JC, Li Y. Hypoxia-induced cancer cell reprogramming: a review on how cancer stem cells arise. Front Oncol. 2023;13:1227884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang L, Zhao S, Wu J, Yu L, Singh N, Yang K, et al. Photodynamic therapy for hypoxic tumors: advances and perspectives. Coord Chem Rev. 2021;438:213888. [Google Scholar]
- 34.Shen Z, Ma Q, Zhou X, Zhang G, Hao G, Sun Y, et al. Strategies to improve photodynamic therapy efficacy by relieving the tumor hypoxia environment. NPG Asia Mater. 2021;13(1):39. [Google Scholar]
- 35.Duong MT-Q, Qin Y, You S-H, Min J-J. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 2019;51(12):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaghoubi A, Ghazvini K, Khazaei M, Hasanian SM, Avan A, Soleimanpour S. The use of Clostridium in cancer therapy: a promising way. Reviews Res Med Microbiol. 2022;33(2):121–7. [Google Scholar]
- 37.Sundaresan A, Le Ngoc M, Wew MU, Ramkumar V, Raninga P, Sum R, et al. A design of experiments screen reveals that Clostridium novyi-NT spore germinant sensing is stereoflexible for valine and its analogs. Commun Biology. 2023;6(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S, Chen Y, Wang J, Tang F, Miao T, Li M. Role of nontoxigenic Clostridium novyi in solid tumor therapy. Reviews Res Med Microbiol. 2014;25(3):71–6. [Google Scholar]
- 39.Dang LH, Bettegowda C, Agrawal N, Cheong I, Huso D, Frost P, et al. Targeting vascular and avascular compartments of tumors with C. novyi-NT and anti-microtubule agents. Cancer Biol Ther. 2004;3(3):326–37. [DOI] [PubMed] [Google Scholar]
- 40.Bettegowda C, Dang LH, Abrams R, Huso DL, Dillehay L, Cheong I et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proceedings of the National Academy of Sciences. 2003;100(25):15083-8. [DOI] [PMC free article] [PubMed]
- 41.Liu G, Bettegowda C, Qiao Y, Staedtke V, Chan KW, Bai R et al. Noninvasive imaging of infection after treatment with tumor-homing bacteria using Chemical Exchange Saturation transfer (CEST) MRI. Magnetic resonance in medicine. 2013;70(6):1690–8. [DOI] [PMC free article] [PubMed]
- 42.Bettegowda C, Huang X, Lin J, Cheong I, Kohli M, Szabo SA, et al. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol. 2006;24(12):1573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takehara M. Study on the interaction between Clostridium perfringens and the host. Nihon Saikingaku Zasshi Japanese J Bacteriol. 2021;76(3):149–60. [DOI] [PubMed] [Google Scholar]
- 44.Monturiol-Gross L, Flores-Díaz M, Pineda-Padilla MJ, Castro-Castro AC, Alape-Giron A. Clostridium perfringens phospholipase C induced ROS production and cytotoxicity require PKC, MEK1 and NFκB activation. PLoS ONE. 2014;9(1):e86475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manni MM, Valero JG, Pérez-Cormenzana M, Cano A, Alonso C, Goñi FM. Lipidomic profile of GM95 cell death induced by Clostridium perfringens alpha-toxin. Chem Phys Lipids. 2017;203:54–70. [DOI] [PubMed] [Google Scholar]
- 46.Navarro MA, McClane BA, Uzal FA. Mechanisms of action and cell death associated with Clostridium perfringens toxins. Toxins. 2018;10(5):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albeituni S, Stiban J. Roles of ceramides and other sphingolipids in immune cell function and inflammation. The Role of Bioactive Lipids in Cancer, Inflammation and Related Diseases. 2019:169 – 91. [DOI] [PubMed]
- 48.Alizadeh J, da Silva Rosa SC, Weng X, Jacobs J, Lorzadeh S, Ravandi A et al. Ceramides and Ceramide synthases in Cancer: focus on apoptosis and autophagy. Eur J Cell Biol. 2023:151337. [DOI] [PubMed]
- 49.Czabotar PE, Garcia-Saez AJ. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat Rev Mol Cell Biol. 2023:1–17. [DOI] [PubMed]
- 50.Abou-Ghali M, Stiban J. Regulation of ceramide channel formation and disassembly: insights on the initiation of apoptosis. Saudi J Biol Sci. 2015;22(6):760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devarajan N, Manjunathan R, Ganesan SK. Tumor hypoxia: the major culprit behind cisplatin resistance in cancer patients. Crit Rev Oncol/Hematol. 2021;162:103327. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Liu T, Wu J, Wang Y, Hong Y, Zhou H. Transferrin receptor-involved HIF-1 signaling pathway in cervical cancer. Cancer Gene Ther. 2019;26(11–12):356–65. [DOI] [PubMed] [Google Scholar]
- 53.Hu K, Babapoor-Farrokhran S, Rodrigues M, Deshpande M, Puchner B, Kashiwabuchi F, et al. Correction: hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget. 2021;12(5):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.