Abstract
Background:
Canine mast cell tumors (MCT) in the skin are classified into cutaneous MCT (cMCT) and subcutaneous MCT (scMCT) types, which exhibit different clinical behaviors. Although these types have been classified only by histology, preoperative differentiation is important for proper surgical planning.
Aim:
To examine the accuracy of differentiating these types based on the gross features before surgery.
Methods:
Gross photographic and histologic features of 52 MCTs (2014–2022) were retrospectively compared between cMCTs and scMCTs. Based on these results, we grossly classified an additional 25 MCTs (2007–2013) into two forms using photographic observations. These observations were then compared with the results of histological classification performed by a blinded pathologist.
Results:
The most notable difference between the two forms was hair loss on the tumor surface. Hair loss was prominent in all 36 cMCTs but minimal or absent in all 16 scMCTs. Histologically, only the cMCT showed prominent follicular reduction due to MCT infiltration. Using the hair loss feature, we classified an additional 25 MCTs: 15 cMCTs, 7 scMCTs, and 3 unclassifiable cases with overlapping features. Agreement with histological classification was 80% (12/15) for cMCT and 100% (7/7) for scMCT. Among the unclassifiable cases, one was cMCT, and two were scMCT. Large tumors (3.5–10.5 cm) were found in two of the three unclassifiable cases and in all three cases without agreement.
Conclusion:
Hair loss on the tumor surface is a distinct feature of cMCT that enables accurate visual differentiation from scMCT, except for some large MCTs. This may assist in surgical planning, specifically for sc-MCT.
Keywords: Dogs, Gross features, Mast cell tumor, Skin
Introduction
Mast cell tumor (MCT) is the most common type of malignant skin tumor in dogs (Willmann et al., 2021; de Nardi et al., 2022). Recently, canine skin MCTs have been categorized into cutaneous MCT (cMCT) and subcutaneous MCT (scMCT) based on their different locations (Willmann et al., 2021). scMCTs reportedly exhibit less aggressive behavior compared to cMCTs (Newman et al., 2007; Thompson et al., 2011; Gill et al., 2020). Additionally, the histological grading used as a crucial prognostic indicator for cMCTs is neither applicable nor useful for scMCTs (Newman et al., 2007; Thompson et al., 2011; Gill et al., 2020; de Nardi et al., 2022). Therefore, scMCT should be classified separately from cMCT in clinical aspects.
Complete surgical excision is the mainstay treatment for both MCT types (de Nardi et al., 2022). When a standard surgical margin was used, the rate of histologically incomplete excision was low (5%–8%) in most low- to intermediate-grade cMCTs (Chu et al., 2020; Saunders et al., 2021), but higher in scMCTs (23%–66%) (Newman et al., 2007; Thompson et al., 2011; Gill et al., 2020; Cherzan et al., 2023; Marconato et al., 2023; Treggiari et al., 2023). Although initial studies suggested that scMCTs rarely recurred (8%–9%) regardless of incomplete excision (Newman et al., 2007; Thompson et al., 2011), recent studies indicated a higher recurrence rate (21%–27%) in incompletely excised cases (Gill et al., 2020; Cherzan et al., 2023). These findings suggest that scMCTs require more careful surgical removal than cMCTs. Furthermore, a recent study reported that 35% of dogs with scMCT had lymph node metastasis at the time of admission (Marconato et al., 2023).
For proper diagnostic or therapeutic planning, clinical diagnosis of scMCTs may be necessary. To date, however, scMCTs have been diagnosed only by histological examination (Willmann et al., 2021), with no description of gross features to assist in clinical diagnosis.
The purpose of this study was to examine the differences in the gross appearance between scMCTs and cMCTs retrospectively and to evaluate the accuracy of the gross diagnosis of scMCTs. In this pilot study, the gross tumor appearance was evaluated by using preserved gross photographs.
Materials and Methods
Histologically diagnosed cases of skin MCTs surgically resected at Aoba Animal Hospital were examined. All multiple or newly developed MCTs in the same dog were included, except for locally recurrent MCTs and multiple skin MCTs after metastatic progression. Cases where gross photographs were not preserved were excluded. Some of the cases have been reported in previous studies with different aims (Itoh et al., 2021).
Study 1
Skin MCTs between November 2014 (the first case of scMCT diagnosis) and December 2022 were classified into cMCT and scMCT based on histopathological diagnosis. Tissue specimens were reviewed to assess the validity of the classification (Willmann et al., 2021). Upon review, two cases originally diagnosed as cMCT were revised to scMCT.
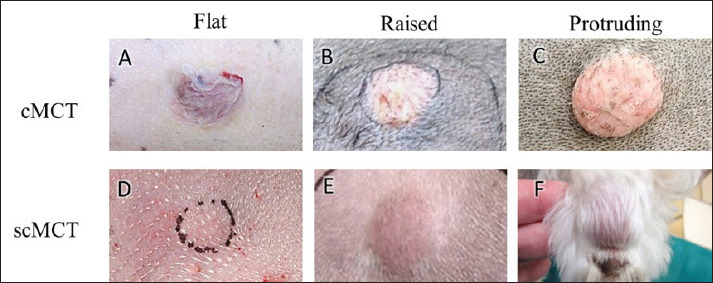
In comparing the two classified groups, we assessed the maximum tumor diameter, gross findings in the photographs, and histological findings. Grossly, tumor elevation from the skin surface was classified into three degrees: 1 (flat), 2 (raised), and 3 (protruding) (Fig. 1). Additionally, we recorded the presence or absence of hair loss, color change, and ulceration on the tumor surface. Histologically, our focus was on the changes in the epidermis and adnexa, and the histological grade (Patnaik’s classification for cMCT, Kiupel’s classification for both cMCT and scMCT) (Patnaik et al., 1984, Kiupel et al., 2011). Loss of hair follicles was classified as follows: 1 (absent), 2 (mild, loss area <25%), 3 (intermediate, loss area 25%–75%), and 4 (severe, loss area >75%).
Fig. 1. Gross images of cMCTs and scMCTs. (A–C) cMCTs with color change and hair loss on the tumor surface, arranged by degree of tumor elevation: flat (A), raised (B), and protruding (C). (D–F) scMCTs with normal appearance on the tumor surface, arranged by degree of tumor elevation: flat (D), raised (E), and protruding (F).
Study 2
Cases histologically diagnosed as skin MCTs between 2007 and 2013, with available gross photographs and tissue specimens, were included. Based on the gross features observed in Study 1, each case was classified into three groups: cMCT, scMCT, and unclassified (cases exhibiting features of both types). Subsequently, a board-certified pathologist (C.J.), blinded to clinical information, classified the tissue specimens into cMCT and scMCT based on microscopic observation, and the concordance rate with the gross classification was assessed.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normal distribution of continuous variables. Normally distributed data are presented as mean ± SD, while non-normally distributed data are presented as median (range). Differences in age, body weight, tumor size, tumor elevation, and hair follicle loss between the cMCT and scMCT groups were analyzed using the Mann-Whitney U test. Hair loss, color change, and ulceration on the tumor surface between the two groups were compared using Fisher’s exact test. Statistical significance was set at p < 0.05. All analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study 1
During the study period, 56 skin MCTs in 38 dogs were diagnosed. Four MCTs (3 cMCTs and 1 dog) were excluded due to the lack of gross photos and one cMCT (1 dog) was excluded due to local recurrence. Thus, 52 skin MCTs in 36 dogs were included in the study: 36 cMCTs in 24 dogs, and 16 scMCTs in 15 dogs. Among these, 3 dogs developed both types at different times.
The signalment for each group is summarized in Table 1. Both groups predominantly consisted of Toy Poodles and Chihuahuas. There was no sex predilection in the cMCT group, while females were predominant (80%) in the scMCT group. No significant differences were observed between the groups in terms of age and body weight.
Table 1. Signalments of dogs with cMCT and scMCT in study-1.
| Dogs with cMCT (n = 24**) |
Dogs with scMCT (n = 15**) |
||
|---|---|---|---|
| Breed* | Toy Poodle Chihuahua Other pure bleeds Mixed breed |
7 4 9 4 |
4 2 5 4 |
| Gender* | male (castrated) female (spayed) |
12 (4) 12 (8) |
3 (2) 12 (10) |
| Age (year) | mean ±SD | 10.1 ± 2.6 | 10.0 ± 3.0 |
| Body weight (kg) | median (range) | 5.7 (2.2–37.8) | 9.8 (3.8–25.3) |
NT: not tested, * indicated by number of dogs, ** Three dogs that developed both MCT types is included in both groups.
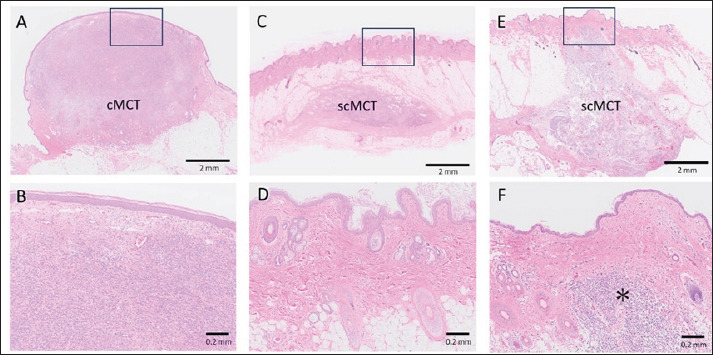
The gross and histological features are shown in Table 2. The tumor size was significantly larger in scMCTs than in cMCTs (p = 0.0083). The degree of tumor elevation tended to be higher in cMCTs but it was not significant. Color change of the tumor surface was more frequent in cMCTs than in scMCTs ( p = 0.0147). The most distinct gross difference between the two groups was hair loss on the tumor surface: hair loss was prominent in all 36 cMCTs but minimal or absent in all 16 scMCTs ( p < 0.0001). Histologically, the majority of tumors in both groups were classified as Kiupel’s low-grade (92% in cMCTs, 88% in scMCTs). In scMCTs, 8 out of 16 (50%) were at least partially adherent to the dermis. Reduction of hair follicles was prominent in cMCTs (moderate in 12%, severe in 88%), while in scMCTs, slight hair follicle reduction was observed only in the two cases that adhered to the dermis and were absent in the others (Fig. 2).
Table 2. Gross and histological features of cMCT and scMCT in study-1.
| cMCT n = 36 |
scMCT n = 16 |
p value | ||
|---|---|---|---|---|
| Gross features | ||||
| Maximal diameter (cm) | Median (range) | 0.9 (0.3–6.8) | 1.95 (0.8–5.1) | 0.0083 |
| Degree of tumor elevation* | 1: flat 2: raised 3: protruding |
6 8 22 |
3 9 4 |
0.0549 |
| Tumor surface change* | Hair loss (%) color change (%) ulcer (%) |
36 (100%) 21 (58%) 8 (22%) |
0 (0%) 3 (19%) 1 (6%) |
<0.0001 0.0147 0.245 |
| Histological features | ||||
| Kiupel's grade* | low (grade1, 2**) high (grade 3**) |
33 (4, 29) 3 (3) |
14 2 |
NT |
| n = 34*** | n = 16 | |||
| Loss of hair follicles* | 1: absent 2: mild or partly 3: intermediate 4: severe (complete) |
0 0 4 30 (15) |
14 2 0 0 |
<0.0001 |
NT: Not tested; * indicated by number of MCTs; ** Patnaik’s grade; *** Two cases could not be evaluated because of inadequate tissue specimen.
Fig. 2. Histological pictures of cMCT (A, B) and scMCT (C–F) (HE stain). (A) Typical lesion of cMCT located in the dermis. (B) Higher magnification of box in A, showing loss of hair follicles due to MCT infiltration. (C) Typical lesion of scMCT located in the subcutis. (D) Higher magnification of box in C, showing normal structure of adnexa. (E) Lesion of scMCT partially attached to the dermis. (F) Higher magnification of box in E. Hair follicles are absent only in the area of tumor infiltration (asterisk).
Study 2
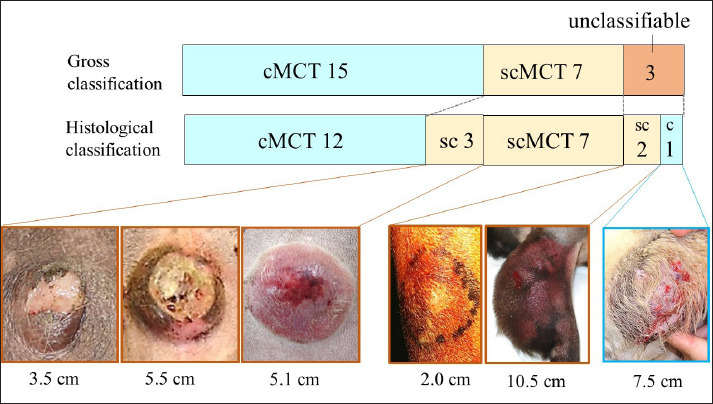
Twenty-five skin MCTs in 23 dogs were included in this study. The results are shown in Figure 3. Focusing on hair loss in the gross photographs, the 25 cases were classified as 15 cMCTs, 7 scMCTs, and 3 cases were deemed unclassifiable due to features of both types. Compared to histological evaluation, the accuracy of these gross classifications was 80% (12/15) for cMCTs and 100% (7/7) for scMCTs, with an overall concordance rate of 86%. Of the three unclassifiable cases, one was histologically determined to be cMCT and two to be scMCT. In the six unclassifiable or non-matching cases, five were large tumors (3.5 to 10.5 cm) with severe changes on the tumor surface.
Fig. 3. Results of comparison between gross and histological classification in study.
Discussion
In Study 1, the majority of both cMCTs and scMCTs were low-grade, as previously reported (Gill et al., 2020; Saunders et al., 2021). The clinical differences between cMCT and scMCT were tumor size, color change, and hair loss, with hair loss being the most distinct feature in cMCT. This can be reasonably explained: cMCTs initially proliferate within the dermis (Willmann et al., 2021), leading to early involvement of hair follicles and subsequent hair loss, whereas scMCTs are typically confined to the subcutaneous tissue (Willmann et al., 2021), preserving the dermal structure. This was histologically evidenced by the prominent reduction of hair follicles observed only in cMCTs.
The gross changes in cMCTs, such as hair loss, likely lead to early detection by owners. In contrast, scMCTs with fewer noticeable skin changes may result in delayed detection. This delay likely explains why scMCTs (median 1.95 cm) were larger than cMCTs (median 0.9 cm). Recent studies supported this observation, reporting larger tumor sizes in scMCTs (median 2.5–3.0 cm) (Cherzan et al., 2023; Marconato et al., 2023; Treggiari et al., 2023) compared to cMCTs (median 1.0 cm) (Chu et al., 2020; Saunders et al., 2021). As larger scMCTs were reportedly associated with shorter survival time (Cherzan et al., 2023; Treggiari et al., 2023), early detection, as well as complete excision, would be clinically important.
In Study 2, a high accuracy rate (86%) for gross diagnosis focusing on hair loss was demonstrated, especially in typical scMCTs without hair loss (7/7). However, diagnosing some large scMCTs was challenging due to skin damage similar to that seen in cMCTs. It has been pointed out that even histology struggles to classify large MCTs, whether they are cMCTs extending deeply or scMCTs reaching the surface (Thompson et al., 2011). Early studies, which focused only on typical scMCTs without dermal invasion, may have been biased towards smaller tumors (Newman et al., 2007; Thompson et al., 2011). Indeed, there are likely larger scMCTs infiltrating into the dermis. For large MCTs with gross skin damage, we think it is important to address them as high-risk cases, regardless of their dermal or subcutaneous origin.
Despite the high rate of incomplete excision in scMCTs (>50%) (Newman et al., 2007; Thompson et al., 2011; Cherzan et al., 2023), the reasons for this have rarely been discussed. Newman et al. (2007) determined that 66% of scMCTs were incompletely excised, consistently at the deep margins. This suggests that scMCTs are located deeper than cMCTs, making it more likely to leave residual deep lesions. In cases clinically diagnosed as scMCT, surgical procedures that ensure the complete excision of deep tumors should be considered.
This pilot study has several limitations. First, the assessment of the tumor appearance was based solely on gross photographs, without direct inspection or palpation. Small scMCTs or those adherent to the dermis (50% in this study) might be difficult to distinguish through palpation. The accuracy of clinical diagnosis based on careful direct inspection and palpation should be examined in prospective studies. Second, the cases in Study 1 were mostly small lesions. Large canine skin MCTs are known to have a wide variety of appearances (de Nardi et al., 2022), so some types may not have been included in this study. Third, given the difficulty in histologically distinguishing large scMCTs (Thompson et al., 2011), it should be considered that the histological classification in some large MCTs may have been quite subjective. Additionally, given the criteria for diagnosing scMCT have shifted from no dermal invasion (Newman et al., 2007; Thompson et al., 2011) to no epidermal involvement (Willmann et al., 2021), the possibility that diagnoses may vary among pathologists should be considered.
Conclusion
The absence of hair loss on the tumor surface is a definitive feature observed only in scMCT, likely serving as a clinical diagnostic indicator for scMCT, except in large MCTs with skin involvement. This may assist in surgical planning, specifically for typical scMCT. To verify the accuracy of clinical diagnosis of scMCTs, further studies involving direct observation and palpation are necessary.
Acknowledgments
Not applicable.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding
No financial support was received for the preparation of this manuscript.
Authors’ contributions
All authors conceived and performed the procedures; drafted and revised the manuscript, and approved the final version.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- doi: 10.1111/vsu.13944. Cherzan, N.L., Fryer, K., Burke, B. and Farrelly, J. 2023. Factors affecting prognosis in canine subcutaneous mast cell tumors: 45 cases. Vet. Surg. 52(4), 531–537. [DOI] [PubMed] [Google Scholar]
- doi: 10.2460/javma.256.5.567. Chu, M.L., Hayes, G.M., Henry, J.G. and Oblak, M.L. 2020. Comparison of lateral surgical margins of up to two centimeters with margins of three centimeters for achieving tumor-free histologic margins following excision of grade I or II cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 256(5), 567–572. [DOI] [PubMed] [Google Scholar]
- doi: 10.3390/cells11040618. de Nardi, A.B., Dos Santos Horta, R., Fonseca-Alves, C.E., de Paiva, F.N., Linhares, L.C.M., Firmo, B.F., Ruiz Sueiro, F.A., de Oliveira, K.D., Lourenço, S.V., De Francisco Strefezzi, R., Brunner, C.H.M., Rangel, M.M.M., Jark, P.C., Castro, J.L.C., Ubukata, R., Batschinski, K., Sobral, R.A., da Cruz, N.O., Nishiya, A.T., Fernandes, S.C., Dos Santos Cunha S.C., Gerardi, D.G., Challoub, G.S.G., Biondi, L.R., Laufer-Amorim, R., de Oliveira Paes, P.R., Lavalle, G.E., Huppes, R.R., Grandi, F., de Carvalho Vasconcellos, C.H., Dos Anjos, D.S., Luzo, Â.C.M., Matera, J.M., Vozdova, M. and Dagli, M.L.Z. 2022. Diagnosis, prognosis and treatment of canine cutaneous and subcutaneous mast cell tumors. Cells 11(4), 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.5326/JAAHA-MS-6960. Gill, V., Leibman, N., Monette, S., Craft, D.M. and Bergman, P.J. 2020. Prognostic indicators and clinical outcome in dogs with subcutaneous mast cell tumors treated with surgery alone: 43 cases. J. Am. Anim. Hosp. Assoc. 56(4), 215–225. [DOI] [PubMed] [Google Scholar]
- doi: 10.1292/jvms.20-0281. Itoh, T., Kojimoto, A., Uchida, K., Chambers, J. and Shii, H. 2021. Long-term postsurgical outcomes of mast cell tumors resected with a margin proportional to the tumor diameter in 23 dogs. J. Vet. Med. Sci. 83(2), 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1177/0300985810386469. Kiupel, M., Webster, J.D., Bailey, K.L., Best, S., DeLay, J., Detrisac, C.J., Fitzgerald, S.D., Gamble, D., Ginn, P.E., Goldschmidt, M.H., Hendrick, M.J., Howerth, E.W., Janovitz, E.B., Langohr, I., Lenz, S.D., Lipscomb, T.P., Miller, M.A., Misdorp, W., Moroff, S., Mullaney, T.P., Neyens, I., O’Toole, D., Ramos-Vara, J., Scase, T.J., Schulman, F.Y., Sledge, D., Smedley, R.C., Smith, K., Snyder, P.W., Southorn, E., Stedman, N.L., Steficek, B.A., Stromberg, P.C., Valli, V.E., Weisbrode, S.E., Yager, J., Heller, J. and Miller, R. 2011. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet. Pathol. 48(1), 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1002/vetr.2991. Marconato, L., Stefanello, D., Solari Basano, F., Faroni, E., Dacasto, M., Giantin, M., Bettini, G., Aresu, L., Bonfanti, U., Bertazzolo, W., Annoni, M., Lecchi, C. and Sabattini, S. 2023. Subcutaneous mast cell tumours: a prospective multi-institutional clinicopathological and prognostic study of 43 dogs. Vet. Rec. 193(1), e2991. [DOI] [PubMed] [Google Scholar]
- doi: 10.1016/j.jcpa.2007.02.003. Newman, S.J., Mrkonjich, L., Walker, K.K. and Rohrbach, B.W. 2007. Canine subcutaneous mast cell tumour: diagnosis and prognosis. J. Comp. Pathol. 136(4), 231–239. [DOI] [PubMed] [Google Scholar]
- doi: 10.1177/030098588402100503. Patnaik, A.K., Ehler, W.J. and MacEwen, E.G. 1984. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet. Pathol. 21(5), 469–474. [DOI] [PubMed] [Google Scholar]
- doi: 10.1111/vco.12630. Saunders, H., Thomson, M.J., O’Connell, K., Bridges, J.P. and Chau, L. 2021. Evaluation of a modified proportional margin approach for complete surgical excision of canine cutaneous mast cell tumours and its association with clinical outcome. Vet. Comp. Oncol. 19(4), 604–615. [DOI] [PubMed] [Google Scholar]
- doi: 10.1177/0300985810387446. Thompson, J.J., Pearl, D.L., Yager, J.A., Best, S.J., Coomber, B.L. and Foster, R.A. 2011. Canine subcutaneous mast cell tumor: characterization and prognostic indices. Vet. Pathol. 48(1), 156–168. [DOI] [PubMed] [Google Scholar]
- doi: 10.1111/vco.12902. Treggiari, E., Valenti, P., Porcellato, I., Maresca, G. and Romanelli, G. 2023. Retrospective analysis of outcome and prognostic factors of subcutaneous mast cell tumours in dogs undergoing surgery with or without adjuvant treatment. Vet. Comp. Oncol. 21(3), 437–446. [DOI] [PubMed] [Google Scholar]
- doi: 10.3389/fvets.2021.755258. Willmann, M., Yuzbasiyan-Gurkan, V., Marconato, L., Dacasto, M., Hadzijusufovic, E., Hermine, O., Sadovnik, I., Gamperl, S., Schneeweiss-Gleixner, M., Gleixner, K.V., Böhm, T., Peter, B., Eisenwort, G., Moriggl, R., Li, Z., Jawhar, M., Sotlar, K., Jensen-Jarolim, E., Sexl, V., Horny, H.P., Galli, S.J., Arock, M., Vail, D.M., Kiupel, M. and Valent, P. 2021. Proposed diagnostic criteria and classification of canine mast cell neoplasms: a consensus proposal. Front. Vet. Sci. 8, 755258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


