Abstract
Background:
Infectious bovine rhinotracheitis (IBR) is a global contagious respiratory disease of ruminants caused by Bovine Herpes virus-1 (BoHV-1). It causes substantial financial losses in the dairy industry worldwide and is considered one of the most important causative agents of abortion and reproductive problems in dairy cattle.
Aim:
This study aimed to estimate the seroprevalence of IBR and the related risk factors in the dairy population in Gharbia governorate, Egypt.
Methods:
A cross-sectional study was conducted to investigate the seroprevalence and associated risk factors of IBR in bovine dairy herds in Qutur district, Gharbia governorate, Egypt from March 2023 to February 2024. A total of 12 smallholder farms and six commercial dairy farms that did not use vaccination protocol against BoHV-1 were randomly selected. Serum samples (n = 400) were collected from 360 cattle and 40 Italian buffaloes and were subjected to evaluation of the serological status of BoHV-1 using indirect ELISA. A multivariate logistic regression model was implemented to evaluate the strength of the risk factors associated with the infection.
Results:
The overall seroprevalence of IBR was 22.5% (95% CI: 18.5%–26.9%). The prevalence of IBR in animals reared under traditional and commercial systems was 28.04% and 21.06%, respectively. The multivariate logistic regression revealed that the risk of infection with IBR in winter months was significantly higher than in autumn [OR = 5.9, CI 95%: 2.22–16.16]. The seroprevalence of IBR was higher in weaned and yearling calves than in adult cattle (p-value = 0.000). The risk of exposure to IBR infection was higher in free stall houses than in tie stall houses [OR = 3.7, CI 95%: 1.11–12.35]. The risk of seropositivity to IBR was significantly higher in animals with a history of recent respiratory manifestation than those without a history of recent respiratory problems (p-value = 0.000).
Conclusion:
This research study revealed that IBR is prevalent among dairy cattle reared under both production systems in the Gharbia governorate. Introducing an appropriate vaccination protocol becomes inevitable to protect our dairy industries from potential economic losses due to this disease.
Keywords: Infectious bovine rhinotracheitis, Risk factors, Egypt, Seroprevalence
Introduction
Livestock animals play a crucial role in the welfare of rural populations worldwide. Bovine respiratory disease (BRD) is a welfare and economic concern for the dairy industry worldwide and is responsible for approximately 50% of total mortalities in feedlot cattle (Ostler and Jones, 2023). It is a multifactorial disease caused by several viral and bacterial pathogens; of them, Bovine Herpes virus-1 (BoHV-1) is considered one of the most globally distributed viral agents (Nagy et al., 2022). BoHV-1 is enrolled within the list of notifiable diseases by the World Organization for Animal Health (OIE) and is considered one of the main causes of trade constraints worldwide (Waldeck et al., 2021). It is endemic in Egypt and it was first described and isolated in the seventies of the 20th century (Sobhy et al., 2014). It belongs to the genus Varicellovirus, in the subfamily Alphaherpesvirinae, under the family Herpesviridae. It is a multi-organ infectious agent of domestic and wild ruminants that is responsible for Infectious bovine rhinotracheitis (IBR), infectious pustular vulvovaginitis (IPV), infectious balanoposthitis, encephalitis, abortion, and infertility problems. IBR remains among the leading causes of economic losses in dairy herds (OIE, 2017). An Irish study estimated a 250 l reduction in annual milk yield per cow in BoHV-1 bulk milk-positive herds (Sayers, 2017). The clinical picture of IBR is represented by respiratory, enteric, and nervous disorders, abortion, reproductive failure, and calf mortalities. After initial infection, it remains latent for life in trigeminal or sciatic ganglia and other non-neural organs such as lymph nodes and reactivates under stressful settings that result in viral excretion to induce primary infection in susceptible animals (Brock et al., 2020). Stress is an important co-factor in the pathogenesis of IBR (Kipyego et al., 2020). Some management and environmental factors contribute to the survival and spread of BHV like group housing, poor bedding, large herd size, overcrowding, transportation, and extreme climatic conditions represented by heat or cold stress (Paudel et al., 2022). Weaning is recognized as a potent stressful time for both beef and dairy calves which arises predominantly from nutritional factors and the breakdown of maternal immunity (Murray et al., 2018). Serological assays such as virus neutralization test (VNT) and ELISA are used broadly for epidemiological surveys. They have the advantage of detecting latent BoHV-1 infection compared with molecular tools. However, ELISA is more sensitive and specific than VNT and is commercially available (Trinidad et al., 2024). Previous studies elucidated the widespread occurrence of BoHV-1 in Egyptian governorates with possible variation in the prevalence status (Elashmawy et al., 2019; Selim et al., 2022). However, the epidemiologic situation of the investigated area is indistinct because of the paucity of studies addressing the seroprevalence and associated risk factors. The epidemiologic data of IBR is greatly important to design suitable control programs for cattle populations in particular areas. Keeping the above points in view, the objective of the present study is to assess the seroprevalence of IBR and identify the related risk factors in the rural cattle populations in Gharbia governorate, Egypt, using indirect ELISA.
Material and Methods
Study design and sample size calculation
This investigation was carried out in Gharbia governorate, particularly in Qutur district from March 2023 to February 2024. Gharbia governorate is located in the northern part of Egypt in the Western Nile Delta (Fig. 1). A cross-sectional study had been conducted on two main rearing systems to investigate the prevalence of IBR. The first was the traditional farm system (smallholder farms), where large ruminants were kept in tie stalls poorly ventilated, closed houses in small groups did not exceed 15 heads. The animals were fed an Egyptian clover twice daily and the concentrate mixture was delivered during the milking time, which was performed manually. The second was the commercial farm dairy system (< 100 heads), where the animals were classified according to age and kept in either open yards or shaded pens. The animals were fed on a total mixed ration, and machine milking was performed. The minimum sample size was calculated presuming, a 95% confidence interval, 50% expected, true population, and a 0.05 margin of error (α) regarding the following formula (Thrusfield, 2018):
Fig. 1. Map of sampling area in Gharbia governorate, Egypt.
where n = Sample size, P exp = Expected true population, and d = Desired margin of error.
Samples and data collection
Six commercial dairy farms and 12 smallholder farms were selected randomly for this survey. All investigated farms did not use vaccination protocol against BoHV-1. A total of 400 blood samples were collected from 360 cattle of different breeds and 40 Italian buffaloes for diagnosis of IBR infection. Out of them, 318 animals were reared in the commercial farm system, and 82 animals were reared in the traditional farm system (Table 1). The blood samples were collected aseptically from the jugular vein in sterile vacutainer tubes without anticoagulant for serum separation. All samples were transported in an ice box to the laboratory of the Virology Department, Animal Health Research Institute, Giza, Egypt. The serum samples were separated and frozen at −20°C until further examination. The data regarding the date of the visit and animals’ breed, age, sex, housing condition, and history of recent respiratory illness were recorded during sample collection.
Table 1. Breeds and numbers of examined animals reared under commercial and production systems in Gharbia governorate, Egypt, for serological investigation of BoHV-1.
| Farm | Animal breeds | No. of examined animals | No. of positive BoHV-1 (%) |
|---|---|---|---|
| Commercial production system | |||
| I | Holstein cattle | 35 | 5 (14.28%) |
| II | Holstein cattle | 90 | 28 (31.1%) |
| III | Italian buffalo | 40 | 14 (35%) |
| IV | Holstein cattle | 70 | 3 (4.28%) |
| V | Holstein cattle | 22 | 4 (18.2%) |
| VI | Holstein cross | 61 | 13 (21.31%) |
| Total | 318 | 67 (21.06%) | |
| Traditional production system* | |||
| Native cattle | 82 | 23 (28.04%) | |
| Total | 400 | 90 (22.5%) | |
*A total of 12 farms that have the same animal breed, housing, and management conditions.
Indirect ELISA
BoHV-1 antibody test kits were obtained from ID vet (Innovative Diagnostics, France, Reference code: IBRS-5P, Lot No: G64). The tests were performed according to manufacturer instructions. Briefly, the antigen-coated microtiter plates were incubated with 10 µl of diluted serum samples (1:10) for 45 minutes at 37°C. The plates were washed three times with at least 300 µl of diluted washing solution (1/20 in distilled water) and incubated with 100 µl of anti-ruminant peroxidase conjugate for 30 minutes at 37°C. The plates were rewashed three times, and 100 µl of substrate solution (TMB) was added to each well for 15 minutes at 21°C. Finally, 100 µl of stop solution was added to stop the reaction. The optical density was measured by a Microtiter plate reader (Biotech 808) at a wavelength of 450 nm. The S/P percentage ≥ 60% is considered positive.
Statistical analysis
The statistical analysis was performed with the SPSS program (SPSS 20 for Windows, SPSS Inc., Chicago, IL, USA). The objected explanatory variables were age, sex, d breeds of the animals, season, housing, type of stall, and history of recent respiratory manifestation. The response variable was the serological status of the examined animals (either seronegative or seropositive) for BoHV-1. The Univariate logistic regression model was used firstly to evaluate the association between each independent variable and the serological status of IBR. The predictors with p values ≤ 0.20 were retained in the final multivariate logistic regression model. The predictors with a p value < 0.05 were considered significant.
Ethical approval
The protocol of animal handling was approved by the Institutional Animal Care and Use Committee at Zagazig University with protocol number (ZU-IACUC/2/F/47/2024).
Results
Seroprevalence of IBR
The seroprevalence of IBR in the Gharbia governorate was 22.5% (90/400) [95% CI: 18.5%–26.9%]. The number of examined animals and the prevalence of the infection in each farm were depicated in (Table 1). In total, 360 cattle of different breeds and 40 Italian buffaloes were tested for BoHV-1 antibodies. As shown in (Table 1), the animal-seroprevalence among the six studied commercial farms was 21.06% (67/318) and 28.04% (23/82) in animals that were reared under the traditional management system. The highest seroprevalence of IBR was reported in farm III, with a percentage of 35% followed by farm II (31.1%). In comparison, the lowest seroprevalence was present in farm IV, with a percentage of 4.28%, (Table 1).
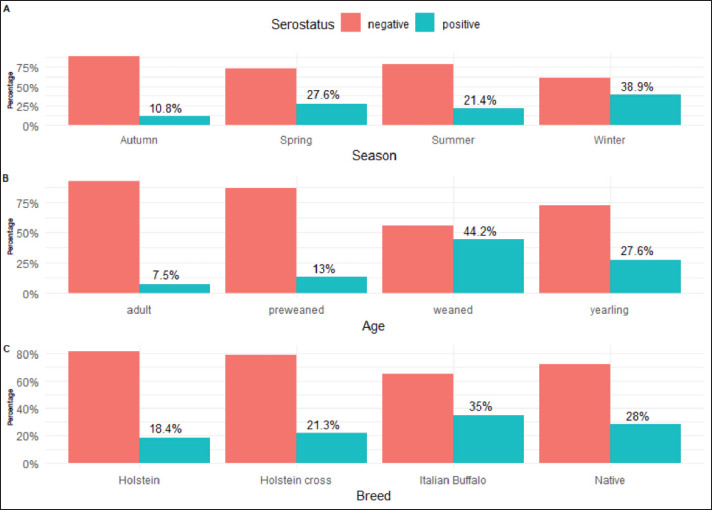
IBR had the highest seroprevalence throughout the winter and spring, with a rate of 38.9% and 27.6%, respectively, whereas the lowest seroprevalence was during autumn, with a rate of 10.79% (Fig. 2A). Regarding the animal’s age, the weaned calves (>3–6 months) represented the highest prevalence for IBR infection with a percentage of 44.2%, followed by yearling animals (>6–18 months) with percentage 27.55% In comparison, the adult animals (≥ 2 years) and pre-weaned calves (≤ 3 months) had the lowest prevalence, with percentages of 7.47% and 13%, respectively (Fig. 2B). The prevalence of IBR was higher in Italian buffaloes (35%) and the native breed (28.04%) and lower in the Holstein breed, with a percentage of 18.43% (Fig. 2C).
Fig. 2. Seroprevalence of BoHV-1 in the studied herds. A: Seroprevalence according to season, B: Seroprevalence according to age, C: Seroprevalence according to breed.
The seroprevalence of IBR was (34.04% and 16.27%) for males and females, respectively (Fig. 3A). Animals that were reared in free stalls had higher seroprevalence than those that were reared in tie stalls with, percentages of 26.54% and 17.24%, respectively (Fig. 3B). On the same line, Animals that were reared in shaded pens or closed houses had higher seroprevalence than those that were reared in open yards, with percentages of 28.78%, 28.04%, and 15.59%, respectively (Fig. 3C). The animals with a history of recent respiratory manifestation had higher seropositivity for BoHV-1 than those without a history of clinical respiratory illness, with percentages of 48.9% and 7.11%, respectively (Table 2).
Fig. 3. Seroprevalence of BoHV-1 in the studied herds. A: Seroprevalence according to sex, B: Seroprevalence according to stall type, C: Seroprevalence according to housing, D: seroprevalence according to history of respiratory illness.
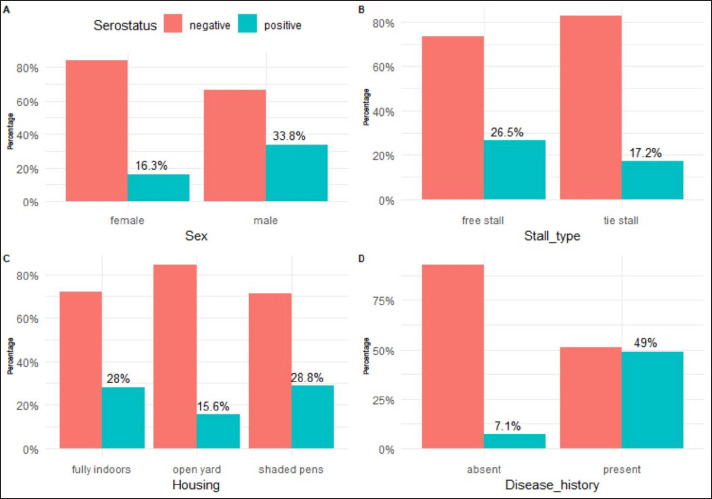
Table 2. The univariate logistic regression model for risk factors associated with BoHV-1 infection.
| Predictor | Level | No. of Ex. animals | No. of positive BoHV-1 (%) | OR1 (95% CI2) | p-value3 |
|---|---|---|---|---|---|
| Environmental factors | |||||
| Season | Autumn | 139 | 15 (10.79%) | Ref | 0.000 |
| Winter | 72 | 28 (38.9%) | 5.26 (2.57–10.75) | ||
| Spring | 105 | 29 (27.6%) | 3.15 (1.58–6.26) | ||
| Summer | 84 | 18 (21.42%) | 2.25 (1.06–4.76) | ||
| Animal factors | |||||
| Age | Adults | 107 | 8 (7.47%) | Ref | 0.000 |
| Pre-weaned | 100 | 13 (13%) | 1.84 (0.73–4.67) | ||
| Weaned | 95 | 42 (44.2%) | 9.8 (4.2–22.4) | ||
| Yearling | 98 | 27 (27.55%) | 4.7 (2.02–10.96) | ||
| Breed | Holstein | 217 | 40 (18.43%) | Ref | 0.07 |
| Italian Buffalo | 40 | 14 (35%) | 2.38 (1.14–4.96) | ||
| Holstein cross | 61 | 13 (21.3%) | 1.19 (0.59–2.41) | ||
| Native | 82 | 23 (28.04%) | 1.72 (0.95–3.11) | ||
| Sex | Females | 258 | 42 (16.27%) | Ref | 0.000 |
| Males | 142 | 48 (34.04%) | 2.51 (1.55–4.05) | ||
| Management factors | |||||
| Stall type | Tie stalls | 174 | 30 (17.24%) | Ref | 0.028 |
| Free stalls | 226 | 60 (26.54%) | 1.73 (1.06–2.83) | ||
| Housing | Open yards | 186 | 29 (15.59%) | Ref | 0.01 |
| Shaded pens | 132 | 38 (28.78%) | 2.18 (1.26–3.78) | ||
| Closed house | 82 | 23 (28.04%) | 2.11 (1.13–3.93) | ||
| History of respiratory illness | Present | 147 | 72 (48.97%) | Ref | 0.000 |
| Absent | 253 | 18 (7.11%) | 0.09 (0.05–0.16) | ||
1OR: Odds ratio; 2CI, confidence interval (95%); 3p-value ≤ 0.20 was used to detect which variables will be retained in the final multivariate model.
Univariate regression analysis
Environmental, animal, and managemental explanatory variables were evaluated separately for their association with the seropositivity of IBR. As shown in Table 2, the animal’s breed, age and sex, season, type of stall, animal housing, and history of respiratory manifestations were considered significant risk factors for infection with IBR (p-value < 0.20). The risk of seasonal occurrence of IBR infection was higher in winter [OR = 5.26, CI 95%: 2.57–10.75] and spring [OR = 3.15, CI 95%: 1.58–6.26] compared with autumn. Both weaned and yearling animals were more susceptible to infection compared to adults.
The Italian buffaloes besides the native and cross-breed cattle were more vulnerable to infection with IBR than Holstein breed (p-value 0.07). Females had a lower risk of infection relative to males [OR = 2.51, CI 95%: 1.55–4.05]. The animals reared in free stalls and closed houses had a higher risk for infection than those reared in tie stall and open yards (p-value 0.01). The odds of infection in animals reared in closed houses or shaded pens were twofold as the infection in animals reared in open yards. The animals with a history of recent respiratory tract infection were 11 times higher for being seropositive for IBR infection compared with their counterparts [OR = 0.09, CI 95%: 0.05–0.16], (Table 2).
Multivariate regression analysis
The final model (Table 3) revealed that the animal’s breed and age besides season, type of stall, and history of respiratory signs were significantly associated with the seroprevalence of IBR (p-value < 0.05). The probability of infection increased by decreasing the ambient temperature. The risk of infection with IBR in the winter and spring months was 5.9 and 2.5 times higher than in autumn. On the other hand, the odds of infection in summer were lower than in autumn by 1.6 times. The weaned and yearling cattle were more exposed to the IBR infection than adult cattle. The risk of exposure to IBR infection was lower in tie stall houses than in free stall houses (p-value 0.03).
Table 3. The final multivariate logistic regression model for risk factors associated with BoHV-1infection.
| Predictor | Level | OR1 (95% CI2) | p-value3 |
|---|---|---|---|
| Environmental factors | |||
| Season | Autumn | Ref | 0.000** |
| Winter | 5.9 (2.22–16.16) | ||
| Spring | 2.5 (0.78–8.0) | ||
| Summer | 0.6 (0.14–2.55) | ||
| Animal factors | |||
| Age | Adults | Ref | 0.000** |
| Pre-weaned | 0.23 (0.03–1.48) | ||
| Weaned | 4.41 (0.7–27.6) | ||
| Yearling | 1.48 (0.47–4.6) | ||
| Breed | Holstein | Ref | 0.02* |
| Buffalo | 7.27 (1.43–26.9) | ||
| Holstein cross | 0.68 (0.17–2.7) | ||
| Native | 0.28 (0.05–1.6) | ||
| Management factors | |||
| Stall type | Tie stalls | Ref | 0.03* |
| Free stalls | 3.7 (1.11–12.35) | ||
| History of respiratory illness | Present | Ref | 0.000** |
| Absent | 0.027 (0.006–0.04) | ||
1OR: Odds ratio; 2CI, confidence interval (95%); 3p-value < 0.05 were considered significant. *p-value < 0.05 were considered significant; **p-value < 0.01 were considered highly significant.
The odds of exposure to the infection in buffaloes were 7.2 times higher than in the Holstein breed. However, the Holstein breed was more exposed to infection than native and cross-breed cattle by 3.5 and 1.4 times (p-value 0.02). The risk of seropositivity to IBR was higher in animals with a history of recent respiratory manifestation than those without a history of recent respiratory problems.
Discussion
The objective of this study was to evaluate the prevalence and potential causes of IBR in Qutur district within Gharbia governorate, Egypt, using indirect ELISA. Qutur is one of the largest districts with the largest number of livestock in Gharbia governorate and one of the largest dairy-producing districts in the Republic.
In this investigation, the seroprevalence of IBR in Gharbia governorate was 22.5%. This finding was consistent with Elashmawy et al. (2019) who found that the seroprevalence of IBR in cattle was 20.13% in Menoufia, Kalubya, and Dakahlia governorates. The obtained results were close to that reported by Zeedan et al. (2018) who found that 27.89% of examined animals were positive for IBR infection in Beni-Suif and El-Fayoum governorates. On the same line, Selim et al. (2022) conducted a serological survey on IBR in Sharkia, Kafr El-Sheikh, and Gharbia governorates and found that the prevalence of the disease was 32.6%, 33.3%, and 26%, respectively. On the contrary, Mahmoud et al. (2009) and El-Shemey and Hassam (2010) reported a higher rate of infection in Giza and El-Behera governorates with percentages of 54.6% and 42.1%, respectively. An earlier study conducted by Fahmy et al. (2006) reported that the seroprevalence of IBR/IPV was 87% in Dakahlia governorate. In Lower Egypt, Ghazy et al. (2007) found that the seroprevalence of IBR was 43.3%. The obtained prevalence did not differ greatly from that reported by previous studies in other countries. In India, the total apparent surveillance of IBR was 61.54% and 30.08% (Krishnamoorthy et al., 2015; Kulkarni et al., 2022), respectively. On the other side, Dima et al. (2024) reported that the prevalence of IBR in Ethiopia was 32.6%. In Saudi Arabia, the sero-surveillance of IBR ranged from 17.4% to 50% (Yousef et al., 2013; Al-Hammadi and Hemida 2014). The difference in animal seroprevalence among different surveys may be due to variations in management systems such as housing type, vaccination protocol, animal breed, herd size, and purpose of animal raising.
The final multivariable model revealed that the animal’s breed and age besides season, type of stall, and history of respiratory illness were considered significant predictors for the infection with IBR (p-value < 0.05). The odds of exposure to the infection in Italian buffaloes were higher than in the Holstein breed. However, the Holstein breed was more exposed to infection than native and Holstein cross breeds. These findings were reciprocally consistent with Ortiz-González et al. (2022) who found that the Holstein breed had the highest seroprevalence of IBR compared to Zebu, Jersy, and Normand cattle breeds. Also, Saravanajayam et al. (2015) found that the native breeds were less susceptible to infection than Jersy and Holstein cross breeds. On the other hand, Selim et al. (2022) who did not find any significant difference between cattle and buffaloes in their susceptibility to the infection. The possible extrapolation from these results is that all cattle and buffalo breeds are susceptible to the infection with IBR; however, the inclination toward a certain breed may be attributed to other circumstances related to the previous exposure to the disease, hygienic and managemental conditions in addition to the immunity status of the examined population (Kaddour et al., 2019).
The probability of infection increased by decreasing the ambient temperature. The risk of infection with IBR in winter and spring was 5.9 and 2.5 times higher than in autumn. This result was consistent with Hashemi et al. (2022) in South Iran who found that the percentage of seropositivity of BoHV-1 was higher in the cold season than warm season. On the contrary, Hekal et al. (2019) did not find any significant difference in the seroprevalence of IBR between summer and winter seasons in imported cattle from Sudan to Egypt. On the other hand, a Sudanese study reported that the seroprevalence of BoHV-1 was significantly higher in the rainy season than winter season (Elhassan et al., 2011). The obtained result may be explained by the fact that the opportunity for survival of BoHV-1 increases when the ambient temperature is lower (Engdawork and Akilu, 2024). In a comprehensive review of the impact of environmental factors on the infection with BRD, Sáfár et al. (2023) concluded that low temperature combined with high relative humidity led to increase the BRD problems within dairy herds. In our country, the nature of fluctuant weather in the spring may be considered as a predisposing factor for infection with IBR which explains the higher odds of infection in spring. This result was boosted by Callan and Gary (2002) who reported that sudden environmental changes increase the probability of infection with respiratory viral diseases.
The age of the animal was considered a potential risk factor for infection with BoHV-1. Most previous studies conveyed that; older animals had higher odds of being positive reactors of BoHV-1 than young animals (Ortiz-González et al., 2022; Barrett et al., 2024). This may be due to the nature of latency of this disease as most of adult animals become seropositive after initial infection (Kalavathi et al., 2024). In contrast, this study revealed that weaned and yearling calves were more exposed to the IBR infection than adult cattle. This result may be imputed to that most of the positive reactors had a near history of BRD infection that was predominantly confined to either pre-weaned, weaned or even fattening calves (Bernal et al., 2023). Also, the age-dependent housing system in dairy herds impedes the spread of the virus between different cattle groups and affects the epidemiologic pattern of BoHV-1 infection within the herds (Waldeck et al., 2021). In the studied commercial herds, calves were classified according to their weaning status and reared in shaded pens while, adult cattle were reared in separated open yards. Another possible explanation was arisen by Woodbine et al. (2009) in West England who conducted a longitudinal sero-epidemiological study of IBR and concluded that the average of seropositivity of adult cattle in unvaccinated herds was less than 50% which may be due to the waning of antibody titer after natural infection over the time.
The risk of exposure to the infection was lower in tie stall houses than in free-stall houses. This result was parallel to what was mentioned by Raaperi et al. (2012) who concluded that loose housing exacerbates the problem of BRD because it permits direct contact between animals and enhances the direct transmission of infectious agents accordingly. Similarly, Maier et al. (2019) reported that calf-to-calf contact in free stall houses was significantly associated with a higher occurrence of BRD in dairy herds. The animals with a history of recent respiratory manifestation had higher odds for being seropositive for IBR infection compared with their counterparts. Many authors demonstrated the alignment between the seropositivity to BoHV-1 and a history of recent respiratory illness. Mahmoud et al. (2009) found a higher seroprevalence of IBR among the clinically diseased cattle compared with the apparently healthy ones. On the same approach, Dima et al. (2024) found that the probability of IBR infection in cattle with respiratory problems was 18 times higher than that in cattle without respiratory problems. This refers to the recrudescence of BoHV-1 as a main cause of BRD infection in cattle.
Although this study focused on the association between respiratory illness and BoHV-1 infection, it neglected other forms of the disease, like reproductive disorders, which may have a great economic impact on the studied herds. Also, Additional studies are required for the molecular diagnosis of BoHV-1. However, the serum dilution of 1:10 may have caused false results due to the probable high concentration of antibodies. Nevertheless, a short incubation period of 45 minutes, as stated in the manufacturer’s protocol, could help mitigate this issue. Buffaloes are a different animal species, so their immune response to IBR can differ slightly from that of cattle. However, the manufacturer protocol does not specialize in certain reassessments for cut-off values for buffaloes. Further investigation using different ELISA kits is recommended to confirm our results and achieve more specificity.
Conclusion
This research study revealed that IBR is prevalent among dairy cattle reared under both production systems in Gharbia governorate. Statistical modeling showed that type of housing, history of recent respiratory illness, age of animals and environmental stressors are the strongest predictors of IBR occurrence. The estimated seroprevalence provides the basis for future surveillance of the disease with recommendations for further comprehensive planned longitudinal researches on BoHV-1 to obtain a complete picture regarding the other forms of the disease and introduce the appropriate vaccination protocol to conserve our dairy industries from potential economic losses due to this disease.
Acknowledgments
The authors are grateful to the animal owners of the private dairy farms and smallholders in Gharbia Governorate, Egypt, for subjecting their animals to the study.
Conflict of interest
No authors declare a conflict of interest.
Funding
This research received no external funding.
Authors’ contributions
Conceptualization, MIE, NZA, MFE, and MIA; methodology, MEE, and MIA. SGY carried out the statistical analysis and interpreted the results. SGY and LB wrote the first draft of the manuscript. MIE, NZA, MFE, and MIA revised and edited the initial manuscript. All authors contributed to the article and approved the submitted version.
Data availability
All findings during our investigation are available in the manuscript.
References
- Al-hammadi, M.A. and Hemida, M.G. 2014. Sero-prevalence of common bovine respiratory viral diseases in Saudi Arabia. AVMJ 60, 76–81. [Google Scholar]
- doi: 10.1038/s41598-023-50433-5. Barrett, D., Lane, E., Lozano, J.M., O’Keeffe, K. and Byrne, A.W. 2024. Bovine herpes virus type 1 (BoHV-1) seroprevalence, risk factor and bovine viral diarrhoea (BVD) co-infection analysis from Ireland. Sci. Rep. 14, 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1016/j.vetmic.2023.109701. Bernal, J.M.C., Fernández, A., Arnal, J.L., Baselga, C., Benito Zuñiga, A., Fernández-Garyzábal, J.F., Vela Alonso, A.I. and Cid, D. 2023. Cluster analysis of bovine respiratory disease (BRD)-associated pathogens shows the existence of two epidemiological patterns in BRD outbreaks. Vet. Microbiol. 280, 109701. [DOI] [PubMed] [Google Scholar]
- doi: 10.1186/s13567-020-00842-5. Brock, J., Lange, M., Guelbenzu-Gonzalo, M., Meunier, N., Vaz, A.M., Tratalos, J.A., Dittrich, P., Gunn, M., More, S.J., Graham, D. and Thulke, H.H. 2020. Epidemiology of age-dependent prevalence of bovine herpes virus type 1 (BoHV-1) in dairy herds with and without vaccination. Vet. Res. 51, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1016/S0749-0720(02)00004-X. Callan, R.J. and Garry, F.B. 2002. Biosecurity and bovine respiratory disease. Vet. Clin. North Am. Food anim. Pract. 18, 57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima, C., Abdesa, K. and Zewde, D. 2024. Bovine Herpesvirus-1 Seroprevalence and its associated risk factors in dairy farms in Holeta Town, Oromia Region, Ethiopia. ASMI. 7, 32-41. [Google Scholar]
- Elashmawy, A., Ali, S., El-Habbaa, A. and Bastawecy, I. 2019. Seroprevalence and molecular detection of isolated BoHV-1 among farm animals. BVMJ 37, 137-–143. [Google Scholar]
- Elhassan, A.M., Fadol, M.A. and El-hussein, A.M. 2011. Seroprevalence of bovine herpes virus-1, bovine herpes virus-4 and bovine viral diarrhea virus in dairy cattle in Sudan. PESR 31, 317–320. [Google Scholar]
- Engdawork, A. and Aklilu, H. 2024. Infectious bovine rhinotracheitis: epidemiology, control, and impacts on livestock production and genetic resources. Vet. Res. Notes. 4, 1–9. [Google Scholar]
- Fahmy, B.G.A., Ali, N.I.L. and Abd el-Fathah, S.A. 2006. Serological and biochemical studies in serum and milk of infected IBR/IPV cattle. AVMJ. 52, 109–127. [Google Scholar]
- Ghazy, A.A., Ahmed, W.M., Mahmoud, M.A. and Lamia A.A. 2007. Prevalence of infectious bovine rhinotracheitis and bovine viral diarrhoea viruses in female buffaloes with reproductive disorders and parasitic infections. Int. J. Dairy Sci. 2, 339–347. [Google Scholar]
- doi: 10.22092/ARI.2022.356904.1941. Hashemi, M., Bakhshesh, M. and Manavian, M. 2022. Bovine viral diarrhea virus and bovine herpes virus-1 in dairy cattle herds in fars province, southern iran: seroprevalence and evaluation of risk factors. Arch. Razi Inst. 77, 1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.5455/javar.2019.f318. Hekal, S.H.A., Al-Gaabary M.H., El-Sayed, M.M., Sobhy, H.M. and Fayed, A.A.A. 2019. Seroprevalence of some infectious transboundary diseases in cattle imported from Sudan to Egypt. J. Adv. Vet. Anim. Res. 15, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.5455/javar.2019.f312. Kaddour, A., Bouyoucef, A., Fernandez, G., Prieto, A., Geda, F. and Moula, N. 2019. Bovine herpesvirus 1 in the northeast of Algiers, Algeria: seroprevalence and associated risk factors in dairy herd. JAVAR 6, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalavathi, S., Kumari, G.D., Latha, K.S., Devi, B.V. and Tanuja, N. 2024. Sero-prevalence of infectious bovine rhinotracheitis in bovines of Palnadu district, Andhra Pradesh. Int. J. Vet. Sci. Anim. Husb. 9(1), 476–479. [Google Scholar]
- doi: 10.1016/j.prevetmed.2019.104863. Kipyego, E.S., Gitau, G., Vanleeuwen, J., Kimeli, P., Abuom, T.O., Gakuya, D., Muraya, J. and Makau, D. 2020. Sero-prevalence and risk factors of infectious bovine rhinotracheitis virus (type 1) in Meru County, Kenya. Prev. Vet. Med. 175, 104863. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy, P., Patil, S., Shome, R. and Rahman, H. 2015. Sero-epidemiology of infectious bovine rhinotracheitis and brucellosis in organized dairy farms in southern India. Indian J. Anim. Sci. 85, 695–700. [Google Scholar]
- Kulkarni, A., Golaviya, A. and Mayur J. 2022. Serological investigation of infectious bovine rhinotracheitis in Bovines in Gujarat state of India. J. Pharm. Innov. 11, 954–957. [Google Scholar]
- Mahmoud, M.A., Mahmoud, N.A. and Allam, A.M. 2009. Investigations on infectious bovine rhinotracheitis in Egyptian Cattle and Buffaloes. Glob. Vet. 3, 335–340. [Google Scholar]
- doi: 10.3168/jds.2018-14773. Maier, G.U., Love, W.J., Karle, B.M., Dubrovsky, S.A., Williams, D.R., Champagne, J.D., Anderson, R.J., Rowe, J.D., Lehenbauer, T.W., Van Eenennaam, A.L. and Aly, S.S. 2019. Management factors associated with bovine respiratory disease in preweaned calves on California dairies: the BRD 100 study. JDS 102, 7288–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1186/s12917-018-1372-9. Murray, G.M., More, S.J., Clegg, T.A., Earley, B., O’Neill, R.G., Johnston, D., Gilmore, J., Nosov, M., McElroy, M.C., Inzana, T.J., and Cassidy, J.P. 2018. Risk factors associated with exposure to bovine respiratory disease pathogens during the peri-weaning period in dairy bull calves. BMC Vet. Res. 14, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1371/journal.pone.0267036. Nagy, A., Abdallah, F., El Damaty, H.M., Tariq, A., Merwad, A.M.A., Alhatlani, B.Y. and Elsohaby, I. 2022. Genetic characterization of upper respiratory tract virome from nonvaccinated Egyptian cow-calf operations. PLoS One. 17, e0267036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE, World Organization for Animal Health. 2017. Infectious bovine rhinotracheitis/ infectious pustular vulvovaginitis. Terrestrial manual. Paris, France: OIE. [Google Scholar]
- doi: 10.3390/v15020552. Ostler, J.B. and Jones, C. 2023. The bovine herpesvirus 1 latency-reactivation cycle, a chronic problem in the cattle industry. Viruses 15(2), 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.14202/vetworld.2022.1550-1556. Ortiz-González, A.D., Buitrago, H.A.L., Bulla-Castañeda, D.M., Lancheros-Buitrago, D.J., Garcia-Corredor, D.J., Díaz-Anaya, A.M., Tobón-Torreglosa, J.C., Ortiz-Ortega, D. and Pulido-Medellín, M.O. 2022. Seroprevalence and risk factors associated with bovine herpesvirus 1 in dairy herds of Colombia. Vet. World. 15, 1550–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel, S., Subedi, D., Shrestha, S., Acharya, M., Chandran, D. and Dhama, K. 2022. Seroprevalence and risk factors of infectious bovine rhinotracheitis in dairy cattle of Chitwan, Nawalpur and Rupandehi Districts of Nepal. J. Exp. Biol. Agric. Sci. 10, 1100–1108. [Google Scholar]
- doi: 10.1186/1751-0147-54-4. Raaperi, K., Bougeard, S., Aleksejev, A., Orro, T. and Viltrop, A. 2012. Association of herd BRSV and BHV-1 seroprevalence with respiratory disease and reproductive performance in adult dairy cattle. Acta Vet. Scand. 54, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáfár, J., Hejel, P., Vass-Bognár, B., Kiss, L., Seregi, B. and Könyves, L. 2023. The impact of environmental factors on bovine respiratory disease complex in dairy calves-a review. Acta Vet. Brno. 92, 213–231. [Google Scholar]
- doi: 10.14202/vetworld.2015.1416-1419. Saravanajayam, M., Kumanan, K. and Balasubramaniam, A. 2015. Seroepidemiology of infectious bovine rhinotracheitis infection in unvaccinated cattle, Vet. World. 8, 1416–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.3168/jds.2016-11113. Sayers, R.G. 2017. Associations between exposure to bovine herpesvirus 1 (BoHV-1) and milk production, reproductive performance, and mortality in Irish dairy herds. J. Dairy Sci. 100, 1340–1352. [DOI] [PubMed] [Google Scholar]
- Selim, A.M., Shoulah, S.A., Mostafa, A.M. and Hamdy, A.S. 2022. Sero-surveillance Infectious Bovine rhinotracheitis in Ruminants and assessment the associated risk factors. BVMJ, 42, 160–163. [Google Scholar]
- Sobhy, N.M., Mor, S.K., Mohammed, M.E.M., Bastawecy, I.M., Fakhry, H.M., Youssef, C.R.B. and Goyal, S.M. 2014. Comparative molecular characterization of bovine herpesvirus-1 strains from Egypt and the United States. Life Sci. 11, 493–499. [Google Scholar]
- doi: 10.2460/ajvr.23.08.0177. Trinidad, S.E.L., Bravo, C.B., Narvasta, S.F., Fuertes, E.H., Trigoso, G.A., Sáenz, F.C. and Quispe-Ccasa, H.A. 2024. Seroprevalence of reproductive and infectious diseases in cattle: the case of Madre de Dios in the Peruvian southeastern tropics. Am. J. Vet. Res. 85(4). [DOI] [PubMed] [Google Scholar]
- Thrusfield, M. 2018. Veterinary epidemiology: Fourth edition, Newyork, NY: John Wiley & Sons Ltd.. [Google Scholar]
- doi: 10.3389/fvets.2021.688935. Waldeck, H.W.F., van Duijn, L., van den Heuvel-van den Broek, K., Mars, M.H., Santman-Berends, I.M.G.A., Biesheuvel, M.M. and van Schaik, G. 2021. Risk factors for introduction of bovine herpesvirus 1 (BoHV-1) into cattle herds: a systematic european literature review. Front. Vet. Sci. 8, 688935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1186/1746-6148-5-5. Woodbine, K.A., Medley, G.F., Moore, S.J., Ramirez-Villaescusa, A.M., Mason, S. and Green, L. E. 2009. A four year longitudinal sero-epidemiological study of bovine herpesvirus type-1 (BHV-1) in adult cattle in 107 unvaccinated herds in south west England. BMC Vet. Res. 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef, M.R., Mahmoud, M.A., Ali, S.M. and AlBlowi, M.H. 2013. Seroprevalence of some bovine viral respiratory diseases among nonvaccinated cattle in Saudi Arabia. Vet. World 6, 1–10. [Google Scholar]
- Zeedan, G.S.G., Abdalhamed, A.M., Ghazy, A.A. and Ghoneim, N.H. 2018. Serological and molecular identification of Infectious Bovine Rhinotracheitis virus isolation and adaptation in embryonated chicken eggs. J. Antivir. Antiretrovir. 10, 12–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All findings during our investigation are available in the manuscript.


