Abstract
Background
Recurrent miscarriage (RM) is defined as the loss of three or more consecutive pregnancies. Further research is required to understand the causes of RM, which remain unknown for many couples. Human chorionic gonadotrophin (hCG) is vital for maintaining the corpus luteum, but may have additional roles during implantation which support its use as a therapeutic agent for RM.
Objectives
To determine the efficacy of hCG in preventing further miscarriage in women with a history of unexplained RM.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 September 2012) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials investigating the efficacy of hCG versus placebo or no treatment in preventing RM. Quasi‐randomised trials are included. Cluster‐randomised trials and trials with a cross‐over design are excluded.
Data collection and analysis
Two review authors independently assessed trials for inclusion and assessed the methodological quality of each study. Date were extracted by two review authors and checked for accuracy.
Main results
We included five studies (involving 596 women). Meta‐analysis suggested a statistically significant reduction in miscarriage rate using hCG.The number of women needed to treat to prevent subsequent pregnancy loss was seven. However, when two studies of weaker methodological quality were removed, there was no longer a statistically significant benefit (risk ratio 0.74; 95% confidence interval 0.44 to 1.23). There were no documented adverse effects of using hCG.
Authors' conclusions
The evidence supporting hCG supplementation to prevent RM remains equivocal. A well‐designed randomised controlled trial of adequate power and methodological quality is required to determine whether hCG is beneficial in RM.
Keywords: Female; Humans; Pregnancy; Abortion, Habitual; Abortion, Habitual/prevention & control; Chorionic Gonadotropin; Chorionic Gonadotropin/therapeutic use; Randomized Controlled Trials as Topic; Tocolytic Agents; Tocolytic Agents/therapeutic use
Plain language summary
Human chorionic gonadotrophin hormone for preventing recurrent miscarriage
Miscarriage is the loss of a pregnancy before 24 weeks of gestation. Recurrent miscarriage (RM) is the loss of three or more consecutive pregnancies, which can cause significant physical and psychological harm with increased depression, anxiety and lowered self‐esteem. RM can be linked to systemic maternal disease, such as diabetes mellitus, thyroid disease and polycystic ovary syndrome. In many cases, the cause of RM may remain unknown despite thorough investigations. Current strategies for preventing RM include the administration of hormones involved in maintaining pregnancy, one of which is human chorionic gonadotrophin (hCG). This hormone is important for the continued production of progesterone from the corpus luteum and may have a role in the implantation of the embryo.
This review included five randomised controlled studies, involving 596 women. When comparing the women who were treated with hCG versus placebo or no treatment, we found a benefit in using hCG. However, when two of the older studies with weaker methodology were excluded, there was no longer evidence of benefit in using hCG for preventing RM. As a result, we were unable to make firm recommendations. There were no documented adverse effects associated with using hCG. More good quality studies with larger sample sizes are needed in order to evaluate the use of hCG compared with other treatments and non‐pharmacological strategies, such as early and accessible carer contact and support.
Background
Description of the condition
Miscarriage is defined by the World Health Organization as the loss of a pregnancy before 24 weeks of gestation (WHO 1992). It has been estimated that 15% to 20% of all clinical pregnancies end in miscarriage (Carrington 2005). These pregnancy losses are usually sporadic, often unavoidable, and may be due to underlying chromosomal or structural abnormalities (Hogge 2003). However, 1% to 3% of couples are affected by recurrent miscarriage (RM), defined as three or more consecutive early pregnancy losses (Berry 1995; Carrington 2005). For couples affected by RM the psychological impact can be profound, with increased depression, anxiety and lowered self‐esteem being reported (Serrano 2006).
The mechanisms underlying RM remain poorly understood. In 50% of cases the cause is unknown (Carrington 2005). As such, further research is needed to explore the mechanisms involved in maintaining a successful pregnancy.
Aetiology of recurrent miscarriage
One cause of RM that cannot be targeted with therapeutics is chromosomal rearrangements. Karyotype abnormalities are thought to occur in approximately 4% of couples, with balanced reciprocal or Robertsonian translocations being the most common (RCOG 2011). Increasing pregnancy losses due to fetal aneuploidy are seen with both advancing maternal and paternal age (Clifford 1997).
Current interventions to prevent further pregnancy losses are centred upon known causes of pathology. Recurrent miscarriage is commonly linked to systemic maternal diseases, such as poorly controlled diabetes mellitus, untreated thyroid disease, obesity, and systemic lupus erythematosus (Tien 2007). Gynaecological factors such as amenorrhoea prior to pregnancy and polycystic ovary syndrome (PCOS) are associated with an increased risk of miscarriage (Balen 1993). The association between PCOS and RM may be attributed to endocrine dysfunction, with insulin resistance, hyperinsulinaemia and hyperandrogenaemia being implicated (RCOG 2011). Anatomical variants, such as a uterine septum can also cause RM (Tan 2010). Prothrombotic factors have been associated with RM. These include inherited thrombophilias, such as activated protein C resistance, deficiency in protein C/S, deficiency in anti‐thrombin III, hyperhomocysteinuria and prothrombin gene variants (Carrington 2005). Additionally, acquired thrombophilias such as antiphospholipid syndrome are also associated with recurrent pregnancy loss (Tan 2010).
Recurrent miscarriage is multifactorial in aetiology, with factors such as maternal smoking, alcohol consumption, illicit drug use, caffeine ingestion and certain prescription medications potentially contributing (RCOG 2011).
More recent research has explored the mechanisms underlying RM of unknown aetiology. One area of interest is the role of the immune system, where a higher predominance of natural killer (NK) cells has been found in the decidual tissue of women with RM (Carrington 2005). However, the levels of NK cells vary widely from person to person, making the development of a measurable assay not viable at present. Conformity between maternal and paternal human leucocyte antigens (HLA) has also been reported in RM, giving rise to ‘HLA‐sharing’ as a potential aetiology (Toth 2010). Other factors under investigation include nuclear hormone receptors, and the presence of circulating microparticles which have already been associated with pre‐eclampsia (Toth 2010).
A long‐standing construct in RM is luteal‐phase insufficiency, where abnormal levels of progesterone and human chorionic gonadotrophin (hCG) hormone result in pregnancy loss (Tan 2010). As a consequence of this, the use of either hormone has been proposed as a means of treating RM.
Description of the intervention
Human chorionic gonadotrophin hormone (hCG) is a glycoprotein hormone secreted by the syncytiotrophoblast (Rosevar 1999). The hormone has been found to impact positively upon the continued production of progesterone and implantation of the embryo (Oon 2000). Endometrial function is closely regulated by the corpus luteum (CL). A suboptimal level of hCG might therefore indirectly affect endometrial receptivity. This supports the role of hCG as a treatment for RM.
How the intervention might work
After ovulation, the Graafian follicle becomes the CL. The endocrine importance of the CL was first demonstrated in early landmark work using rabbit embryos (Corner 1929). A later review of studies in humans and other primates found that luteectomy prior to seven weeks of gestation consistently resulted in miscarriage (Csapo 1978). The CL is known to have an active role in secreting oestradiol, progesterone, relaxin and inhibins, in preparation for implantation of the fertilised ovum (Licht 2001). With successful fertilisation and implantation, trophoblast cells secrete hCG into the maternal circulation (Stocco 2006). Serum levels of hCG rise rapidly following implantation, resulting in persistence of the CL (Lustbader 1998). This is necessary to maintain progesterone levels and promote a stable endometrium until the placenta can support the pregnancy alone by six to eight weeks after implantation (Oon 2000). Human chorionic gonadotrophin production peaks at eight to 10 weeks of gestation and plateaus as pregnancy continues (Lustbader 1998). If no conception occurs, the CL is unsupported, resulting in diminished production of progesterone and commencement of menses.
Interestingly, the levels of hCG present in the circulation after implantation are in excess of those required for the maintenance of the CL (Lustbader 1998). This has led to recent speculation that hCG may have other functions, including a more direct involvement in implantation. Successful implantation is a two‐way process involving both the embryo and endometrium. Post‐fertilisation, the developing blastocyst sheds its surrounding zona pellucida in preparation for adherence to the endometrium (Diedrich 2007). By the eight‐cell stage of development, embryonic cells already transcribe hCG‐subunits, one of the earliest endocrine products. Syncytiotrophoblast cells then begin secreting hCG as part of trophoblastic differentiation in the blastocyst. As such, paracrine effects of hCG on the endometrium may precede its classical role of CL ‘rescue’ (Licht 2001).
At the endometrial level, the presence of oestrogen and progesterone creates an environment suitable for implantation between days five and 10 post‐ovulation: the ‘implantation window’ (Diedrich 2007). Human chorionic gonadotrophin secreted by the blastocyst can bind to the endometrium at G‐protein coupled surface receptors. Their appearance corresponds to the secretory phase of the endometrium, with glandular and stromal tissue expressing these receptors during the implantation window. Insulin‐like growth factor binding protein‐1 (IGFBP‐1) is secreted by endometrial stromal cells predominantly during the implantation window, with peak levels occurring just prior to the end of the fertile period (Licht 2007). IGFBP‐1 is therefore a good marker for decidualisation. In experimental work, hCG has been demonstrated to significantly inhibit IGFBP‐1. As such, hCG may be secreted by the blastocyst to forestall the production of IGFBP‐1 associated with the close of the implantation window, thus prolonging the time period available for potential implantation. Human chorionic gonadotrophin may also have a direct impact upon implantation through the stimulation of protease enzymes implicated in endometrial breakdown (Licht 2007).
During the decidualisation process, cytokines contribute to angiogenesis, which is essential for supporting pregnancy and assisting placental development (Zygmunt 2003). At a molecular level hCG shares sequence homology with several of these cytokines, such as vascular endothelial growth factor (VEGF) (Oon 2000). The presence of hCG has been found to induce significant increases in endometrial VEGF levels (Licht 2007).
It therefore appears that hCG has a pivotal role in early pregnancy, ensuring effective maintenance of the CL, as well as preparation for implantation and endometrial support.
Why it is important to do this review
Recurrent miscarriage is a relatively common condition and is associated with both physical and psychological morbidity. Regardless of the presence of specific pathology, all pregnancies are underpinned by elements of endometrial receptivity, implantation and hormonal secretion, in each of which hCG has a key role. As such, this review investigates the use of hCG as a prophylactic agent to prevent further pregnancy loss in women with a history of RM of unknown aetiology. This review has excluded studies where hCG has been used to treat women with symptoms of a threatened miscarriage with the objective of preventing subsequent pregnancy loss, as this is covered elsewhere (Devaseelan 2010).
Objectives
To assess the efficacy and safety of prophylactic hCG in women with a history of recurrent miscarriage (RM) of unknown aetiology.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials evaluating the efficacy of human chorionic gonadotrophin (hCG) in the prevention of further miscarriage in women with a history of unexplained RM of unknown aetiology. In a departure from the protocol, this review includes studies only investigating hCG for the prevention of RM rather than its management. The Cochrane review by Devaseelan 2010 presents the results of hCG for the treatment of threatened miscarriage. Quasi‐randomised trials are included in this review. Cluster‐randomised trials and trials with a cross‐over design are excluded.
Types of participants
Women with a history of three or more consecutive unexplained miscarriages prior to 24 weeks of gestation, who had a confirmed pregnancy. The target population of this review were women with truly unexplained miscarriage after routine investigations. In a change from the protocol, this review has not included studies where participants had any factors known to contribute to RM. As such, participants in the included studies were investigated for factors contributing to RM prior to their involvement. This included pelvic imaging, screening for systemic diseases, testing for immunological abnormalities, endocrine assays, bacteriological and virology testing, and chromosomal analysis. Any studies involving patients with known causes of miscarriage, such as thrombophilia or PCOS, were excluded from the review. Studies including patients undergoing in vitro fertilisation (IVF) cycles were also excluded from the analysis since this population of subfertile patients may have additional risk factors for RM over and above women conceiving spontaneously. None of the included studies involved multiple pregnancies.
Types of interventions
Randomised controlled trials investigating the efficacy of hCG versus placebo or no treatment in preventing RM. Studies comparing hCG to any active treatment were excluded. Studies included used hCG as an injectable preparation, administered in any dose and regimen. Studies using hCG prior to conception were to be included as long as they continued hCG treatment into pregnancy with the specific aim of trying to prevent miscarriage, although no relevant studies were identified.
Types of outcome measures
Primary outcomes
First trimester pregnancy loss (less than 12 completed weeks of gestation)
Second trimester pregnancy loss (12 to 24 completed weeks of gestation)
Stillbirth (greater than 24 completed weeks of gestation)
Secondary outcomes
Threatened miscarriage
Low birthweight (less than 2500 g)
Prematurity (gestation less than 37 completed weeks)
Neonatal death (less than 28 days of delivery)
Adverse effects: maternal and fetal
Cost
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 September 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We retrieved additional references, cited in papers identified through the above search strategy. These references have been used in the background and discussion sections of this review. We did not apply language restrictions.
Data collection and analysis
Selection of studies
Studies were selected by reviewing all of the studies identified as a result of the search strategy. A decision to select the study was made if its content met the defined inclusion and exclusion criteria of this review.
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. The review authors were blinded to the journal and institution of origin. We resolved any disagreement through discussion. Where necessary, we contacted the authors of the research in question to provide further information. We used a language translation service where necessary.
Data extraction and management
We collected data from the selected studies using a proforma designed by the review authors. For eligible studies, two review authors extracted data to each proforma, in accordance with Cochrane Collaboration guidelines. We resolved discrepancies through discussion. We entered data into Review Manager (RevMan 2011) software and checked it for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. If disagreements could not be resolved, we planned to involve the third author, although this was not required.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. more than 20% missing data; numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made judgements about whether studies were at high risk of bias according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratios with 95% confidence intervals.
Continuous data
For continuous data, we planned to use mean difference if outcomes were measured in the same way between trials, and standardised mean difference to combine trials that measured the same outcome, but used different methods. However, there were no continuous data to analyse.
Unit of analysis issues
Cluster‐randomised trials and cross‐over studies were not eligible for inclusion.
In future updates, if we include trials involving women with multiple pregnancies the unit of analysis will be the pregnancy.
Dealing with missing data
For included studies, we noted levels of attrition. In all the included studies, all of the women who were randomised, completed the study.
In future updates of this review, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity between studies using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes, we will use the test proposed by Egger 1997, and for dichotomous outcomes, we will use the test proposed by Harbord 2006. If asymmetry is detected in any of these tests or is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. Where the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we undertook subgroup and sensitivity analyses. We considered whether the overall summary was meaningful, and used random‐effects analysis.
We planned to carry out the following subgroup analyses, although we were restricted by the small number of included studies. As more data become available, future updates of this Cochrane review will include these analyses.
Duration of hCG administration (long course verus short course)
Total dose of hCG administered (low dose versus high dose)
Use of pre‐conceptual hCG versus no prior use of hCG
Age of participants (below 20 years versus 21 to 30 years versus 31 to 40 years versus 41 years plus)
Body mass index of participants (underweight versus normal versus overweight versus obese)
Multiple versus singleton pregnancies
Sensitivity analysis
We performed sensitivity analysis based upon the quality of the trials. We defined high‐quality trials as having low risk of bias for allocation concealment, blinding and randomisation technique.
Results
Description of studies
SeeCharacteristics of included studies; Characteristics of excluded studies.
Results of the search
The search strategy retrieved 13 trial reports relating to 12 studies. Five studies (involving 596 women) are included, five are excluded, and two trials are awaiting classification
Studies awaiting classification
Two trials are awaiting classification (El‐Zibdeh 1998; El‐Zibdeh 2002). The 1998 study reports the results of a randomised controlled trial (RCT) comparing progesterone with human chorionic gonadotrophin (hCG) and placebo. These results are given in an abstract of the proceedings of the 16th World Congress on Fertility and Sterility 1998. The 2002 study details the findings of a RCT comparing dydrogesterone, hCG and placebo. These results are given as an abstract of a poster presentation at the 10th World Congress on Menopause. The full texts of these studies were not available to review. We have contacted the authors to establish more information about these studies. A later study by the same author (El‐Zibdeh 2005) is included in the review.
Included studies
SeeCharacteristics of included studies.
Participants
Five studies (involving 596 women) met the inclusion criteria (El‐Zibdeh 2005; Harrison 1985; Harrison 1992; Quenby 1994; Svigos 1982).
Three of the studies defined recurrent miscarriage (RM) as three or more consecutive pregnancy losses, while the studies by Quenby 1994 and Svigos 1982 included women with two or more miscarriages. Across each of the included studies, a total of 302 women were randomised with 151 women receiving hCG and 151 women in the control group. Four of the studies took place in single centres, while the Harrison 1992 study presented the results of an international 10‐centre RCT (seeTable 1).
1. Demographic details of included studies.
| Study | Country | Number of hCG participants | Number of control participants | Age of hCG participants (years) | Age of control participants (years) |
| El‐Zibdeh 2005 | Jordan | 50 | 48 | 27.6 | 27.5 |
| Harrison 1985 | Ireland | 10 | 10 | 30.7 | 33.4 |
| Harrison 1992 | 10 centre | 36 | 39 | Unknown | Unknown |
| Quenby 1994 | UK | 42 | 39 | 29.1 | 29.65 |
| Svigos 1982 | Australia | 13 | 15 | 29 | 29 |
The overall age of participants was equivalent between the studies. Only the study by El‐Zibdeh 2005 excluded women of over 35 years from participating. The ages of hCG‐treated women and those in the control arms were comparable. The study by Quenby 1994 specified that only women with a body mass index of under 25kg/m² be included. Svigos 1982 measured plasma progesterone levels in the women allocated to the treatment arm of their study. Using the progesterone values dictated by Broom 1981, women in this study were only given hCG if their serum progesterone was outside the lowest value of the accepted normal range. Accordingly, we included only the participants in the control arm and those who received hCG.
The aim of each study analysed in this review was to assess the efficacy of prophylactically‐administered hCG in women with RM of unknown aetiology. As such, participants had been investigated for any identifiable causes of RM prior to involvement. This included chromosome analysis, sex hormone assays (follicle‐stimulating hormone (FSH), luteinizing hormone (LH), oestradiol, prolactin, plasma progesterone between days 20 and 24 of the cycle) and bacteriological culture of semen and cervical secretions. Systemic disease potentially contributing to miscarriage was excluded in patients, for example, diabetes mellitus, thyroid dysfunction, autoimmune disease and inherited thrombophilias. Women included in the El‐Zibdeh 2005, Harrison 1985 and Harrison 1992 studies additionally underwent hysterosalpingography.
Intervention
Different preparations and dosages of hCG were used in each of the included studies. The authors also varied in their hCG supplementation regimens, ranging from 5000 units weekly up to 9000 units three times each week (seeTable 2).
2. hCG regimens used in included studies.
| Study | hCG preparation | hCG regimen | Duration of hCG | Total hCG units |
| El‐Zibdeh 2005 | Profasi (Serono Welwyn Garden City, UK) or Pregnyl (Organon, Oss, The Netherlands) | 5000 units IM every 4 days | Diagnosis of pregnancy to 12 weeks | 140,000 |
| Harrison 1985 | Profasi (Serono Welwyn garden City, UK) | Loading dose of 10,000 units, 5000 twice weekly to 12 weeks, 5000 units once weekly to 16 weeks | Diagnosis of pregnancy to 16 weeks | 170,000 |
| Harrison 1992 | Pregnyl (Organon, Oss, The Netherlands) | Loading dose of 10,000 units, 5000 twice weekly to 12 weeks, 5000 units once weekly to 16 weeks | Diagnosis of pregnancy to 16 weeks | 170,000 |
| Quenby 1994 | Profasi (Serono Welwyn Garden City, UK) | Loading dose of 10,000 units, 5000 units twice weekly | Diagnosis of pregnancy to 14 weeks | 130,000 |
| Svigos 1982 | Pregnyl (Organon, Oss, The Netherlands) | 9000 units IM 3 times per week | Diagnosis of pregnancy to 12 weeks | 175,500 |
IM: intramuscular
In each study, treatment with hCG was commenced soon after the diagnosis of pregnancy. Prior to commencing hCG, the studies by Harrison 1985; Harrison 1992 required ultrasound confirmation of a pregnancy of less than eight weeks of gestation, while Quenby 1994 included pregnancies of under six weeks diagnosed using ultrasound. In the Svigos 1982 study pregnancy was diagnosed with a βhCG assay at five to six weeks, with ultrasound performed at six to seven weeks and repeated at 16 weeks. El‐Zibdeh 2005 commenced treatment “as soon as possible after confirmation of pregnancy” with hCG.
Comparison
When comparing hCG against a control, the Harrison 1985; Harrison 1992 and Quenby 1994 studies used an identically‐packaged placebo. El‐Zibdeh 2005 and Svigos 1982 gave no intervention to the control group. The authors of this study compared hCG against no treatment or oral dydrogesterone supplementation, which was administered to a third treatment arm. As such, only the data from participants in the hCG or control groups were included in this review.
All studies, except the Harrison 1985 study, documented the supportive care that both the hCG and control arms received. However, upon contacting the author, Harrison reports that the same methodology was employed in both Harrison studies, with the patients in the 1992 study attending for two‐weekly appointments. In the El‐Zibdeh 2005 trial supportive care included recommending bed rest and multivitamin supplements. The patients in the Quenby 1994 study had a review and reassurance ultrasound scan at two‐weekly intervals between weeks six and 14. Svigos 1982 measured patients' plasma progesterone levels twice weekly from diagnosis of pregnancy.
Excluded studies
Five studies were excluded (Baber 1988; Blumenfeld 1992; Nagpal 2001; Qureshi 2005; Shu 2002).
One study (Baber 1988) was excluded because participants had undergone fertility treatment rather than conceiving in spontaneous cycles. Blumenfeld 1992 was not included due to selection bias, since patients were given information about the possibility of an increased rate of miscarriage in infertile patients and were able to choose whether to join control or treatment arms. The study by Nagpal 2001 was not suitable for inclusion as patients were also administered with progesterone and tocolytic agents. Qureshi 2005 investigated hCG as a treatment for threatened miscarriage as opposed to prophylaxis in women with a prior history of RM. Shu 2002 was excluded as the trial compared hCG with a Chinese herbal remedy, rather than placebo or no treatment.
Risk of bias in included studies
The risk of bias is discussed for each included study and summarised in Figure 1 and Figure 2. Where necessary, the review authors contacted the study authors by e‐mail to clarify aspects of the methodology which were unclear. Harrison 1985; Harrison 1992 and Svigos 1982 answered our queries.
1.
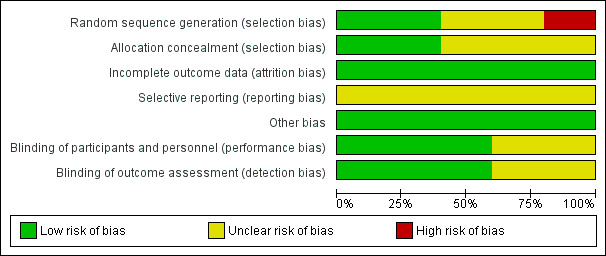
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
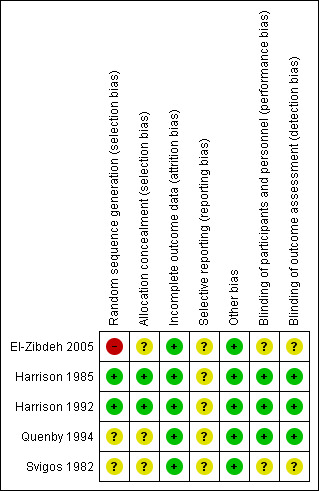
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In both Harrison 1985; Harrison 1992 studies, the pharmacist kept details of the study randomisation which was supplied directly by the manufacturer in a sealed envelope. The adequacy of allocation concealment in the other three studies remained unclear.
The method of randomisation was not stated in the Svigos 1982 study. The author responded that randomisation to either arm of the trial was achieved by generating a random allocation sequence, although the method by which this was generated remains unclear. In the El‐Zibdeh 2005 study, patients were assigned according to the day of the week women attended the clinic (quasi‐randomised).
Blinding
The studies by Harrison 1985; Harrison 1992 and Quenby 1994 were reported as being double‐blind, employing an identically packaged placebo. The El‐Zibdeh 2005 and Svigos 1982 study compared hCG with no treatment.
Incomplete outcome data
All of the women who were randomised completed each study, with no patients dropping out. The attrition rate was therefore 0%.
Selective reporting
Inadequate information from available studies to assess reporting bias, as such the risk of reporting bias has been assessed as 'unclear' for all included studies.
Other potential sources of bias
We identified no other potential sources of bias during the course of this review.
Effects of interventions
Primary outcomes
First trimester miscarriage
The meta‐analysis suggested a statistically significant benefit in using hCG (risk ratio (RR) 0.51, 95% confidence interval (CI) 0.32 to 0.81; five studies, 302 women Analysis 1.1). The mean number needed to treat to prevent subsequent pregnancy loss was seven.
1.1. Analysis.
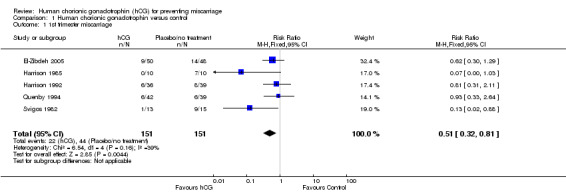
Comparison 1 Human chorionic gonadotrophin versus control, Outcome 1 1st trimester miscarriage.
There was, however, statistical heterogeneity in the combined comparison (I² = 39%). This statistic suggests a lack of combinability in the data, as evidenced by the wide‐ranging confidence intervals between the studies illustrated. The protocol for this review stated that in cases of heterogenous results, the random‐effects statistical model would be applied to the results in order to measure the average of the range of possible treatment effects. However, when the random‐effects model is used the heterogeneity remains the same (I2 = 39%). With the random‐effects model applied to all five studies, the risk ratio was 0.55 (95% CI 0.28 to 1.09), indicating that although there is a trend towards a benefit in using hCG, the result is non‐significant.
A potential reason for the heterogeneity may have been the trials by Harrison 1985 and Svigos 1982 which produced the results in strongest favour of hCG (RR 0.07, CI 0.00 to 1.03; RR 0.13, CI 0.02 to 0.88 respectively). Given that these two studies represented the oldest and those with the least power, a sensitivity analysis was performed excluding these data.
When including only the data from El‐Zibdeh 2005, Harrison 1992 and Quenby 1994, the pooled RR was 0.74 (CI 0.44 to 1.23). Here, the RR suggests a trend toward a benefit in using hCG, although the result is statistically non‐significant. The I² value is now 0%, suggesting greater homogeneity between these results (Analysis 1.2). The heterogeneity statistic remained 0% when either the fixed‐effect or random‐effects tests were used. The result was almost identical; fixed‐effect: RR 0.74 (CI 0.44 to 1.23), random‐effects: RR 0.74 (CI 0.44 to 1.22). As such, the value of administering hCG remains uncertain with a lack of sufficient evidence to support its use.
1.2. Analysis.
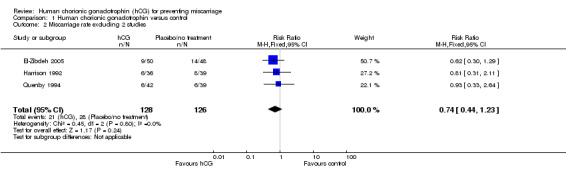
Comparison 1 Human chorionic gonadotrophin versus control, Outcome 2 Miscarriage rate excluding 2 studies.
Across the included studies, there were insufficient data to present results for the other pre‐determined primary outcomes (second trimester miscarriage or stillbirths).
Secondary outcomes
Adverse effects
Overall, the studies indicated that using hCG in pregnancy was safe for both mother and baby. None of the studies reported any adverse effects from the use of hCG. Harrison 1992 and Svigos 1982 reported that there were no significant differences in antenatal and intrapartum complications between the hCG and control groups.
Threatened miscarriage
Two of the included studies recorded threatened miscarriage in women being treated with hCG versus placebo or no treatment (Analysis 1.3). El‐Zibdeh 2005 found threatened miscarriage in 6% (3/50) of women given hCG and 10.4% (5/48) in the control group. The Harrison 1985 study had no women experiencing threatened miscarriage in the hCG arm (0/10) and one patient in the placebo or no treatment arm (1/10). When these data were pooled, the RR was 0.52 (CI 0.15 to 1.82), indicating no statistically significant difference in risk of threatened miscarriage when hCG was used.
1.3. Analysis.
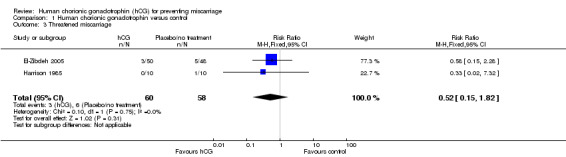
Comparison 1 Human chorionic gonadotrophin versus control, Outcome 3 Threatened miscarriage.
Congenital defects
Three studies recorded the presence of congenital defects in children born after using hCG in pregnancy (Analysis 1.4). El‐Zibdeh 2005 found 2% (1/50) of live born children to be affected by congenital defects in the hCG‐treated group and 2.08% (1/48) in the control group. Svigos 1982 calculated 7.7% (1/13) affected pregnancies to occur in the hCG group versus 6.6% (1/15) in the control group. Of the neonatal deaths presented in this review, there was one case of sepsis secondary to premature rupture of membranes, two cases of respiratory distress syndrome and one case with multiple congenital abnormalities. Harrison 1985 also recorded congenital defects in their study, although there were no cases in either arm. As such, their findings could not contribute to a meta‐analysis. The RR calculated from the results of El‐Zibdeh 2005 and Svigos 1982 was 1.05 (CI 0.16 to 7.12), suggesting no increased risk of congential defects when using hCG.
1.4. Analysis.
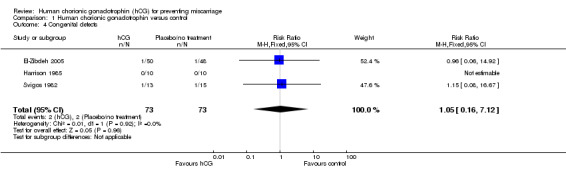
Comparison 1 Human chorionic gonadotrophin versus control, Outcome 4 Congenital defects.
There were not enough data in the included studies to obtain results for the other secondary outcomes: low birthweight (less than 2500 g), prematurity (gestation less than 37 completed weeks), neonatal death (less than 28 days of delivery) and cost.
Discussion
Summary of main results
The initial results of this review demonstrated significant benefit in using human chorionic gonadotrophin (hCG) to prevent recurrent miscarriage (RM) when all of the included studies were analysed. However, the value of this result remains uncertain since the application of the random‐effects statistical test produced a non‐significant result. Additionally, the sensitivity analysis which excluded two older studies with lower power suggested that there was no significant difference in miscarriage rate between patients treated with hCG or control. However, removing these studies reduced the data pool from which conclusions could be drawn, thus limiting the power of this meta‐analysis to exclude any possible treatment effect.
All of the included studies recorded miscarriage rate as their primary outcome. The intended parameters stated as our secondary outcome measures were seldom reported. The studies by Harrison 1985 and El‐Zibdeh 2005 provided data concerning threatened miscarriage, with no statistically significant difference between the intervention and control groups (Analysis 1.3). The Harrison 1992 paper reported that there were no differences in birth weight, placental weight or Apgar scores between the hCG or control arms (P > 0.20). The Svigos 1982 study also indicated that there were no significant differences in obstetric complications between the groups, although no data were provided.
Each study indicated that hCG was safe, with no adverse events reported as a direct result of hCG.
Overall completeness and applicability of evidence
The small number of studies available for inclusion limits the ability of this review to draw definitive conclusions as to the efficacy and safety of hCG in preventing RM. Efforts to conduct larger randomised controlled trials (RCTs) have failed to recruit adequate participant numbers. A large, multi‐centre study conducted by Harrison 1992 (n = 75) was discontinued after an interim analysis showed a lack of efficacy of hCG alongside “escalating costs and diminishing participation”. As such, data from this international study were only available for those patients recruited prior to the discontinuation. As such, the small sample sizes presented in this review demonstrate the need for an up‐to‐date RCT with adequate power.
Higher‐powered studies would enable subgroup analysis in future meta‐analyses. The Quenby 1994 study comprised women with regular menstrual cycles and a group with oligomenorrhoea, defined as variation of more than seven days in menstrual cycle length. In the regular cycle group, hCG conferred no benefit on the miscarriage rate. However, the oligomenorrhoea subgroup experienced a statistically significant benefit with hCG supplementation: miscarriage rate of 3.3/13 (25%) in hCG group versus 3/10 (30%) in placebo group. It would seem reasonable to identify these groups of women with RM that may that may derive additional benefit from hCG (Cocksedge 2008).
The methodology of the included studies exhibited differences, such as disparities in the definition of RM, investigations of miscarriage aetiology, timing of hCG commencement, type of hCG preparation and regimen used. There was also clinical heterogeneity within the population of patients included in this review. Although the age of patients was equivalent, there may have been differences in, for example, BMI, ethnicity and numbers of previous miscarriages. Future studies investigating hCG would therefore benefit from standardisation of methodology and improved patient selection.
One difference between the included studies was the method of diagnosis of pregnancy. In four of the studies, (Harrison 1985; Harrison 1992; Quenby 1994; Svigos 1982), an ultrasound was used to confirm the pregnancy prior to commencing hCG, whereas the El‐Zibdeh 2005 study used βhCG measurements. There is a potential risk associated with the use of hCG when the location of the pregnancy is unknown. For example, in the case of a suspected ectopic pregnancy, the use of serial measurement of βhCG would not be possible if the patient had been treated with exogenous hCG. The mean gestational age of the pregnancy in women presenting with symptoms of ectopic pregnancy is around seven weeks (range five to 11 weeks) (Pradhan 2006). As such, women with RM being treated with hCG may benefit from early pregnancy scanning, prior to eight weeks of gestation. However, the risk needs to be balanced with the possibility that greater benefit from the use of hCG could be achieved from its commencement at the earliest opportunity in pregnancy or even prior to conception.
Quality of the evidence
The quality of evidence obtained from the studies has been presented as an Assessment of risk of bias in included studies. SeeFigure 2. The search strategy for this review produced 13 studies for potential inclusion. Five studies met the inclusion criteria (n= 596). Within the included studies, El‐Zibdeh 2005 was judged to be at risk of selection bias as the study had a quasi‐randomised design, where women were randomised on the basis of the day they attended the clinic. Harrison 1985; Harrison 1992; Quenby 1994; Svigos 1982 were rated as unclear risk due to inadequate information. Harrison 1992 was at low risk of bias for allocation concealment as a detailed account of their protocol is given. This information is not provided in Harrison 1985, although the author responded that the methodology was the same for both trials. El‐Zibdeh 2005; Quenby 1994 and Svigos 1982 did not state their method of allocation concealment. There was a 0% attrition bias in the included studies, with all patients completing the trials and no losses to follow‐up. Harrison 1985 and Harrison 1992 documented their blinding protocol and use of an identically packaged placebo. Quenby 1994 stated that their trial was 'double blind'. El‐Zibdeh 2005 and Svigos 1982 did not supply this information. No selection bias or other forms of bias were detected.
The body of evidence from which to draw conclusions is small; the main limitation of this review being small sample size. In addition, within the included studies relevant information relating to methodological quality was missing. This may, in part, be due to the age of these studies. In summary, the available evidence in the included studies is adequate for drawing clinical conclusions regarding the use of HCG. However, the field of RM would benefit from a large, well‐conducted RCT to add to the literature.
Potential biases in the review process
We identified no biases in our review process. SeeMethods.
Agreements and disagreements with other studies or reviews
Analysing the results of this review has identified the need for a large, up‐to‐date RCT investigating hCG and RM. Other well‐powered studies in RM are being achieved. A recent study of unexplained RM (the ALFIE trial) investigated pregnancy outcomes in 364 women using anticoagulation as a therapeutic agent (Kaandorp 2011). The trial compared women randomly assigned to receive aspirin combined with low‐molecular‐weight heparin, aspirin alone or placebo. It found that none of the intervention strategies produced a significant improvement in the live birth rate.
A subsequent cohort study following the women enrolled in the ALFIE trial (n = 251) found that 50% of these patients had achieved a live birth after attempting to conceive after 41 weeks. Similarly, a large cohort study of 987 Danish women investigated the proportion of women with RM, due to any aetiology, who achieved a live birth after referral to a tertiary miscarriage centre (Lund 2011). Women in the RM cohort were 3.8 (3.4 to 4.3) times as likely to have a live birth within one year of clinic referral compared with the general population. The interpretation of cohort studies such as these is complicated by the additional desire to have children and the support provided by the clinic over and above the general population. However, these data do suggest that despite the lack of evidence supporting the use of pharmacologic intervention in unexplained RM, the likelihood of these women achieving a live birth remains good.
In addition to hCG and anticoagulation, progesterone supplementation has also been considered as an intervention to prevent RM. The PROgesterone in recurrent MIScarriagE (PROMISE 2009) study is currently underway. This is a large randomised double‐blind, placebo‐controlled, multi‐centre trial investigating progesterone therapy in women with a history of RM. A study of this nature would be ideally placed to accurately assess the efficacy of hCG. The use of hCG as a therapeutic agent has certain practical advantages over progesterone including uniformity of administration and consistency of preparation. As such, the field of RM would benefit from a future meta‐analysis comparing the use of hCG versus progesterone.
Authors' conclusions
Implications for practice.
The results of this review have demonstrated that there is a trend towards a benefit in the use of hCG to prevent further pregnancy loss in women with a history of RM. However, this trend is non‐significant. As such, the results remain equivocal and as yet, there is not enough evidence to support the use of hCG in women with RM of unknown aetiology in clinical practice.
The results also showed that there were no side‐effects or adverse effects secondary to the use of hCG in any of the included studies. As such hCG is safe to be used within the context of future RCTs further exploring the potential efficacy of this intervention.
Implications for research.
A well‐designed RCT of adequate power and methodology is required to establish whether hCG may have an evidence‐based role in preventing miscarriage and improving pregnancy outcomes. A subgroup analysis should be performed to investigate the use of hCG in prognostic subgroups, for example, maternal age and BMI. Future studies could explore the optimum timing of commencement of hCG to achieve a possible benefit, such as the use of pre‐conception hCG. An economic evaluation should be undertaken to establish the cost‐effectiveness of hCG. The efficacy of hCG in preventing RM should be compared to other interventions, such as progesterone.
History
Protocol first published: Issue 7, 2010 Review first published: Issue 1, 2013
| Date | Event | Description |
|---|---|---|
| 3 March 2011 | Amended | Contact details edited. |
| 8 November 2010 | Amended | Minor edits made to 'Criteria for considering studies for this review' and 'Data collection and analysis' sections. |
Acknowledgements
The authors wish to thank the Cochrane Pregnancy and Childbirth Group for their assistance with developing this systematic review. They also thank Professor Harrison and Professor Svigos for answering email queries regarding their studies.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Human chorionic gonadotrophin versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1st trimester miscarriage | 5 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.81] |
| 2 Miscarriage rate excluding 2 studies | 3 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.44, 1.23] |
| 3 Threatened miscarriage | 2 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.15, 1.82] |
| 4 Congenital defects | 3 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.16, 7.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
El‐Zibdeh 2005.
| Methods | Country carried out: Jordan. Date conducted: 1994‐2000. Type of trial design: RCT. Unit of randomisation: recurrent miscarriage. Method of randomisation: according to day of the week women attended clinic. Timing of randomisation: N/A. Number of centres: 1. Source of funding: N/A. |
|
| Participants | Total number randomised: 98. Total number for analysis: 98. Inclusions:
Exclusions: > 35 years. |
|
| Interventions | Treatment arm: IM hCG (Profasi, Serono; Pregnyl, Organon). 5000 IU every 4 days. Control arm: no treatment. Additional interventions: both arms received supportive care; multivitamins, bedrest, standard antenatal follow‐up. Duration: from diagnosis of pregnancy to 12 weeks. |
|
| Outcomes | Pregnancy loss. Threatened miscarriage. Obstetric complications. Delivery details. Congenital abnormalities. Neonatal death. Adverse effects. |
|
| Notes | This study assessed the efficacy of hCG versus dydrogesterone compared to a control group. Only the data pertaining to the hCG and control groups have been included here. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women randomised on day of clinic attendance; quasi‐randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not stated in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the patients randomised completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No selective reporting apparent in the study. |
| Other bias | Low risk | No other bias apparent in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of blinding methodology not stated in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of blinding methodology not stated in the study. |
Harrison 1985.
| Methods | Authors do not give details of their randomisation and allocation concealment methods in this study. However, in the 1992 study by the same author, the methodology is discussed in more depth and is stated to be the same as in the previous study. SeeHarrison 1992. Country carried out: Ireland. Date conducted: Type of trial design: RCT. Method of randomisation: women were randomised after their pregnancy had been formally diagnosed with USS. Number of centres: 1. Source of funding: Serono supplied drugs for the study. |
|
| Participants | Total number randomised: 20. Total number for analysis: 20. Inclusions:
Exclusions: none given. |
|
| Interventions | Treatment arm: IM hCG 10,000 IU loading dose, 5000 IU twice weekly to week 12, 5000 units weekly to week 16 (Profasi, Serono, UK). Control arm: placebo. Additional interventions: Duration: from USS detection of a pregnancy of under 8 weeks' gestation until 16 weeks. |
|
| Outcomes | Miscarriage. Premature rupture of membranes. Pregnancy complications. Method of delivery. Neonatal death. Congenital abnormalities. Adverse effects. |
|
| Notes | Completed a pilot study prior to commencing the RCT. 2 out of 20 patients were not investigated for the cause of miscarriage prior to commencing the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors do not give details of their randomisation and allocation concealment methods in this study. However, in the 1992 study by the same author, the methodology is discussed in more depth and is stated to be the same as in the previous study. SeeHarrison 1992. |
| Allocation concealment (selection bias) | Low risk | Authors do not give details of their randomisation and allocation concealment methods in this study. However, in the 1992 study by the same author, the methodology is discussed in more depth and is stated to be the same as in the previous study. SeeHarrison 1992. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias apparent in the study. |
| Other bias | Low risk | No other bias apparent in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study, using identically packaged placebo in the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind study. |
Harrison 1992.
| Methods | Country carried out: Ireland, Norway, The Netherlands, France, Sweden, England, Scotland, Belgium. Date conducted: Type of trial design: RCT. Method of randomisation: randomised on formal diagnosis of pregnancy on USS. Timing of randomisation: Number of centres: 10. Source of funding: Organon Int organised and funded the study. |
|
| Participants | Total number randomised:75. Total number for analysis:75. Inclusions:
Exclusions: none given. |
|
| Interventions | Treatment arm: IM hCG 10,000 unit loading dose, 5000 units twice weekly to week 12, 5000 units once weekly to week 16 (Pregnyl, Organon). Control arm: placebo. Additional interventions: Duration: from USS detection of a pregnancy of under 8 weeks gestation until 16 week. |
|
| Outcomes | Pregnancy success (beyond 28 weeks). | |
| Notes | Trial was discontinued due to non‐significant results at interim analysis, escalating costs and diminishing participation. Results were gathered for 75 patients already in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated to group using a paired sequential design randomised in blocks of 6. Each women entering the study was one of a pair, consisting of a woman receiving hCG and a woman receiving placebo. |
| Allocation concealment (selection bias) | Low risk | The study randomisation was supplied by Serono directly to the hospital pharmacist in a sealed envelope. The pharmacist alone had the key to the randomisation protocol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias apparent in the study. |
| Other bias | Low risk | No other bias apparent in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study, using identical packaging of the placebo in the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind study. |
Quenby 1994.
| Methods | Country carried out: UK. Date conducted: 1989‐1992. Type of trial design: RCT. Method of randomisation: randomised on formal diagnosis of pregnancy on USS prior to 6 weeks' gestation. Number of centres: 1. Source of funding: none given. |
|
| Participants | Total number randomised: 81. Total number for analysis: 81. Inclusions:
Exclusions: BMI > 25. |
|
| Interventions | Treatment arm: IM hCG 10,000 units loading dose, 5000 units twice weekly to week 14. Control arm: placebo (normal saline). Additional interventions: all patients received supportive care. Duration: from USS detection of a pregnancy of under 8 weeks' gestation until 16 weeks. |
|
| Outcomes | Early pregnancy loss. Late pregnancy loss. |
|
| Notes | This study compared hCG versus placebo in patients with regular menstrual cycles and those with oligomenorrhoea. In this study the patients with oligomenorrhoea are able to conceive unaided, rather than patients with polycystic ovaries syndrome, rendering it suitable for inclusion in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias apparent in the study. |
| Other bias | Low risk | No other bias apparent in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double bind study. |
Svigos 1982.
| Methods | Country carried out: Australia. Date conducted: Type of trial design: RCT. Method of randomisation: randomised on formal diagnosis of pregnancy on USS at 6‐7 weeks. Number of centres: 1. Source of funding: none given. |
|
| Participants | Total number randomised: 32. Total number for analysis: 16. Inclusions:
Exclusions: none given. |
|
| Interventions | Treatment arm: IM hCG 9000 units 3 times per week until week 12. Control arm: no treatment. Additional interventions: Duration: from USS confirmation of pregnancy at 6‐7 weeks until week 12. |
|
| Outcomes | Early pregnancy loss. Pregnancy complications. Labour complications. Congenital defects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not presented in the paper. However, the author responded that randomisation to either arm of the trial was achieved by generating a random allocation sequence. However, the method of sequence generation is unknown. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias apparent in the study. |
| Other bias | Low risk | No other forms of bias apparent in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method of blinding not stated in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding not stated in the study. |
BMI: body mass index hCG: human chorionic gonadotrophin IM: intramuscular IU: international units RCT: randomised controlled trial USS: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baber 1988 | The participants in this study had achieved their pregnancies by in‐vitro fertilisation, which does not meet the selection criteria for this review. |
| Blumenfeld 1992 | In this study, each patient was given information about the possibility of an increased rate of miscarriage in infertile patients and was able to choose whether to join the control or treatment arms. In cases where the patient was indifferent, they were prospectively randomised. The study therefore has to be excluded due to selection bias. |
| Nagpal 2001 | This study also included patients with congenital malformations of the uterus as well as a those with a history of recurrent miscarriage. Progesterone and tocolytic agents were given to the control group and alongside human chorionic gonadotrophin in the treatment group. As such, there are confounding variables preventing this study from being comparable to the other trials. |
| Qureshi 2005 | This study deals with threatened rather than recurrent miscarriage. |
| Shu 2002 | This study observed the effects of Chinese herbal medicine plus human chorionic gonadotropin and progesterone in treating anticardiolipin antibody‐positive recurrent miscarriage. |
Characteristics of studies awaiting assessment [ordered by study ID]
El‐Zibdeh 1998.
| Methods | Randomised controlled trial. |
| Participants | 114 women with a history of recurrent unexplained recurrent miscarriage. All women were under 35 years of age. The mean number of previous abortions was 3.3. |
| Interventions | 48 women received progesterone therapy. 36 women received human chorionic gonadotrophin (5000 IU IM every 4 days). 30 women received no treatment. The interventions were stopped at 12 weeks of gestation. |
| Outcomes | In the human chorionic gonadotrophin group, 31 (81.5%) women achieved live births. In the no treatment arm, 20 (68%) of women achieved live births. |
| Notes | This study was presented in abstract form following a presentation at the 16th World Congress on Fertility and Sterility (1998). We have contacted the author for further information about the study and await their reply. |
El‐Zibdeh 2002.
| Methods | Randomised controlled trial. |
| Participants | 114 women randomised to 3 groups. |
| Interventions | Participants received either dydrogesterone (n = 48), human chorionic gonadotrophin (n = 36) or no treatment (n = 30). |
| Outcomes | In the human chorionic gonadotrophin group, 30 (83.4%) women achieved live births. In the no treatment arm, 24 women achieved live births (80%). |
| Notes | This study was presented in abstract form following a presentation at the 10th World Congress on Menpause. We have contacted the author for further information about the study and await their reply. |
IM: intramuscular IU: international units
Differences between protocol and review
The methods have been updated to reflect the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
LC Morley and T Tang wrote protocol and prepared this manuscript.
N Simpson commented on and revised the protocol and manuscript.
T Tang acts as the guarantor of the review.
Declarations of interest
None known.
New
References
References to studies included in this review
El‐Zibdeh 2005 {published data only}
- El‐Zibdeh MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. Journal of Steroid Biochemistry & Molecular Biology 2005;97(5):431‐4. [DOI] [PubMed] [Google Scholar]
Harrison 1985 {published data only}
- Harrison RF. Hormonal treatment with hCG. Contraception, Fertilite, Sexualite 1991;19:373‐6. [Google Scholar]
- Harrison RF. Treatment of habitual abortion with human chorionic gonadotropin: results of open and placebo‐controlled studies. European Journal of Obstetrics & Gynecology and Reproductive Biology 1985;20:159‐68. [DOI] [PubMed] [Google Scholar]
Harrison 1992 {published data only}
- Harrison RF. Human chorionic gonadotrophin (hCG) in the management of recurrent abortion; results of a multi‐centre placebo‐controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1992;47(3):175‐9. [DOI] [PubMed] [Google Scholar]
Quenby 1994 {published data only}
- Quenby S, Farquharson RG. Human chorionic gonadotropin supplementation in recurring pregnancy loss: a controlled trial. Fertility and Sterility 1994;62(4):708‐10. [DOI] [PubMed] [Google Scholar]
Svigos 1982 {published data only}
- Svigos J. Preliminary experience with the use of human chorionic gonadotrophin therapy in women with repeated abortion. Clinical Reproduction and Fertility 1982;1:131‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Baber 1988 {published data only}
- Baber R, Kuan R, Porter R, Saunders D. Early pregnancy support in an in‐vitro fertilization program: does human chorionic gonadotropin reduce the miscarriage rate?. Asia‐Oceania Journal of Obstetrics & Gynaecology 1988;14(4):453‐5. [DOI] [PubMed] [Google Scholar]
Blumenfeld 1992 {published data only}
- Blumenfeld Z, Ruach M. Early pregnancy wastage: the role of repetitive human chorionic gonadotropin supplementation during the first 8 weeks of gestation. Fertility and Sterility 1992;58:19‐23. [DOI] [PubMed] [Google Scholar]
Nagpal 2001 {published data only}
- Nagpal M, Malhotra R. Should human chorionic gonadotropin supplementation be used as a routine prophylaxis in high risk pregnancies?. Journal of Obstetrics and Gynecology of India 2001;51(4):65‐7. [Google Scholar]
Qureshi 2005 {published data only}
- Qureshi NS, Edi‐Osagie EC, Ogbo V, Ray S, Hopkins RE. First trimester threatened miscarriage treatment with human chorionic gonadotrophins: a randomised controlled trial. BJOG: An International Journal of Obstetrics & Gynaecology 2005;112(11):1536‐41. [DOI] [PubMed] [Google Scholar]
Shu 2002 {published data only}
- Shu J, Miao P, Wang RJ. Clinical observation on effect of Chinese herbal medicine plus human chorionic gonadotropin and progesterone in treating anticardiolipin antibody‐positive early recurrent spontaneous abortion. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi//Chinese Journal of Integrated Traditional and Western Medicine 2002;22(6):414‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
El‐Zibdeh 1998 {published data only}
- El‐Zibdeh MY. Randomised controlled trial comparing the efficacy of reducing spontaneous abortion following treatment with progesterone and human chorionic gonadotrophin hormone (hCG). Fertility and Sterility 1998;70(3 Suppl 1):S77‐S78. [Google Scholar]
El‐Zibdeh 2002 {published data only}
- El‐Zibdeh MY. Randomized clinical trial comparing the efficacy of dydrogesterone and human chorionic gonadotropin. Climacteric 2002;5(Suppl 1):136. [Google Scholar]
Additional references
Balen 1993
- Balen AH, Tan SL, MacDougall J, Jacobs S. Miscarriage rates following in‐vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Human Reproduction 1993;8:959‐64. [DOI] [PubMed] [Google Scholar]
Berry 1995
- Berry CW, Bramabati B, Eskes TK, Exalto N, Fox H, Geraedts JP, et al. The Euro‐Team early pregnancy (ETEP) protocol for recurrent miscarriage. Human Reproduction 1995;10(6):1516‐20. [DOI] [PubMed] [Google Scholar]
Broom 1981
- Broom TJ, Matthew CD, Cooke ID, Ralph MM, Seamark RF, Cox LW. Endocrine profiles and fertility status of human menstrual cycles of varying follicular phase length. Fertility and Sterility 1981;3:194‐200. [DOI] [PubMed] [Google Scholar]
Carrington 2005
- Carrington B, Sacks G, Regan L. Recurrent miscarriage: pathophysiology and outcome. Current Opinion in Obstetrics and Gynecology 2005;17:591‐7. [DOI] [PubMed] [Google Scholar]
Clifford 1997
- Clifford K, Rai R, Regan L. Future pregnancy outcome in unexplained recurrent first trimester miscarriage. Human Reproduction 1997;12:387‐9. [DOI] [PubMed] [Google Scholar]
Cocksedge 2008
- Cocksedge KA, Li TC, Saravelos SH, Metwally M. A reappraisal of the role of polycystic ovary syndrome in recurrent miscarriage. Reproductive Biomedicine Online 2008;17:151‐60. [DOI] [PubMed] [Google Scholar]
Corner 1929
- Corner GW, Allen WM. Physiology of the corpus luteum, II: production of a special uterine reaction (progestational proliferation) by extracts of the corpus luteum. American Journal of Physiology 1929;88:340‐6. [Google Scholar]
Csapo 1978
- Csapo AI, Pulkkinen M. Indespensibility of the human corpus luteum in the maintenance of early pregnancy luteectomy evidence. Obstetrical and Gynecological Survey 1978;33(2):69‐81. [DOI] [PubMed] [Google Scholar]
Devaseelan 2010
- Devaseelan JP, Fogarty PP, Regan L. Human chorionic gonadotrophin for threatened miscarriage. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007422.pub2] [DOI] [PubMed] [Google Scholar]
Diedrich 2007
- Diedrich K, Fauser BCJM, Devroey P, Griesinger G, Evian Annual Reproduction (EVAR) Workshop Group. The role of the endometrium and embryo in human implantation. Human Reproduction Update 2007;13(4):365‐77. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hogge 2003
- Hogge WA, Byrnes AL, Lanasa MC, Surti U. The clinical use of karyotyping spontaneous abortions. American Journal of Obstetrics and Gynecology 2003;189(2):397‐400. [DOI] [PubMed] [Google Scholar]
Kaandorp 2011
- Kaandorp SP, Mens T, Post JAM, Hutten BA, Buller HR, Veen F, et al. Time to pregnancy resulting in a live birth in women with unexplained recurrent miscarriage. Abstracts of the 27th Annual Meeting of ESHRE; 2011 July 3‐6; Stockholm, Sweden. 2011.
Licht 2001
- Licht P, Russu V, Wildt L. On the role of human chorionic gonadotrophin (hCG) in the embryo‐endometrial microenvironment: implications for differentiation and implantation. Seminars in Reproductive Medicine 2001;19(1):37‐47. [DOI] [PubMed] [Google Scholar]
Licht 2007
- Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotrophin directly involved in the regulation of human implantation?. Molecular and Cellular Endocrinology 2007;15:85‐92. [DOI] [PubMed] [Google Scholar]
Lund 2011
- Lund M, Kamper‐Jorgensen M, Nielson HS, Lidegard O, Nybo‐Andersen AM, Christiansen OB. Long‐term prognosis for live birth in women with recurrent miscarriage: a descriptive follow up study of a cohort of 987 women. Abstracts of the 27th Annual Meeting of ESHRE; 2011 July 3‐6; Stockholm, Sweden. 2011.
Lustbader 1998
- Lustbader JW, Lobel L, Wu H, Elliott MM. Structural and molecular studies of human chorionic gonadotrophin and its receptor. Recent Progress in Hormone Research 1998;53:395‐424. [PubMed] [Google Scholar]
Oon 2000
- Oon VJ, Johnson MR. The regulation of the human corpus luteum steroidogenesis: a hypothesis. Human Reproduction Update 2000;6(5):519‐29. [DOI] [PubMed] [Google Scholar]
Pradhan 2006
- Pradhan P, Thapamagar SB, Maskey S. A profile of ectopic pregnancy at Nepal medical college teaching hospital. Nepal Medical College Journal 2006;8:238‐42. [PubMed] [Google Scholar]
PROMISE 2009
- Coomarasamy A. First trimester progesterone therapy in women with a history of unexplained recurrent miscarriages: a randomised double‐blind placebo‐controlled multi‐centre trial (The PROMISE [PROgesterone in recurrent MIScarriagE] Trial). http://www.controlled‐trials.com/ISRCTN92644181/ISRCTN92644181 (accessed 11 January 2012). [DOI] [PMC free article] [PubMed]
RCOG 2011
- RCOG. The investigation and treatment of couples with recurrent first‐trimester and second‐trimester miscarriage (Green‐top Guideline Number 17). 3rd Edition. London (UK): RCOG Press, 2011 April. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosevar 1999
- Rosevar S. Bleeding in early pregnancy. In: Weiner CP, Gonik B editor(s). High Risk Pregnancy Management Options. 2nd Edition. London: W.B. Saunders, 1999:61‐89. [Google Scholar]
Serrano 2006
- Serrano F, Lima ML. Recurrent‐miscarriage: psychological and relational consequences for couples. Psychology and Psychotherapy 2006;79(Pt 4):585‐94. [DOI] [PubMed] [Google Scholar]
Stocco 2006
- Stocco C, Tellerian T, Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocrine Reviews 2006;28(1):117‐49. [DOI] [PubMed] [Google Scholar]
Tan 2010
- Tan AW, Quenby S. Recent thoughts on management and prevention of recurrent early pregnancy loss. Current Opinion in Obstetrics and Gynaecology 2010;22:446‐51. [DOI] [PubMed] [Google Scholar]
Tien 2007
- Tien JC, Tan TY. Non‐surgical interventions for threatened and recurrent miscarriages. Singapore Medical Journal 2007;48(12):1074‐90. [PubMed] [Google Scholar]
Toth 2010
- Toth B, Jeschke U, Rogenhofer N, Scholz C, Wurfel W, Thaler CJ, et al. Recurrent miscarriage: current concepts in diagnosis and treatment. Journal of Reproductive Immunology 2010;85:25‐32. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10. Vol. 1, Geneva: World Health Organization, 2004. [Google Scholar]
Zygmunt 2003
- Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. European Journal of Obstetrics & Gynaecology and Reproductive Biology 2003;110(Suppl 1):S10‐S18. [DOI] [PubMed] [Google Scholar]


