Abstract
1. In an attempt to define the importance of acetate as a metabolic precursor, the activities of acetyl-CoA synthetase (EC 6.2.1.1) and acetyl-CoA hydrolase (Ec 3.1.2.1) were assayed in tissues from rats and sheep. In addition, the concentrations of acetate in blood and liver were measured, as well as the rates of acetate production by tissue slices and mitochondrial fractions of these tissues. 2. Acetyl-CoA synthetase occurs at high activities in heart and kidney cortex of both species as well as in rat liver and the sheep masseter muscle. The enzyme is mostly in the cytosol fraction of liver, whereas it is associated with the mitochondrial fraction in heart tissue. Both mitochondrial and cytosol activities have a Km for acetate of 0.3mm. Acetyl-CoA synthetase activity in liver was not altered by changes in diet, age or alloxan-diabetes. 3. Acetyl-CoA hydrolase is widely distributed in rat and sheep tissues, the highest activity being found in liver. Essentially all of the activity in liver and heart is localized in the mitochondrial fraction. Hepatic acetyl-CoA hydrolase activity is increased by starvation in rats and sheep and during the suckling period in young rats. 4. The concentrations of acetate in blood are decreased by starvation and increased by alloxan-diabetes in both species. The uptake of acetate by the sheep hind limb is proportional to the arterial concentration of acetate, except in alloxan-treated animals, where uptake is impaired. 5. Acetate is produced by liver and heart slices and also by heart mitochondrial fractions that are incubated with either pyruvate or palmitoyl-(—)-carnitine. Liver mitochondrial fractions do not form acetate from either substrate but instead convert acetate into acetoacetate. 6. We propose that acetate in the blood of rats or starved sheep is derived from the hydrolysis of acetyl-CoA. Release of acetate from tissues would occur under conditions when the function of the tricarboxylic acid cycle is restricted, so that the circulating acetate serves to redistribute oxidizable substrate throughout the body. This function is analogous to that served by ketone bodies.
Full text
PDF


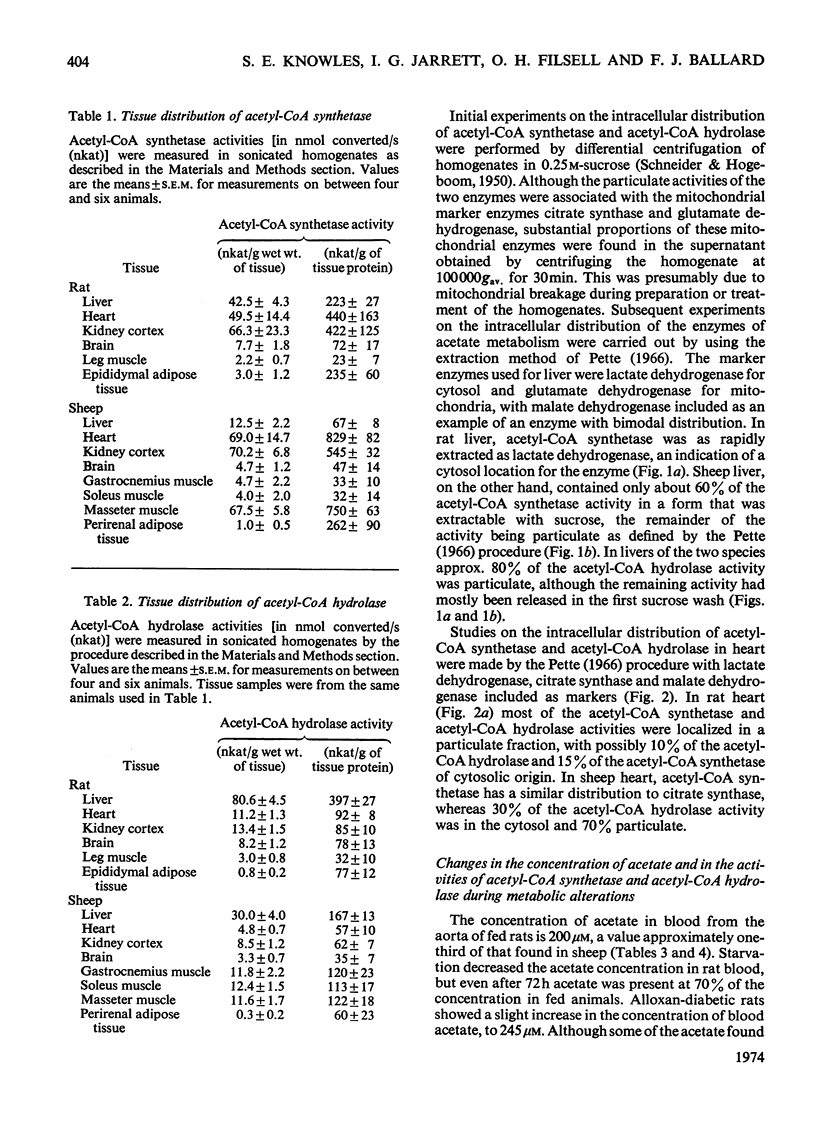
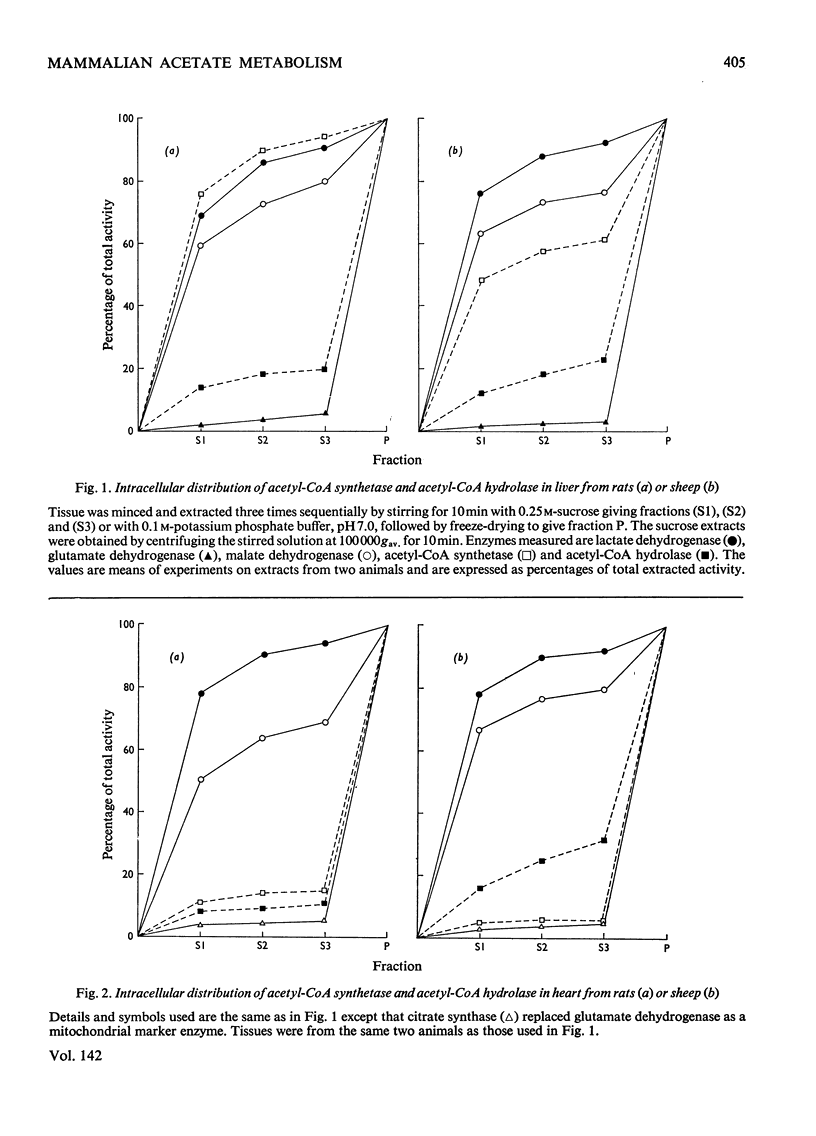
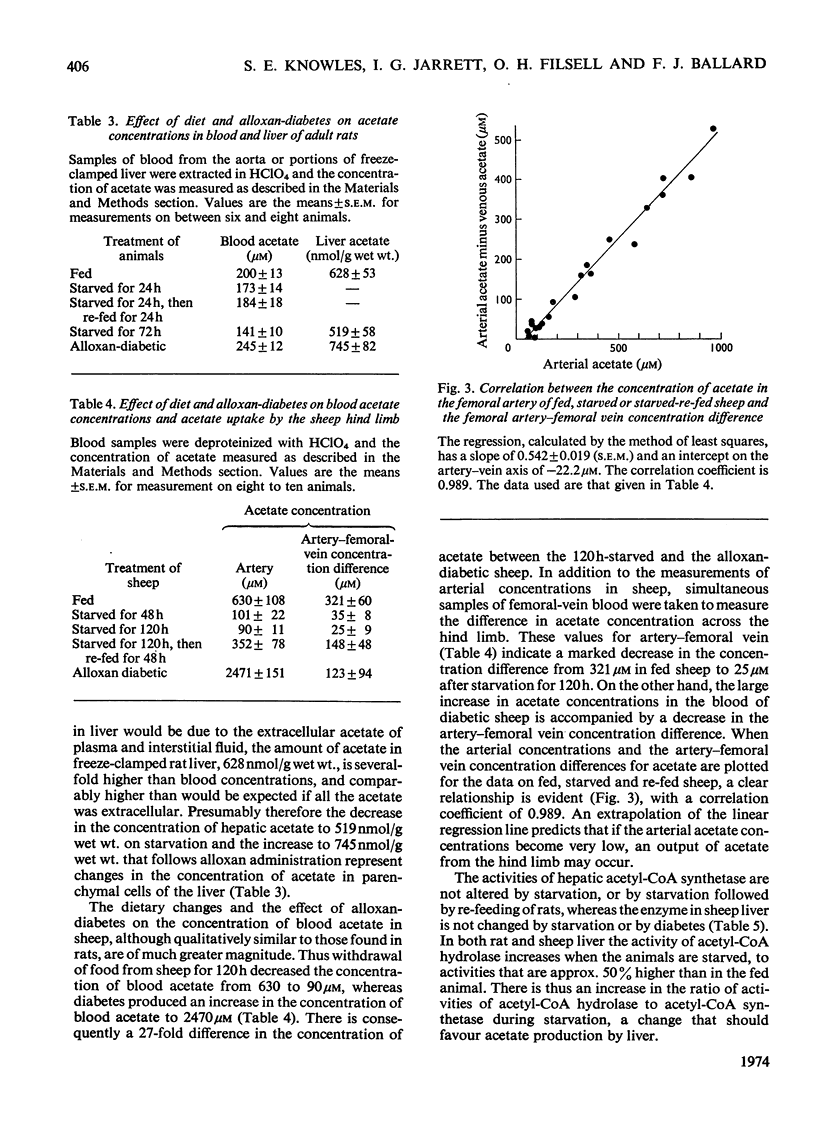
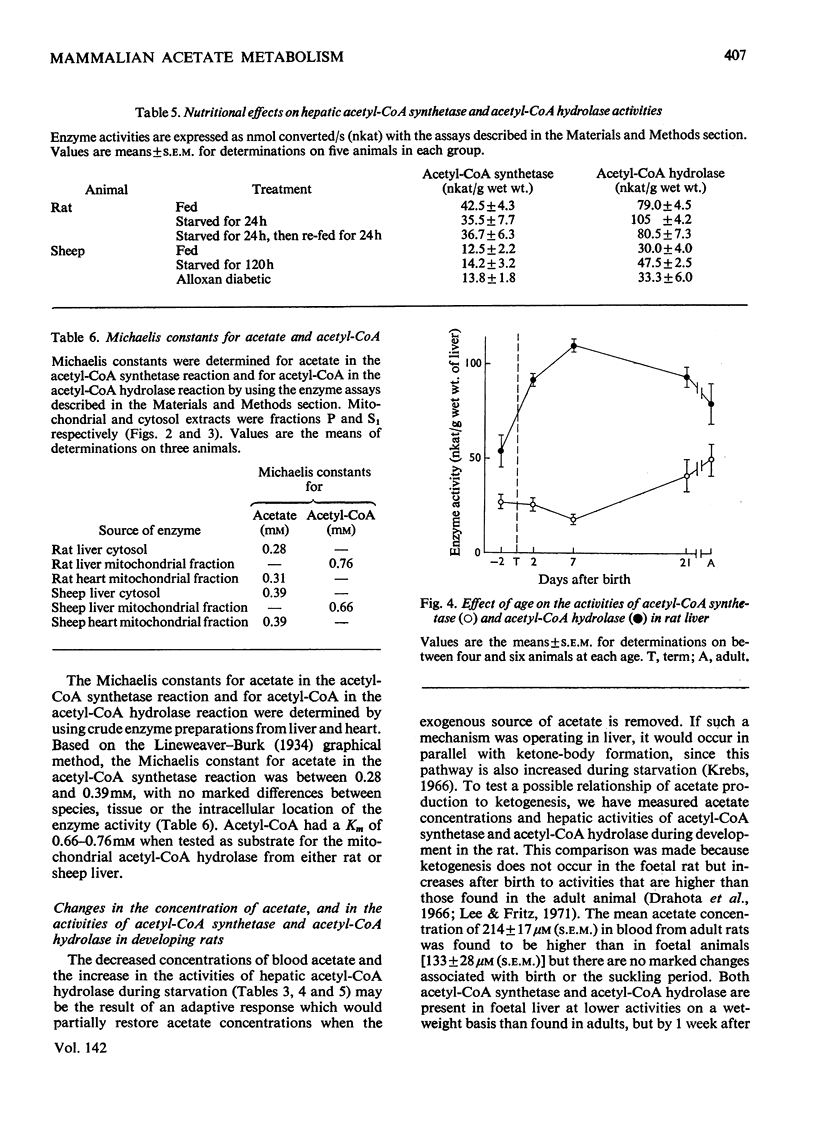



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Bremer J. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim Biophys Acta. 1968 Oct 22;164(2):157–166. doi: 10.1016/0005-2760(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Aas M. Organ and subcellular distribution of fatty acid activating enzymes in the rat. Biochim Biophys Acta. 1971 Feb 2;231(1):32–47. doi: 10.1016/0005-2760(71)90253-0. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Filsell O. H., Jarrett I. G. Effects of carbohydrate availability on lipogenesis in sheep. Biochem J. 1972 Jan;126(1):193–200. doi: 10.1042/bj1260193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Ballard F. J., Oliver I. T. Ketohexokinase, isoenzymes of glucokinase and glycogen synthesis from hexoses in neonatal rat liver. Biochem J. 1964 Feb;90(2):261–268. doi: 10.1042/bj0900261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J. Regulation of gluconeogenesis during exposure of young rats to hypoxic conditions. Biochem J. 1971 Jan;121(2):169–178. doi: 10.1042/bj1210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J. Supply and utilization of acetate in mammals. Am J Clin Nutr. 1972 Aug;25(8):773–779. doi: 10.1093/ajcn/25.8.773. [DOI] [PubMed] [Google Scholar]
- Ballard F. J. The development of gluconeogenesis in rat liver. Controlling factors in the newborn. Biochem J. 1971 Sep;124(2):265–274. doi: 10.1042/bj1240265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C., Sladek M., Decker K. The subcellular distribution of short-chain fatty acyl-CoA synthetase activity in rat tissues. Biochim Biophys Acta. 1971 Oct 5;248(1):24–33. doi: 10.1016/0005-2760(71)90071-3. [DOI] [PubMed] [Google Scholar]
- CIARANFI E., FONNESU A. Time-course of injected acetate in normal and depancreatized dogs. Biochem J. 1954 May;57(1):171–175. doi: 10.1042/bj0570171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. J. On the nature of malonate-insensitive oxidation of pyruvate and glutamate by heart sarcosomes. Biochim Biophys Acta. 1968 Jul 16;162(1):1–10. doi: 10.1016/0005-2728(68)90208-9. [DOI] [PubMed] [Google Scholar]
- Drahota Z., Hahn P., Honová E. Acetoacetate formation by livers of young and adult rats. Biol Neonat. 1965;9(1):124–131. doi: 10.1159/000239984. [DOI] [PubMed] [Google Scholar]
- GRUNERT R. R., PHILLIPS P. H. A modification of the nitroprusside method of analysis for glutathione. Arch Biochem. 1951 Feb;30(2):217–225. [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. W., Ballard F. J. The relative significance of acetate and glucose as precursors for lipid synthesis in liver and adipose tissue from ruminants. Biochem J. 1967 Nov;105(2):529–536. doi: 10.1042/bj1050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson N. S. Intracellular location and genetic control of isozymes of NADP-dependent isocitrate dehydrogenase and malate dehydrogenase. Ann N Y Acad Sci. 1968 Jun 14;151(1):429–440. doi: 10.1111/j.1749-6632.1968.tb11906.x. [DOI] [PubMed] [Google Scholar]
- JARRETT I. G., FILSELL O. H. Acetate tolerance in the young lamb. Nature. 1960 Oct 29;188:418–419. doi: 10.1038/188418a0. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Perkins J. R. The physiological role of liver alcohol dehydrogenase. Biochem J. 1970 Jul;118(4):635–644. doi: 10.1042/bj1180635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The regulation of the release of ketone bodies by the liver. Adv Enzyme Regul. 1966;4:339–354. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee L. P., Fritz I. B. Hepatic ketogenesis during development. Can J Biochem. 1971 May;49(5):599–605. doi: 10.1139/o71-086. [DOI] [PubMed] [Google Scholar]
- Mayfield E. D., Bensadoun A., Johnson B. C. Acetate metabolism in ruminant tissues. J Nutr. 1966 Jun;89(2):189–196. doi: 10.1093/jn/89.2.189. [DOI] [PubMed] [Google Scholar]
- McCARTHY R. D., SHAW J. C., LAKSHMANAN S. Metabolism of volatile fatty acids by the perfused goat liver. Proc Soc Exp Biol Med. 1958 Dec;99(3):560–562. doi: 10.3181/00379727-99-24419. [DOI] [PubMed] [Google Scholar]
- Palmquist D. L. Palmitic acid as a source of endogenous acetate and -hydroxybutyrate in fed and fasted ruminants. J Nutr. 1972 Nov;102(11):1401–1406. doi: 10.1093/jn/102.11.1401. [DOI] [PubMed] [Google Scholar]
- Pearson D. J., Tubbs P. K. Carnitine and derivatives in rat tissues. Biochem J. 1967 Dec;105(3):953–963. doi: 10.1042/bj1050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishi S., Cook R. M. Utilization of volatile fatty acids in ruminants. IV. Relative activities of acetyl CoA synthetase and acetyl CoA hydrolase in mitochondria and intracellular localization of acetyl CoA synthetase. J Agric Food Chem. 1972 Jan-Feb;20(1):91–95. doi: 10.1021/jf60179a003. [DOI] [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regen D. M., Terrell E. B. Effects of glucagon and fasting on acetate metabolism in perfused rat liver. Biochim Biophys Acta. 1968 Nov 12;170(1):95–111. doi: 10.1016/0304-4165(68)90164-5. [DOI] [PubMed] [Google Scholar]
- STACEY R. E., LATIMER S. B., TOVE S. B. DETERMINATION OF ACYL-COA SYNTHETASE ACTIVITY FOR VOLATILE FATTY ACIDS. Biochim Biophys Acta. 1964 Apr 20;84:192–195. doi: 10.1016/0926-6542(64)90077-0. [DOI] [PubMed] [Google Scholar]
- Smyth D. H. The rate and site of acetate metabolism in the body. J Physiol. 1947 Jan 15;105(4):299–315. [PMC free article] [PubMed] [Google Scholar]
- Snoswell A. M., Koundakjian P. P. Relationships between carnitine and coenzyme A esters in tissues of normal and alloxan-diabetic sheep. Biochem J. 1972 Mar;127(1):133–141. doi: 10.1042/bj1270133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff R. W. Substrate transformations dependent on respiratory states of mitochondria. Changes in metabolic control sites of rabbit heart mitochondria. Nature. 1967 Apr 1;214(5083):23–26. doi: 10.1038/214023a0. [DOI] [PubMed] [Google Scholar]
- WADA H., MORINO Y. COMPARATIVE STUDIES ON GLUTAMIC-OXALACETIC TRANSAMINASES FROM THE MITOCHONDRIAL AND SOLUBLE FRACTIONS OF MAMMALIAN TISSUES. Vitam Horm. 1964;22:411–444. doi: 10.1016/s0083-6729(08)60346-5. [DOI] [PubMed] [Google Scholar]
- Wieland O., Weiss L., Eger-Neufeldt I. Enzymatic regulation of liver acetyl-CoA metabolism in relation to ketogenesis. Adv Enzyme Regul. 1964;2:85–99. doi: 10.1016/s0065-2571(64)80007-8. [DOI] [PubMed] [Google Scholar]


