Abstract
Florida is the second largest producer of strawberries in the United States. However, the production system faces numerous challenges, especially Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) infestations. Management of this pest involves applying insecticides and use of predatory mites, particularly Amblyseius swirskii Athias-Henriot, Neoseiulus cucumeris Oudemans, and Neoseiulus californicus McGregor (Mesostigmata: Phytoseiidae). Strawberry growers in Florida are concerned about the compatibility of the commercial formulations of insecticides used in strawberry pest management with predatory mites. This study assessed the residual effect of commercial insecticides used in strawberry production on the survival, feeding, and oviposition of the 3 predators. Using Munger cells, predators were exposed to commercial formulations of spinetoram, cyantraniliprole, azadirachtin + pyrethrin, Beauveria bassiana, Cordyceps javanica, capsicum, garlic, and canola oil extracts, and water control. There was a gradual decline in the survival and feeding of predatory mites when exposed to all insecticides. Spinetoram had the highest impact on the survival and feeding of all predators compared to other insecticides, while C. javanica had the lowest impact. Cyantraniliprole and azadirachtin + pyrethrin significantly reduced predator survival after 72 h of exposure, whereas capsicum, garlic, and canola oil extracts caused a similar reduction after 96 h. All predators consumed low proportions of S. dorsalis across all treatments. Oviposition was low in all treatments, with no discernable variation among treatments. These results highlight the potential of using entomopathogenic fungi in conjunction with A. swirskii, N. cucumeris, and N. californicus for the management of S. dorsalis and T. urticae in strawberries.
Keywords: Scirtothrips dorsalis, Amblyseius swirskii, Neoseiulus cucumeris, Neoseiulus californicus, nontarget effects
Graphical Abstract
Graphical Abstract.
Introduction
Strawberry Fragaria × ananassa (Rosaceae) production significantly contributes to the US economy, especially in California and Florida, the top-producing states. The state of Florida is the second-largest producer and the overall top producer of winter strawberries (Guan et al. 2016, Huang et al. 2022). Similar to other agricultural sectors, strawberry production faces significant challenges, particularly from a variety of arthropod pests. In Florida, the primary strawberry pest complex includes various thrips species such as Scirtothrips dorsalis Hood, Frankliniella occidentalis Pergande, and Frankliniella bispinosa Morgan (Thysanoptera: Thripidae), of which the S. dorsalis is the most severe pest (Lahiri and Panthi 2020, Panthi and Renkema 2020, Panthi et al. 2021). The pest complex also contains a wide range of phytophagous mite species, such as Tetranychus urticae Koch (Trombidiformes: Tetranychidae), Polyphagotarsonemus latus Banks, and Phytonemus pallidus Banks (Trombidiformes: Tarsonemidae), with T. urticae being the most prevalent mite pest (Akyazi and Liburd 2019, Lahiri et al. 2022, 2024, Montemayor et al. 2023, Busuulwa et al. 2024). In some strawberry fields, it is possible to find co-occurring infestations of S. dorsalis and T. urticae (Lahiri et al. 2024).
To manage S. dorsalis and T. urticae, the majority of strawberry growers in Florida rely on insecticide applications (Lahiri and Panthi 2020, Panthi and Renkema 2020, Gireesh et al. 2022, Lahiri et al. 2022, Lahiri 2023). Some of the most commonly used insecticides in strawberry production include broad-spectrum reduced-risk synthetic insecticides such as spinetoram and cyantraniliprole. Plant-derived insecticides such as capsicum oleoresin, garlic oil, and canola oil extracts, and azadirachtin + pyrethrin are also widely used. Additionally, entomopathogenic fungi especially Beauveria bassiana strain GHA and Cordyceps javanica (formally known as Isaria fumosorosea) are used by some growers during the strawberry season. However, due to increasing concerns about the development of resistance to some of the reduced-risk insecticides (Kaur et al. 2023), the augmentative release of biological control agents, particularly phytoseiid mites, has become a common practice among growers (Lahiri et al. 2022, 2024, Lahiri 2023).
Currently, the most commonly used predatory mites include Neoseiulus cucumeris Oudemans, Neoseiulus californicus McGregor, Amblyseius swirskii Athias-Henriot, and Phytoseiulus persimilis Athias-Henriot (Mesostigmata: Phytoseiidae). Neoseiulus cucumeris, N. californicus, and A. swirskii are generalist predators (McMurtry and Croft 1997, 2003, McMurtry et al. 2013) that can feed on a variety of prey species in addition to pollen. In contrast, P. persimilis is a specialist predator of spider mites. Amblyseius swirskii and N. cucumeris have been used to control important agricultural pests such as Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) (Nomikou et al. 2002, Li et al. 2017), and thrips (Zilahi-Balogh et al. 2007, Arthurs et al. 2009, Kakkar et al. 2016, Lahiri and Yambisa 2021, Schoeller et al. 2022) while N. californicus has been extensively used to manage T. urticae (Rhodes et al. 2006, Gotoh et al. 2007, Rahmani et al. 2016). The ability of A. swirskii, N. cucumeris, and N. californicus to feed on various mite species has significantly enhanced their mass-rearing and facilitated their commercialization on a large scale (Massaro et al. 2016). Since these predators can also survive on pollen (McMurtry et al. 2013), they are able to maintain stable populations in the field even when pest populations are low, thereby providing constant pest suppression. As a result, these qualities have made them the preferred augmentative biocontrol agent for S. dorsalis management in strawberries.
However, it is still common to encounter strawberry growers applying insecticides and releasing predatory mites concurrently in the same field, a practice done as part of their integrated pest management (IPM) strategy to effectively suppress S. dorsalis populations. Several laboratory studies have shown that most of the insecticides used in various cropping systems negatively affect many species of phytoseiid predatory mites by reducing their survival rate, predation, and in some instances oviposition. For example, imidacloprid, fenpyroximate, and lambda-cyhalothrin were found to be extremely toxic to A. swirskii, P. persimilis and Amblyseius andersoni (Chant) (Fiedler and Sosnowska 2014).
Fenazaquin, an acaricide with both contact and ovicidal activity, was reported to decrease the developmental time of A. swirskii, and that of its successive generations, while acetamiprid caused a significant decline in survival and fecundity of the predatory mite (Shahbaz et al. 2019). Similarly, high mortality of A. swirskii was observed when it was exposed to fenpyroximate (Fiedler and Sosnowska 2014). Although a combination of fenpyroximate and thiacloprid at their reduced rate was reported to be moderately toxic to A. swirskii (Ghasemzadeh and Qureshi 2018), applications of thiacloprid alone significantly reduced the survival and oviposition of the predatory mite.
Exposure of A. swirskii to abamectin and pyridaben was reported to result into high mortality rates for all developmental stages of the predator, with the highest mortality occurring in adult females (Döker and Kazak 2019). Similarly, exposure of Iphiseius degenerans (Berlese) (Mesostigmata: Phytoseiidae) to spinetoram resulted in high mortality of the predator (Döker et al. 2015). High acetamiprid concentrations were reported to heavily reduce feeding, oviposition, and survival of N. cucumeris (Cheng et al. 2018). Azadirachtin, a biorational insecticide although reported to be nontoxic to Stratiolaelaps scimitus (Womersley) (Mesostigmata: Laelapidae) was found to be moderately toxic to Galendromus occidentalis (Nesbitt) (Mesostigmata: Phytoseiidae) (Yanar 2019), while spinetoram applications were found to cause high mortality of the predatory mite (Beers and Schmidt 2014).
Considering that strawberry growers in Florida perform weekly insecticide and fungicide applications in their fields alongside the release of predatory mites, it is essential to examine the effects of commonly used insecticides to assess their compatibility with these beneficial predators. Such research would offer the foundational information necessary for developing IPM programs that allow for the incorporation of predatory mites. Thus, the main aim of this study was to determine the compatibility of commonly used conventional and biorational pesticides with predatory mites by comparing their effect on the feeding, oviposition, and survival of A. swirskii, N. cucumeris, and N. californicus.
Materials and Methods
Predatory Mite Rearing
Amblyseius swirskii, N. cucumeris, and N. californicus, used in the experiment, were initially sourced from Arbico Organics (Tucson, AZ, USA) and then placed in laboratory culture. To start the laboratory colonies used in the bioassays, 200 gravid females of each predator species were transferred onto separate rearing arenas using a fine paint brush. Gravid females were identified by their distinctly enlarged, round-shaped opisthosomas.
The rearing arenas used in this experiment were similar to those described by Helle and Sabelis (1985). Each arena comprised a plastic dish pan (35.6 × 29 × 12 cm, Greenbrier International, Inc., USA) half-filled with distilled water. Large multipurpose sponges (19 × 14 × 2.5 cm, QEP, Boca Raton, FL, USA) were placed in the pans on which a black polystyrene flexible plastic board (12 × 8 cm, MEGA Format, Brooklyn, NY, USA) was placed. The edges of the plastic boards were lined with moist, nonsterile cotton (Fisher Scientific, NJ, USA) to prevent the predators from escaping.
To facilitate oviposition, triangular structures were created from small plastic sheets, and cotton fibers were then adhered to the underside of these structures, which were then placed on the arena. These structures provided suitable spots for the predators to lay their eggs. Once prepared, the arenas were transferred to a growth chamber, maintained at 25 ± 1 °C, 70 ± 5% RH, and 14:10 h L:D. To sustain the established colonies, a mixture of ~300–400 first and second-instar larvae of S. dorsalis were provided as a food source every 48 h, by gently brushing them onto the arena using a paintbrush. Both first and second instar larvae of S. dorsalis were provided because of the predators’ capability to feed on both developmental stages (Arthurs et al. 2009). Scirtothrips dorsalis larvae used as a food source were obtained from laboratory colonies raised on cotton plants in a growth room, where the conditions were kept at 25 ± 1 °C, 65 ± 5% RH, and 14:10 h L:D.
To obtain predators of the same age, 120 gravid female predatory mites were randomly selected from the primary colony and placed into individual rearing arenas for egg-laying. After a 24-h oviposition period, the females were removed, and the arenas with the eggs were kept in a growth chamber at 26 ± 1 °C, 70 ± 5% RH, and 14:10 h L:D to ensure optimal conditions for the eggs to hatch. Upon hatching, the predatory mite nymphs were provided with first and second instar larvae of S. dorsalis by brushing approximately 200 larvae onto each rearing arena. This procedure was repeated at 48-h intervals, culminating when the predatory mites matured into adults and commenced oviposition, which occured 8 days after hatching. This predatory mite generation was then used for all following experiments.
Insecticides
Six insecticides commonly used in S. dorsalis management in strawberry production in Florida were tested (Table 1). The insecticides were categorized into 2 broad groups: reduced-risk insecticides (spinetoram and cyantraniliprole) and biopesticides (Beauveria bassiana, Cordyceps javanica, azadirachtin + pyrethrin and capsicum oleoresin, garlic oil, and canola oil extracts (Leahy et al. 2014). The biopesticides were further divided into 2 categories: the entomopathogenic fungi (Beauveria bassiana and Cordyceps javanica), and plant extracts (azadirachtin + pyrethrin and capsicum oleoresin, garlic oil, and canola oil extracts).
Table 1.
List of insecticides tested on predatory mites, including their trade names, active ingredients, and the maximum recommended application rates for strawberries specified by the manufacturers
| Trade name | Active ingredient (AI) and percentage composition | Chemical class | Insecticide type | Application rate |
|---|---|---|---|---|
| Radiant SC | Spinetoram (11.7%) | Spinosyns | Reduced risk | 0.88 L/ha |
| Exirel | Cyantraniliprole (10.2%) | Diamides | Reduced risk | 1.5 L/ha |
| Azera | Azadirachtin (1.20%) and Pyrethrin (1.40%) | Pyrethrin | Plant extract | 4.1 L/ha |
| Captiva Prime | Capsicum oleoresin (7.60%), garlic oil (23.40%), and canola oil (55.00%) extracts | Botanical essence | Plant extract | 2.4 L/ha |
| Mycotrol ESO | Beauveria bassiana strain GHA (11.30%) | Fungal agents | Entomopathogenic fungi | 4.7 L/ha |
| PFR-97 20% WDG | Cordyceps javanica formally Isaria fumosorosea Apopka Strain 97 (20.0%) | Fungal agents | Entomopathogenic fungi | 2.24 g/ha |
Strawberry Plants
“Brilliance” cultivar strawberry transplants were grown in plastic pots inside an insect-rearing cage. The cage was kept in a growth chamber with the temperature set at 25 ± 1 °C, relative humidity at 65 ± 5%, and a light-dark cycle of 14:10 L:D. Plants were watered and fertilized as needed. The plants were grown for 6 weeks before being used in the experiments.
Residual Contact Toxicity of Insecticides to Predatory Mite Adult Females
Leaf discs measuring 12 mm in diameter were cut from S. dorsalis-free plants from the growth chamber. The leaf discs were then immersed for 10 s in an insecticide solution that had been prepared using the manufacturer’s maximum strawberry recommended application rate for the management of S. dorsalis (Table 1). A control treatment, created by dipping the leaf discs in distilled water for 10 s, was included in the experiment. After the dipping process, the treated leaf discs were left to air dry for 1.5 h before being used in the experiment. Experimental arenas used were similar to those used by Busuulwa et al. (2024), which were closely modeled after those described by Helle and Sabelis (1985) and Argolo et al. (2020).
In brief, the arenas were constructed using 2 transparent acrylic glass plates, each measuring 75 mm by 26 mm. One of the glass plates had a central circular hole with a diameter of 12.7 mm, designed to fit within the outline of the leaf disc used in the experiment. The second glass plate, identical in size, served as the base of the setup. A layer of moist cotton was placed on this base plate, on top of which a leaf disc with the abaxial surface facing downward was placed. The glass plate with the hole was then carefully placed on top of the leaf disc, creating a sandwich-like structure.
In each arena, a single 10-day-old female predator was randomly selected from the age-synchronized colony and carefully placed onto the treated strawberry leaf disc. To serve as a food source, 10 S. dorsalis larvae (first and second instar) were introduced into the same arena with the predatory mite. Each treatment (insecticides and the control) consisted of 10 replicates. After the experimental setup, the arenas were transferred to a growth chamber maintained at 25 ± 1 °C, 65 ± 5% RH, and 14:10 h L:D.
Scirtothrips dorsalis larvae were added to the arenas every 24 h to replenish those consumed by the predatory mites. Data on the number of predatory mites alive (survival), the number of S. dorsalis larvae consumed (feeding), and the total number of egg produced by the predators (oviposition rate) was recorded at 24-h intervals for 120 h. Scirtothrips dorsal larvae that had been fed on by the predators were easily distinguishable from those that had died of other causes given that the former were desiccated. During the course of the experiment, eggs laid by the predators were not removed from the experimental arena to avoid disturbing the adult females and to prevent the potential escape of S. dorsalis larval prey. As a result, the number of eggs laid during each period was determined by subtracting the egg count from the previous day. Nevertheless, the viability of the eggs was not assessed, as it was beyond the scope of this study. The whole experimental setup was conducted twice to ensure consistency and reliability of the results obtained.
Statistical Analysis
The Bayesian framework (Ellison 2004) was utilized to test our hypothesis that both conventional and biopesticides possess some negative effects on predatory mites. This approach was chosen primarily for the fact that it allows the use of regularizing priors, which can improve parameter identifiability and generate more robust estimates compared with maximum-likelihood based methods (McElreath 2020). Overall, the experiment was structured as a completely randomized design with a split-plot restriction on randomization, wherein there were 2 replicates of the main plot factor (predatory mite species) and 10 replicates for each insecticide and control treatment (subplot factor). In addition, the study involved repeated measures on each individual leaf disc taken at 5 time points. Separate analyses, described below, were conducted for predatory mite survival, feeding, and oviposition. For each model, we executed 8 chains and performed 25,000 iterations, with 20,000 of those iterations designated as warm-up iterations. All analyses were conducted in R version 4.0.3 (R Core Team 2024) and Stan (version 2.30) (Bürkner 2021, Stan Development Team 2022, Guo Jiqiang et al. 2024).
Predatory mite survival was modeled using ordinal logistic regression, with mite species treated as a fixed effect while the effects of insecticides, the insecticide-by-species interaction, and the main plot experimental units (“2 trials,” the whole experimental repeated twice), were treated as random effects. The former 2 random effects were treated as such to generate partially pooled estimates (Hobbs and Hooten 2015), which were especially desirable because for some combinations of predator and insecticide, no predators survived to the first observation period. The proportion of S. dorsalis consumed by the predators throughout the 120-h period of observation was assumed to be binomially distributed, and thus predatory mite feeding was modeled using a generalized linear mixed-effects model (GLMM) (Bolker et al. 2009), with the specification of fixed and random effects the same as in the analysis of mite survival. The proportion of S. dorsalis larvae consumed by the predators was calculated as number of S. dorsalis consumed every 24 h divided by the total number of S. dorsalis larvae provided (10 larvae). Given that predators consistently consumed low proportions of S. dorsalis over the entire observation period, a regression model was fitted, slope calculated, and comparisons between the slopes made using 120-h as the cutoff point.
Predatory mite oviposition, recorded as the daily number of eggs produced (oviposition rate) was also modeled using a GLMM, but with the assumption that egg production had a Poisson distribution and with a First-order Autoregressive Covariance Structure (AR1) among measures taken from the same leaf disc over time. A Poisson distribution was chosen in this case because using a negative binomial and an autoregressive correlation structure rendered the model overparameterized and unidentifiable. The predatory mite species were treated as fixed effects. In all cases, fixed effects were given weakly informative normal priors with mean zero. Random effect standard deviations were given weakly informative half-Cauchy priors, and the cut points in the ordinal logistic regression were given induced Dirichlet priors with concentration parameters equal to one (for details, see Betancourt (2019)).
After fitting the models, preplanned orthogonal contrasts were used to estimate, compare, and test the effects of different groups of insecticides on the survival, feeding, and oviposition of the different predatory mites, as shown in (Table 2). Such contrasts provide more focused and meaningful comparisons than those achieved via all pairwise comparisons (Saville and Graham 2012). Therefore, preplanned orthogonal contrast that leveraged relationships between the insecticides and predatory mites were developed. These comparisons assessed the probability of predatory mites surviving, the proportion of prey consumed, and the rate of oviposition for 120 h under treatment, considering the demonstrated residual activity of the insecticides used, especially spinetoram, which lasts between 3 and 7 days (Shimokawatoko et al. 2012, Depalo et al. 2016). To detect significant differences between contrasts, a comparison of posterior distributions was performed. This was done by computing the product of the Lower and Upper Credible Interval (LCL/UCL) and determining whether it overlaps with zero (LCL*UCL > 0).
Table 2.
Preplanned orthogonal contrasts designed to compare the percentage of predatory mites alive (survival), number of S. dorsalis consumed by the predatory mites (feeding), and daily number of eggs laid by the predatory mites (oviposition rate) after exposure to different groups of insecticide treatments
| Contrast | Name | Description |
|---|---|---|
| C1 | Control—Insecticide | Predatory mites on insecticide-treated leaf discs vs. those in the control treatment |
| C2 | Biopesticide—Reduced-risk insecticide | Predatory mites on leaf discs treated with a reduced-risk insecticide (cyantraniliprole or spinetoram) vs. those on leaf discs treated with a biopesticide (azadirachtin + pyrethrin, capsicum canola, and garlic oil extracts, Beauveria bassiana, or Cordyceps javanica) |
| C3 | Plant Extract—Entomopathogenic insecticide | Predatory mites on leaf discs treated with an entomopathogenic insecticide (Beauveria bassiana or Cordyceps javanica) vs. those on leaf discs treated with a plant extract-based insecticide (azadirachtin + pyrethrin or capsicum canola and garlic oil extracts) |
| C4 | Spinetoram—Cyantraniliprole (between reduced-risk insecticides) | Predatory mites on leaf discs treated with cyantraniliprole vs. those on leaf discs treated with spinetoram |
| C5 | Beauveria bassiana—Cordyceps javanica. (between entomopathogenic insecticide) | Predatory mites on leaf discs treated with Cordyceps javanica vs. those on leaf discs treated with Beauveria bassiana |
| C6 | Azadirachtin + Pyrethrin—Capsicum, garlic, and canola oil extracts (between plant extracts insecticides) | Predatory mites on leaf discs treated with capsicum canola and garlic oil extracts vs. those on leaf discs treated with azadirachtin + pyrethrin |
The “Contrast” column contains the abbreviation /code for the contrast. The “Name” column lists the conditions being contrasted, with the first stated category regarded as the first condition and the second category as the second condition. For example, for C1, “Control” is Condition 1, and “Insecticide” is Condition 2; similarly, for C2, “Biopesticide” is Condition 1, and “Reduced risk insecticide” is Condition 2. The “Description” column provides details of the contrast.
Results
Overall Survival
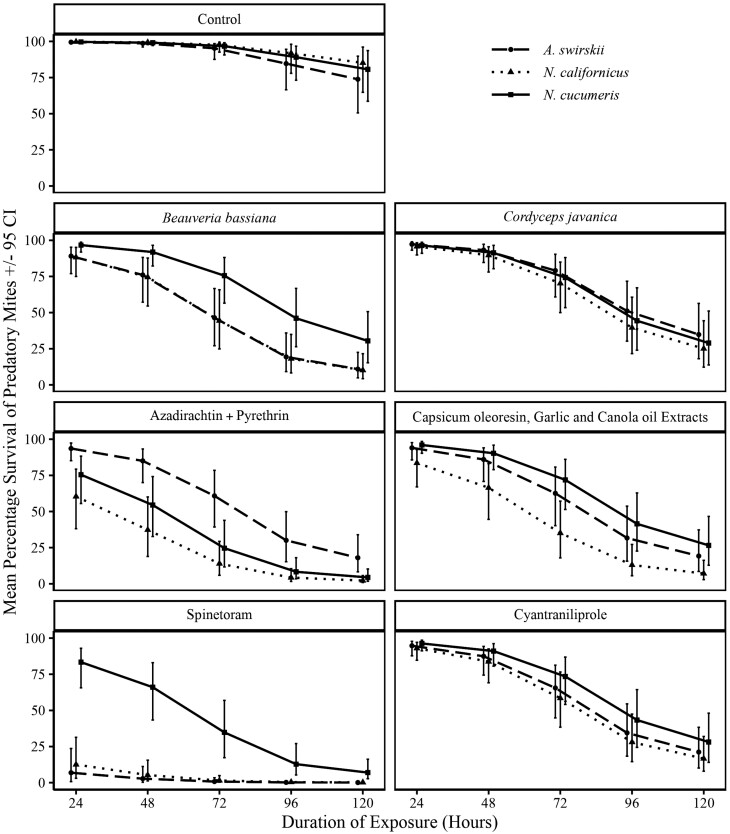
After 120 h, predatory mites exposed to reduced-risk insecticides had the lowest survival, 12.5%, with a 95% credible interval (CI) of 8.4%–18.0% compared to those exposed to the 2 types of biopesticides (18.5%, CI: 13.7%–24.3%). Predators exposed to plant extracts had lower survival (13.1%, CI: 8.7%–19.0%) than those exposed to entomopathogenic fungal insecticides (23.7%, CI: 17.3%–31.9%). The highest predatory mite survival was observed in the control group at 81.6% (CI: 69.6%–90.5%) compared to all other treatments (16.5%, CI: 12.5%–21.3%). However, the analysis also revealed significant variation within each insecticide type (Table 3).
Table 3.
Percentage of predatory mites alive after 120 h of exposure to different groups of insecticide treatments. Comparisons are based on the preplanned contrasts
| Contrast | Predatory mite survival marginal means (%) | Difference between survival marginal means (Δμ) | Δμ LCL | Δμ UCL | UCL*LCL > 0 | |
|---|---|---|---|---|---|---|
| C1 | 79.17 | 16.73(%) | 62.44 | 52.8 | 74.2 | * |
| C2 | 18.78 | 12.58 | 6.2 | 0.7 | 11.3 | * |
| C3 | 13.41 | 23.99 | −10.58 | 17.9 | −3.6 | * |
| C4 | 2.47 | 22.27 | −19.8 | 29.6 | −12.1 | * |
| C5 | 17.61 | 30.16 | −12.55 | 24.7 | −1.1 | * |
| C6 | 8.44 | 18.10 | −9.66 | 18.6 | −0.22 | * |
The “Predatory mite survival marginal means (%)” column includes 2 subcolumns that show the mean percentage survival of predatory mites across 3 species for the 2 conditions being compared. The conditions are listed in the same order as described in Table 2. The differences in marginal means (Δ μ) were calculated by subtracting the mean of condition 1 from that of condition 2 in each contrast. Positive values indicate higher percentage survival of predatory mites for condition 1 of the contrast, while negative values indicate higher survival for condition 2. The “LCL” and “UCL” columns show the lower and upper credible intervals of Δμ, respectively. Asterisks (*) indicate significant differences between the contrast comparisons. Significance was computed by establishing whether the product of UCL and LCL overlap with zero.
On average, among reduced-risk insecticides, spinetoram had the lowest predatory mite survival (2.5% CI: 1.0%–5.4%) compared to cyantraniliprole (22.3% CI 14.7%–32.7%) after 120 h of exposure. Between the plant extract group, azadirachtin + pyrethrin had the lowest predator survival (8.2%, CI: 4.5%–13.8%) compared to capsicum oleoresin, garlic, and canola oil extracts (18.0%, CI: 11.1%–27.1%). Upon comparing the entomopathogenic fungal insecticides, B. bassiana had lower predator survival (17.5%, CI: 11.1%–26.3%) compared to C. javanica (18.0% CI: 11.1%–27.1%).
Survival by Predator Species
The impact of insecticides on the survival of predators varied across predatory mite species. In all treatments, we observed a decrease in predator survival with prolonged exposure to insecticides (Fig. 1). When exposed to spinetoram, A. swirskii and N. californicus had very low survival (6.8%, CI: 0.7%–20.4% and 12.5%, CI: 3.1%–31.4%, respectively), compared to N. cucumeris (83.4%, CI: 65.6%–92.9%). However, there was a substantial decline in N. cucumeris survival by 72 h of exposure (34.8%, CI: 17.3%–56.8%).
Fig. 1.
Percentage of A. swirskii, N. californicus, and N. cucumeris alive at various time points following exposure to insecticide treatments.
When exposed to azadirachtin + pyrethrin, A. swirskii consistently had higher survival even at 72 h (60.8%, CI: 39.3%–78.5%) compared to N. californicus (13.9%, CI: 5.9%–29.3%) and N. cucumeris (24.6%, CI: 11.6%–43.9%). Neoseiulus cucumeris exhibited higher survival in the B. bassiana treatment compared to A. swirskii and N. californicus, especially after 72 h (N. cucumeris: 75.5%, CI: 56.6%–88.1%; A. swirskii: 46.4%, CI: 27.1%–66.6%; N. californicus: 44.4%, CI: 24.9%–65.8%), and 120 h of exposure (N. cucumeris: 30.4%, CI: 15.3%–50.1%; A. swirskii: 10.9%, CI: 4.8%–22.5%; N. californicus: 10.1%, CI: 4.3%–21.5%). However, there were no differences in predatory mite survival when exposed to C. javanica, cyantraniliprole, capsicum oleoresin, garlic, and canola oil extracts.
Feeding (Proportion of S. dorsalis Consumed)
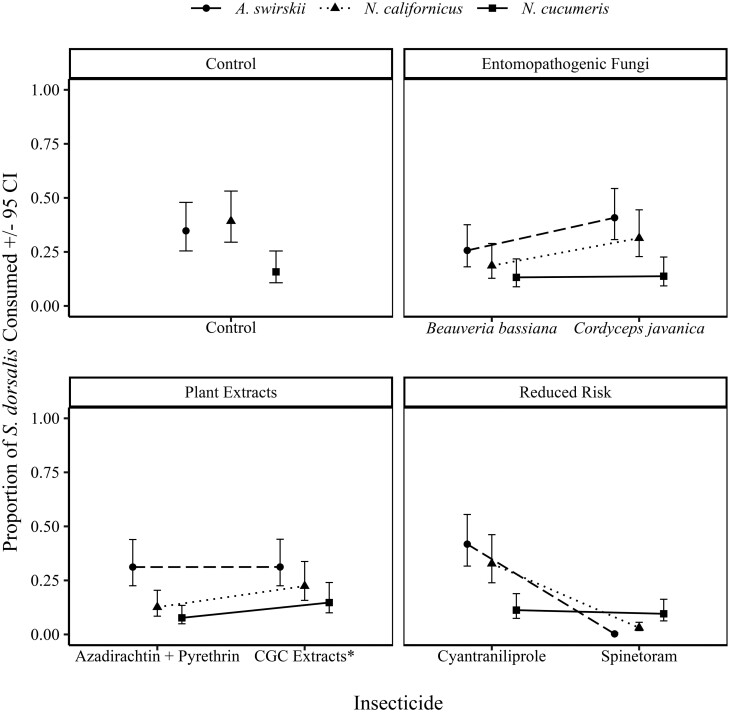
The highest proportion of prey consumed averaged across all 3 predators was observed in the control (0.30, CI: 0.26–0.382). Predators exposed to entomopathogenic had higher proportions of prey consumed compared to those exposed to plant extracts (0.20, CI: 0.17–0.27). Reduced-risk insecticides had the lowest proportion of S. dorsalis consumed (0.17, CI: 0.14–0.21) compared to all other treatments (Table 4).
Table 4.
Pooled proportion of S. dorsalis larvae (prey) consumed by the predatory mites after exposure to different insecticide treatments. Comparisons are based on the preplanned contrasts
| Contrast | Proportion of prey consumed | Difference between proportion of prey consumed (Δμ) | Δμ LCL | Δμ UCL | UCL*LCL > 0 | |
|---|---|---|---|---|---|---|
| C1 | 0.30 | 0.20(%) | 0.097 | 0.057 | 0.138 | * |
| C2 | 0.22 | 0.17 | 0.054 | 0.027 | 0.085 | * |
| C3 | 0.20 | 0.24 | −0.039 | −0.073 | −0.006 | * |
| C4 | 0.04 | 0.28 | −0.238 | −0.301 | −0.189 | * |
| C5 | 0.19 | 0.29 | −0.092 | −0.140 | −0.044 | * |
| C6 | 0.17 | 0.22 | −0.054 | −0.102 | −0.011 | * |
The “Proportion of prey consumed” column contains 2 subcolumns that show the proportion (out of 10) of S. dorsalis larvae consumed averaged across the 3 predatory mite species for the 2 conditions being contrasted. The conditions are listed in the same order as described in Table 2. Differences in the proportion of prey consumed (Δμ) were calculated by subtracting the mean of condition 1 from that of condition 2 in each contrast. Positive values indicate a higher proportion of prey consumed for condition 1, while negative values indicate a higher proportion of prey consumed for condition 2. The “LCL” and “UCL” columns show the lower and upper credible intervals of Δμ, respectively. Asterisks (*) indicate significant differences between the contrast comparisons, determined by whether the product of UCL and LCL overlap with zero.
The results also indicated that by 120 h, within the control treatment, N. californicus and A. swirskii had the highest proportions of prey consumed (N. californicus: 0.40, CI: 0.28–0.52; A. swirskii: 0.35, CI: 0.25–0.48) compared to N. cucumeris (0.16 CI: 0.10–0.26). Within the entomopathogenic group, A. swirskii had a higher proportion of prey consumption (0.25, CI: 0.17–0.37) in comparison to N. californicus (0.18, CI: 0.12–0.29) and N. cucumeris (0.13, CI: 0.08–0.21), when exposed to B. bassiana (Fig. 2). A similar trend was observed when predators were exposed to C. javanica where A. swirskii had a higher proportion of prey consumed (0.40, CI: 0.29–0.54) in comparison to N. californicus (0.31, CI: 0.22–0.44) and N. cucumeris (0.14, CI: 0.09–0.23).
Fig. 2.
The average proportion of S. dorsalis consumed by A. swirskii, N. californicus, and N. cucumeris throughout the experiment *CGC extracts represents capsicum, garlic, and canola oil extracts.
When exposed to azadirachtin + pyrethrin, A. swirskii consumed a higher proportion of consumed S. dorsalis (0.31, CI: 0.21–0.42) compared to N. californicus (0.13, CI: 0.08–0.21), and N. cucumeris (0.08, CI: 0.08–0.04). Similarly, in the capsicum oleoresin, garlic, and canola oil extracts treatment, A. swirskii had the highest proportion of consumed prey (0.31, CI: 0.21–0.43) compared to N. californicus (0.22, CI: 0.15–0.34), and N. cucumeris (0.15, CI: 0.09–0.24) (Fig. 2).
Oviposition
Analysis of the oviposition rate revealed that N. cucumeris in the control treatment initially (24 h) exhibited a relatively high oviposition rate (0.52 eggs per day, CI: 0.18–1.56) compared to N. californicus and A. swirskii, which initially had lower oviposition rates (0.01 per day, CI: 0.007–0.3, and 0.07, CI: 0.02–0.22, respectively) (Table 5). However, the rate of oviposition of both predators increased over time (0.036 per hour, CI: 0.0203–0.0539, respectively). On leaf discs treated with insecticides, oviposition decreased to 0.017% (CI: 0.0038–0.0329) in N. californicus and 0.0013 (CI: 0.0133–0.0102) in A. swirskii. Nonetheless, there were no discernable differences in the rate of oviposition among predatory mite species in insecticide treatments (Table 5).
Table 5.
Daily number of eggs laid by the predatory mites (oviposition rate) following exposure to different insecticide treatments. Comparisons are based on the preplanned contrasts
| Contrast | Predatory mite oviposition rate | Difference in daily predatory mite oviposition rate (Δμ) | Δμ LCL | Δμ UCL | UCL*LCL > 0 | |
|---|---|---|---|---|---|---|
| C1 | 0.286 | 0.184(%) | 0.097 | 0.075 | 0.55 | * |
| C2 | 0.218 | 0.108 | 0.105 | −0.0271 | 0.41 | |
| C3 | 0.165 | 0.259 | −0.086 | −0.398 | 0.17 | |
| C4 | 0.027 | 0.032 | −0.005 | −0.485 | −0.03 | |
| C5 | 0.169 | 0.334 | −0.154 | −0.673 | 0.12 | |
| C6 | 0.229 | 0.089 | 0.132 | −0.058 | 0.81 | |
The “Predatory mite oviposition rate” column includes 2 subcolumns displaying the daily number of eggs laid averaged across the 3 predatory mite species for the 2 conditions being compared. The conditions are listed in the same order as described in Table 2. Differences in daily predatory mite oviposition rates (Δμ) were calculated by subtracting the mean of condition 1 from condition 2 for each contrast. Positive values indicate higher daily oviposition for condition 1, while negative values indicate higher daily oviposition for condition 2. The “LCL” and “UCL” columns represent the lower and upper credible intervals of Δμ, respectively. Asterisks (*) denote significant differences between the contrast comparisons based on whether the product of UCL and LCL overlap with zero.
Discussion
Insecticides are key to managing S. dorsalis in strawberries, but they can harm predatory mites used for pest control. This study found that spinetoram significantly reduced the survival of A. swirskii, N. cucumeris, and N. californicus. Overall, A. swirskii and N. californicus were most affected, while N. cucumeris was the least affected. Fungal insecticides (B. bassiana and C. javanica) had the least impact on the survival or the predatory mites. Furthermore, the results of this study highlight substantial variability in response to insecticides among predatory mite species, not only within the same genus but also across the entire family.
To minimize the impact of insecticides on nontarget organisms, many companies in the insecticide industry are focusing on developing chemistries that have a lower impact on beneficial insect species (Sparks et al. 2021). This shift is partly driven by changes in government regulations, which now mandate that all new insecticides undergo testing on beneficial insects during their development (Leahy et al. 2014). Reduced-risk insecticides such as spinetoram and cyantraniliprole are expected to have a low impact on beneficial organisms such as bees (Besard et al. 2011, Kim et al. 2022). However, many studies have shown that these insecticides can negatively affect other beneficial organisms, such as predatory mites (Duso et al. 2008, Kim et al. 2018, Barroso et al. 2022).
Predatory mites are particularly vulnerable to insecticides due to the multiple routes of exposure, including direct contact, exposure to insecticide residuals, and the ingestion of prey that may harbor residual insecticides (Gentz et al. 2010). However, as this study demonstrates, different predatory mite species exhibit varying susceptibility to insecticides. This variability is as a result of differences in the kinetics and dynamics of toxicological processes among these predators (Feyereisen et al. 2015, Van Leeuwen and Dermauw 2016, Duso et al. 2020).
Research on the acaricidal effects of spinosyns against Acari has yielded some conflicting results depending on the Acari group being studied. In the Tetranychidae family, some studies report no acaricidal effects, while others demonstrate significant acaricidal activity of spinosyns. For example, Cowles (1998) found that spinosad had little to no activity against T. urticae when applied directly to the leaves of plants in a nursery setting. In contrast, van Leeuwen et al. (2005) reported that applying spinosad directly to the roots of tomatoes grown in rockwool (systemic application) and directly onto the leaves (contact application) provided excellent control of T. urticae. Wang et al. (2016) found that applications of spinetoram, an analog of spinosad, reduced the developmental time of T. urticae from egg to adult. Additionally, Wang et al. (2016) reported that the fecundity, intrinsic rate of increase, and net reproductive rate of T. urticae increased, leading to outbreaks of this pest.
In contrast, the effects of spinosyns on the Phytoseiidae family have generally been negative. The consensus indicates that spinosyns are harmful to predatory mites (Schmidt‐Jeffris et al. 2021), with most studies showing that spinetoram is more harmful than spinosad. For example, Kim et al. (2018) reported high mortality rates for P. persimilis (97.0%) and A. swirskii (90.7%) following exposure to spinetoram residues. Similarly, studies by Beers and Schmidt (2014, 2016), Shearer et al. (2016), Bergeron and Schmidt‐Jeffris (2023), Mills et al. (2015) and Döker et al. (2015) found comparable levels of adult mortality in G. occidentalis and I. degenerans when exposed to spinetoram. On the other hand, spinosad has been reported to have varying effects on the survival of adult phytosiide mites, with effects ranging from harmless to harmful (Fountain and Medd 2015). For example, Kim et al. (2018) reported that spinosad had a low effect on the survival of adults of N. cucumeris, while a meta-analysis by Schmidt‐Jeffris et al. (2021) showed that spinosad was highly toxic to larvae of many phytoseiid mites. This, therefore, shows that direct integration of spinosyns with phytoseiids used as biological control agents can reduce their efficacy, disrupting biological control.
Diamides have been reported to be harmful to predatory mites under laboratory and field conditions (Mills et al. 2015, Beers et al. 2016, Shearer et al. 2016, Bergeron and Schmidt‐Jeffris 2023). However, in this study, more than 50% of the predatory mites survived even after 72 h of exposure to cyantraniliprole. This suggests that while cyantraniliprole may be harmful to predatory mites initially, its harmfulness appears to decrease with prolonged exposure. This opens up the possibility of integrating cyantraniliprole into pest management strategies for S. dorsalis in strawberries by utilizing a temporal separation period of at least 72 h. By carefully timing the release of predatory mites after the application of cyantraniliprole, its impact on phytoseiid mites could be minimized. Additionally, establishing pesticide-free areas (predatory mite refuge sites) could provide a hiding place for predators to escape the adverse effects of diamides. This approach will further enhance the efficacy of predatory mites in the presence of this active ingredient (Duso et al. 2020).
In this study, azadirachtin + pyrethrin was found to be less harmful to A. swirskii compared to N. cucumeris and N. californicus, suggesting that this insecticide could be effectively combined with A. swirskii in pest management strategies. While azadirachtin has been reported to be selective and less harmful to certain predators (Castagnoli et al. 2002, Duarte et al. 2020), pyrethrin, an active ingredient in Azera, has been found to be harmful to predators (Duso et al. 2008). Although the exact mode of action of azadirachtin is still unclear (Sparks and Nauen 2015), this active ingredient has been reported to have acaricidal properties that could be harmful to some predators in this case N. cucumeris and N. californicus (Marčić and Međo, 2015, Thao and Thuy, 2023). Additionally, azadirachtin functions as an antifeedant, oviposition deterrent, metamorphosis inhibitor, and an effective insect repellent (Mordue (Luntz) and Nisbet 2000, Trumm and Dorn 2000, Ditzen et al. 2008,Adusei and Azupio 2022). These combined effects could potentially be detrimental to predatory mites, particularly with prolonged exposure beyond 72 h.
When exposed to capsicum oleoresin, garlic, and canola oil extracts, there was a rapid decline in the survival of predatory mites especially beyond 72 h. Similar findings were reported when Orius insidiosus (Say) (Hemiptera: Anthocoridae) was exposed to capsicum oleoresin, garlic oil, and soybean oil extracts (Herrick and Cloyd 2017, Cloyd and Herrick 2018). According to the label information, capsicum oleoresin + garlic and canola oil extract is a product designed to repel insects (Gowan Company 2024). However, research has shown that most of the above components do not pose a direct threat to the majority of natural predators (Bostanian et al. 2005, Cloyd et al. 2009, Cloyd and Herrick 2018). This is probably because they are designed to repel insects from feeding on plants rather than predators feeding on prey. This suggests that these extracts could be effectively integrated into a management program involving A. swirskii, N. cucumeris and N. californicus. Capsicum oleoresin + garlic and canola oil extracts have been reported to be effective in suppressing S. dorsalis populations in strawberries (Lahiri et al. 2024). Therefore, combining these extracts with predatory mites could further enhance S. dorsalis management in strawberries, especially when pest densities become too high for predatory mites to control effectively. This approach could be particularly useful in Florida strawberry fields during February to March when (Rahmani et al. 2015) S. dorsalis populations rapidly increase.
Commercial formulations of entomopathogenic fungi such as B. bassiana and C. javanica have been successfully used as an alternative to the chemical for the management of many agricultural pests, including various phytophagous mite species such Tetranychus evansi Baker & Pritchard (Trombidiformes: Tetranychidae) (Wekesa et al. 2005), and T. urticae (Sáenz-de-Cabezón Irigaray et al. 2003). However, since predatory mites share many evolutionary similarities with phytophagous mites, entomopathogenic fungi can also be detrimental to these beneficial organisms. In this study, we observed that exposure of N. californicus and A. swirskii to B. bassiana for more than 72 h led to a drastic decline in their survival. Beauveria bassiana is one of the many toxigenic entomopathogenic fungi that produce mycotoxins, especially beauvericin. These mycotoxins cause significant cytotoxicity in cells and also induce oxidative stress, ultimately leading to the death of the host (Mallebrera et al. 2018). Secondly, B. bassiana conidia produce chitinase and Pr1–Pr2 proteases as part of the epicuticle penetration process (Kim et al. 2010) to accelerate conidia-host penetration, which can also affect predatory mites. Therefore, the secretion of toxins and cuticle degradation of the predators could explain the observed decline in rapid decline in survival especially after 72 h of exposure.
Different strains of B. bassiana have been reported to be infectious to many predatory mites. For example, 3 strains of B. bassiana (DEBI008, F, and J.B.) were reported to cause significant mortality to A. swirskii especially after 72 h (Seiedy et al. 2015). Other studies reported similar findings when A. swirskii and N. californicus were exposed to B. bassiana (Castagnoli et al. 2005, Numa Vergel et al. 2011, Midthassel et al. 2016). Additionally, B. bassiana has been reported to affect the survival of P. persimilis when the predator was exposed to topical treatments and dry residues (Duso et al. 2008, Pozzebon and Duso 2010, Numa Vergel et al. 2011). Nonetheless, we observed that N. cucumeris was the least affected predator when exposed to B. bassiana. Similar observations were made by Jacobson et al. (2001) when B. bassiana was used in conjunction with N. cucumeris under greenhouse and laboratory settings.
Avertedly, when predators were exposed to C. javanica, there was a rapid decline in their survival after 96 h of exposure. Cordyceps javanica has been shown to possess low toxicity to the predatory mite N. cucumeris (Chen et al. 2020), N. californicus (Castillo-Ramírez et al. 2020), and A. swirskii (Zhang et al. 2015). The decline in survival observed beyond 96 can be attributed to the reported low toxicity of C. javanica and the fact that these entomopathogenic fungi require a longer time to kill their host (Inglis et al. 2001, Shah and Pell 2003). This provides an opportunity of conducting concurrent applications of C. javanica and predatory mite releases. This strategy could be implemented at the start of the season (October to December), when S. dorsalis populations are low, allowing for the use of stronger chemistries later in the season as pest pressures increase.
The low predation by N. cucumeris observed in this study could be as a result of the quality of predators obtained from commercial suppliers. Variations in commercial rearing conditions, especially the nutritional history of the predators, can significantly impact their performance. However, these effects can be reversed in successive generations if the predators are provided with more than one food source. (Dicke et al. 1989, Lopez and Smith 2016, Vangansbeke et al. 2023). Additionally, the provision of a food source that is not nutritionally ideal for the predators (such as thrips) can lead to low predation rates (Eubanks and Denno 2000, Wimmer et al. 2008, Schmidt et al. 2012), which would also explain the low proportions of S. dorsalis consumed by the predators in this study. This further emphasizes the importance of providing generalist predators with alternative food sources such as pollen even when target prey is in abundance, as this approach has been shown to enhance their efficacy in controlling pests (Beltrà et al. 2017, Benson and Labbe 2021, Etienne et al. 2021).
Although N. californicus prefers feeding on spider mites in its natural habitat (McMurtry and Croft 2003, McMurtry et al. 2013), in this study, N. californicus consumed the highest proportion of S. dorsalis larvae. The ability of N. californicus to feed on thrips has been demonstrated (Rahmani et al. 2015). Additionally, the possibility of developing a strain of N. californicus capable of feeding on thrips has also been demonstrated to be possible (Castagnoli and Simoni 1999). Early exposure of N. californicus to S. dorsalis as a food source could have also facilitated the predation rate observed (Zhu et al. 2022) or that the quality of N. californicus received from the commercial insectary was better than that of A. swirskii and N. cucumeris.
However, it is crucial to recognize that the insecticides tested could have direct or indirect impacts on predation, which are not yet fully understood. The literature on the effects of some of the insecticides tested in this study on the feeding behavior of other predators suggests that these insecticides do not significantly impact feeding. For instance, exposure of G. occidentalis to cyantraniliprole had no impact on its predation capability (Schmidt-Jeffris and Beers 2017). Exposure of Delphastus catalinae (Horn) (Coleoptera: Coccinellidae) to C. javanica had no impact on its capability to feed on Aleurothrixus trachoides Back (Hemiptera: Aleyrodidae) (Avery et al. 2020). Similarly, when Thalassa montezumae Mulsant (Coleoptera: Coccinellidae) fed on eggs of Phalacrococcus howertoni Hodges and Hodgson (Hemiptera Coccidae) that had been sprayed with C. javanica, its predation capability was not affected (Barahona et al. 2018). Although B. bassiana has been shown to have minimal effect on N. cucumeris when released to suppress F. occidentalis (Jacobson et al. 2001). It can negatively affect other predatory mites in the Neoseiulus genus (Michereff-Filho et al. 2022). For instance, feeding Neoseiulus barkeri (Hughes) on F. occidentalis treated with B. bassiana led to reduced longevity and fecundity of the predatory mite (Wu et al. 2015). Another study reported observing P. persimilis avoiding leaves that had been treated with B. bassiana and exhibiting heightened grooming behavior and prolonged foraging, which directly impacted its predation (Zhang et al. 2021).
Oviposition in many phytoseiids mites is closely linked to prey consumption (Sabelis 1990) and the predator’s ability to digest prey (Janssen and Sabelis 1992). Thus, any factor that limits prey consumption, for example exposure to insecticides, indirectly impacts oviposition. Our findings indicate that the oviposition rates of the 3 predators did not vary when exposed to different types of insecticides. However, the impact of some tested insecticides on the oviposition of predatory mites is well documented in existing literature. For instance, azadirachtin was reported to cause a significant reduction in oviposition of N. californicus and Phytoseiulus macropilis (Banks) (Bernardi et al. 2013). Comparable outcomes were reported with exposure of P. persimilis to bean leaves treated with azadirachtin (Duso et al. 2008).
Broad-spectrum entomopathogenic fungi like B. bassiana have been shown to affect the oviposition of both phytophagous (Shi and Feng 2009) and predacious mites (Thoeming and Poehling 2006, Wu et al. 2015, 2018, Ullah and Lim 2017, Michereff-Filho et al. 2022). For example, in a laboratory study, B. bassiana was reported to cause a significant reduction in oviposition of A. swirskii (Midthassel et al. 2016), while another study reported similar findings when Typhlodromalus aripo De Leon (Mesostigmata: Phytoseiidae) was exposed to the entomopathogenic fungus Neozygites tanajoae (Agboton et al. 2013). Additionally, the fecundity of P. persimilis was reduced when the predator was exposed to C. javanica. (Numa Vergel et al. 2011). Therefore, although entomopathogenic fungi have a lesser impact on the survival and feeding of predatory mites and can be directly integrated into a pest management program involving predators, their application could still affect predator oviposition, potentially reducing overall efficacy. To mitigate this, establishing oviposition sites in the form of pesticide-free zones could provide refuges where predators can safely lay their eggs.
In conclusion, findings from this study indicate that the insecticides used to manage S. dorsalis in strawberry production affect the survival and feeding of N. cucumeris, N. californicus, and A. swirskii. Among all the tested insecticides, spinetoram had the most significant impact on feeding and oviposition, suggesting an incompatibility between this active ingredient and predatory mites. Additionally, this research highlights that there might be a potential for integrating cyantraniliprole, azadirachtin + pyrethrin, capsicum, garlic, canola oil extracts, and C. javanica in an S. dorsalis IPM program that involves the use of predatory mites. However, additional research on the ideal time to release these predators after insecticide application needs to be fully studied. Proper timing of when to release predators following insecticide application can minimize the impact of these chemistries on predatory mites, allowing for efficient suppression of targeted pests. Nonetheless, the transgenerational effects of these insecticides on these predatory mites remain to be fully studied.
Acknowledgments
The authors would like to express their sincere gratitude to Dr. Vance Whitaker for his generous provision of the strawberry transplants essential for this study. Additionally, heartfelt thanks are extended to Sherline Estaing for her invaluable assistance in the execution of the project. We are also thankful to the various pesticide manufacturers who donated their products for this study.
Contributor Information
Allan Busuulwa, Entomology and Nematology Department, Gulf Coast Research and Education Center, University of Florida, Wimauma, FL, USA.
Simon S Riley, Statistical Consulting Unit, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL, USA.
Alexandra M Revynthi, Entomology and Nematology Department, Tropical Research and Education Center, University of Florida, Homestead, FL, USA.
Oscar E Liburd, Entomology and Nematology Department, University of Florida, Gainesville, FL, USA.
Sriyanka Lahiri, Entomology and Nematology Department, Gulf Coast Research and Education Center, University of Florida, Wimauma, FL, USA.
Author contributions
Allan Busuulwa (Conceptualization [equal], Data curation [lead], Formal analysis [lead], Investigation [lead], Methodology [equal], Writing—original draft [lead]), Simon Riley (Formal analysis [equal], Writing—original draft [equal]), Alexandra Revynthi (Conceptualization [equal], Methodology [equal], Writing—review & editing [equal]), Oscar Liburd (Conceptualization [equal], Writing—review & editing [equal]), and Sriyanka Lahiri (Conceptualization [lead], Funding acquisition [lead], Project administration [lead], Writing—review & editing [equal])
Funding
This project was funded by the Florida Strawberry Growers Association and the USDA National Institute of Food and Agriculture Hatch Project No. FLA-GCR-005888.
References
- Adusei S, Azupio S.. 2022. Neem: a novel biocide for pest and disease control of plants. J. Chem. 2022(e6778554):1–12. https://doi.org/ 10.1155/2022/6778554 [DOI] [Google Scholar]
- Agboton BV, Hanna R, Onzo A, et al. 2013. Interactions between the predatory mite Typhlodromalus aripo and the entomopathogenic fungus Neozygites tanajoae and consequences for the suppression of their shared prey/host Mononychellus tanajoa. Exp. Appl. Acarol. 60(2):205–217. https://doi.org/ 10.1007/s10493-012-9630-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akyazi R, Liburd OE.. 2019. Biological control of the twospotted spider mite (Trombidiformes: Tetranychidae) with the predatory mite Neoseiulus californicus (Mesostigmata: Phytoseiidae) in blackberries. Fla. Entomol. 102(2):373–381. https://doi.org/ 10.1653/024.102.0217 [DOI] [Google Scholar]
- Argolo PS, Revynthi AM, Canon MA, et al. 2020. Potential of predatory mites for biological control of Brevipalpus yothersi (Acari: Tenuipalpidae). Biol. Control 149(10):104330. https://doi.org/ 10.1016/j.biocontrol.2020.104330 [DOI] [Google Scholar]
- Arthurs S, McKenzie CL, Chen J, et al. 2009. Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol. Control 49(1):91–96. https://doi.org/ 10.1016/j.biocontrol.2009.01.002 [DOI] [Google Scholar]
- Avery PB, Kumar V, Francis A, et al. 2020. Compatibility of the Predatory Beetle, Delphastus catalinae, with an entomopathogenic fungus, Cordyceps fumosorosea, for biocontrol of invasive pepper whitefly, Aleurothrixus trachoides, in Florida. Insects 11(9):590. https://doi.org/ 10.3390/insects11090590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahona CFS, Threlkeld BS, Avery PB, et al. 2018. Compatibility and efficacy of the lady beetle Thalassa montezumae and the entomopathogenic fungus Isaria fumosorosea for biological control of the green croton scale: laboratory and greenhouse investigations. Arthropod. Plant Interact. 12(5):715–723. https://doi.org/ 10.1007/s11829-018-9618-9 [DOI] [Google Scholar]
- Barroso G, Pazini JB, Iost Filho FH, et al. 2022. Are pesticides used to control Thrips Harmonious with soil-dwelling predatory mite Cosmolaelaps sabelis (Mesostigmata: Laelapidae)? J. Econ. Entomol. 115(1):151–159. https://doi.org/ 10.1093/jee/toab219 [DOI] [PubMed] [Google Scholar]
- Beers EH, Mills NJ, Shearer PW, et al. 2016. Nontarget effects of orchard pesticides on natural enemies: Lessons from the field and laboratory. Biol. Control 102:44–52. https://doi.org/ 10.1016/j.biocontrol.2016.04.010 [DOI] [Google Scholar]
- Beers EH, Schmidt RA.. 2014. Impacts of orchard pesticides on Galendromus occidentalis: lethal and sublethal effects. Crop Prot. 56(2):16–24. https://doi.org/ 10.1016/j.cropro.2013.10.010 [DOI] [Google Scholar]
- Beltrà A, Calabuig A, Navarro-Campos C, et al. 2017. Provisioning of food supplements enhances the conservation of phytoseiid mites in citrus. Biol. Control 115(12):18–22. https://doi.org/ 10.1016/j.biocontrol.2017.09.007 [DOI] [Google Scholar]
- Benson CM, Labbe RM.. 2021. Exploring the role of supplemental foods for improved greenhouse biological control. Ann. Entomol. Soc. Am. 114(3):302–321. https://doi.org/ 10.1093/aesa/saab005 [DOI] [Google Scholar]
- Bergeron PE, Schmidt‐Jeffris RA. 2023. Updating integrated mite management 50 years later: comparing laboratory pesticide susceptibility of a ‘new’ generalist predatory mite to a cornerstone specialist predator. Pest Manage. Sci. 79(10):3451–3458. https://doi.org/ 10.1002/ps.7518 [DOI] [PubMed] [Google Scholar]
- Bernardi D, Botton M, Silva da Cunha U, et al. 2013. Effects of azadirachtin on Tetranychus urticae (Acari: Tetranychidae) and its compatibility with predatory mites (Acari: Phytoseiidae) on strawberry. Pest Manag. Sci. 69(1):75–80. https://doi.org/ 10.1002/ps.3364 [DOI] [PubMed] [Google Scholar]
- Besard L, Mommaerts V, Abdu‐Alla G, et al. 2011. Lethal and sublethal side‐effect assessment supports a more benign profile of spinetoram compared with spinosad in the bumblebee Bombus terrestris. Pest Manag. Sci. 67(5):541–547. https://doi.org/ 10.1002/ps.2093 [DOI] [PubMed] [Google Scholar]
- Betancourt M. Ordinal regression. 2019. [accessed 2024 January 19]https://betanalpha.github.io/assets/case_studies/ordinal_regression.html#1_Clear_Cut [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, et al. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24(3):127–135. https://doi.org/ 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Bostanian NJ, Akalach M, Chiasson H.. 2005. Effects of a Chenopodium-based botanical insecticide/acaricide on Orius insidiosus (Hemiptera: Anthocoridae) and Aphidius colemani (Hymenoptera: Braconidae). Pest Manag. Sci. 61(10):979–984. https://doi.org/ 10.1002/ps.1065 [DOI] [PubMed] [Google Scholar]
- Bürkner P-C. 2021. Bayesian item response modeling in R with brms and Stan. J. Stat. Softw. 100(5):1. https://doi.org/ 10.18637/jss.v100.i05 [DOI] [Google Scholar]
- Busuulwa A, Revynthi A, Liburd O, et al. 2024. Residual effect of commonly used fungicides in strawberries on Amblyseius swirskii, Neoseiulus cucumeris, and Neoseiulus californicus (Mesostigmata: Phytoseiidae). Exp. Appl. Acarol 93(2):253. https://doi.org/ 10.1007/s10493-024-00928-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnoli M, Simoni S.. 1999. Effect of long-term feeding history on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 23(3):217–234. https://doi.org/ 10.1023/A:1006066930638 [DOI] [Google Scholar]
- Castagnoli M, Angeli G, Liguori M, et al. 2002. Side effects of botanical insecticides on predatory mite Amblyseius andersoni (Chant). Anz. Schädlingskd. 75(5):122–127. https://doi.org/ 10.1046/j.1472-8206.2002.02035.x [DOI] [Google Scholar]
- Castagnoli M, Liguori M, Simoni S, et al. 2005. Toxicity of some insecticides to Tetranychus urticae, Neoseiulus californicus and Tydeus californicus. BioControl 50(4):611–622. https://doi.org/ 10.1007/s10526-004-8121-7 [DOI] [Google Scholar]
- Castillo-Ramírez O, Guzmán-Franco AW, Santillán-Galicia MT, et al. 2020. Interaction between predatory mites (Acari: Phytoseiidae) and entomopathogenic fungi in Tetranychus urticae populations. BioControl 65(4):433–445. https://doi.org/ 10.1007/s10526-020-10004-3 [DOI] [Google Scholar]
- Chen X, Sun L, Zhang YX, et al. 2020. Differing infection of Isaria fumosorosea (Wize) Brown & Smith in an aphid (Myzus persicae [Sulzer]) and predatory mite (Neoseiulus cucumeris [Oudemans]) under a scanning electron microscope. Syst. Appl. Acarol. 25(12):2263–2272. https://doi.org/ 10.11158/saa.25.12.9 [DOI] [Google Scholar]
- Cheng S, Lin R, Lin T, et al. 2018. Effects of acetamiprid on life cycle development of predatory mite Amblyseius cucumeris (Acari: Phytoseiidae) after contact exposure. Chemosphere 210(11):889–895. https://doi.org/ 10.1016/j.chemosphere.2018.07.096 [DOI] [PubMed] [Google Scholar]
- Cloyd RA, Herrick NJ.. 2018. Effects of pesticides on the survival of rove beetle (Coleoptera: Staphylinidae) and insidious flower bug (Hemiptera: Anthocoridae) Adults. J. Econ. Entomol. 111(1):78–88. https://doi.org/ 10.1093/jee/tox280 [DOI] [PubMed] [Google Scholar]
- Cloyd RA, Galle CL, Keith SR, et al. 2009. Effect of commercially available plant-derived essential oil products on arthropod pests. J. Econ. Entomol. 102(4):1567–1579. https://doi.org/ 10.1603/029.102.0422 [DOI] [PubMed] [Google Scholar]
- Cowles RS. 1998. Effect of spinosad formulations and other miticides on twospotted spider mite, 1995. Arthropod. Manag. Tests 23(1):342–343. https://doi.org/ 10.1093/amt/23.1.342 [DOI] [Google Scholar]
- Depalo L, Masetti A, Avilla J, et al. 2016. Toxicity and residual activity of spinetoram to neonate larvae of Grapholita molesta (Busck) and Cydia pomonella (L.) (Lepidoptera: Tortricidae): semi-field and laboratory trials. Crop Prot. 89(11):32–37. https://doi.org/ 10.1016/j.cropro.2016.06.019 [DOI] [Google Scholar]
- Dicke M, de Jong M, Alers MPT, et al. 1989. Quality control of mass‐reared arthropods: nutritional effects on performance of predatory mites. J. Appl. Entomol. 108(1-5):462–475. https://doi.org/ 10.1111/j.1439-0418.1989.tb00480.x [DOI] [Google Scholar]
- Ditzen M, Pellegrino M, Vosshall LB.. 2008. Insect odorant receptors are molecular targets of the insect repellent deet. Science 319(5871):1838–1842. https://doi.org/ 10.1126/science.1153121 [DOI] [PubMed] [Google Scholar]
- Döker I, Kazak C.. 2019. Non-target effects of five acaricides on a native population of Amblyseius swirskii (Acari: Phytoseiidae). Int. J. Acarol. 45(1-2):69–74. https://doi.org/ 10.1080/01647954.2018.1542457 [DOI] [Google Scholar]
- Döker I, Pappas ML, Samaras K, et al. 2015. Compatibility of reduced-risk insecticides with the non-target predatory mite Iphiseius degenerans (Acari: Phytoseiidae). Pest Manag. Sci. 71(9):1267–1273. https://doi.org/ 10.1002/ps.3921 [DOI] [PubMed] [Google Scholar]
- Duarte A da F, de Bastos Pazini J, Duarte JLP, et al. 2020. Compatibility of pesticides used in strawberry crops with predatory mites Stratiolaelaps scimitus (Womersley) and Cosmolaelaps brevistilis (Karg). Ecotoxicology 29(2):148–155. https://doi.org/ 10.1007/s10646-020-02164-w [DOI] [PubMed] [Google Scholar]
- Duso C, Malagnini V, Pozzebon A, et al. 2008. Comparative toxicity of botanical and reduced-risk insecticides to Mediterranean populations of Tetranychus urticae and Phytoseiulus persimilis (Acari Tetranychidae, Phytoseiidae). Biol. Control 47(1):16–21. https://doi.org/ 10.1016/j.biocontrol.2008.06.011 [DOI] [Google Scholar]
- Duso C, Van Leeuwen T, Pozzebon A.. 2020. Improving the compatibility of pesticides and predatory mites: recent findings on physiological and ecological selectivity. Curr. Opin. Insect Sci. 39(6):63–68. https://doi.org/ 10.1016/j.cois.2020.03.005 [DOI] [PubMed] [Google Scholar]
- Ellison AM. 2004. Bayesian inference in ecology. Ecology Lett. 7(6):509–520. https://doi.org/ 10.1111/j.1461-0248.2004.00603.x [DOI] [Google Scholar]
- Etienne L, Bresch C, van Oudenhove L, et al. 2021. Food and habitat supplementation promotes predatory mites and enhances pest control. Biol. Control 159(8):104604. https://doi.org/ 10.1016/j.biocontrol.2021.104604 [DOI] [Google Scholar]
- Eubanks MD, Denno RF.. 2000. Health food versus fast food: the effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecol. Entomol. 25(2):140–146. https://doi.org/ 10.1046/j.1365-2311.2000.00243.x [DOI] [Google Scholar]
- Feyereisen R, Dermauw W, Van Leeuwen T.. 2015. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic. Biochem. Physiol. 121(6):61–77. https://doi.org/ 10.1016/j.pestbp.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Fiedler Z, Sosnowska D.. 2014. Side effects of fungicides and insecticides on predatory mites, in laboratory conditions. J. Plant Prot. Res. 54(4):349–353. https://doi.org/ 10.2478/jppr-2014-0052 [DOI] [Google Scholar]
- Fountain MT, Medd N.. 2015. Integrating pesticides and predatory mites in soft fruit crops. Phytoparasitica 43(5):657–667. https://doi.org/ 10.1007/s12600-015-0485-y [DOI] [Google Scholar]
- Gentz MC, Murdoch G, King GF.. 2010. Tandem use of selective insecticides and natural enemies for effective, reduced-risk pest management. Biol. Control 52(3):208–215. https://doi.org/ 10.1016/j.biocontrol.2009.07.012 [DOI] [Google Scholar]
- Ghasemzadeh S, Qureshi JA.. 2018. Demographic analysis of fenpyroximate and thiacloprid exposed predatory mite Amblyseius swirskii (Acari: Phytoseiidae). PLoS One 13(11):e0206030. https://doi.org/ 10.1371/journal.pone.0206030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gireesh M, Perry C, Lahiri S.. 2022. Understanding the efficacy of hexythiazox against twospotted spider mites in strawberry. Arthropod. Manag. Tests 47(1):1–2. https://doi.org/ 10.1093/amt/tsac117 [DOI] [Google Scholar]
- Gotoh T, Tsuchiya A, Kitashima Y.. 2007. Influence of prey on developmental performance, reproduction, and prey consumption of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 40(3-4):189–204. https://doi.org/ 10.1007/s10493-006-9032-3 [DOI] [PubMed] [Google Scholar]
- Gowan Company. Captiva prime label. 2024. [accessed 2023 June 17] https://www.gowanco.com/sites/default/files/2023-10/captiva_prime_10163-336_02-r0819.pdf [Google Scholar]
- Guan Z, Wu F, Whidden AJ. Top challenges facing the Florida strawberry industry. EDIS; 2016. [accessed 2023 Dec 7]. https://edis.ifas.ufl.edu/publication/FE972 [Google Scholar]
- Guo J, Gabry J, Goodrich B, et al. Rstan: R interface to Stan (version 2.32.5). 2024. [accessed 2024 March 20]https://CRAN.R-project.org/package=rstan [Google Scholar]
- Helle W and Sabelis M.. Spider mites: their biology, natural enemies, and control. Vol. 1B. Amsterdam: Elsevier; 1985. p. 458. [Google Scholar]
- Herrick NJ, Cloyd RA.. 2017. Direct and indirect effects of pesticides on the Insidious flower bug (Hemiptera: Anthocoridae) under laboratory conditions. J. Econ. Entomol. 110(3):931–940. https://doi.org/ 10.1093/jee/tox093 [DOI] [PubMed] [Google Scholar]
- Hobbs NT, and Hooten MB.. Bayesian Models: a statistical primer for ecologists. Princeton, New Jersey, USA: Princeton University Press; 2015. p. 1–320. [Google Scholar]
- Huang K-M, Guan Z, Hammami A.. 2022. The U.S. fresh fruit and vegetable industry: an overview of production and trade. Agriculture 12(10):1719. https://doi.org/ 10.3390/agriculture12101719 [DOI] [Google Scholar]
- Jacobson RJ, Chandler D, Fenlon J, et al. 2001. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Sci. Technol. 11(3):391–400. https://doi.org/ 10.1080/09583150120055808 [DOI] [Google Scholar]
- Janssen A, Sabelis MW.. 1992. Phytoseiid life-histories, local predator-prey dynamics, and strategies for control of tetranychid mites. Exp. Appl. Acarol. 14(3-4):233–250. https://doi.org/ 10.1007/bf01200566 [DOI] [Google Scholar]
- Kakkar G, Kumar V, Seal DR, et al. 2016. Predation by Neoseiulus cucumeris and Amblyseius swirskii on Thrips palmi and Frankliniella schultzei on cucumber. Biol. Control 92(1):85–91. https://doi.org/ 10.1016/j.biocontrol.2015.10.004 [DOI] [Google Scholar]
- Kaur G, Stelinski LL, Martini X, et al. 2023. Reduced insecticide susceptibility among populations of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in strawberry production. J. Appl. Entomol. 147(4):271–278. https://doi.org/ 10.1111/jen.13108 [DOI] [Google Scholar]
- Kim JS, Roh JY, Choi JY, et al. 2010. Correlation of the aphicidal activity of Beauveria bassiana SFB-205 supernatant with enzymes. Fungal Biol. 114(1):120–128. https://doi.org/ 10.1016/j.mycres.2009.10.011 [DOI] [PubMed] [Google Scholar]
- Kim SY, Ahn HG, Ha PJ, et al. 2018. Toxicities of 26 pesticides against 10 biological control species. J. Asia-Pac. Entomol. 21(1):1–8. https://doi.org/ 10.1016/j.aspen.2017.10.015 [DOI] [Google Scholar]
- Kim J, Chon K, Kim B, et al. 2022. Assessment of acute and chronic toxicity of cyantraniliprole and sulfoxaflor on honeybee (Apis mellifera) larvae. Pest Manag. Sci. 78(12):5402–5412. https://doi.org/ 10.1002/ps.7162 [DOI] [PubMed] [Google Scholar]
- Lahiri S. 2023. Arthropod pest management practices of strawberry growers in Florida: a survey of the 2019-2020 field season. EDIS 2023(1):ENY2097. https://doi.org/ 10.32473/edis-in1391-2023 [DOI] [Google Scholar]
- Lahiri S, Panthi B.. 2020. Insecticide efficacy for chilli thrips management in strawberry, 2019. Arthropod. Manag. Tests 45(1):1–2. https://doi.org/ 10.1093/amt/tsaa046 [DOI] [Google Scholar]
- Lahiri S, Yambisa A.. 2021. Efficacy of a biopesticide and predatory mite to manage chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in strawberry. Fla. Entomol. 104(4):322–324. https://doi.org/ 10.1653/024.104.0410 [DOI] [Google Scholar]
- Lahiri S, Smith HA, Gireesh M, et al. 2022. Arthropod pest management in strawberry. Insects 13(5):475. https://doi.org/ 10.3390/insects13050475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Kaur G, Busuulwa A.. 2024. Field efficacy of a biopesticide and a predatory mite for suppression of Scirtothrips dorsalis (Thysanoptera: Thripidae) in strawberry. J. Econ. Entomol. 117(4):1623–1627. https://doi.org/ 10.1093/jee/toae144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy J, Mendelsohn M, Kough J, et al. 2014. Biopesticide oversight and registration at the U.S. Environmental Protection Agency. In: Biopesticides: state of the art and future opportunities. Washington (DC): American Chemical Society; p. 3–18. https://doi.org/ 10.1021/bk-2014-1172.ch00 [DOI] [Google Scholar]
- Li M, Yang N, Wan F, et al. 2017. Functional response of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) to Bemisia tabaci (Gennadius) on tomato leaves. Biocontrol Sci. Technol. 27(5):677–685. https://doi.org/ 10.1080/09583157.2017.1328484 [DOI] [Google Scholar]
- Lopez L, Smith HA.. 2016. Quality assessment of the commercially available predator Amblyseius swirskii (Acari: Phytoseiidae). Plant Health Prog. 17(3):206–210. https://doi.org/ 10.1094/PHP-RS-16-0040 [DOI] [Google Scholar]
- Mallebrera B, Prosperini A, Font G, et al. 2018. In vitro mechanisms of Beauvericin toxicity: a review. Food Chem. Toxicol. 111(1):537–545. https://doi.org/ 10.1016/j.fct.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Marčić D, Međo I.. 2015. Sublethal effects of azadirachtin-A (NeemAzal-T/S) on Tetranychus urticae (Acari: Tetranychidae). Syst. Appl. Acarol. 30(1):25. https://doi.org/ 10.11158/saa.20.1.4 [DOI] [Google Scholar]
- Massaro M, Martin JPI, de Moraes GJ.. 2016. Factitious food for mass production of predaceous phytoseiid mites (Acari: Phytoseiidae) commonly found in Brazil. Exp. Appl. Acarol. 70(4):411–420. https://doi.org/ 10.1007/s10493-016-0087-5 [DOI] [PubMed] [Google Scholar]
- McElreath R. 2020. Ulysses’ compass. In: Statistical rethinking: a Bayesian course with examples in R and Stan. 2nd ed. Boca Raton, New York, USA: CRC Press. [Google Scholar]
- McMurtry JA, Croft BA.. 1997. Lifestyles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 42(1):291–321. https://doi.org/ 10.1146/annurev.ento.42.1.291 [DOI] [PubMed] [Google Scholar]
- McMurtry JA, Croft BA.. 2003. Lifestyles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 42(1):291–321. https://doi.org/ 10.1146/annurev.ento.42.1.291 [DOI] [PubMed] [Google Scholar]
- McMurtry JA, De Moraes GJ, Sourassou NF.. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 18(4):297–320. https://doi.org/ 10.11158/saa.18.4.1 [DOI] [Google Scholar]
- Michereff-Filho M, Navia D, Quevedo IA, et al. 2022. The effect of spider mite-pathogenic strains of Beauveria bassiana and humidity on the survival and feeding behavior of Neoseiulus predatory mite species. Biol. Control 176(12):105083. https://doi.org/ 10.1016/j.biocontrol.2022.105083 [DOI] [Google Scholar]
- Midthassel A, Leather SR, Wright DJ, et al. 2016. Compatibility of Amblyseius swirskii with Beauveria bassiana: two potentially complimentary biocontrol agents. BioControl 61(4):437–447. https://doi.org/ 10.1007/s10526-016-9718-3 [DOI] [Google Scholar]
- Mills NJ, Beers EH, Shearer PW, et al. 2015. Comparative analysis of pesticide effects on natural enemies in western orchards: A synthesis of laboratory bioassay data. Biol. Control 102:17–25. https://doi.org/ 10.1016/j.biocontrol.2015.05.006 [DOI] [Google Scholar]
- Montemayor JD, Smith HA, Peres NA, et al. 2023. Potential of UV-C for management of two-spotted spider mites and thrips in Florida strawberry. Pest Manag. Sci. 79(2):891–898. https://doi.org/ 10.1002/ps.7263 [DOI] [PubMed] [Google Scholar]
- Mordue (Luntz) AJ, Nisbet AJ.. 2000. Azadirachtin from the neem tree Azadirachta indica: its action against insects. Anais Soc. Entomol. Bras. 29(4):615–632. https://doi.org/ 10.1590/S0301-80592000000400001 [DOI] [Google Scholar]
- Nomikou M, Janssen A, Schraag R, et al. 2002. Phytoseiid predators suppress populations of Bemisia Tabaci on cucumber plants with alternative food. Exp. Appl. Acarol. 27(1-2):57–68. https://doi.org/ 10.1023/a:1021559421344 [DOI] [PubMed] [Google Scholar]
- Numa Vergel SJ, Bustos RA, Rodríguez CD, et al. 2011. Laboratory and greenhouse evaluation of the entomopathogenic fungi and garlic–pepper extract on the predatory mites, Phytoseiulus persimilis and Neoseiulus californicus and their effect on the spider mite Tetranychus urticae. Biol. Control 57(2):143–149. https://doi.org/ 10.1016/j.biocontrol.2011.02.007 [DOI] [Google Scholar]
- Panthi B, Renkema J.. 2020. Managing Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in Florida strawberry with Flupyradifurone. Int. J. Fruit Sci. 20(sup1):967–977. https://doi.org/ 10.1080/15538362.2020.1755768 [DOI] [Google Scholar]
- Panthi BR, Renkema JM, Lahiri S, et al. 2021. The short-range movement of Scirtothrips dorsalis (Thysanoptera: Thripidae) and rate of spread of feeding injury among strawberry plants. Environ. Entomol. 50(1):12–18. https://doi.org/ 10.1093/ee/nvaa149 [DOI] [PubMed] [Google Scholar]
- Pozzebon A, Duso C.. 2010. Pesticide side-effects on predatory mites: the role of trophic interactions. In: Sabelis M, Bruin J, editors. Trends in acarology. Dordrecht: Springer Netherlands; p. 465–469. https://doi.org/ 10.1007/978-90-481-9837-5_77 [DOI] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. 2024. [accessed 2023 June 20]https://www.r-project.org/ [Google Scholar]
- Rahmani H, Fathipour Y, Kamali K. 2009. Life history and population growth parameters of Neoseiulus californicus (Acari: Phytoseiidae) fed on Thrips tabaci (Thysanoptera: Thripidae) in laboratory conditions. System. Appl. Acarol. 12(2):91–91. https://doi.org/ 10.11158/saa.14.2.2 [DOI] [Google Scholar]
- Rahmani H, Hoseini M, Saboori A, et al. 2016. Prey preference of the predatory mite Neoseiulus californicus (Mesostigmata: Phytoseiidae) when offered two major pest species, the two spotted spider mite and the onion thrips. Int. J. Acarol. 42(6):319–323. https://doi.org/ 10.1080/01647954.2016.1191540 [DOI] [Google Scholar]
- Rhodes EM, Liburd OE, Kelts C, et al. 2006. Comparison of single and combination treatments of Phytoseiulus persimilis, Neoseiulus californicus, and Acramite (bifenazate) for control of twospotted spider mites in strawberries. Exp. Appl. Acarol. 39(3-4):213–225. https://doi.org/ 10.1007/s10493-006-9005-6 [DOI] [PubMed] [Google Scholar]
- Sabelis MW. 1990. How to analyse prey preference when prey density varies? A new method to discriminate between effects of gut fullness and prey type composition. Oecologia 82(3):289–298. https://doi.org/ 10.1007/BF00317473 [DOI] [PubMed] [Google Scholar]
- Sáenz-de-Cabezón Irigaray FJ, Marco-Mancebón V, Pérez-Moreno I.. 2003. The entomopathogenic fungus Beauveria bassiana and its compatibility with triflumuron: effects on the twospotted spider mite Tetranychus urticae. Biol. Control 26(2):168–173. https://doi.org/ 10.1016/s1049-9644(02)00123-8 [DOI] [Google Scholar]
- Saville JD, Graham R.. Statistical methods: the geometric approach. 2nd ed. New York. Springer Texts in Statistics; 2012. p. 561 [Google Scholar]
- Schmidt JM, Sebastian P, Wilder SM, et al. 2012. The nutritional content of prey affects the foraging of a generalist arthropod predator. PLoS One 7(11):e49223. https://doi.org/ 10.1371/journal.pone.0049223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Jeffris RA, Beers EH. 2017. Potential impacts of orchard pesticides on Tetranychus urticae: A predator-prey perspective. Crop Protect. 103:56–64. https://doi.org/ 10.1016/j.cropro.2017.09.009 [DOI] [Google Scholar]
- Schmidt‐Jeffris RA, Beers EH, Sater C.. 2021. Meta‐analysis and review of pesticide non‐target effects on phytoseiids, key biological control agents. Pest Manag. Sci. 77(11):4848–4862. https://doi.org/ 10.1002/ps.6531 [DOI] [PubMed] [Google Scholar]
- Schoeller EN, McKenzie CL, Osborne LS.. 2022. Chilli thrips rose management using an Amblyseius swirskii or Amblydromalus limonicus (Acari: Phytoseiidae) pepper banker plant. J. Appl. Entomol. 146(10):1281–1292. https://doi.org/ 10.1111/jen.13066 [DOI] [Google Scholar]
- Seiedy M, Tork M, Deyhim F.. 2015. Effect of the entomopathogenic fungus Beauveria bassiana on the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) as a nontarget organism. Syst. Appl. Acarol. 20(3):241–250. https://doi.org/ 10.11158/saa.20.3.2 [DOI] [Google Scholar]
- Shah PA, Pell JK.. 2003. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 61(5-6):413–423. https://doi.org/ 10.1007/s00253-003-1240-8 [DOI] [PubMed] [Google Scholar]
- Shahbaz M, Khoobdel M, Khanjani A, et al. 2019. Sublethal effects of acetamiprid on biological aspects and life table of Amblyseius swirskii (Acari: Phytoseiidae) fed on Aleuroclava jasmini (Hemiptera: Aleyrodidae). Biocontrol Sci. Technol. 22(12):1398–1416. https://doi.org/ 10.11158/saa.24.5.7 [DOI] [Google Scholar]
- Shearer PW, Amarasekare KG, Castagnoli SP, et al. 2016. Large-plot field studies to assess impacts of newer insecticides on non-target arthropods in Western U.S. orchards. Biol. Control 102:26–34. https://doi.org/ 10.1016/j.biocontrol.2016.05.004 [DOI] [Google Scholar]
- Shi WB, Feng MG.. 2009. Effect of fungal infection on reproductive potential and survival time of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 48(3):229–237. https://doi.org/ 10.1007/s10493-009-9238-2 [DOI] [PubMed] [Google Scholar]
- Shimokawatoko Y, Sato N, Yamaguchi T, et al. Development of the novel insecticide spinetoram (Diana®). Sumitomo Chemical Co., Ltd; 2012. [accessed 2023 May 5] https://www.sumitomo-chem.co.jp/english/rd/report/files/docs/01_2012e.pdf [Google Scholar]
- Sparks TC, Nauen R.. 2015. IRAC: mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 121(6):122–128. https://doi.org/ 10.1016/j.pestbp.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Sparks TC, Crouse GD, Benko Z, et al. 2021. The spinosyns, spinosad, spinetoram, and synthetic spinosyn mimics – discovery, exploration, and evolution of a natural product chemistry and the impact of computational tools. Pest Manag. Sci. 77(8):3637–3649. https://doi.org/ 10.1002/ps.6073 [DOI] [PubMed] [Google Scholar]
- Stan Development Team. Stan modeling language users guide and reference manual (version 2.30). 2022. [accessed 2023 Feburary 5]https://mc-stan.org [Google Scholar]
- Thao NTP, Thuy NT.. 2023. Effects of certain pesticides on the predatory mite Typhlodromus ndibu Pritchard and Baker (Acari: Phytoseiidae). Agriculture 13(9):1776. https://doi.org/ 10.3390/agriculture13091776 [DOI] [Google Scholar]
- Thoeming G, Poehling HM.. 2006. Integrating soil-applied azadirachtin with Amblyseius cucumeris (Acari: Phytoseiidae) and Hypoaspis aculeifer (Acari: Laelapidae) for the management of Frankliniella occidentalis (Thysanoptera: Thripidae). Environ. Entomol. 35(3):746–756. https://doi.org/ 10.1603/0046-225x-35.3.746 [DOI] [Google Scholar]
- Trumm P, Dorn A.. 2000. Effects of azadirachtin on the regulation of midgut peristalsis by the stomatogastric nervous system in Locusta migratoria. Phytoparasitica 28(1):7–26. https://doi.org/ 10.1007/bf02994020 [DOI] [Google Scholar]
- Ullah MS, Lim UT.. 2017. Laboratory evaluation of the effect of Beauveria bassiana on the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). J. Invertebr. Pathol. 148(9):102–109. https://doi.org/ 10.1016/j.jip.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Vangansbeke D, Van Doren E, Duarte MVA, et al. 2023. Why are phytoseiid predatory mites not effectively controlling Echinothrips americanus? Exp. Appl. Acarol. 90(1-2):1–17. https://doi.org/ 10.1007/s10493-023-00803-5 [DOI] [PubMed] [Google Scholar]
- van Leeuwen T, Dermauw W, Van De Veire M, et al. 2005. Systemic use of spinosad to control the two-spotted spider mite (Acari: Tetranychidae) on tomatoes grown in rockwool. Exp. Appl. Acarol. 37(1–2):93–105. https://doi.org/ 10.1007/s10493-005-0139-8 [DOI] [PubMed] [Google Scholar]
- Van Leeuwen T, Dermauw W.. 2016. The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu. Rev. Entomol. 61(1):475–498. https://doi.org/ 10.1146/annurev-ento-010715-023907 [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Y, Xie W, et al. 2016. Sublethal effects of spinetoram on the twospotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Pestic. Biochem. Physiol. 132(9):102–107. https://doi.org/ 10.1016/j.pestbp.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Wekesa VW, Maniania NK, Knapp M, et al. 2005. Pathogenicity of Beauveria bassiana and Metarhizium anisopliae to the tobacco spider mite Tetranychus evansi. Exp. Appl. Acarol. 36(1-2):41–50. https://doi.org/ 10.1007/s10493-005-0508-3 [DOI] [PubMed] [Google Scholar]
- Wimmer D, Hoffmann D, Schausberger P.. 2008. Prey suitability of western flower thrips, Frankliniella occidentalis, and onion thrips, Thrips tabaci, for the predatory mite Amblyseius swirskii. Biocontrol Sci. Technol. 18(6):533–542. https://doi.org/ 10.1080/09583150802029784 [DOI] [Google Scholar]
- Wu S, Gao Y, Xu X, et al. 2015. Feeding on Beauveria bassiana-treated Frankliniella occidentalis causes negative effects on the predatory mite Neoseiulus barkeri. Sci. Rep. 5(1):1–12. https://doi.org/ 10.1038/srep12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Xing Z, Sun W, et al. 2018. Effects of Beauveria bassiana on predation and behavior of the predatory mite Phytoseiulus persimilis. J. Invertebr. Pathol. 153(3):51–56. https://doi.org/ 10.1016/j.jip.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Yanar D. 2019. Side effects of different doses of azadirachtin on predatory mite Metaseiulus occidentalis (Nesbitt) (Acari: Phytoseiidae) under laboratory conditions. Appl. Ecol. Environ. Res. 17(2):3433–3440. https://doi.org/ 10.15666/aeer/1702_34333440 [DOI] [Google Scholar]
- Zhang YX, Sun L, Lin GY, et al. 2015. A novel use of predatory mites for dissemination of fungal pathogen for insect biocontrol: the case of Amblyseius swirskii and Neoseiulus cucumeris (Phytoseiidae) as vectors of Beauveria bassiana against Diaphorina citri (Psyllidae). Syst. Appl. Acarol. 20(2):177–187. https://doi.org/ 10.11158/saa.20.2.4 [DOI] [Google Scholar]
- Zhang X, Wu S, Reitz SR, et al. 2021. Simultaneous application of entomopathogenic Beauveria bassiana granules and predatory mites Stratiolaelaps scimitus for control of western flower thrips, Frankliniella occidentalis. J. Pest Sci. 94(1):119–127. https://doi.org/ 10.1007/s10340-020-01227-5 [DOI] [Google Scholar]
- Zhu JY, Liu J, Qin L, et al. 2022. Learning behavior of Neoseiulus californicus (Acari: Phytoseiidae) can help in adapting from feeding on alternative prey to target prey. Syst. Appl. Acarol. 27(10):1467–1482. https://doi.org/ 10.11158/saa.27.10.8 [DOI] [Google Scholar]
- Zilahi-Balogh GMG, Shipp JL, Cloutier C, et al. 2007. Predation by Neoseiulus cucumeris on western flower thrips, and its oviposition on greenhouse cucumber under winter vs. summer conditions in a temperate climate. Biol. Control 40(2):160–167. https://doi.org/ 10.1016/j.biocontrol.2006.10.011 [DOI] [Google Scholar]