Abstract
Background
Drug rash with eosinophilia and systemic symptoms (DRESS) is a life-threatening severe cutaneous adverse reaction.
Objective
This study aims to study fatal DRESS cases using FAERS database and systematic review.
Methods
Data of the FDA Adverse Event Reporting System (FAERS) database were extracted and manipulated. Articles from Pubmed, Embase and CINAHL databases were screened.
Results
0.13% of the adverse events submitted to FAERS was identified as DRESS and the percentage of fatal cases was up to 6.62%. The top five drugs calculated to induce DRESS with the highest number of reported cases were allopurinol, lamotrigine, vancomycin, amoxicillin and carbamazepine. The top five drugs statistically related to fatal outcome with the highest number of reported cases were allopurinol, vancomycin, trimethoprim, sulfamethoxazole and lamotrigine. Skin manifestations remained the main reason for admission and the average time from dose to rash onset was 27.19 days. The most commonly cited culprit medication type were antibiotics (50.00%), anti-gout agents (15.38%) and anti-epileptic drug (11.54%).
Conclusions
We discussed fatal cases of DRESS through FAERS system and case reports, hoping to raise awareness when using relevant drugs.
Keywords: drug reaction with eosinophilia and systemic symptoms, drug-induced hypersensitivity syndrome, FDA adverse event reporting system, fatal cases, drug adverse reaction, systematic review, disproportionality analysis
1. Introduction
Drug-induced hypersensitivity syndrome (DIHS), also referred to as drug reaction with eosinophilia and systemic symptoms (DRESS), is a severe cutaneous adverse reaction (SCAR) characterized by the presence of a rash, fever, and involvement of hematologic and visceral organs. The etiology of DRESS syndrome is currently not well-defined. Proposed mechanisms include aberrations in drug detoxification enzymes leading to the buildup of reactive drug metabolites, sequential reactivation of herpesviruses, such as cytomegalovirus, Epsteine-Barr virus (EBV), human herpesvirus (HHV) -6 and -7, and genetic predisposition linked to specific human leukocyte antigen alleles. However, a comprehensive understanding of the pathogenesis of DRESS syndrome is still lacking (1).
DIHS/DRESS is a highly debilitating and potentially life-threatening condition, historically associated with a mortality rate estimated at 10%, while recent studies have indicated mortality rates ranging from 1.2% to 6.1% (2). In the pediatric population, systematic reviews have reported mortality rates ranging from 3% to 5.4% (2).
To our best knowledge, so far there has been no article summarizing reports of death cases of DRESS. Therefore, we analyzed the signals of DRESS in FDA Adverse Event Reporting System (FAERS) and made a systematic review of the death case reports on DRESS, in order to provide reference for the security of drug usage in clinical practice.
2. Methods
2.1. Analysis of FAERS database
The FAERS system serves as a spontaneous adverse event reporting mechanism, gathering reports from diverse sources such as healthcare professionals, patients, and manufacturers on a global scale. Its significance lies in its ability to identify safety concerns promptly, particularly pertaining to newly introduced medications and infrequent adverse events. OpenVigil 2, a pioneering web-based tool for pharmacovigilance analysis, utilizes the open FDA online interface provided by the FDA to access pharmacovigilance data from FAERS in the United States. Our study employed OpenVigil 2 to extract the FAERS data from 2003Q4 to 2023Q3. “Drug reaction with eosinophilia and systemic symptoms” was searched as preferred term (PT) for targeted adverse events. Arbitrary drug names (generic names, brand names, abbreviations, and so on) was mapped to unique drug names by using Pubchem and Drugs@FDA. We attempted to identify same active ingredient in different drugs according to the WHO Anatomical Therapeutic Chemical (ATC) classification.
We did the disproportionality analyses through OpenVigil 2 and Microsoft® Excel 2016. Proportional reporting ratio (PRR) and reporting odds ratio (ROR) was calculated to assess the association between drug and events. Higher PRR or ROR suggests stronger association. Roughly, PRR/ROR values greater than 2 indicate that this drug-adverse event-combination is 2-fold more likely than all other combinations. Adverse events reported to FAERS database will be considered as positive signals if fulfilled the criteria: (1) PRR ≥2, χ2 ≥4 and ≥3 case; and (2) reporting odds ratio (ROR)>1; and (3) 95% confidence interval (CI) of ROR>1.
2.2. Systematic review
2.2.1. Search strategy
A comprehensive literature search was conducted in Pubmed, Embase, and CINAHL databases, encompassing articles published on or before August 2023. The objective was to identify case reports and case series of deaths pertaining to adverse drug reactions (ADR) associated with DRESS. Additionally, the reference lists of the identified articles were scrutinized. The search strategy employed a combination of search terms including ‘drug reaction with eosinophilia and systemic symptoms’, ‘DRESS’, ‘drug-induced hypersensitivity syndrome’, ‘DIHS’, ‘case report’, ‘case reports’, ‘case series’, ‘case study’, ‘case studies’ and other related terms, connected using logical operators ‘AND’ and ‘OR.’ Both MeSH and text-word searching methods were utilized in the search process.
2.2.2. Selection criteria
The selection process involves the inclusion of studies that satisfy the subsequent criteria: (1) the publication type is either a case report or case series of human, (2) the studies describe cases involving mortality subsequent to the dress diagnosis, and (3) the studies provide comprehensive information regarding patients and their adverse reactions. Conversely, studies that cannot conform to the aforementioned study type, uncertain to be caused by other drugs, constitute secondary literature, or lack access to full-text or essential information are excluded. According to the RegiSCAR score, DRESS can be categorized into four levels: no case (score < 2), possible case (score 2~3), probable case (score 4~5), and definitive case (score > 5) (3). For our study, we specifically focused on probable and definitive DRESS cases, which were identified based on a RegiSCAR score of 4 and above. Additionally, The selection of publications is conducted autonomously by two reviewers, with any discrepancies being resolved through consensus.
2.2.3. Data extraction
The extracted information encompasses various aspects, namely: (1) personal details of the patient such as age, gender, past medical history, time and place of death, and cause of death; (2) medication-related information including the specific drug responsible for DRESS, the commencement and cessation dates of medication administration; and (3) pertinent details pertaining to DRESS, such as diagnostic test indicators, affected organs or systems, and the Regi S-CAR scores.
3. Results
3.1. DRESS in FAERS
From 2003 Q4 to 2023 Q3, 0.13% of adverse events submitted to FAERS (15,751 of 11,737,133) was identified as DRESS. The percentage of fatal cases was up to 6.62% (1,043 of 15,751). Among all DRESS patients, there were slightly more females than males (53.9% vs 46.1%), with an age of 47.5 ± 22.4 years Among all DRESS patients who died as a result, there were slightly more females than males (53.3% vs 46.1%), with an age of 54.3 ± 24.4 years. There was no significant difference in the gender ratio between the deceased patients and all patients (p>0.01), but there was a statistical difference in age (p<0.001). During the period evaluated, 2324 different drugs were considered as suspected drugs, while 618 of them was positive signals, namely statistically related to induce DRESS, and 191 of them was statistically related to death. The top five drugs calculated to induce DRESS with the highest number of reported cases were allopurinol (n=2375), lamotrigine (n=1732), vancomycin (n=1564), amoxicillin (n=1175) and carbamazepine (n=1163) ( Table 1 ).
Table 1.
Association analysis of DRESS and suspected drugs, sorted by the number of reported cases.
| Drugs | Cases (n1) | PRR | χ2 | Drugs with fatal outcome | Deaths (n2) | PRR | χ2 |
|---|---|---|---|---|---|---|---|
| Allopurinol | 2375 | 35.04 | 66753.98 | Allopurinol | 280 | 50.19 | 9847.91 |
| Lamotrigine | 1732 | 19.30 | 26762.54 | Vancomycin | 104 | 27.91 | 2407.23 |
| Vancomycin | 1564 | 47.95 | 64803.64 | Trimethoprim | 87 | 12.80 | 857.66 |
| Amoxicillin | 1175 | 15.58 | 14846.29 | Sulfamethoxazole | 84 | 13.33 | 869.99 |
| Carbamazepine | 1163 | 24.44 | 24222.75 | lamotrigine | 84 | 15.89 | 1064.69 |
| Trimethoprim | 1067 | 14.25 | 12262.20 | Minocycline | 74 | 126.80 | 8471.92 |
| Sulfamethoxazole | 1040 | 15.09 | 12779.84 | Phenytoin | 68 | 30.44 | 1784.42 |
| Rifampicin | 1038 | 54.46 | 50904.99 | Levetiracetam | 68 | 14.48 | 785.87 |
| Levetiracetam | 797 | 7.28 | 4098.65 | Furosemide | 63 | 3.10 | 82.23 |
| Valproic acid | 760 | 7.35 | 3969.42 | Rifampicin | 62 | 38.74 | 2110.72 |
| Phenytoin | 753 | 23.04 | 15115.38 | Ceftriaxone | 58 | 23.23 | 1145.17 |
| Ceftriaxone | 697 | 26.96 | 16650.70 | Carbamazepine | 55 | 18.96 | 870.07 |
| Piperacillin | 650 | 31.62 | 18473.30 | Pantoprazole | 52 | 4.28 | 121.11 |
| Isoniazid | 635 | 44.70 | 26030.60 | Amoxicillin | 48 | 13.93 | 537.83 |
| Tazobactam | 629 | 30.24 | 17069.80 | Methylprednisolone | 48 | 4.95 | 140.65 |
| Furosemide | 571 | 2.96 | 710.62 | Prednisolone | 44 | 3.40 | 69.09 |
| Pyrazinamide | 553 | 69.42 | 35962.90 | Omeprazole | 43 | 3.11 | 57.07 |
| Ethambutol | 550 | 54.98 | 28116.87 | Phenobarbital | 42 | 41.76 | 1566.11 |
| Ciprofloxacin | 527 | 8.11 | 3169.73 | Isoniazid | 37 | 29.39 | 952.50 |
| Levofloxacin | 524 | 6.29 | 2252.83 | Saccharated iron oxide | 36 | 23.64 | 732.42 |
The top five drugs statistically related to fatal outcome with the highest number of reported cases was allopurinol, vancomycin, trimethoprim, sulfamethoxazole and lamotrigine, and the number of dead cases were 280, 104, 87, 84 and 84, respectively ( Table 1 ). The top five drugs analyzed by PRR most likely to induce DRESS were oxypurinol (PRR=745.31), aurotioprol (PRR=638.96), naproxen (PRR=212.99), iomeprol (PRR=199.96) and iobitridol (PRR=195.10) ( Table 2 ). The top five drugs analyzed by PRR most likely to induce DRESS-related death was almotriptan, iobitridol, prasterone, canrenone and rifater (the combination of rifampicin, pyrazinamide and isoniazid), with the PRR of 736.38, 450.41, 320.16, 270.14 and 165.944 respectively ( Table 2 ).
Table 2.
Association analysis of DRESS and suspected drugs, sorted by proportional reporting ratio (PRR).
| Drugs | Cases (n1) | PRR | χ2 | Drugs with fatal outcome | Deaths (n2) | PRR | χ2 |
|---|---|---|---|---|---|---|---|
| Oxypurinol | 3 | 745.31 | 1549.52 | Almotriptan | 7 | 736.38 | 4406.07 |
| Aurotioprol | 6 | 638.96 | 3213.51 | Iobitridol | 6 | 450.41 | 2248.98 |
| Naproxen | 6 | 212.99 | 1063.85 | Prasterone | 7 | 320.16 | 1908.37 |
| Iomeprol | 87 | 199.96 | 16953.51 | Canrenone | 11 | 270.14 | 2660.83 |
| Iobitridol | 41 | 195.10 | 7714.77 | Combination of rifampicin, pyrazinamide and isoniazid (Rifater) | 6 | 165.94 | 821.67 |
| Influenza vaccines | 11 | 139.03 | 1373.38 | Minocycline | 74 | 126.80 | 8471.92 |
| Ethacrynic acid | 38 | 117.29 | 4261.26 | Chlormezanone | 3 | 125.75 | 256.51 |
| Lysozyme | 3 | 101.63 | 207.00 | Siltuximab | 4 | 99.90 | 298.11 |
| Sertaconazole | 3 | 89.44 | 181.57 | Iopamidol | 11 | 91.46 | 887.18 |
| Sultamicillin | 6 | 73.32 | 359.10 | Trimebutine | 8 | 87.74 | 597.98 |
| Oxacillin | 45 | 73.26 | 3130.79 | Clemastine | 8 | 77.99 | 529.89 |
| Pyrazinamide | 553 | 69.42 | 35962.85 | Remifentanil | 11 | 77.43 | 747.98 |
| Cloxacillin | 56 | 64.52 | 3431.34 | Propylthiouracil | 7 | 75.14 | 438.04 |
| Cefotaxime | 189 | 58.08 | 10561.15 | Phloroglucinol | 7 | 65.74 | 381.69 |
| Kanamycin | 27 | 58.59 | 1470.68 | Dapsone | 19 | 61.45 | 1051.65 |
| Combination of rifampicin, pyrazinamide and isoniazid (Rifater) | 6 | 165.94 | 821.67 | Mianserin | 18 | 56.11 | 904.91 |
| Ethambutol | 550 | 54.98 | 28116.87 | Streptomycin | 5 | 54.12 | 209.45 |
| Rifampicin | 1038 | 54.46 | 50904.99 | Allopurinol | 280 | 50.19 | 9847.91 |
| Vancomycin | 1564 | 47.95 | 64803.64 | Oxacillin | 3 | 45.56 | 89.81 |
| Teicoplanin | 125 | 45.77 | 5393.47 | Phenobarbital | 42 | 41.76 | 1566.11 |
3.2. Death of DRESS reviewed in case reports
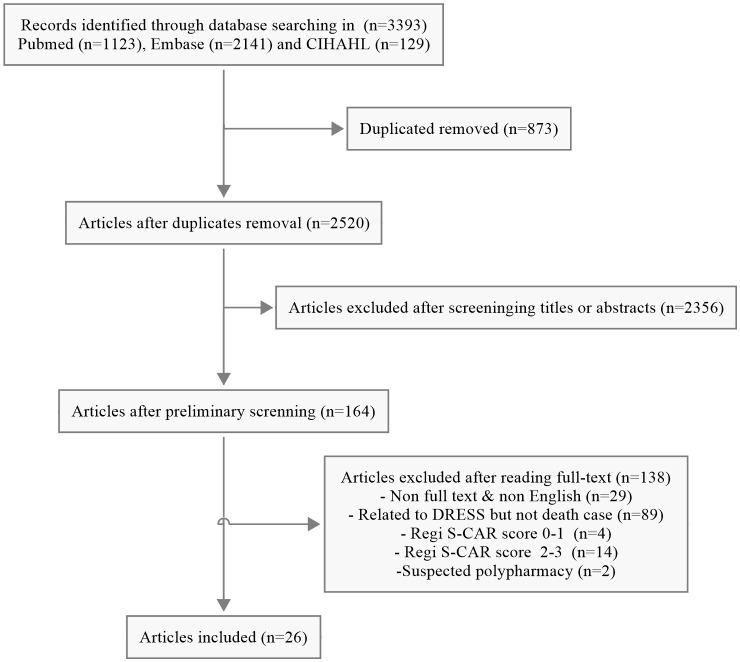
A total of 3393 unique articles were initially retrieved. According to the inclusion and exclusion criteria, 26 individual cases from 26 publications were pooled for further analysis after screening ( Figure 1 ).
Figure 1.
The detailed flow chart of the literature search.
3.3. Demographics and comorbidities
The age of patients in our systematic review ranged from 12–84 years (mean 55.13 years) for men and 1–86 years for women (mean 42.36 years). The number of male patients is slightly higher than that of female patients. The reported races included Caucasian, African, Asian, Turkish, Biracial, et al. In terms of comorbidities, there were relatively more patients combine with cardiac (7/26, 26.92%), endocrine and metabolic diseases (7/26, 26.92%).
3.4. Latency and reasons for hospitalization
DRESS patients characteristically experience a rash or fever before admission. The average time from dose to rash onset was 27.19 days (except for 4 patients not mentioned the exact time). Skin manifestations remained the main reason for admission (24/26, 92.31%), and the next was whole body issues (21/26, 80.77%).
3.5. Outcome
The majority of patients died in the hospital (23/26, 88.46%). Multi-organ problems were the most frequent cause of death (8/26, 30.77%), followed by respiratory (7/26, 26.92%), renal and liver (7/26, 26.92%). A complete list of the place of death and cause of death were presented in Table 3 .
Table 3.
Demographics, comorbidities, reasons for hospitalization, outcomes of the patients.
| Gender | No. of cases |
Age range (years) |
Mean age (years) |
|---|---|---|---|
| Male | 15 (57.69%) | 12-84 | 55.13 |
| Female | 11 (42.31%) | 1-86 | 42.36 |
| Total | 26 | 1-86 | 49.73 |
| Race | |||
| Caucasian | 4 (15.38%) | Caucasian | |
| African | 2 (7.69%) | African | |
| Asian | 1 (3.85%) | Asian | |
| Turkish | 1 (3.85%) | Turkish | |
| Biracial | 1 (3.85%) | Biracial | |
| Not reported | 17 (66.38%) | Not reported | |
| Comorbidities | |||
| Cardiac | 7 (26.92%) | ||
| Endocrine and metabolic | 7 (26.92%) | ||
| Renal and liver | 6 (23.08%) | ||
| Infection | 6 (23.08%) | ||
| Skin | 5 (19.23%) | ||
| Whole body | 5 (19.23%) | ||
| Respiratory | 3 (11.54%) | ||
| Immune | 3 (11.54%) | ||
| Brain | 2 (7.69%) | ||
| Blood | 2 (7.69%) | ||
| Bone | 2 (7.69%) | ||
| Nervous | 1 (3.85%) | ||
| Gastrointestinal | 1 (3.85%) | ||
| Urinary | 1 (3.85%) | ||
| Reasons for hospitalization | |||
| Skin | 24 (92.31%) | ||
| Whole body | 21 (80.77%) | ||
| Gastrointestinal | 5 (19.23%) | ||
| Respiratory | 4 (15.38%) | ||
| Immune | 2 (7.69%) | ||
| Urinary | 2 (7.69%) | ||
| Mental | 1 (3.85%) | ||
| Brain | 1 (3.85%) | ||
| Blood | 1 (3.85%) | ||
| Eye | 1 (3.85%) | ||
| Cardiac | 1 (3.85%) | ||
| Renal and liver | 1 (3.85%) | ||
| Not reported | 1 (3.85%) | ||
| Places of death | |||
| In hospital | 23 (88.46%) | ||
| Not in hospital | 3 (11.54%) | ||
| Direct causes of death | |||
| Multi-organ | 8 (30.77%) | ||
| Respiratory | 7 (26.92%) | ||
| Renal and liver | 7 (26.92%) | ||
| Infection | 6 (23.08%) | ||
| Cardiac | 6 (23.08%) | ||
| Brain | 1 (3.85%) | ||
| Skin | 1 (3.85%) | ||
| Time from the onset of the rash to the death | |||
| ≤30 days | 12 (46.15%) | ||
| 30<t<100 days | 6 (23.08%) | ||
| ≥100 days | 5 (19.23%) | ||
| Not mentioned | 3 (11.54%) | ||
| Organ involvement | Involved | ||
| Skin | 25 (96.15%) | ||
| Liver | 20 (76.92%) | ||
| Pulmonary | 9 (34.62%) | ||
| Kidney | 8 (30.77%) | ||
| Cardiac | 7 (26.92%) | ||
| Pancreatic | 1 (3.85%) | ||
| Others | 4 (15.38%) | ||
| Laboratory indicators | Yes | No | N/A |
| Temperature (>38.5°C) | 17 (65.38%) | 4 (15.38%) | 5 (19.23%) |
| Lymphomegaly | 13 (50.00%) | 2 (7.69%) | 11 (42.31%) |
| Average value | Range | Number of patients | |
| Leukocytes (*10e9/L) | 861.35 (8 N/A) | ≤10 | 5 (19.23%) |
| 10~30 | 9 (34.62%) | ||
| ≥30 | 5 (19.23%) | ||
| N/A | 7 (26.92%) | ||
| Eosinophile granulocytes(*10e9/L) | 111.51 (5 N/A) | ≤0.5 | 2 (7.69%) |
| 0.5~1.5 | 4 (15.38%) | ||
| ≥1.5 | 15 (57.69%) | ||
| N/A | 5 (19.23%) | ||
| Alanine aminotransferase (ALT, IU/L) | 594.37 (11 N/A) | ≤40 | 1 (3.85%) |
| 40~120 | 6 (23.08%) | ||
| ≥120 | 9 (34.62%) | ||
| N/A | 10 (38.46%) | ||
| Aspartate aminotransferase (AST, IU/L) | 1650.83 (11 N/A) | ≤40 | 1 (3.85%) |
| 40~120 | 6 (23.08%) | ||
| ≥120 | 8 (30.77%) | ||
| N/A | 10 (38.46%) | ||
| Alkaline phosphatase (ALP, IU/L) |
466.05 (18 N/A) | ≤150 | 0 |
| 150~450 | 5 (19.23%) | ||
| ≥450 | 4 (15.38%) | ||
| N/A | 17 (65.38%) | ||
| Direct bilirubin (DBil, mg/dl) |
5.52 (20 N/A) | ≤0.25 | 0 |
| 0.25~0.75 | 0 | ||
| ≥0.75 | 7 (26.92%) | ||
| N/A | 19 (73.08%) | ||
| Total bilirubin (TBil, mg/dl) |
9.61 (18 N/A) | ≤1 | 0 |
| 1~3 | 1 (3.85%) | ||
| ≥3 | 8 (30.77%) | ||
| N/A | 17 (65.38%) | ||
3.6. Organ involvement
Multi-organ involvement was common (16/26, 61.54%). The most frequently affected visceral organs being the skin (25/26, 96.15%), while liver involvement occurred in (20/26, 76.92%). Pulmonary (9/26, 34.62%), kidney (8/26, 30.77%), cardiac (7/26, 26.92%) involvement was relatively common. Pancreatic occurred in only 1 out of 26 patients (3.85%). Others included esophagus, stomach, duodenum, sigmoid colon, digestive, eyes, colon, re-epithelialized mucosa of the descending colon, sigmoid colon, and rectum, were less common.
3.7. Laboratory indicators
In our systematic review, 18 of 27 patients (66.67%) presents fever (>38.5°C), while almost half patients showed lymphomegaly (48.15%). The leukocyte count was 861.35*10e9/L, ranging from 0.0121 to 16,000 cells/L. The eosinophile granulocyte count was 111.51*10e9/L, ranging from 0 to 2240 cells/L. One patient had normal ALT and AST, while 9 (34.62%), 8 (30.77%) and 4 (15.38%) patients had ALT, AST and ALP three times higher than normal, respectively. Seven patients with abnormal DBil values had DBil values more than three times normal. As for Tbil, 30% of patients had Tbil values three times greater than normal.
3.8. Putative causative agents
As shown in Figure 2 and Table 4 , the most commonly suspected medications were antibiotics (13/26, 50.00%), followed by anti-gout agents (4/26, 15.38%) and anti-epileptic drugs (AEDs) (3/26, 11.54%). Others including NSAIDs, anti-HIV agents, anti-acid and antiulcer drugs, proton pump inhibitors (PPIs), disease-modifying anti-rheumatic drugs (DMARDs) and antiosteoporotic agents, only reported one case respectively (1/26, 3.85%). More details of the case reports included in the systematic review are shown in Table 5 .
Figure 2.
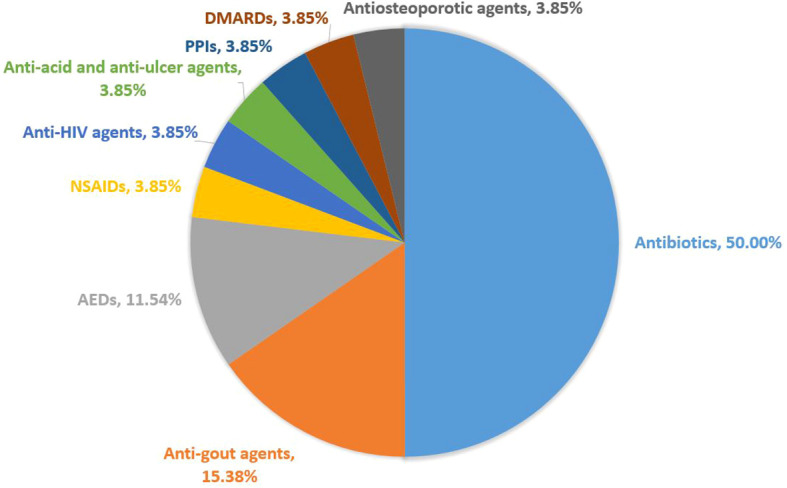
Causative drugs related to fatal outcome of DRESS cases.
Table 4.
List of the culprit medication in patients with DRESS syndrome ended in death.
| Culprit Medication | Number of cases |
|---|---|
| Allopurinol | 4 (15.38%) |
| Minocycline | 3 (11.54%) |
| Vancomycin | 2 (7.69%) |
| Amoxicillin and clavulanate, azithromycin, clindamycin, colistimethate, dolutegravir, lansoprazole, leflunomide, levetiracetam, nabumetone, omeprazole, oxacillin, phenytoin, pyrazinamide, strontium ranelate, sulfasalazine, sultamicillin, zonisamide | 17 (65.38%) |
| Total | 26 |
Table 5.
Details of DRESS related death reported in case reports and case series.
| No. | Articles | Age | Gender | Medicine | Comorbidities | Cause of death | RegiSCAR criteria |
|---|---|---|---|---|---|---|---|
| 1 | Yaylacı, S., 2012 (4) | 70 | M | Allopurinol | Gout | Acute renal failure and acute liver failure | 7 |
| 2 | Choi, H. G., 2014 (5) | 84 | M | Allopurinol | Stage 3 chronic renal disease | Sudden cardiac arrest | 4 |
| 3 | Uppalapati, A., 2014 (6) | 40 | F | Lansoprazole | Liver cirrhosis | Multiorgan failure | 5 |
| 4 | Sato, H., 2022 (7) | 59 | M | Levetiracetam | Aphasia | Multiple organ system failure secondary to acute fulminant liver injury from atypical DIHS | 6 |
| 5 | Desai, A. S., 2022 (8) | 76 | M | Oxacillin | New-onset heart failure in the context of S. Aureus bacteremia | Extensive pneumonia | 6 |
| 6 | Boumitri, C., 2017 (9) | 64 | M | Vancomycin | Infected right hip prosthesis | Cytomegalovirus infection | >5 |
| 7 | Martin, C., 2018 (10) | 59 | M | Dolutegravir | HIV-positive, pulmonary tuberculosis | Liver decompensation | 9 |
| 8 | Sibille, L., 2017 (11) | 72 | M | Nabumetone | Gastrointestinal and bone issues | Lung infiltrate | 6 |
| 9 | Kombila, U. D., 2018 (12) | 35 | M | Pyrazinamide | First episode of a smear positive pulmonary tuberculosis | Liver failure | 4 |
| 10 | Gude, D., 2011 (13) | 86 | F | Phenytoin | Stroke,seizure disorder | Severe sepsis, respiratory failure, cardiac arrest | 4 |
| 11 | Montecchiani, V., 2017 (14) | 40 | F | Allopurinolinduzierte | Active pulmonary tuberculosis (TB) | Multi-organ failure | 6 |
| 12 | He, Q., 2020 (15) | 66 | F | Omeprazole | Abdominal distention | Renal failure | 9 |
| 13 | Drago, F., 2016 (16) | 71 | F | Strontium ranelate | Osteoporosis | Cardiac and respiratory insufficiencies | 7 |
| 14 | Plost, G., 2017 (17) | 12 | M | Minocycline | Folliculitis | Complications of multisystem organ failure | 6 |
| 15 | Miller Quidley, A., 2012 (18) | 63 | F | Clindamycin | Methicillin-susceptible Staphylococcus aureus (MSSA) prosthetic hip joint infection | Life-sustaining measures were withdrawn at the family’s request | 9 |
| 16 | Micozzi, S., 2015 (19) | 20 | F | Minocycline | Acne | Primary graft failure | 6 |
| 17 | Pursnani, A., 2009 (20) | 48 | M | Azithromycin | Upper respiratory tract infection | Sepsis | 5 |
| 18 | Numéro, N. J. R. A., 2011 (21) | 19 | F | Minocycline | Acne | Heart transplantation | 4 |
| 19 | Ardérius, M., 2018 (22) | 28 | F | Sulfasalazine | Psoriatic arthritis | Cardiogenic shock and multiorgan failure;anoxic encephalopathy | 6 |
| 20 | Coban-Karatas, M., 2012 (23) | 31 | M | Amoxicillin and clavulanate | Flu | Progressive liver damage and fulminant hepatitis | 6 |
| 21 | Karimpour, H., 2020 (24) | 28 | M | Colistimethate sodium, phenytoin, meropenem, quetiapine, levofloxacin, vancomycin | Decreased levels of consciousness | Septic shock | 5 |
| 22 | Kitcharoensakkul, M., 2012 (25) | 1.8 | F | Vancomycin | Methicillin-resistant Staphylococcus aureus pericarditis with bacteremia status post subtotal pericardiectomy | Respiratory and cardiac arrest | 6 |
| 23 | Kato, M., 2016 (26) | 68 | M | Allopurinol | Hyperuricaemia | Septic shock | 7 |
| 24 | Takamiyagi, S., 2022 (27) | 66 | M | Zonisamide | Numbness after thalamic hemorrhage | Respiratory failure caused by pneumonia | 7 |
| 25 | Chen, H. C., 2022 (28) | 55 | M | Sultamicillin | Eosinophilia and Systemic Symptoms Syndrome Patients With Lymph Node | Septic shock | 6 |
| 26 | Fatima, M., 2023 (29) | 32 | F | Leflunomide | Joint pains | Worsening skin lesions | 7 |
4. Discussion
DRESS is a life-threatening multi-organ system reaction. DRESS was often overlooked in clinical practice due to its unique clinical presentation, such as delayed onset and similarity to symptoms of other conditions. DRESS has a heterogenous clinical presentation making it challenging to diagnose, typically developed by assessing the timing of drug exposure in relation to the onset of skin rash and the resolution of symptoms following discontinuation of the offending drug. Recently, Brüggen et al. (30), reported a delphi-based international consensus on diagnostic workup, severity assessment, and management of DRESS, but it did not cover all aspects of DRESS management in detail and some topics remained controversial. Therefore, the development of more researches and future guidelines are necessary. The main advantages of our research is that we integrated the FAERS database analysis with case report assessments and a systematic review, effectively reducing the risk of bias that might come from using just one single database.
Results showed that allopurinol has the highest probability to induce DRESS and related death statistically. A comprehensive analysis (31) including 52 case reports revealed a mortality rate of 25.0% of allopurinol-induced DRESS, with septic shock, gastrointestinal bleeding, and multiple organ failure identified as the primary causes of death. Factors associated with increased risk of mortality included advanced age, underlying cardiovascular disease, chronic kidney disease, high allopurinol dosage, infection, and internal organ involvement such as the kidney, heart, lung, and gastrointestinal tract. In our systematic review, two patients (50%) had chronic renal failure, that may be related to their poor prognosis. The most common visceral manifestation were liver and kidney injuries. The human leukocyte antigen (HLA) class I alleles HLA-B*58:01 and HLA-C*03:02 have been implicated in allopurinol-induced SCAR, with HLA-C*03:02 being predominantly associated with DRESS (32). These findings underscore the importance of pre-treatment HLA genotyping in patients with compromised renal function to mitigate the risk of DRESS. Chronic renal failure was the cause of 2 out of 4 deaths caused by allopurinol in our systematic review. HLA associations with DRESS have provided insights into immunopathogenesis. In some SCAR cases, HLA oligoclonal CD8+ T-cell responses restricted by HLA are observed at the tissue level (33). Nevertheless, for the majority of drugs, the specific HLA risk alleles and antigens that drive this response remain unidentified. Additionally, HLA risk alleles exhibit incomplete positive and negative predictive values, rendering comprehensive screening a current challenge. Recently, single-cell RNA sequencing has been proved helpful in guiding therapeutic decisions on DRESS patients (34).
A systematic review (35) identified 1072 cases of psychotropic drug-induced DRESS indicated that carbamazepine, lamotrigine, phenytoin, valproate, and phenobarbital were the most implicated drugs. Studies have examined the genetic contributions to cutaneous adverse drug reactions caused by AEDs and discovered significant associations with HLA molecule genes, the cytochrome P450 enzyme and complement factor H genes (36). One case (1/26, 3.85%) in our systematic review were related to phenytoin, but the patient did not do any genetic test. Significant genetic factors have been identified in association with phenytoin-related SCAR. Patients with SCAR, particularly those carrying the CYP2C9*3 allele, exhibited delayed plasma phenytoin clearance (37). The alleles HLA-B*13:01, HLA-B*15:02, and HLA-B*51:01 demonstrated significant associations with phenytoin hypersensitivity, each exhibiting distinct phenotypic specificities (38). Therefore, integrating the evaluation of HLA and CYP2C9 risk alleles enhances the efficacy of predictive genetic testing for the prevention of phenytoin hypersensitivity in Asians. Although there were no deaths caused by lamotrigine among the deaths included in this article, lamotrigine ranked second in drugs calculated to induce DRESS and fifth statistically related to fatal outcome. In Asian populations, HLA-B*1502 increases the risk of lamotrigine- induced bullous lesions, while HLA-A*2402 is linked to susceptibility to SCARs (39). Conversely, HLA-A*3303 serves as a protective allele against lamotrigine- induced SCARs (39).
A review conducted in the USA (1980 to 2016) found that antibiotics, particularly vancomycin (39%), β-lactams (23%), fluoroquinolones (4%), tetracyclines (4%), and sulfonamides (3%), were implicated in total 74% of DRESS syndrome cases (40). In our study, vancomycin ranked third among the drugs related to DRESS and ranked second among the drugs statistically related to death. Vancomycin related DRESS syndrome happened within 21 days (41). A systematic case review (42) which analysis the immune-mediated reactions to vancomycin indicated that the incidence of DRESS syndrome was 16/71, 23%, men represented a majority (56%) with median latency of 21 days [IQR 17 days, 28 days] for DRESS syndrome. Our results showed that the time from the onset of the rash to the death with vancomycin were 40 to 56 days, which means once rash begins to appear, the next 20-30 days are very valuable for treatment. It is noteworthy that other glycopeptide antibiotics are also possible to cause DRESS syndrome in limited number of patients and may lead to allergic cross-reactivity with vancomycin (43). Vancomycin-associated DRESS may be due to genetic factors. A study identified that 20% of HLA-A*32:01 patients would develop vancomycin DRESS if exposed for more than two weeks (44). Another study reported the cross-reactivity of alternative glycopeptide antibiotics, namely teicoplanin and telavancin, in patients exhibiting HLA-A*32:01–restricted vancomycin-induced DRESS, who also share the expression of an HLA class II haplotype, specifically DQA1*01:01 and DQB1*05:03 (45). A PCR-based assay specific to HLA-A*32:01 has been developed to screen patients for the risk of vancomycin-induced DRESS (46).
Among the deaths included in this article, 3 patients were related to DRESS of minocycline, and 2 of them had cardiac involvement. Dyspnea and chest pain are the most prevalent symptoms in patients with DRESS and cardiac involvement, with minocycline being identified as one of the most frequently implicated medications (47). Due to the high mortality rate of DRESS with cardiac involvement (45%) (47), doctors and pharmacists should pay special attention to the chest pain or abnormal heartbeats of patients. In the systematic review, the cardiac damage lead to death in DRESS cases included sudden cardiac arrest, heart transplantation after giant cell myocarditis (GCM), cardiac insufficiencies and cardiogenic shock. When dealing with DRESS patients, ECG, echocardiogram, cardiac enzymes and troponin should be performed, If necessary, perform endomyocardial biopsy as well. Physicians should pay attention to the occurrence of chest pain, sinusoidal, ischemic, depressed injection fraction, giant cell myopathy, and fulminant eosinophilic necrotizing myopathy, which may be associated with a higher possibility of death.
The antibiotics mentioned in this study include minocycline (3/13, 23%), vancomycin (2/13, 15%), amoxicillin and clavulanate, azithromycin, clindamycin, colistimethate, oxacillin, pyrazinamide, sulfasalazine and sultamicillin (1/13, 0.69% each). Previous research (41) has shown that the severity of antibiotic-induced DRESS syndrome compared to other triggers remains a topic of debate, but our research suggests that antibiotic related DRESS has a relatively high mortality rate (50%). Therefore, clinicians should exercise caution when prescribing antibiotics to patients with a history of DRESS syndrome.
So far, rash remained the most frequent clinical findings. The skin disorders in fatal cases were mostly severe, systemic and diffuse, with features that included rash, erythema, maculopapulars, urticarial rash, pustules. The generalized rash could be with or without itching, edema, esquamation, blisters, scabs, erosions. The rash could appear on the trunk, extremities and face, and could started from one part of the body and spread to the entire body. Out of 26 cases, 10 have undergone skin biopsy and all were consistent with DRESS. Six skin biopsy (6/10, 60%) mentioned dermatitis or perivascular inflammatory infiltrate with elevated eosinophils. One skin lesion (1/10, 10%) showed liquefaction degeneration in the epidermis. One skin biopsy (110, 10%) for direct and indirect immunofluorescence revealed linear C3 deposits and IgG deposits, respectively. ELISA testing for BP 180 and 230 was positive, consistent with a diagnosis of bullous pemphigoid. A study retrospective study on 50 skin biopsies from 36 patients with DRESS syndrome, and demonstrated that 28% with typical lymphocytes (most with CD8 expressed) and 6% with T-cell (48). Different types of SCARs may differ in terms of the specific effector T cells involved and the chemokines/cytokines released, resulting in cell homing to skin and specific tissue targeting (33).
Virus reactivation were observed in 4 fatal cases in the systematic review, of which 2 cases referred to HHV reactivation (including HHV-6 and HHV-7), and 2 cases mentioned cytomegalovirus (CMV) reactivation. In addition, there were 5 cases reported as virus infection. A study prospectively assessed 40 patients exhibiting DRESS found that EBV, HHV-6 or HHV-7 reactivation in 76% of the patients (49). In our systematic review, this proportion was lower, that possibly due to two reasons. On the one hand, some cases did not undergo virus testing, and on the other hand, some cases that tested positive for the virus were not determined whether they belonged to reactivation. Virus reactivation is related to the onset of DRESS, because the viral reactivation in DRESS cases is thought to lead to the circulating CD8+ T lymphocytes activation (49). HHV reactivation is potentially linked to increased severity of reactions, with viral reactivation correlating to the level of inflammation (50). A reactivation can be asymptomatic, or it may cause prolonged symptoms in DRESS, long after stopping the causative drugs (50).
Among the drugs mentioned in this article, allopurinol, minocycline, vancomycin, amoxicillin and clavulanate, leflunomide, levetiracetam, nabumetone, sulfasalazine, zonisamide and azithromycin have warning signs for DRESS in their drug instructions, while colistimethate, dolutegravir, lansoprazole, omeprazole, oxacillin, phenoin, pyrazinamide do not have warning signs in their drug instructions. It is necessary to remind patients in the drug labels that serious skin reactions, including dress may develop, and advise patients to stop taking suspect drug immediately if they develop any type of rash or fever. The patients should discontinue the suspected drug immediately if symptoms occur and contact their healthcare provider as soon as possible.
The initial step in managing DRESS involves discontinuing the suspected drug responsible. Systemic corticosteroids continue to be the preferred treatment option. Systemic corticosteroids continue to be the preferred treatment option. However, several retrospective studies indicated that systemic corticosteroids associated with higher risk of DRESS relapse (51), viral reactivation (51, 52), various infections (53), and development of autoimmune diseases (53). Although the role of steroid-sparing therapies in DRESS is still not well-established yet, there is increasing evidence supporting the use of steroid-sparing therapies such as cyclosporine, intravenous immunoglobulin (IVIG), interleukin (IL)-5 axis inhibitors, and Janus kinase (JAK) inhibitors (54). Although biomarkers not measured in the cases reviewed in this article, in light of the ongoing diagnostic challenges associated with DRESS, there is a growing interest in investigating biomarkers. Ogawa’s study revealed a significant increase in serum thymus and activation-regulated chemokine (TARC/CCL17) levels among patients with DRESS compared to those with morbilliform drug eruption and Stevens-Johnson syndrome/Toxic epidermal necrolysis (SJS/TEN) (55). In fact, a prospective case control study demonstrated comparable efficacy between serum TARC levels and a RegiSCAR score (≥2) in distinguishing cases of DRESS from controls with maculopapular drug rash (56). Miyagawa reported that Th2-associated chemokines were markedly upregulated in DIHS/DRESS (57). IFN-γ ELISpot assay (58, 59) and IFN-γ combined with IL-4/IL-2 (56, 60) could offer potential for use as rapid diagnostic tests.
It must be admitted that the study encountered various limitations. Firstly, the FAERS data utilized in our analysis were subject to voluntary reporting by healthcare professionals and consumers, potentially resulting in variations in report quality due to the skills and diligence of the reporters. This could introduce bias into our findings through missing, inadequate, or incomplete reports. Additionally, FAERS lacks comprehensive information on patient morbidity related to drug use, as it does not provide data on the total number of patients using the drug. Furthermore, the small sample size of case reports limited our ability to do risk factor analysis. Lastly, methodological constraints prevented us from establishing a causal relationship between drug and the observed positive signals or reported cases.
5. Conclusion
To our knowledge, this article is the first study that focus on fatal DRESS cases. Our research indicates a relatively high mortality rate associated with antibiotic-related DRESS. Cardiac involvement is the most common comorbidities among DRESS deaths, and patients taking high-risk medication should be carefully monitoring.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. National Natural Science Foundation of China (82304456) from C-SL, National Natural Science Foundation of China (82304638) from XL and National High Level Hospital Clinical Research Funding (2022-PUMCH-B-059)from BZ.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
C-SL: Data curation, Funding acquisition, Writing – original draft, Writing – review & editing. P-JA: Formal analysis, Methodology, Writing – review & editing. Y-ZZ: Data curation, Formal analysis, Writing – original draft. XL: Conceptualization, Funding acquisition, Writing – review & editing. BZ: Funding acquisition, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. (2013) 68:e1–14. doi: 10.1016/j.jaad.2013.01.033 [DOI] [PubMed] [Google Scholar]
- 2. Wei BM, Fox LP, Kaffenberger BH, Korman AM, Micheletti RG, Mostaghimi A, et al. Drug-induced hypersensitivity syndrome / drug reaction with eosinophilia and systemic symptoms. Part I. Epidemiology, pathogenesis, clinicopathological features, and prognosis. J Am Acad Dermatol. (2024) 90(5):885–908. doi: 10.1016/j.jaad.2023.02.072 [DOI] [PubMed] [Google Scholar]
- 3. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. (2007) 156:609–11. doi: 10.1111/j.1365-2133.2006.07704.x [DOI] [PubMed] [Google Scholar]
- 4. Yaylacı S, Demir MV, Temiz T, Tamer A, Uslan MI. Allopurinol-induced DRESS syndrome. Indian J Pharmacol. (2012) 44:412–4. doi: 10.4103/0253-7613.96351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi HG, Byun J, Moon CH, Yoon JH, Yang KY, Park SC, et al. Allopurinol-induced DRESS syndrome mimicking biliary obstruction. Clin Mol Hepatol. (2014) 20:71–5. doi: 10.3350/cmh.2014.20.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uppalapati A, Gogineni S, Koneru S, Kamel G. Are proton pump inhibitors(PPI) naive? A case of drug reaction with eosinophilia and systemic symptom (DRESS) secondary to lansoprazole. J Allergy Clin Immunol. (2014) 133:AB273. doi: 10.1016/j.jaci.2013.12.968 [DOI] [Google Scholar]
- 7. Sato H, Takase K, Harada A, Ozono I, Kodama Y, Ishitobi T, et al. Atypical, levetiracetam-induced hypersensitivity syndrome complicated by fulminant liver failure in a patient undergoing hemodialysis. Internal Med (Tokyo Japan). (2022) 61:2911–6. doi: 10.2169/internalmedicine.8985-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desai AS, Dudzinski DM, Stib MT, Chen ST, Newton-Cheh C, Blumenthal KG, et al. Case 32-2022: A 76-year-old man with postoperative cardiogenic shock and diffuse rash. New Engl J Med. (2022) 387:1502–13. doi: 10.1056/NEJMcpc2201245 [DOI] [PubMed] [Google Scholar]
- 9. Boumitri C, Rao SC, Tahan V. A case of severe erosive enterocolitis secondary to cytomegalovirus reactivation in a patient with DRESS syndrome: 1474. Am J Gastroenterol. (2017) 112:S806–7. doi: 10.1038/ajg.2017.312 [DOI] [Google Scholar]
- 10. Martin C, Payen MC, De Wit S. Dolutegravir as a trigger for DRESS syndrome? Int J STD AIDS. (2018) 29:1036–8. doi: 10.1177/0956462418764973 [DOI] [PubMed] [Google Scholar]
- 11. Sibille L, Jean-Christophe M, Grégoire W. DRESS syndrome following exposure to Nabumétone. Acta Clinica Belgica: Int J Clin Lab Med. (2017) 72:37. doi: 10.1080/17843286.2017.1401314 [DOI] [Google Scholar]
- 12. Kombila UD, Ka W, Mbaye FBR, Diouf NF, Fall L, Ouedraogo P, et al. DRESS syndrome secondary to pyrazinamide: An uncommon complication of tuberculosis treatment. Rev Des maladies respiratoires. (2018) 35:69–73. doi: 10.1016/j.rmr.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 13. Gude D, Chennamsetty S, Jha R, Rajitha K. DRESSing up to phenytoin. Indian J Pharmacol. (2011) 43:617–8. doi: 10.4103/0253-7613.84991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montecchiani V, Zurbano Azqueta L, De Las Vecillas Sanchez L, Anton M. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome and tuberculosis: Case report. Allergy: Eur J Allergy Clin Immunol. (2017) 72:694. doi: 10.1111/all.13252 [DOI] [Google Scholar]
- 15. He Q, Ying G, Fei X, Zha C, Chen Z, Bao Y, et al. Drug rash with eosinophilia and systemic symptoms and severe renal injury induced by proton pump inhibitor therapy: A case report. Medicine. (2020) 99:e22509. doi: 10.1097/md.0000000000022509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drago F, Cogorno L, Broccolo F, Ciccarese G, Parodi A. A fatal case of DRESS induced by strontium ranelate associated with HHV-7 reactivation. Osteoporosis international: J established as result cooperation between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA. (2016) 27:1261–4. doi: 10.1007/s00198-015-3384-7 [DOI] [PubMed] [Google Scholar]
- 17. Plost G, Gurnee E, Rice Z, Stoff B, Spraker M, Lawley L. A fatal case of drug-induced hypersensitivity syndrome complicated by multiple autoimmune disorders including bullous pemphigoid. Pediatr Dermatol. (2017) 34:S26–7. doi: 10.1111/pde.13194 [DOI] [Google Scholar]
- 18. Miller Quidley A, Bookstaver PB, Gainey AB, Gainey MD. Fatal clindamycin-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Pharmacotherapy. (2012) 32:e387–92. doi: 10.1002/phar.1142 [DOI] [PubMed] [Google Scholar]
- 19. Micozzi S, Pinto C, Seoane M, Carbone J, Tornero P. Giant cell myocarditis in hypersensitivity reactions: is an early diagnose possible? Ann allergy Asthma immunology: Off Publ Am Coll Allergy Asthma Immunol. (2015) 115:247–8. doi: 10.1016/j.anai.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 20. Pursnani A, Yee H, Slater W, Sarswat N. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Internal Med. (2009) 150:225–6. doi: 10.7326/0003-4819-150-3-200902030-00027 [DOI] [PubMed] [Google Scholar]
- 21. San Nicasio CS, Fernández CM, Carrero EB, Hernanz Hermosa JM, De La Cueva Dobao P. Is minocycline a safe treatment for acne? J Am Acad Dermatol. (2011) 64(2-supp-S1):AB111. Numéro, N. J. R. A. doi: 10.1016/j.jaad.2010.09.740 [DOI] [Google Scholar]
- 22. Ardérius M, Menezes MN, Marques T, Brito D, Veiga F. Late recurrence of fulminant myocarditis related to HSS/DRESS. Cor et Vasa. (2018) 60(6):e635–7. doi: 10.1016/j.crvasa.2017.12.009 [DOI] [Google Scholar]
- 23. Coban-Karatas M, Turunc T, Altan-Yaycioglu R. Purtscher-like retinopathy related to drug-induced hypersensitivity syndrome. Ocular Immunol Inflammation. (2012) 20:475–7. doi: 10.3109/09273948.2012.714441 [DOI] [PubMed] [Google Scholar]
- 24. Karimpour H, Shojaei L, Shahbazi F. Probable drug eruption eosinophilia and systemic symptoms due to colistimethate sodium: A challenging case report. Infect Dis Clin Pract. (2020) 28:e58–60. doi: 10.1097/IPC.0000000000000898 [DOI] [Google Scholar]
- 25. Kitcharoensakkul M, Ree N, Bloomberg GR, Dehner LP, Heidingsfelder JA, White AJ, et al. Vancomycin-induced DRESS with evidence of T-cell activation in a 22-month-old patient. Ann allergy Asthma immunology: Off Publ Am Coll Allergy Asthma Immunol. (2012) 109:280–1. doi: 10.1016/j.anai.2012.07.016 [DOI] [PubMed] [Google Scholar]
- 26. Kato M, Kano Y, Sato Y, Shiohara T. Severe agranulocytosis in two patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. Acta dermato-venereologica. (2016) 96:842–3. doi: 10.2340/00015555-2420 [DOI] [PubMed] [Google Scholar]
- 27. Takamiyagi S, Iriki H, Asahina Y, Furuichi Y, Funakoshi T, Ichikawa M, et al. Severe graft-versus-host disease-like enterocolitis accompanied with cytomegalovirus-reactivation in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J Dermatol. (2022) 49:796–9. doi: 10.1111/1346-8138.16415 [DOI] [PubMed] [Google Scholar]
- 28. Chen HC, Wang RC, Tsai HP, Medeiros LJ, Chang KC. Morphologic spectrum of lymphadenopathy in drug reaction with eosinophilia and systemic symptoms syndrome. Arch Pathol Lab Med. (2022) 146:1084–93. doi: 10.5858/arpa.2021-0087-OA [DOI] [PubMed] [Google Scholar]
- 29. Fatima M, Azimi SS, Ashwini S, Radhakrishna MH. Leflunomide induced atypical DRESS: A case report and literature review. Mediterr J Rheumatol. (2023) 34:91–6. doi: 10.31138/mjr.34.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brüggen M-C, Walsh S, Ameri MM, Anasiewicz N, Maverakis E, French LE, et al. Management of adult patients with drug reaction with eosinophilia and systemic symptoms: A delphi-based international consensus. JAMA Dermatol. (2024) 160:37–44. doi: 10.1001/jamadermatol.2023.4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Q, Zhao S, Chen W. Clinical features, treatment outcomes and prognostic factors of allopurinol-induced DRESS in 52 patients. J Clin Pharm Ther. (2022) 47:1368–78. doi: 10.1111/jcpt.13667 [DOI] [PubMed] [Google Scholar]
- 32. Ha Pham TT, Tran QB, Chu CH, Nga Do TQ, Nguyen HA, Nguyen DV, et al. Allopurinol-induced severe cutaneous adverse reactions in Vietnamese: the role of HLA alleles and other risk factors. Pharmacogenomics. (2022) 23:303–13. doi: 10.2217/pgs-2021-0156 [DOI] [PubMed] [Google Scholar]
- 33. Gibson A, Deshpande P, Campbell CN, Krantz MS, Mukherjee E, Mockenhaupt M, et al. Updates on the immunopathology and genomics of severe cutaneous adverse drug reactions. J Allergy Clin Immunol. (2023) 151:289–300. doi: 10.1016/j.jaci.2022.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim D, Kobayashi T, Voisin B, Jo J-H, Sakamoto K, Jin S-P, et al. Targeted therapy guided by single-cell transcriptomic analysis in drug-induced hypersensitivity syndrome: a case report. Nat Med. (2020) 26:236–43. doi: 10.1038/s41591-019-0733-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bommersbach TJ, Lapid MI, Leung JG, Cunningham JL, Rummans TA, Kung S. Management of psychotropic drug-induced DRESS syndrome: A systematic review. Mayo Clinic Proc. (2016) 91:787–801. doi: 10.1016/j.mayocp.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 36. Mullan KA, Anderson A, Illing PT, Kwan P, Purcell AW, Mifsud NA. HLA-associated antiepileptic drug-induced cutaneous adverse reactions. HLA. (2019) 93:417–35. doi: 10.1111/tan.13530 [DOI] [PubMed] [Google Scholar]
- 37. Chung W-H, Chang W-C, Lee Y-S, Wu Y-Y, Yang C-H, Ho H-C, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. (2014) 312:525–34. doi: 10.1001/jama.2014.7859 [DOI] [PubMed] [Google Scholar]
- 38. Su S-C, Chen C-B, Chang W-C, Wang C-W, Fan W-L, Lu L-Y, et al. and CYP2C9*3 as predictors of phenytoin hypersensitivity in east asians. Clin Pharmacol Ther. (2019) 105:476–85. doi: 10.1002/cpt.1190 [DOI] [PubMed] [Google Scholar]
- 39. Deng Y, Li S, Zhang L, Jin H, Zou X. Association between HLA alleles and lamotrigine-induced cutaneous adverse drug reactions in Asian populations: A meta-analysis. Seizure. (2018) 60:163–71. doi: 10.1016/j.seizure.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 40. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. (2019) 7:633–40. doi: 10.1016/j.jaip.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. (2021) 77:275–89. doi: 10.1007/s00228-020-03005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Minhas JS, Wickner PG, Long AA, Banerji A, Blumenthal KG. Immune-mediated reactions to vancomycin: A systematic case review and analysis. Ann allergy Asthma immunology: Off Publ Am Coll Allergy Asthma Immunol. (2016) 116:544–53. doi: 10.1016/j.anai.2016.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miyazu D, Kodama N, Yamashita D, Tanaka H, Inoue S, Imakyure O, et al. and subsequent teicoplanin administration: A case report. Am J Case Rep. (2016) 17:625–31. doi: 10.12659/ajcr.899149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Konvinse KC, Trubiano JA, Pavlos R, James I, Shaffer CM, Bejan CA, et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol. (2019) 144:183–92. doi: 10.1016/j.jaci.2019.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakkam N, Gibson A, Mouhtouris E, Konvinse KC, Holmes NE, Chua KY, et al. Cross-reactivity between vancomycin, teicoplanin, and telavancin in patients with HLA-A∗32:01-positive vancomycin-induced DRESS sharing an HLA class II haplotype. J Allergy Clin Immunol. (2021) 147:403–5. doi: 10.1016/j.jaci.2020.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rwandamuriye FX, Chopra A, Konvinse KC, Choo L, Trubiano JA, Shaffer CM, et al. A rapid allele-specific assay for HLA-A*32:01 to identify patients at risk for vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Mol Diagnostics: JMD. (2019) 21:782–9. doi: 10.1016/j.jmoldx.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Radovanovic M, Jevtic D, Calvin AD, Petrovic M, Paulson M, Rueda Prada L, et al. Heart in DRESS": cardiac manifestations, treatment and outcome of patients with drug reaction with eosinophilia and systemic symptoms syndrome: A systematic review. J Clin Med. (2022) 11:704. doi: 10.3390/jcm11030704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, Wechsler J, de Feraudy S, Duong TA, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. (2015) 173:50–8. doi: 10.1111/bjd.13683 [DOI] [PubMed] [Google Scholar]
- 49. Picard D, Janela B, Descamps V, D'Incan M, Courville P, Jacquot S, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Trans Med. (2010) 2:46ra62. doi: 10.1126/scitranslmed.3001116 [DOI] [PubMed] [Google Scholar]
- 50. Schrijvers R, Gilissen L, Chiriac AM, Demoly P. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Trans Allergy. (2015) 5:31. doi: 10.1186/s13601-015-0073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Funck-Brentano E, Duong T-A, Bouvresse S, Bagot M, Wolkenstein P, Roujeau J-C, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. (2015) 72:246–52. doi: 10.1016/j.jaad.2014.10.032 [DOI] [PubMed] [Google Scholar]
- 52. Tohyama M, Hashimoto K, Oda F, Namba C, Sayama K. Influence of corticosteroid therapy on viral reactivation in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J Dermatol. (2020) 47:476–82. doi: 10.1111/1346-8138.15294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ushigome Y, Kano Y, Ishida T, Hirahara K, Shiohara T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. (2013) 68:721–8. doi: 10.1016/j.jaad.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 54. Wei BM, Fox LP, Kaffenberger BH, Korman AM, Micheletti RG, Mostaghimi A, et al. Drug-induced hypersensitivity syndrome / drug reaction with eosinophilia and systemic symptoms. Part II. Diagnosis and management. J Am Acad Dermatol. (2024) 90(5):911–26. doi: 10.1016/j.jaad.2023.02.073 [DOI] [PubMed] [Google Scholar]
- 55. Ogawa K, Morito H, Hasegawa A, Daikoku N, Miyagawa F, Okazaki A, et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS). J Dermatol Sci. (2013) 69:38–43. doi: 10.1016/j.jdermsci.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 56. Kumkamthornkul P, Udnaen S, Tansit T, Tuchinda P, Srinoulprasert Y. Evaluation of a lymphocyte transformation test and cytokine detection assay to identify phenytoin and carbamazepine provoked DRESS or SJS/TEN in epilepsy patients. Int Immunopharmacol. (2018) 63:204–10. doi: 10.1016/j.intimp.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 57. Miyagawa F, Hasegawa A, Imoto K, Ogawa K, Kobayashi N, Ito K, et al. Differential expression profile of Th1/Th2-associated chemokines characterizes Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) and drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS) as distinct entities. Eur J Dermatology: EJD. (2015) 25:87–9. doi: 10.1684/ejd.2014.2477 [DOI] [PubMed] [Google Scholar]
- 58. Suthumchai N, Srinoulprasert Y, Thantiworasit P, Rerknimitr P, Tuchinda P, Chularojanamontri L, et al. The measurement of drug-induced interferon γ-releasing cells and lymphocyte proliferation in severe cutaneous adverse reactions. J Eur Acad Dermatol Venereology: JEADV. (2018) 32:992–8. doi: 10.1111/jdv.14890 [DOI] [PubMed] [Google Scholar]
- 59. Klaewsongkram J, Thantiworasit P, Suthumchai N, Rerknimitr P, Sukasem C, Tuchinda P, et al. In vitro test to confirm diagnosis of allopurinol-induced severe cutaneous adverse reactions. Br J Dermatol. (2016) 175(5):994–1002. doi: 10.1111/bjd.14701 [DOI] [PubMed] [Google Scholar]
- 60. Polak ME, Belgi G, McGuire C, Pickard C, Healy E, Friedmann PS, et al. In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensitivity reactions. Br J Dermatol. (2013) 168:539–49. doi: 10.1111/bjd.12109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.