Abstract
In a series of studies on blood-brain barrier transportable peptides, a soybean dipeptide, Tyr-Pro, penetrated the mouse brain parenchyma after oral intake and improved short and long memory impairment in acute Alzheimer’s model mice. Here, we aimed to clarify the anti-dementia effects of this peptide administered to SAMP8 mice prior to dementia onset. At the end of the 25-week protocol in 16-week-old SAMP8 mice, Tyr-Pro (10 mg/kg/day) significantly improved the reduced spatial learning ability compared with that in the control and amino acid (Tyr + Pro) groups as indicated by the results of Morris water maze tests conducted for five consecutive days. The hippocampus and cortex regions of SAMP8 harvested after the test showed lower amyloid ß (Aß) accumulation in the Tyr-Pro group than those in the control and amino acid groups. Consistent with the lower level of Aß, decreased expression of ß-secretase (BACE1) and markedly increased expression (4-times higher) of insulin degrading enzyme (IDE) were obtained compared to those in the control group. Collectively, we demonstrated that long-term daily intake of the dipeptide Tyr-Pro in SAMP8 mice may be sufficient for maintaining cognitive ability by preventing excess Aß accumulation through downregulated BACE1 and particularly upregulated IDE.
Subject terms: Peptides, Diseases
Introduction
Alzheimer’s disease (AD), currently the most prevalent cause of dementia, is a neurodegenerative condition characterized by the gradual deterioration of cognitive abilities, such as memory impairment, compromised thinking skills, and alterations in behavior and personality1. The presence of amyloid plaques and neurofibrillary tangles is a typical histopathological feature in the brains of individuals with AD2. Plaques composed of aggregated insoluble amyloid-ß (Aß) neurotoxic peptides are generated outside the cells by cleavage of amyloid precursor protein (APP)3. Amyloid plaques trigger inflammation, oxidative stress, and neurotoxicity4. The development of AD drugs has been a great challenge to demonstrate clear clinical evidence and to reduce the drug’s high5. In 2021, the US Food and Drug Administration (FDA) approved aducanumab a monoclonal antibody for early-stage Alzheimer’s disease. However, the clinical evidence of the drug was not clear for drug’s cognitive benefit and the price of the drug was set at a staggering $56,000 per year. Finally, the manufacturer decided to discontinue Alzheimer’s drug.
Thus, much attention has been given to the prevention of AD onset by consuming functional foods, such as epicatechins6, quercetin7, chlorogenic acid7, and peptides8, from the perspective of quality of life (QOL). Among these, peptide intake can compensate for AD prevention. In a human study, Maebuchi et al.9 reported that daily intake of soybean hydrolysate (8 g/day) for 8 weeks was efficient at improving the delayed memory scores in 30 subjects with mild cognitive impairment. Yuda et al.10 also reported that intake of the casein tripeptide Met-Lys-Pro for 24 weeks improved orientation in 268 adults without dementia. However, the critical evidence on their bioavailability and blood-brain barrier (BBB) permeability has not been clarified.
In a series of previous studies, we thus focused on brain-health peptides that can cross the BBB in intact forms. We successfully identified that the soybean dipeptide Tyr-Pro reached the mouse brain parenchyma across the BBB and accumulated in regions of the hippocampus, hypothalamus, and cerebral cortex by conducting ex vivo mouse in situ perfusion experiments11. Furthermore, the transport of intact Tyr-Pro to the brain was also achieved by oral administration in ICR mice at 0.34 ± 0.11 pmol·min/mg-dry brain tissue after administration (10 mg/kg body weight)12. Based on the evidence of bioavailability and BBB transport of Tyr-Pro, a 2-week oral administration of the dipeptide Tyr-Pro was conducted in Aß25-35-induced acute AD in mice to improve the impaired short- and long-term memory impairment through the Y-maze test and passive avoidance test, respectively13. However, considering the potential for AD prevention by the daily intake of foods, a long-term study of Tyr-Pro administration prior to AD onset in spontaneous acceleration in senescence is necessary to demonstrate its anti-dementia effect. Therefore, in this study, we evaluated the preventive effect of long-term intake of Tyr-Pro at a dose of 0.1 g/kg diet (equiv. ~10 mg/kg/day, which was set based on the evidence of the aforementioned bioavailability test) in senescence-accelerated mouse-prone 8 (SAMP8, 16–25 weeks).
Results
Effects of Tyr-Pro Administration on Growth Parameters and on Memory and Learning in SAMP8 Mice
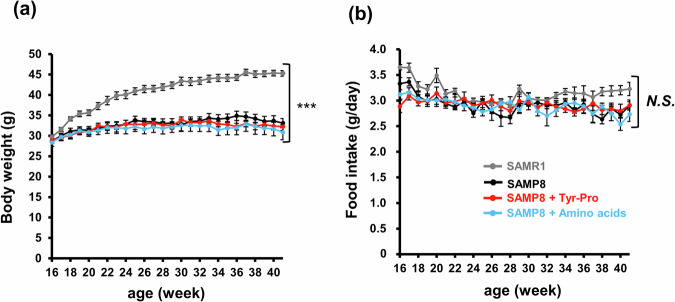
SAMP8 mice showing cognitive dysfunction because of aging were administered with 0.1 g/kg Tyr-Pro containing diet (equiv. a daily dose of ~10 mg/kg) to evaluate its effect on spatial memory and learning abilities using the Morris water maze test. During the period of Tyr-Pro administration (10 mg/kg/day) to 16-week-old SAMP8 mice for 25 weeks, no significant differences were found in body weight and food intake between SAMP8 mice fed with casein (control), amino acids (Tyr + Pro), or Tyr-Pro (Fig. 1). The significantly lower body weight of SAMP8 mice compared to that of SAMR1 mice suggests that growth was influenced by spontaneously accelerated aging in SAMP8 mice. During the 25-week protocol, one mouse each in the SAMP8 group fed with casein and with amino acids died because of accelerated aging, whereas none of the mice in the SAMP8 group fed with Tyr-Pro and in the SAMR1 died (data were not shown).
Fig. 1. Changes in the body weight and food intake of SAMP8 mice during the 25-week protocol.
a Changes in the body weight and b food intake of SAMR1 and SAMP8 mice fed with AIN-93M diet with or without 0.1 g/kg Tyr-Pro, or 0.05 g/kg Tyr + 0.05 g/kg Pro. Data are expressed as the mean ± SEM (SAMR1 group n = 11, control group n = 9, Tyr-Pro group n = 8, amino acid group n = 7). Significant differences were evaluated using a two-way repeated ANOVA. Statistical significance at ***p < 0.001.
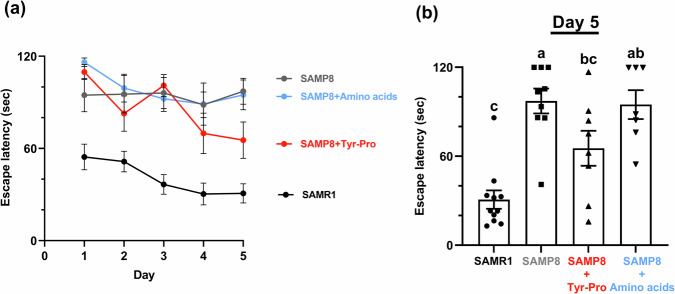
Figure 2 shows the escape latency of 41-week-old mice to a platform location in the Morris water maze test during training trials conducted for five consecutive days and on day 5. As shown in Fig. 2, accelerated aging in all three groups of SAMP8 mice apparently resulted in degraded spatial memory and learning abilities, compared to that in SAMR1 mice. However, among the SAMP8 groups, mice fed with Tyr-Pro showed improved abilities in the training trials conducted for five consecutive days in the Morris water maze test; further, on day 5 the mice fed with Tyr-Pro rapidly reached the platform location with their enhanced spatial memory and learning abilities (escape latency of SAMP8, 97.21 ± 8.41 s; SAMP8 with Tyr-Pro, 65.36 ± 11.83 s, p < 0.05).
Fig. 2. Effect of Tyr-Pro intake on escape latency of 41-week-old SAMP8 mice in the Morris water maze test.
a The escape latency of 41-week-old SAMP8 mice in the Morris water maze test with trials conducted for five consecutive days. b Effect of Tyr-Pro intake on day-5 escape latency of the SAMR1, SAMP8 control, Tyr-Pro, and amino acids groups. Data are expressed as the mean ± SEM (SAMR1 group n = 11, control group n = 9, Tyr-Pro group n = 8, amino acid group n = 7). Significant differences on day 5 results were evaluated using a Kruskal-Wallis test followed by Dunn’s test. Different letters (a–c on the histogram) represent the statistical significance at p < 0.05.
Effect of Tyr-Pro Administration on Amyloid ß Accumulation in the Brain of SAMP8 Mice
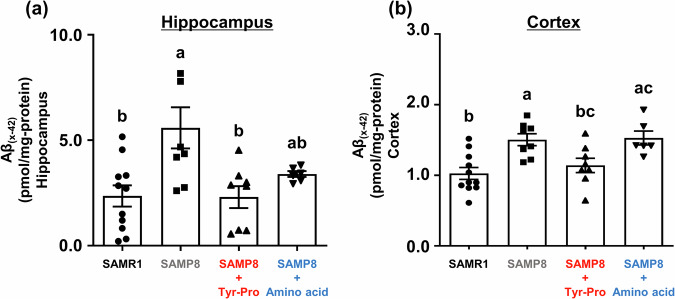
Considering that the neurodegenerative AD pathology is closely associated with progressive Aß accumulation in the brain prior to AD symptoms, including tau protein hyperphosphorylation and neurofibrillary tangles14,15, we focused on Aß accumulation in the hippocampus and cortex regions of 41-week-old SAMP8 mice in this study. Thus far, Aß accumulation in the hippocampus and cerebral cortex of SAMP8 mice at the age of six months16–18 and tau hyperphosphorylation in the cortex, hippocampus, and striatum of SAMP8 mice at the age of three months19 have been reported. As shown in Fig. 3, Aß levels in the hippocampus and cortex regions of SAMP8 mice fed with Tyr-Pro were significantly lower than those in the SAMP8 control groups; Aß levels in the hippocampus were 5.59 ± 0.98 pmol/mg-protein and 2.30 ± 0.52 pmol/mg-protein in the control and Tyr-Pro groups, respectively (p < 0.05), while Aß levels in the cortex were 1.50 ± 0.85 pmol/mg-protein and 1.14 ± 0.10 pmol/mg-protein in the control and Tyr-Pro groups, respectively (p < 0.05).
Fig. 3. Effect of Tyr-Pro intake on amyloid ß levels in the hippocampus and cortex of SAMP8 mice.
a The Aß levels in the hippocampus. b The Aß levels in the cortex. Aß levels in each region were measured using an Aß ELISA kit. The hippocampus and cortex were collected from the brains of mice after the Morris water maze trial. Data are expressed as the mean ± SEM (SAMR1 group n = 11, control group n = 8, Tyr-Pro group n = 8, amino acid group n = 6). Significant differences were evaluated using a one-way ANOVA followed by Tukey’s test. Different letters (a–c on the histogram) represent the statistical significance at p < 0.05.
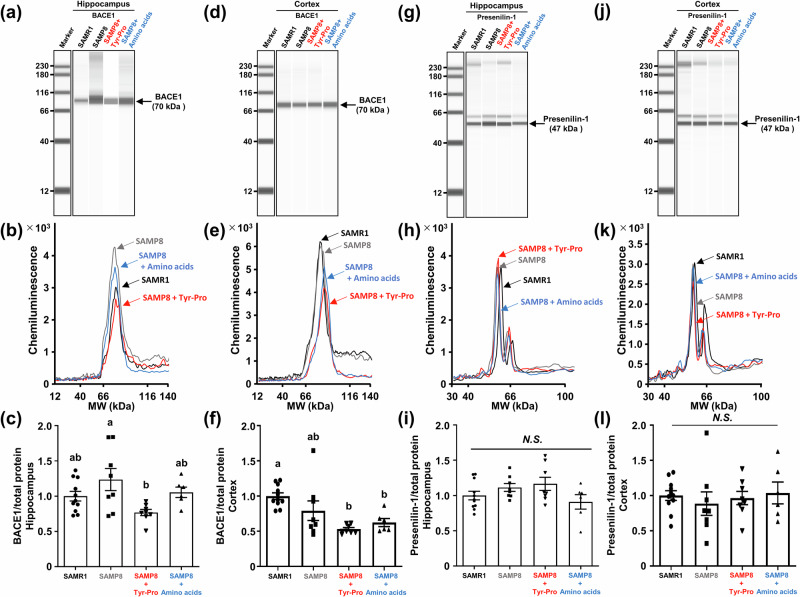
The reduced Aß levels in SAMP8 fed with Tyr-Pro for 25 weeks (Fig. 3) led us to further investigate the mechanism underlying the Aß production (amyloidogenesis) or degradation cascade. After the Morris water maze trial, mice were sacrificed to obtain the hippocampal and cortical tissues from the brains. For amyloidogenesis, ß-secretase (BACE1) and γ-secretase (presenilin-1) enzymes involved in the Aß production from the APP were evaluated by a Wes automated capillary electrophoresis protein expression analysis. BACE1, a pivotal enzyme, catalyzes APP cleavage to generate the N-terminus of Aß. It initiates the amyloidogenic pathway by precisely cleaving APP positioned at Asp1 via BACE1, producing soluble APPß and C99 fragments20, and Presenilin-1 mediates cleavage of the C99 fragment, releasing Aß1-40 or Aß1-4221. As shown in Fig. 4, BACE1 expression in the hippocampus and cortex tended to decrease in the Tyr-Pro group, but this reduction was not apparent compared to that of the control and amino acid groups. No significant changes in presenilin-1 activity were observed in any of the four groups. This suggests that long-term administration of Tyr-Pro did not severely cause downregulation of amyloidogenesis enzymes in the brain of SAMP8 mice; in other words, Aß production from APP in the brain was not greatly influenced by Tyr-Pro intake.
Fig. 4. Effect of Tyr-Pro intake on the protein expression of BACE1 and presenilin-1 in the hippocampus and cortex of SAMP8 mice.
Protein expression in each region was measured by a Wes analysis. a, d, g, j Virtual blot-like images; b, e, h, k electropherograms; c, f, i, l protein expression normalized by the electropherogram peak area of the total protein in each sample. Each region was collected from the brain of mice after the Morris water maze trial. Data are expressed as the mean ± SEM (SAMR1 group n = 11, control group n = 8, Tyr-Pro group n = 8, amino acid group n = 6). Significant differences were evaluated using a one-way ANOVA followed by Tukey’s test. Different letters (a and b on the histogram) represent the statistical significance at p < 0.05.
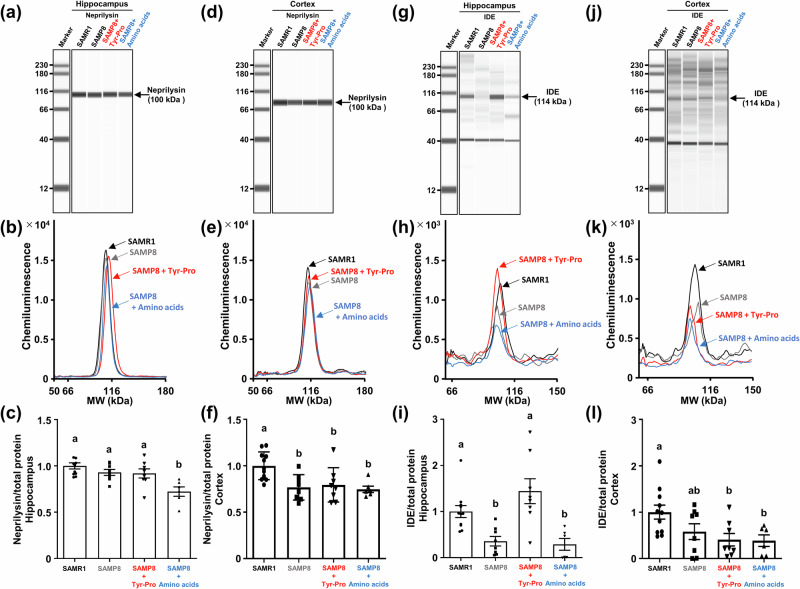
For Aß degradation, neprilysin and IDE involved in Aß hydrolysis were also evaluated using the Wes protein expression analysis. As shown in Fig. 5, the Wes analysis revealed no significant increase of neprilysin expression in the three SAMP8 groups, suggesting that Tyr-Pro did not accelerate Aß degradation through neprilysin upregulation. In contrast, 4-times higher IDE expression was observed in the hippocampus of Tyr-Pro-administered SAMP8 mice compared to that of the control and amino acid groups, which was comparable to the level in SAMR1, whereas there was no change in the cortical IDE expression among the three SAMP8 groups (Fig. 5). The results led us to hypothesize that the significantly lower Aß level in the hippocampus of SAMP8 mice fed with Tyr-Pro (Fig. 3) may have resulted from the Aß degradation facilitated by the upregulated IDE expression (Fig. 5); however, further studies are needed on the mechanism underlying IDE upregulation in the hippocampus or no IDE change in the cortex to clarify the role of Tyr-Pro role in the brain.
Fig. 5. Effect of Tyr-Pro intake on the protein expression of neprilysin and IDE in the hippocampus and cortex of SAMP8 mice.
Protein expression in each region was measured by a Wes analysis. a, d, g, j Virtual blot-like images; b, e, h, k electropherograms; c, f, i, l protein expression normalized by the electropherogram peak area of the total protein in each sample. Each region was collected from the brain of mice after the Morris water maze trial. Data are expressed as the mean ± SEM (SAMR1 group n = 11, control group n = 8, Tyr-Pro group n = 8, amino acid group n = 6). Significant differences were evaluated using a one-way ANOVA followed by Tukey’s test. Different letters (a and b on the histogram) represent the statistical significance at p < 0.05.
Discussion
The soybean dipeptide Tyr-Pro is a potential BBB transportable penetrant upon oral intake and accumulates in the hippocampus and cortex of ICR mice, facilitating improvement of impaired short- and long-term memory in Aß-induced AD in mice11,13. An explored goal of our studies is to prevent AD pathology using brain-health functional foods, as a radical treatment for AD pathology remains unavailable, which may potentially facilitate AD progression, which is possible via diet, but not using drugs in the pre-symptomatic stage. In this study, we evaluated the preventive effect of long-term Tyr-Pro intake on AD pathology in 16-week-old pre-symptomatic SAMP8 mice. After a 25-week intake of Tyr-Pro at 10 mg/kg/day in SAMP8 mice showing AD-like cognitive dysfunction, such as impaired learning and memory abilities because of aging, impaired abilities in the SAMP8 and SAMP8 fed with Tyr + Pro groups were significantly improved in the Tyr-Pro group as observed in the Morris water maze trial (Fig. 2). It has been reported that key pathological features of AD include not only the accumulation of Aß but also the hyperphosphorylation of tau proteins. Considering that an early rise in Aß levels, rather than tau hyperphosphorylation, is typically observed during the natural aging process of SAMP8 mice22, this study primarily focused on investigating Aß accumulation. Among Aß proteins, detection of the Aß monomer rather than the oligomer form is more appropriate for the evaluation of early stages of cognitive impairment or AD. Moreover, Aß42 is the most neurotoxic form, most likely to aggregate into insoluble amyloid plaques, and is closely associated with AD23. Thus, we evaluated the monomer type of Aß42 by ELISA kit as shown in the Methods part. In line with the improved abilities of SAMP8 mice fed with Tyr-Pro, Aß level in the hippocampus was also reduced (Fig. 3), which could be explained by the upregulation of IDE activity, but not marked downregulation of BACE1 and presenilin-1 enzymes, which are involved in Aß production from APP (Figs. 4–6). Moreover, although the Aß in the hippocampus of SAMP8 mice fed with Tyr + Pro showed a downward trend (no significant difference), it was concluded that SAMP8 mice fed amino acids did not show improved behavior of memory impairment based on their behavior tests and BACE1 and IDE protein tests results. These results further demonstrated that Tyr-Pro as a peptide form possesses the potential to enhance spatial learning ability in SAMP8 mice.
Fig. 6. Schematic representation of the Tyr-Pro-induced Aß signaling pathway in SAMP8 mice.
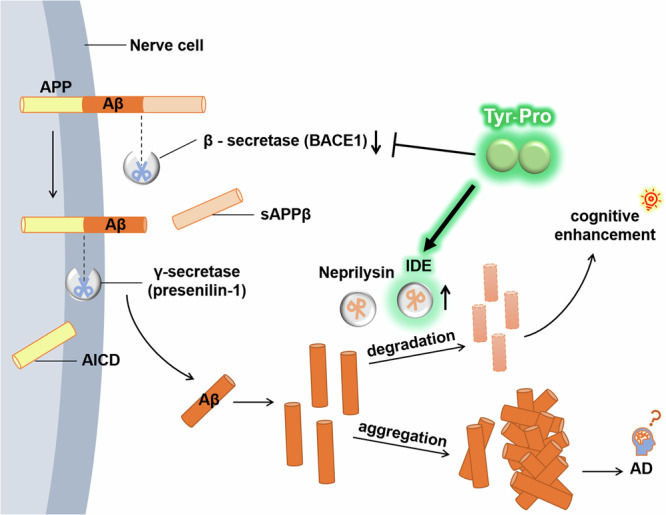
Aß amyloid beta, AD Alzheimer’s disease, AICD amyloid precursor protein intracellular domain, APP amyloid precursor protein, BACE1 ß-secretase enzyme, presenilin-1 γ-secretase enzyme, IDE insulin-degrading enzyme.
To evaluate the anti-cognitive decline effects of food compounds, studies have been conducted using SAMP8 mice, as they display progressive impairment of recognition with accelerated aging24. Using the Morris water maze test, Wang et al.25 reported the anti-cognitive decline effect of 3-n-butylphthalide after 60-day intake in 16-week-old SAMP8 mice; they showed that 3-n-butylphthalide activated the ACh nervous system by enhancing ChAT RNA gene expression. Reduction of Aß accumulation by the intake of Fuzhisan, a Chinese herbal complex prescription, through BACE1 downregulation26 and by the intake of Dendrobium nobile Lindl. alkaloids through neprilysin upregulation27 were also observed in SAMP8 mice. Additionally, fermented natto stimulated the CREB/BDNF signaling pathway after a 14-week oral administration in SAMP8 mice28. In this study, we demonstrated the anti-cognitive decline effect of Tyr-Pro in SAMP8 mice and the reduction of Aß levels mainly via activation of the Aß degradation pathway. Within the present experimental conditions in SAMP8 mice, the amyloidogenesis pathway was not predominantly involved in the reduction of Aß levels by the dipeptide (Fig. 4). Although Leu-Asn29 and Cys-Ile-Lys30 could inhibit BACE1 in cell experiments, their poor inhibitory activity (e.g., IC50 of Cys-Ile-Lys against BACE1, 34.48 μmol/L30) could be excluded from the mechanism underlying Aß reduction in the present in vivo study of Tyr-Pro intake in SAMP8 mice, as Tyr-Pro accumulation in the brain after oral administration in mice was approximately 0.34 pmol·min/mg-dry brain (at a dose of 10 mg/kg)30.
In this study, we found a marked IDE upregulation in the hippocampus of SAMP8 fed with Tyr-Pro (Fig. 5), indicating the involvement of the administered Tyr-Pro in accelerated Aß degradation or reduced Aß accumulation. A human study on IDE, a regulator of insulin levels in tissues, demonstrated that reducing IDE activity in the brain was closely related to AD pathology in 210 patients with advanced AD31. Corraliza-Gomez et al.32 found that hippocampal-dependent memory tests in mice were influenced by IDE gene dose. Shen et al.33 demonstrated that an alternative IDE action in the brain was the preferable hydrolysis of Aß peptides (Aß1-40) by cleavage of multiple sites in Aß. The current studies on IDE have allowed us to further understand the close relationship between IDE and neurodegenerative diseases, including AD and Perkinson’s34,35. In studies regarding the expression of IDE by food compounds, α-linolenic acid was reported to upregulate IDE expression in Aß25−35-infused mice36 and a normal chow diet containing 0.4% curcumin was found to stimulate IDE expression in C57BL/6 mice37. According to a report by Zhao et al.38, IDE expression may be modulated by the AMPK-related phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling pathway. It should be noted that we clarified Tyr-Pro exhibited AdipoR1 agonistic effects by in vitro L6 cells and in silico molecular dynamics simulation experiments39. Moreover, Tyr-Pro also exerted AdipoR1-induced AMPK activation to stimulate acetylcholine secretion in nerve NE-4C cells40. Thus, Tyr-Pro-induced IDE expression in SAMP8 mice might be mediated by the activation of AMPK-PI3K/PKB signaling pathway. However, the reasons for enhanced IDE expression only in the hippocampus and no change in neprilysin expression observed in the present Tyr-Pro-SAMP8 experiments needed to be resolved by further experiments in NE-4C cells and RNA-seq analysis of the SAMP8 brain.
In conclusion, the present study provides the first finding that long-term intake of the soybean dipeptide Tyr-Pro (10 mg/kg/day) for 25 weeks was effective at improving impaired spatial learning and memory in 41-week-old SAMP8 mice. The reduced Aß levels and upregulation of IDE expression by Tyr-Pro intake are an appropriate AD preventive feature prior to AD symptoms; this dipeptide can thus be considered as an efficient anti-cognitive decline functional food that can be administered through a diet.
Materials and methods
Chemicals and Reagents
Tyr (Lot: M2A5719) and Pro (Lot: M1R4497) were purchased from Nacalai Tesque Co. (Kyoto, Japan). Tyr-Pro (Lot: SQ18973) was purchased from Scrum, Inc., Japan (Tokyo, Japan). Distilled water, methanol (MeOH), acetonitrile (ACN), and formic acid (FA) were of MS grade (Merck, Darmstadt, Germany). All other reagents were of analytical grade and were used without further purification.
Animal Experiments
Sixteen-week-old male SAMP8 and SAMR1 mice (Japan SLC Inc., Shizuoka, Japan) were used. The SAMP8 mice were randomly divided into control, Tyr-Pro, and amino acids groups to show similar average body weight. All mice were housed separately and maintained in a controlled environment with a temperature of 23 ± 2 °C, humidity ranging from 60 ± 10%, and a light/dark cycle of 12 h each (lights on at 7:00 a.m.). Mice were provided with distilled water ad libitum during the experimental protocol. Experimental diets (Table 1) were composed of AIN-93M (Oriental Yeast Co., Tokyo, Japan) for control group, or AIN-93M containing either 0.1 g/kg Tyr-Pro (Tyr-Pro group), or 0.05 g/kg Tyr + 0.05 g/kg Pro (amino acids group). Body weight and food intake were measured weekly during a 25-week protocol period. At 41 weeks of age, all the mice were subjected to a Morris water maze trial for five consecutive days. After the day-5 trial, all mice were fasted for 24 h and were anesthetized with sevoflurane (Lot: WTQ1642, Wako Pure Chemicals, Tokyo, Japan); a brain sample was collected, immediately frozen with liquid N2, and stored at -30 °C. All methods were reported in accordance with ARRIVE guidelines. All procedures involving animal care and use were performed in accordance with the regulations stipulated by the Experimental Animal Care and Use Committee of Fukuoka University (#1913104, #2112098).
Table 1.
Composition of the experimental diets (g/kg)
| Ingredient | Control diet | Tyr-Pro diet | Amino acid diet |
|---|---|---|---|
| Casein | 140.0 | 140.0 | 140.0 |
| Tyr-Pro | – | 0.01 | – |
| Tyr + Pro | – | – | 0.01 |
| l-Cystine | 1.8 | 1.8 | 1.8 |
| Corn starch | 465.6 | 465.6 | 465.6 |
| Alpha cornstarch | 155.0 | 155.0 | 155.0 |
| Sucrose | 100.0 | 100.0 | 100.0 |
| Soybean oil | 40.0 | 40.0 | 40.0 |
| Cellulose powder | 50.0 | 50.0 | 50.0 |
| AIN-93M mineral | 35.0 | 35.0 | 35.0 |
| AIN-93 vitamins | 10.0 | 10.0 | 10.0 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Butylhydroquinone | 0.008 | 0.008 | 0.008 |
The Morris Water Maze Test
The Morris water maze test41 to assess spatial learning and memory activities was performed in a trial conducted for five consecutive days for 41-week-old mice according to a previously published protocol42 with some modifications. The swimming pool (Neuroscience, Tokyo, Japan), 150 cm in diameter and 45 cm in depth was a circular water tank. The pool was filled with water at 23 ± 2 °C, and a 12-cm diameter platform was placed inside the pool. The top surface was 1 cm below the surface, and the position of the platform remained unchanged during the test. The pool was evenly divided into four quadrants. External cues are attached to the walls around the pool, which were visible from within the pool and could be used by the mice for spatial location. Each mouse underwent three trials daily for five consecutive days. In a trial, each mouse was placed at one of the three starting positions in different quadrants that did not contain the platform and was given 120 s to locate the platform. Once the mouse found the platform, it was forced to remain there for 30 s. The results are expressed as the average escape latency of three trials per day.
Measurement of Amyloid ß and Protein Expression in the Brain of SAMP8 Mice
A quantitative assay of Aß levels in the hippocampus and cortex of SAMP8 mice was performed using an Aß ELISA kit (Human/Rat ß Amyloid(42) ELISA Kit Wako, High Sensitive; Cat No 292-64501; FUJIFILM Wako Co.; Osaka, Japan). Briefly, the hippocampus and cortex were collected from the brains of SAMP8 mice after the Morris water maze trial. The hippocampal and cerebral cortical brain tissues were homogenized in 1.0 mL and 1.5 mL, respectively, of Tris buffer containing 150 mM NaCl, 100 mM Tris (pH 7.4), 1 mM EGTA, 1 mM EDTA, 1.0% TrironX-100, and 0.5% sodium deoxycholate, supplemented with 1 mM phenylmethylsulfonyl fluoride and 1% protease inhibitor cocktail (Nacalai Tesque Co.). Homogenization was performed using a Polytron homogenizer (KINEMATICA AG, Luzern Switzerland) at a speed of 20,000 rpm for 90 s × 2 at a temperature of 4 °C. Following centrifugation of the homogenate at a speed of 14,000× g for 30 min at 4 °C, an aliquot of the supernatant was used for the Aß assay and protein expression assay. According to the manufacturer’s instructions, the amount of Aß in each region was determined at 450 nm (FlexStation 3, Molecular Devices LLC., San Jose, CA, USA).
Protein levels in the hippocampal and cortical regions of the SAMP8 brain were measured using a Wes instrument based on capillary electrophoresis immunoassay (ProteinSimple Co., San Jose, CA, USA). The supernatants obtained from the cortex and hippocampus were diluted with 0.1 × sample buffer (ProteinSimple Co.) and 5 × fluorescent master mix denaturing buffer, followed by denaturation at 95 °C for 5 min. The supernatant for the BACE1 protein assay was set at 0.5 mg/mL and that for presenilin-1, neprilysin, and IDE was set at 1 mg/mL. The Wes measurement was conducted using a separation module (12–230 kDa) with an 8 × 25 mm capillary cartridge. The biotinylated ladder and primary antibodies were dispensed in a microplate and subjected to automated capillary electrophoresis using a Wes system. An automated immune detection process was performed using a horseradish peroxidase-conjugated secondary antibody and a chemiluminescent substrate. A pentafluorophenyl ester-biotin labeling reagent capable of attaching to all applied proteins was used for total protein detection. At the end of the run, ProteinSimple Compass software displayed the chemiluminescent signal as both a virtual blot-like image and an electropherogram based on molecular weight. Normalized protein expression was determined by analyzing the electropherogram peak area of the total protein in each lane.
The primary antibodies used were as follows: BACE1 (BACE1 [D10E5], 5606 T, Lot: 6, 1:50 dilution; Cell Signaling Technology, Massachusetts, USA), presenilin-1 (Presenilin 1 [D-10], sc-365450, Lot: I1622, 1:50 dilution; Santa Cruz Biotechnology, Texas, USA), neprilysin (Mouse Neprilysin/CD10 Antibody, AF1126-SP, Lot: JG10227051, 1:250 dilution; R&D systems, Minnesota, USA), and IDE (IDE [F-9], sc-393887, Lot: E2621, 1:50 dilution; Santa Cruz Biotechnology).
Statistical Analysis
Data are presented as the mean ± SEM. Statistical analysis in this present study was performed using GraphPad Prism (ver. 9.4.0, GraphPad; La Jolla, CA, USA). Data obtained from regular monitoring of body weight and food intake were analyzed using two-way repeated measures analysis of variance (ANOVA) to understand if there is an interaction between the two independent variables (i.e. group and age) on the dependent variable (weight or food intake). The statistical significance of differences among groups was determined using a one-way ANOVA, followed by Tukey’s post hoc analyses. Regarding escape latency in a Morris water maze trial, the statistical significances were determined by Kruskal-Wallis test, followed by Dunn’s test. p < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
This study was funded by JSPS KAKENHI [Grant No. JP21H04863 (T.M.) and JP21F21386 (T.M. and L.C.)] and JST SPRING [Grant No. JPMJSP2136 (X.L.)].
Author contributions
X.L., Y.I., and T.W. contributed equally to this study. T.W. and K.I. performed all the animal experiments. X.L., Y.I., T.W., A.Y., L.C., and Y.N. assayed Aβ-related parameters in the brains. X.L., Y.I., T.W., A.Y., L.C., Y.N., F.T., S.D., K.I., M.T., and T.M. analyzed and discussed the results and designed the experimental strategy. X.L. wrote the manuscript. T.W., F.T., S.D., K.I., M.T., and T.M. designed the study. M.T. and T.M. revised the manuscript and supervised the study. All authors have read and approved the final manuscript for publication.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xixi Li, Yuka Ichiba, Takuya Watanabe.
Contributor Information
Mitsuru Tanaka, Email: mitsurut@agr.kyushu-u.ac.jp.
Toshiro Matsui, Email: tmatsui@agr.kyushu-u.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41538-024-00360-0.
References
- 1.Knopman, D. S. et al. Alzheimer disease. Nat. Rev. Dis. Prim.7, 33 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeTure, M. A. & Dickson, D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener.14, 32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien, R. J. & Wong, P. C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci.34, 185–204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agostinho, P., Cunha, R. A. & Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of alzheimers disease. Curr. Pharm. Des.16, 2766–2778 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Brockmann, R., Nixon, J., Love, B. L. & Yunusa, I. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer’s disease: patient outcomes, healthcare costs, and drug development. Lancet Reg. Health Am.20, 100467 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haskell-Ramsay, C. F., Schmitt, J. & Actis-Goretta, L. The impact of epicatechin on human cognition: The role of cerebral blood flow. Nutrients10, 986 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maccioni, R. B., Calfío, C., González, A. & Lüttges, V. Novel nutraceutical compounds in Alzheimer prevention. Biomolecules12, 249 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baig, M. H., Ahmad, K., Rabbani, G. & Choi, I. Use of peptides for the management of Alzheimer’s disease: Diagnosis and inhibition. Front. Aging Neurosci.10, 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maebuchi, M., Kishi, Y., Koikeda, T. & Furuya, S. Soy peptide dietary supplementation increases serum dopamine level and improves cognitive dysfunction in subjects with mild cognitive impairment. Jpn. Pharmacol. Ther.41, 67–73 (2013). [Google Scholar]
- 10.Yuda, N. et al. Effect of the Casein-Derived Peptide Met-Lys-Pro on Cognitive Function in Community-Dwelling Adults Without Dementia: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Interv. Aging15, 743–754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka, M. et al. Brain-transportable dipeptides across the blood-brain barrier in mice. Sci. Rep.9, 5769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng, L. et al. A memory-improving dipeptide, Tyr-Pro, can reach the mouse brain after oral administration. Sci. Rep.13, 16908 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka, M. et al. Brain-transportable soy dipeptide, Tyr-Pro, attenuates amyloid β peptide25-35-induced memory impairment in mice. npj Sci. Food4, 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso, A. C., Grundke-Iqbal, I. & Iqbal, K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med.2, 783–787 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Iqbal, K., Liu, F. & Gong, C.-X. Tau and neurodegenerative disease: the story so far. Nat. Rev. Neurol.12, 15–27 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Del Valle, J. et al. Early amyloid accumulation in the hippocampus of SAMP8 mice. J. Alzheimers Dis.19, 1303–1315 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Pačesová, A. et al. Age-related metabolic and neurodegenerative changes in SAMP8 mice. Aging (Albany NY)14, 7300–7327 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gómez-Oliva, R. et al. Rescue of neurogenesis and age-associated cognitive decline in SAMP8 mouse: role of transforming growth factor alpha. Aging Cell.22, e13829 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canudas, A. M. et al. Hyperphosphorylation of microtubule-associated protein tau in senescence-accelerated mouse (SAM). Mech. Ageing Dev.126, 1300–1304 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Vassar, R. & Citron, M. Abeta-generating enzymes: recent advances in beta- and gamma-secretase research. Neuron27, 419–422 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Chen, G.-F. et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin.38, 1205–1235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiguchi, I. et al. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology37, 293–305 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Song, C., Zhang, T. & Zhang, Y. Conformational essentials responsible for neurotoxicity of Aβ42 aggregates revealed by antibodies against oligomeric Aβ42. Molecules27, 6751 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butterfield, D. A. & Poon, H. F. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol.40, 774–783 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Wang, F., Chen, H., Sun, X. J. & Ke, Z. J. Improvement of cognitive deficits in SAMP8 mice by 3-n-butylphthalide. Neurol. Res.36, 224–233 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Z.-X., Zhao, R.-P., Wang, D.-S. & Li, Y.-B. Fuzhisan ameliorates the memory deficits in aged SAMP8 mice via decreasing Aβ production and tau hyperphosphorylation of the hippocampus. Neurochem. Res.41, 3074–3082 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Lv, L.-L. et al. Dendrobium nobile Lindl. Alkaloids ameliorate cognitive dysfunction in senescence accelerated SAMP8 mice by decreasing amyloid-β aggregation and enhancing autophagy activity. J. Alzheimers Dis.76, 657–669 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Zheng, Y. et al. Fermented soybean foods (natto) ameliorate age-related cognitive decline by hippocampal TAAR1-mediated activation of the CaMKII/CREB/BDNF signaling pathway in senescence-accelerated mouse prone 8 (SAMP8). Food Funct.14, 10097–10106 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Lee, J. K. et al. Neuroprotective Effect of β-secretase Inhibitory Peptide from Pacific Hake (Merluccius productus) Fish Protein Hydrolysate. Curr. Alzheimer Res.16, 1028–1038 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Yu, Z. et al. Identification of ovalbumin-derived peptides as multi-target inhibitors of AChE, BChE, and BACE1. J. Sci. Food Agric.100, 2648–2655 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Bian, L. et al. Insulin-degrading enzyme and Alzheimer disease: a genetic association study in the Han Chinese. Neurology63, 241–245 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Corraliza-Gomez, M. et al. Insulin-degrading enzyme (IDE) as a modulator of microglial phenotypes in the context of Alzheimer’s disease and brain aging. J. Neuroinflam.20, 233 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen, Y., Joachimiak, A., Rosner, M. R. & Tang, W.-J. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature443, 870–874 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sousa, L., Guarda, M., Meneses, M. J., Macedo, M. P. & Vicente Miranda, H. Insulin-degrading enzyme: an ally against metabolic and neurodegenerative diseases. J. Pathol.255, 346–361 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Takamatsu, Y. et al. Combined immunotherapy with “anti-insulin resistance” therapy as a novel therapeutic strategy against neurodegenerative diseases. npj Parkinsons Dis.3, 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, A. Y., Lee, M. H., Lee, S. & Cho, E. J. Alpha-Linolenic Acid from Perilla frutescens var. japonica Oil Protects Aβ-Induced Cognitive Impairment through Regulation of APP Processing and Aβ Degradation. J. Agric. Food Chem.65, 10719–10729 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Lee, S.-J. et al. Dietary curcumin restores insulin homeostasis in diet-induced obese aged mice. Aging (Albany NY)14, 225–239 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao, L. et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease intervention. J. Neurosci.24, 11120–11126 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, Y. et al. In vitro and in silico characterization of adiponectin-receptor agonist dipeptides. npj Sci. Food5, 29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng, L. et al. Adiponectin-receptor agonistic dipeptide Tyr-Pro stimulates the acetylcholine nervous system in NE-4C cells. J. Agric. Food Chem.72, 7121–7129 (2024). [DOI] [PubMed] [Google Scholar]
- 41.Morris, R. G. M. Spatial localization does not require the presence of local cues. Learn. Motiv.12, 239–260 (1981). [Google Scholar]
- 42.Kubota, K. et al. The traditional Japanese herbal medicine hachimijiogan elicits neurite outgrowth effects in PC12 cells and improves cognitive in AD model rats via phosphorylation of CREB. Front. Pharmacol.8, 850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.