Abstract
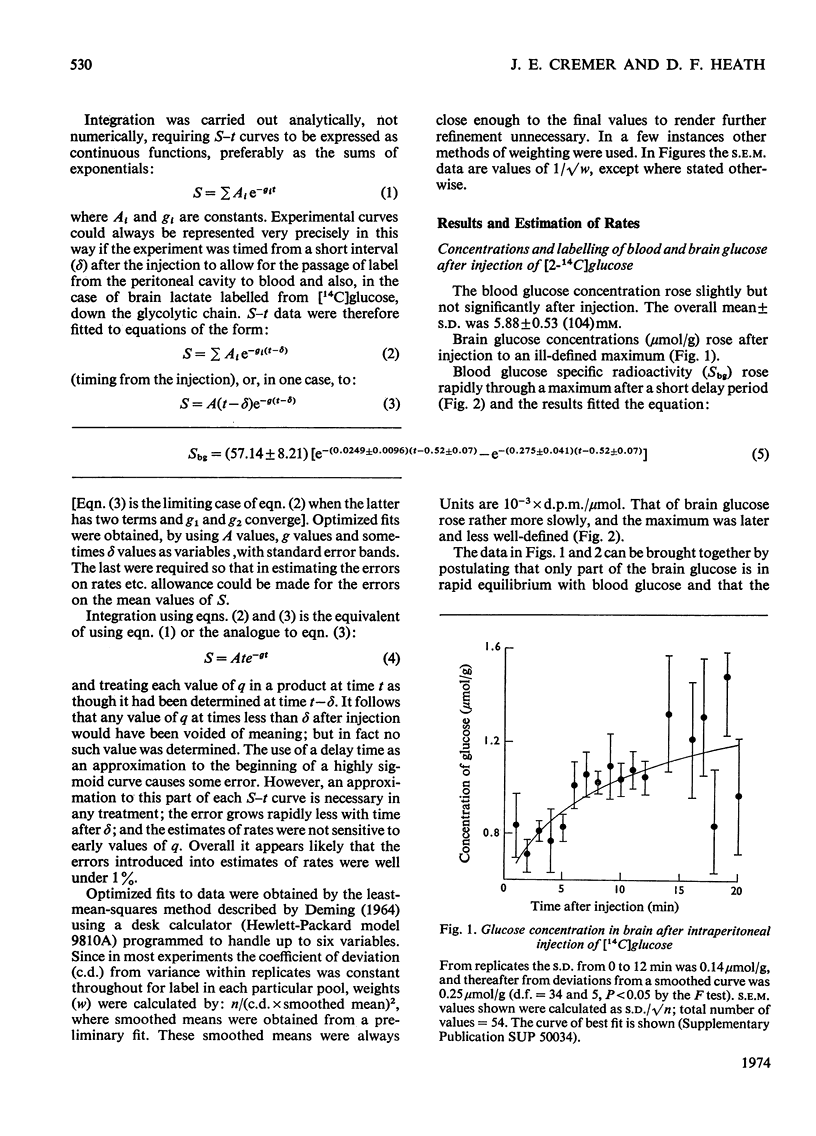
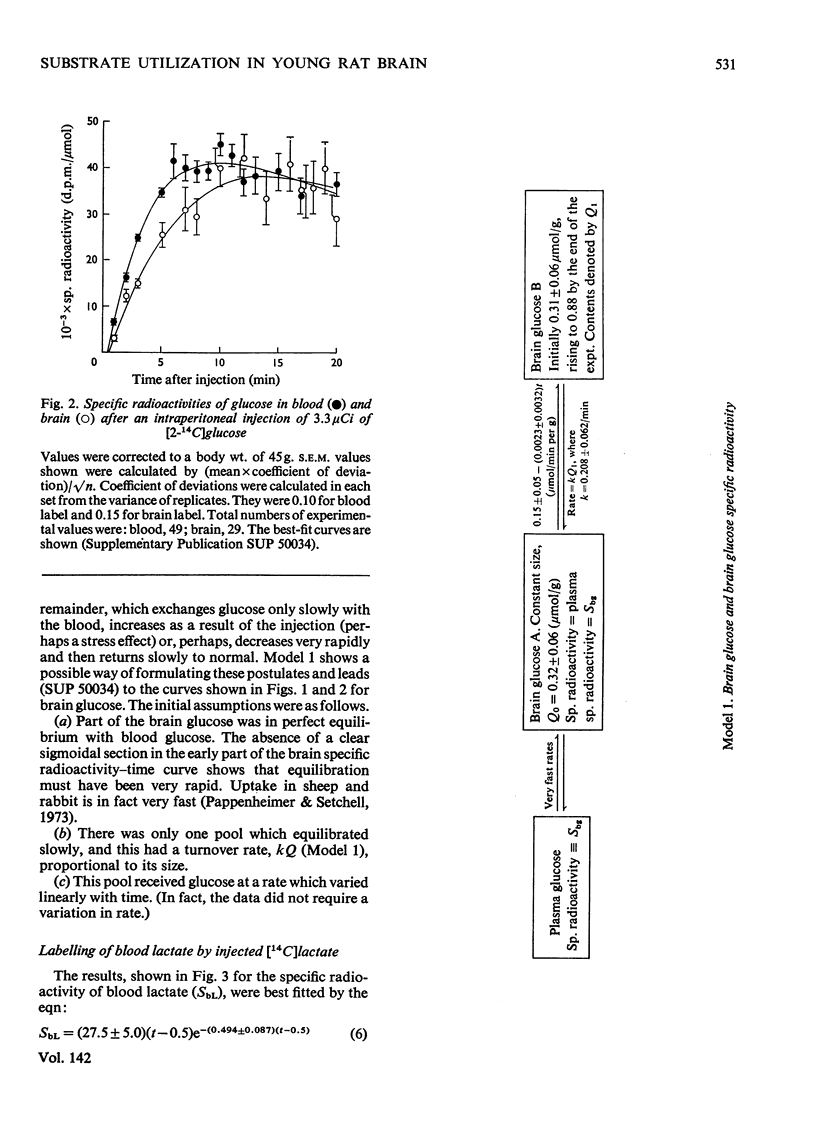
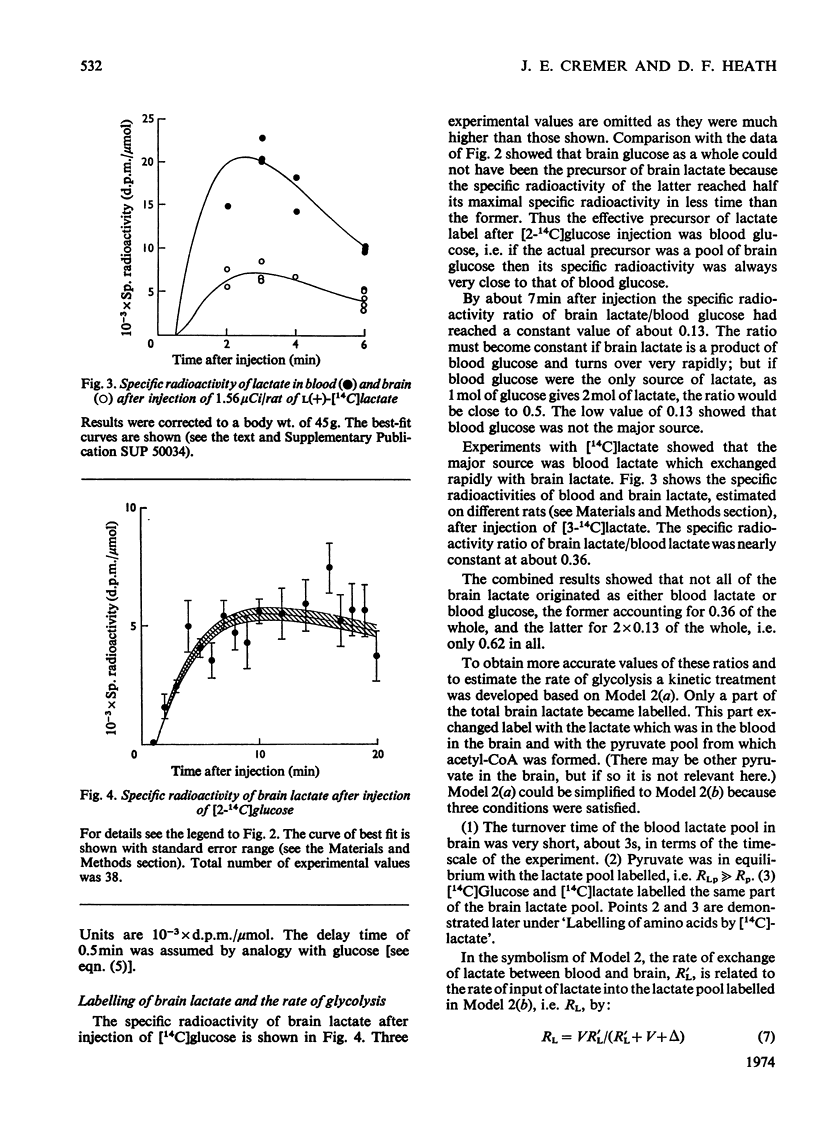
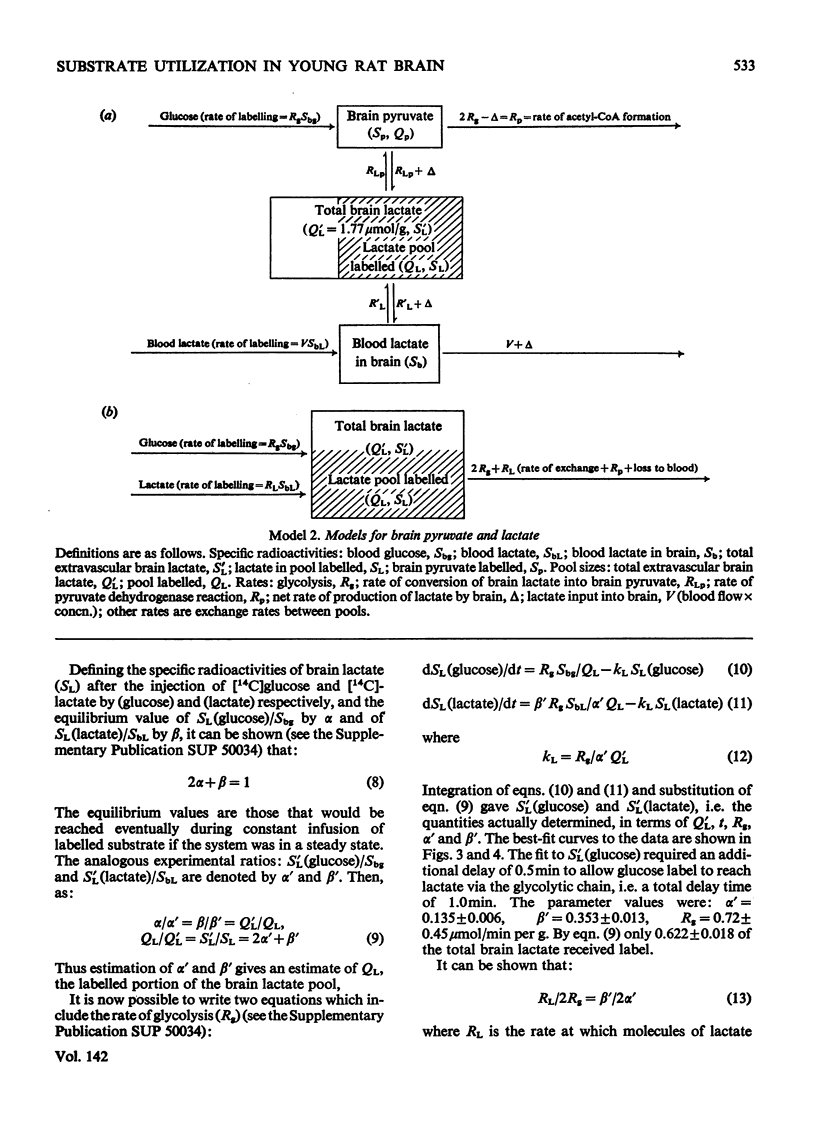
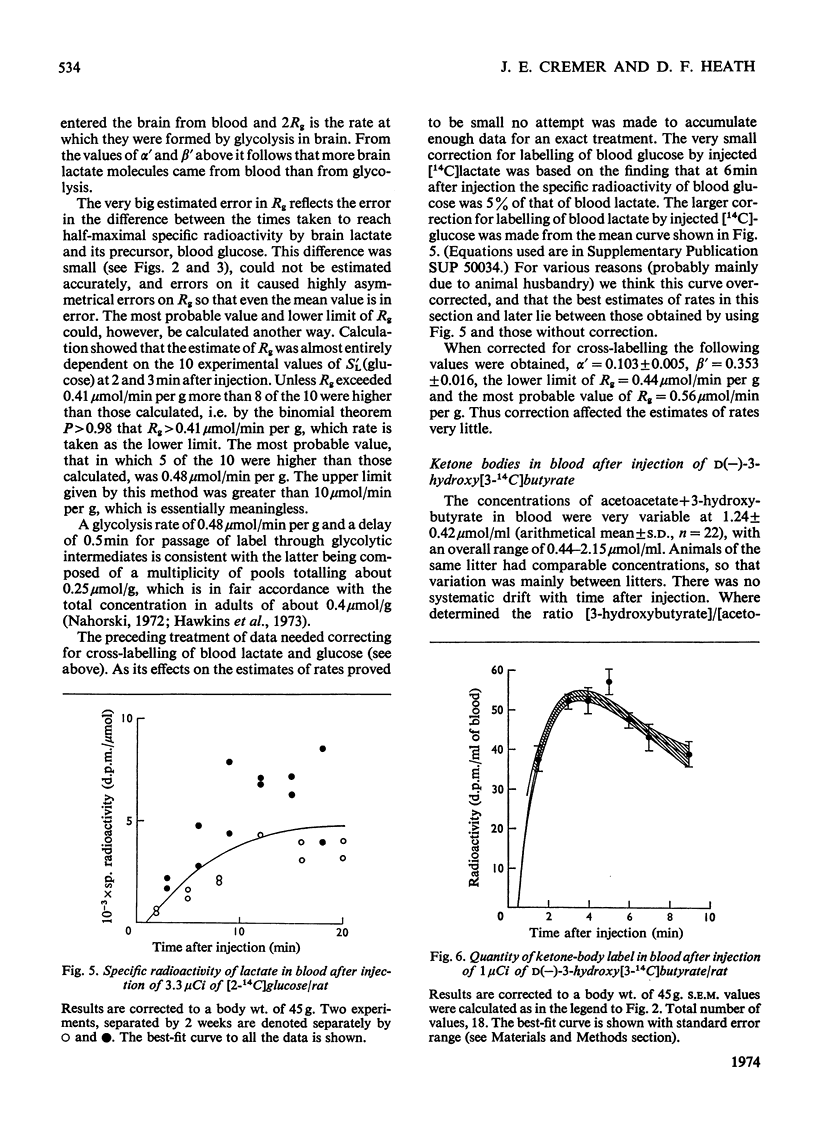
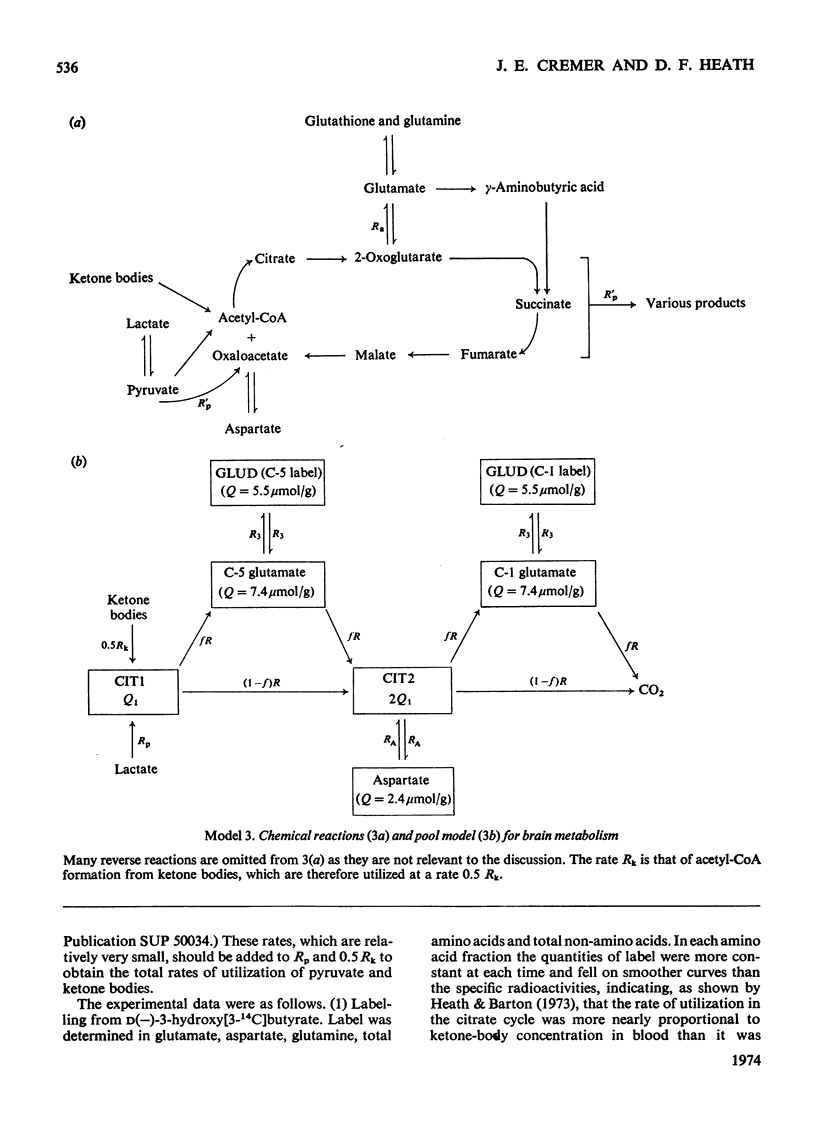
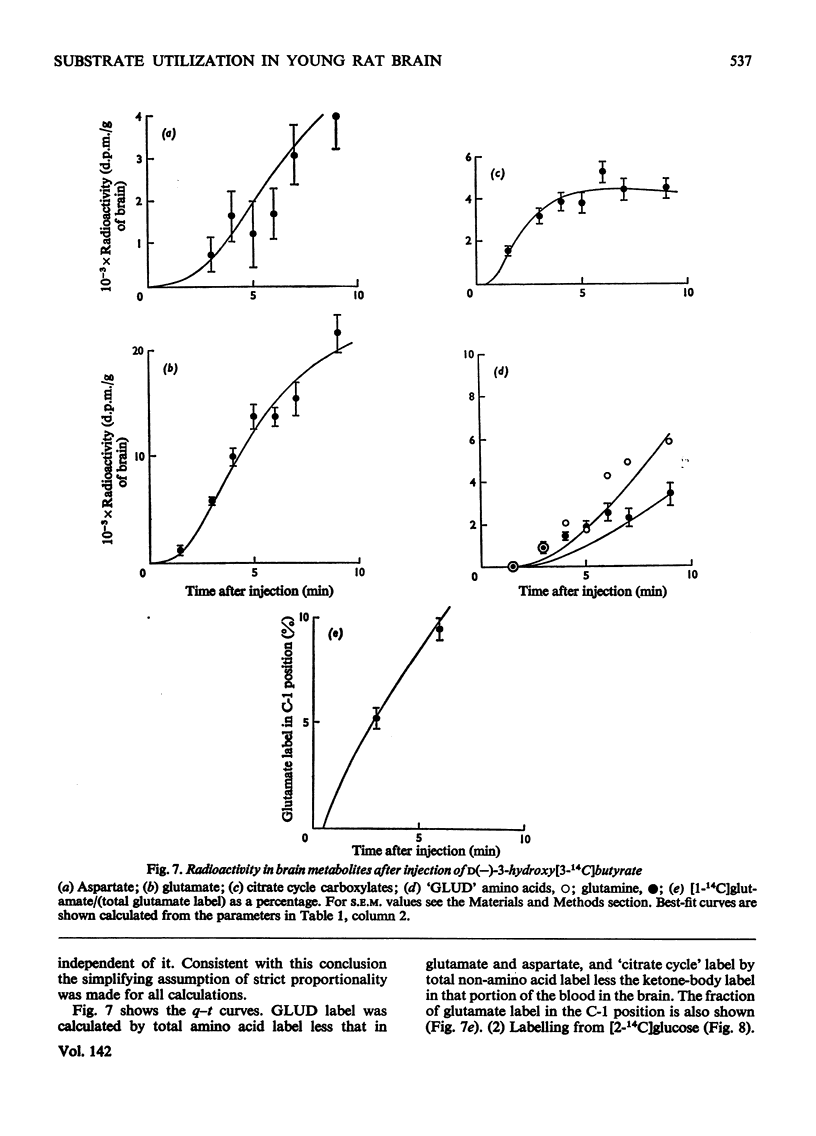
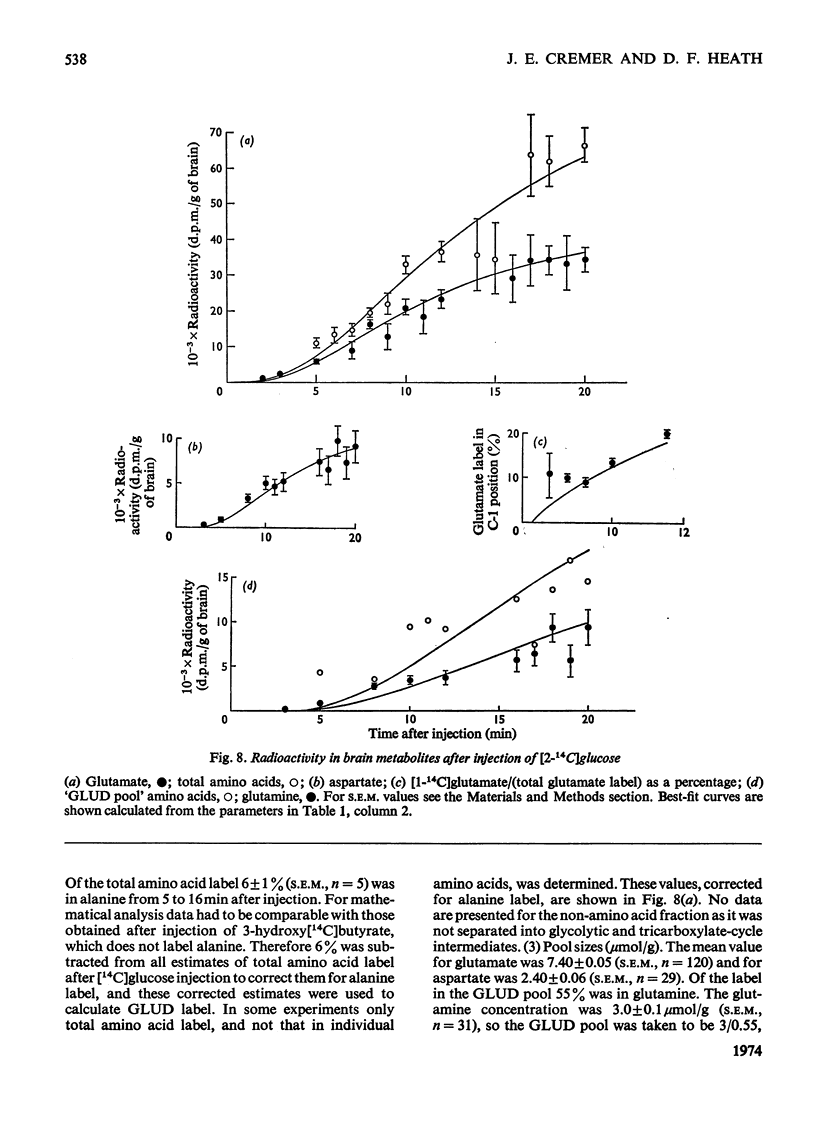
The brains of 18-day-old rats utilize glucose and ketone bodies. The rates of acetyl-CoA formation from these substrates and of glycolysis were determined in vivo from the labelling of intermediary metabolites after intraperitoneal injection of d-[2-14C]glucose, l(+)-[3-14C]- and l(+)-[U-14C]-lactate and d(−)-3-hydroxy[14C]butyrate. Compartmental analysis was used in calculating rates to allow for the rapid exchange of blood and brain lactate, the presence in brain of at least two pools each of glucose and lactate, and the incomplete equilibration of oxaloacetate with aspartate and of 2-oxoglutarate with glutamate. Results were as follows. 1. Glucose and ketone bodies labelled identical pools of tricarboxylate-cycle metabolites, and were in every way alternative substrates. 2. The combined rate of oxidation of acetyl-CoA derived from pyruvate (and hence glucose) and ketone bodies was 1.05μmol/min per g. 3. Ketone bodies contributed 0.11–0.53μmol/min per g in proportion to their concentration in blood, with a mean rate of 0.30μmol/min per g at 1.24mm. 4. Pyruvate and ketone bodies were converted into lipid at 0.018 and 0.008μmol/min per g respectively. 5. Glycolysis, at 0.48μmol/min per g, was more rapid in most rats than pyruvate utilization by oxidation and lipid synthesis, resulting in a net output of lactate from brain to blood. 6. Rates of formation of brain glutamate, glutamine and aspartate were also measured. Further information on the derivation of the models has been deposited as Supplementary Publication SUP 50034 (18 pages) at the British Library, Lending Division (formerly the National Lending Library for Science and Technology), Boston Spa, Yorks. LS23 7QB, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1973) 131, 5.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton R. N. The interconversion and disposal of ketone bodies in untreated and injured post-absorptive rats. Biochem J. 1973 Nov;136(3):531–543. doi: 10.1042/bj1360531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R. N. The interconversion and disposal of ketone bodies in untreated and injured post-absorptive rats. Biochem J. 1973 Nov;136(3):531–543. doi: 10.1042/bj1360531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer S. M., McMurray W. C. The metabolism of amino acids in developing rat brain. J Neurochem. 1967 Jul;14(7):695–706. doi: 10.1111/j.1471-4159.1967.tb10303.x. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Gilboe D. D., Drewes L. R. Effects of anoxia on net uptake and unidirectional transport of glucose into the isolated dog brain. Brain Res. 1974 Feb 22;67(2):307–316. doi: 10.1016/0006-8993(74)90280-7. [DOI] [PubMed] [Google Scholar]
- CREMER J. E. AMINO ACID METABOLISM IN RAT BRAIN STUDIED WITH 14C-LABELLED GLUCOSE. J Neurochem. 1964 Mar;11:165–185. doi: 10.1111/j.1471-4159.1964.tb06127.x. [DOI] [PubMed] [Google Scholar]
- Cremer J. E. Incorporation of label from D- -hydroxy( 14 C)butyrate and (3- 14 C)acetoacetate into amino acids in rat brain in vivo. Biochem J. 1971 Apr;122(2):135–138. doi: 10.1042/bj1220135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer J. E. Selective inhibition of glucose oxidation by triethyltin in rat brain in vivo. Biochem J. 1970 Aug;119(1):95–102. doi: 10.1042/bj1190095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer J. E., Teal H. M. The activity of pyruvate dehydrogenase in rat brain during postnatal development. FEBS Lett. 1974 Feb 1;39(1):17–20. doi: 10.1016/0014-5793(74)80006-2. [DOI] [PubMed] [Google Scholar]
- DYMSZA H. A., CZAJKA D. M., MILLER S. A. INFLUENCE OF ARTIFICIAL DIET ON WEIGHT GAIN AND BODY COMPOSITION OF THE NEONATAL RAT. J Nutr. 1964 Oct;84:100–106. doi: 10.1093/jn/84.2.100. [DOI] [PubMed] [Google Scholar]
- Drahota Z., Hahn P., Kleinzeller A., Kostolánská A. Acetoacetate formation by liver slices from adult and infant rats. Biochem J. 1964 Oct;93(1):61–65. doi: 10.1042/bj0930061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzubow L. M., Garfinkel D. A simulation study of brain compartments. II. Atom-by-atom simulation of the metabolism of specifically labeled glucose and acetate. Brain Res. 1970 Oct 28;23(3):407–417. doi: 10.1016/0006-8993(70)90066-1. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Freminet A., Bursaux E., Poyart C. F. An improved micromethod for the determination of L-lactate 14 C in blood. Biochem Med. 1972 Feb;6(1):72–76. doi: 10.1016/0006-2944(72)90062-2. [DOI] [PubMed] [Google Scholar]
- GAITONDE M. K. RATE OF UTILIZATION OF GLUCOSE AND 'COMPARTMENTATION' OF ALPHA-OXOGLUTARATE AND GLUTAMATE IN RAT BRAIN. Biochem J. 1965 Jun;95:803–810. doi: 10.1042/bj0950803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman H., Sapirstein L. A. Brain blood flow in the conscious and anesthetized rat. Am J Physiol. 1973 Jan;224(1):122–126. doi: 10.1152/ajplegacy.1973.224.1.122. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Miller A. L., Nielsen R. C., Veech R. L. The acute action of ammonia on rat brain metabolism in vivo. Biochem J. 1973 Aug;134(4):1001–1008. doi: 10.1042/bj1341001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. F., Barton R. N. The design of experiments using isotopes for the determination of the rates of disposal of blood-borne substrates in vivo with special reference to glucose, ketone bodies, free fatty acids and proteins. Biochem J. 1973 Nov;136(3):503–518. doi: 10.1042/bj1360503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. F., Phillips J. C. Disequilibrium in the malate dehydrogenase reaction in rat liver mitochondria in vivo. Biochem J. 1972 Apr;127(3):453–470. doi: 10.1042/bj1270453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. F., Rose J. G. The distribution of glucose and [14C]glucose between erythrocytes and plasma in the rat. Biochem J. 1969 Apr;112(3):373–377. doi: 10.1042/bj1120373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEPPE R. E., HAHN C. H. Concerning pyruvate metabolism in rat brain. J Biol Chem. 1962 Apr;237:1026–1028. [PubMed] [Google Scholar]
- Kanazawa I., Ueta N., Yamakawa T. The incorporation of labelled acetate into cerebroside and other lipids of the developing mouse brain. J Neurochem. 1972 Jun;19(6):1483–1494. doi: 10.1111/j.1471-4159.1972.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Lund P. Control of glutamine synthesis in rat liver. Biochem J. 1971 Sep;124(3):653–660. doi: 10.1042/bj1240653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel H. M., Hammes G. G. Purification and characterization of a lecithin-D(-)- -hydroxybutyrate dehydrogenase complex. J Biol Chem. 1973 Jul 25;248(14):4885–4889. [PubMed] [Google Scholar]
- Middleton B. The acetoacetyl-coenzyme A thiolases of rat brain and their relative activities during postnatal development. Biochem J. 1973 Apr;132(4):731–737. doi: 10.1042/bj1320731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. J., Lione A. P., Regen D. M., Tarpley H. L., Raines P. L. Brain glucose metabolism in the newborn rat. Am J Physiol. 1971 Dec;221(6):1746–1753. doi: 10.1152/ajplegacy.1971.221.6.1746. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R. Biochemical effects of the anticonvulsants trimethadione, ethosuximide and chlordiazepoxide in rat brain. J Neurochem. 1972 Aug;19(8):1937–1946. doi: 10.1111/j.1471-4159.1972.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer J. R., Setchell B. P. Cerebral glucose transport and oxygen consumption in sheep and rabbits. J Physiol. 1973 Sep;233(3):529–551. doi: 10.1113/jphysiol.1973.sp010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. J., Balázs R. Manifestation of metabolic compartmentation during the maturation of the rat brain. J Neurochem. 1970 Jul;17(7):955–971. doi: 10.1111/j.1471-4159.1970.tb02249.x. [DOI] [PubMed] [Google Scholar]
- Tarkowski S., Cremer J. E. Metabolism of glucose and free amino acids in brain, studies with 14C-labelled glucose and butyrate in rats intoxicated with carbon disulphide. J Neurochem. 1972 Nov;19(11):2631–2640. doi: 10.1111/j.1471-4159.1972.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Tildon J. T., Sevdalian D. A. CoA transferase in the brain and other mammalian tissues. Arch Biochem Biophys. 1972 Feb;148(2):382–390. doi: 10.1016/0003-9861(72)90155-5. [DOI] [PubMed] [Google Scholar]
- van den Berg C. J., Garfinkel D. A simulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. Biochem J. 1971 Jun;123(2):211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]


