Abstract
Background
Allergic bronchopulmonary aspergillosis (ABPA) is a disease resulting from an overactive type 2 response to Aspergillus. Initial studies suggest that asthma biologics can effectively treat ABPA, but it is unclear which biologic class is superior.
Objective
We sought to compare the effectiveness of asthma biologics in the treatment of ABPA.
Methods
We performed a retrospective analysis of patients with ABPA treated with asthma biologics, and measured outcomes of respiratory exacerbations, daily oral corticosteroids, and antifungals. We assessed these variables while individuals were treated with 1 of 3 biologic classes: anti-IgE, anti–IL-5/IL-5 receptor alpha (IL-5Ra), anti–IL-4 receptor alpha (IL-4Ra).
Results
A total of 21 patients were included in our analysis. Anti–IL-4Ra was associated with a significantly lower number of exacerbations and oral corticosteroid use compared with anti-IgE or anti–IL-5/IL-5Ra therapies. Anti–IL-4Ra also had significantly lower antifungal use than anti-IgE, and there was a trend toward lower antifungal use when compared with anti–IL-5/IL-5Ra. In a subgroup of 10 patients treated with 2 or more biologics sequentially, we found that 8 of them achieved clinical control on anti–IL-4Ra therapy after failing anti-IgE and/or anti–IL-5/IL-5Ra therapies.
Conclusions
Dupilumab blocks the IL-4Ra, resulting in the downstream inhibition of both IL-4 and IL-13 effector pathways. Dupilumab may benefit patients with ABPA by inhibiting the generation of airway mucus (IL-13), and by reducing local B-cell differentiation into IgE antibody–secreting cells (IL-4). On the basis of our findings and with the known molecular mechanisms of dupilumab, we believe that anti–IL-4Rα–targeted therapy may be more effective than anti-IgE or anti–IL-5/IL-5Rα therapies to treat ABPA.
Key words: ABPA, allergic bronchopulmonary aspergillosis, asthma biologics, dupilumab, severe asthma
Allergic bronchopulmonary aspergillosis (ABPA) is a disease resulting from airway colonization with Aspergillus species that induces an overactive immune response involving cellular and humoral type-2 (T2) inflammation promoting secretion of IL-4, IL-5, IL-10, and IL-13.1 ABPA most frequently presents in a subset of patients with severe asthma and/or cystic fibrosis (CF).2 Moreover, ABPA and asthma share some clinical features and underlying pathological mechanisms.3
Oral corticosteroids (OCSs) and antifungal therapy are the mainstay of ABPA treatment.4 However, treatment response is variable, and some patients continue to have uncontrolled symptoms despite treatment. Well-known risks associated with long-term systemic corticosteroid therapy include osteoporosis, cataracts, weight gain, and diabetes.5,6 Patients with ABPA often receive antifungal medications, which carry the risk of inducing a selection pressure on fungal species that can result in the evolution of resistant strains. In addition, antifungals have side effects and interact with many other medications.4 For these reasons, it is imperative to find alternative treatment options that prevent respiratory exacerbations in patients with ABPA and reduce prolonged and repeated OCS and antifungal use.
Biologic medications targeting the T2 inflammation pathway can improve symptoms, preserve lung function, reduce exacerbations, and decrease OCS dependence in patients with severe asthma.7,8 T2 inflammation is a shared pathway for many patients with severe asthma9 and ABPA.1 Because many patients with ABPA also have severe asthma with T2 biomarkers such as elevated blood eosinophils or IgE, they frequently qualify for biologic therapies based on their asthma.10 The long-term effectiveness of biologic therapies for ABPA is unknown. However, the use of these medications may be considered for patients with ABPA with recurrent exacerbations. It is still unclear whether sustained asthma control with biologic therapies will modify ABPA progression; however, small studies suggest that there may be a clinical benefit.
Because IgE is involved in the pathogenesis of ABPA, some studies have examined the effect of omalizumab (an anti-IgE mAb) in the treatment of this disease. A small randomized controlled trial of 13 participants showed that patients with ABPA on omalizumab had fewer exacerbations when compared with those on placebo.11 One of the main issues with studying omalizumab in ABPA is that this drug is dosed on the basis of pretreatment IgE levels, and because IgE levels tend to be extremely high in ABPA, the appropriated treatment dose is often higher than the maximum recommended dose.12 This likely results in frequent underdosing of omalizumab when treating ABPA. In addition, the effectiveness of omalizumab in treating ABPA in patients with underlying CF is even less clear.13 Anti–IL-5 and anti–IL-5 receptor alpha (IL-5Ra) therapies for ABPA have also shown promise in case series.14,15 Both omalizumab and IL-5 therapies may benefit symptoms in ABPA, but only IL-5 signaling blockade has been shown to clear mucus plugs.3,15 The biologic dupilumab is a human monoclonal IgG4 antibody against the IL-4 receptor alpha (IL-4Ra) subunit that blocks the activity of both IL-4 and IL-13 cytokine signaling pathways.7 Our group and others have shown improvement in symptoms, reduction in exacerbations, and a decrease in OCS use with dupilumab in case reports and series of patients with ABPA.16, 17, 18, 19, 20, 21, 22 A few cases have also been identified where dupilumab effectively treated patients with ABPA who had previously failed to achieve control using other biologic therapies.18,22 Tezepelumab is the latest asthma biologic to have been approved by the US Food and Drug Administration (FDA) and targets thymic stromal lymphopoietin (TSLP), an upstream alarmin involved in asthma pathogenesis. Tezepelumab has been effective in treating patients with ABPA in 2 case reports.23,24 Although most reports have focused on exacerbation reduction, a recent retrospective study showed that these asthma biologics are effective in reducing the use of OCSs in patients with ABPA.25
With several FDA-approved asthma biologics that differ in mechanisms of action,7 one important question is whether one biologic class is superior for managing ABPA. To address this gap in knowledge, we conducted a retrospective analysis of patients with ABPA to compare the effectiveness of different asthma biologics.
Methods
Patient enrollment
We searched the Emory Healthcare medical record database for allergic bronchopulmonary aspergillosis (International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code B44.81) and the use of any asthma biologic: omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, or tezepelumab from January 2006 to September 2022. Patients with a diagnosis of ABPA and the use of at least 1 biologic for at least 1 month were included in the analysis. Study subjects were enrolled under a protocol approved by the Emory University Institutional Review Board.
Clinical data collection
We collected the following clinical data variables from the electronic medical chart: demographics (age, sex, race, ethnicity), underlying clinical condition predisposing ABPA (asthma and/or CF), absolute eosinophil count, total IgE, Aspergillus fumigatus–specific IgE, Aspergillus fumigatus–specific IgG, fungal culture isolates, skin prick or intradermal testing for Aspergillus, FEV1, fractional exhaled nitric oxide, asthma control test score, radiographic findings (presence of bronchiectasis, mucus plugging, or high attenuation mucus in the radiology report), duration in months of daily systemic corticosteroid use, duration in months of antifungal use (including itraconazole, voriconazole, or isavuconazonium), duration in months of use of each asthma biologic, and number of respiratory exacerbations (defined as worsened respiratory symptoms and use of OCS (at least 40 mg of prednisone or equivalent per day) for 5 or more days). Exacerbations that happened during the same month were counted as 1 month with at least 1 exacerbation given that it is hard to assess whether it was the same unresolved exacerbation or a new one. When an exacerbation straddled 2 months, it was counted as only 1 month (the month during which the exacerbation started). If more than 5 days in a month there was a use of a medication it was counted as positive during that month.
Study variables and outcomes
We examined 3 main outcomes in this study: (1) number of respiratory exacerbations, defined as a worsening of respiratory symptoms and use of OCS (at least 40 mg of prednisone or equivalent per day) for 5 or more days, (2) duration of use of daily OCS, defined as a daily dose of at least 5 mg of prednisone, and (3) duration of antifungal use for the treatment of ABPA. As for study exposures, we gathered clinical information on each outcome during the treatment of each biologic class (anti-IgE: omalizumab, anti–IL-5/IL-5Ra: mepolizumab/benralizumab/reslizumab, anti–IL4Ra: dupilumab, anti-TSLP: tezepelumab). Mepolizumab, reslizumab, and benralizumab were analyzed together because they have similar mechanisms of actions, inhibiting the IL-5 signaling pathway. The first 2 months on each biologic were removed from the analysis to eliminate carryover effect from previous biologic or the no-biologic condition.
Data analysis and statistical tests
We modeled the binary outcome variable (exacerbation/OCS/antifungal) on the basis of a longitudinal repeated-measures model. Generalized estimating equations were used to analyze the binary response variable involving repeated-measures data, fitted through logistic regression. Specifically, we used SAS’s PROC GENMOD procedure, modeling the relationship between the outcome and predictor variables using a logit link function. We designated patient ID as the subject for repeated measures. A compound symmetry covariance structure was used, assuming identical correlations across all observation points. This assumption provides more robust estimates by accounting for the correlation among all observation points. Our analysis focused on comparing the effects of different treatments. The results of these comparisons are expressed as odds ratios (ORs), obtained by exponentiating the estimated coefficients in the model. We provided the 95% CI for OR for each contrast estimate, calculated by exponentiating the CIs of the estimated coefficients (L’Beta estimates). The significance of these comparisons was assessed using Wald’s chi-square test, with statistical significance reported using P values (Pr > ChiSq / Pr > |Z|). We used Adobe Illustrator 2024 to build figures (Adobe, San Jose, Calif).
Results
Study subject characteristics
We found 62 patients with the diagnosis of ABPA in the Emory Healthcare electronical medical record system from January 2006 to September 2022. Of the 62 patients, 21 had been treated with at least 1 asthma biologic and were included in the analysis. Table I presents the demographic and relevant clinical characteristics of the study participants. Seven (33.3%) subjects were male and 14 (66.6%) were female. The average age at the start of the first asthma biologic was 46 years, with a range from 18 to 75 years. Twelve subjects self-identified as White or Caucasian, 8 as Black or African American, and 1 as Hispanic. All 21 subjects had the diagnosis of asthma in the electronic medical record, and 7 subjects also had the diagnosis of CF. Seventeen of 21 (81%) patients had evidence of bronchiectasis on chest imaging. The average absolute blood eosinophil count before starting biologics was 1465 cells/μL (range, 90-4400 cells/μL). The average serum level of total IgE was 2176 IU/mL (range, 199-5000 IU/mL).
Table I.
Clinical characteristics and demographics of research subjects
| No. of subjects | 21 |
|---|---|
| Sex, n (%) | |
| Male | 7 (33) |
| Female | 14 (67) |
| Age (y), mean | |
| (range) | 46 (18-75) |
| Race & ethnicity, n (%) | |
| White/Caucasian | 12 (57) |
| Black/African American | 8 (38) |
| Hispanic | 1 (5) |
| Underlying diagnosis, n (%) | |
| Asthma | 21 (100) |
| CF | 7 (33) |
| Clinical characteristics, n (%) or n (range) | |
| Bronchiectasis | 17 (81) |
| Blood absolute eosinophils (cells/μL) | 1465 (90-4400) |
| Serum total IgE average (IU/mL) | 2176 (199-5000∗) |
| ABPA diagnostic criteria, n (%) | |
| Positive for Asano criteria | 16 (76.2) |
| Positive for Modified ISHAM criteria | 14 (66.6) |
| Positive for both criteria | 10 (47.6) |
| Positive for either criterion | 20 (95.2) |
| ABPA diagnosis made outside Emory | 1 (4.8) |
Upper limit of detection.
Clinical criteria for the diagnosis of ABPA
There is no one specific test for the diagnosis of ABPA. This disease is a continuum of different degrees of severity with a variable clinical presentation that has a common underlying pathogenesis of hypersensitivity to aspergillus in a susceptible patient population with concomitant severe asthma or CF. The diagnosis of ABPA is based on meeting certain clinical criteria, and these criteria have evolved over time. The 2 latest and best-validated criteria are the Asano26 and the Modified International Society for Human and Animal Mycology (ISHAM)-ABPA Working Group (AWG).27,28 In our study, 16 (76.2%) subjects met the Asano criteria, 14 (66.6%) subjects met the Modified ISHAM-AWG criteria, 20 (95.2%) subjects met either one of these diagnostic criteria, and 10 (47.6%) subjects met both diagnostic criteria. Only 1 patient (4.8%) was diagnosed with ABPA outside of the Emory Healthcare system by an allergy/immunology specialist, and we do not have clinical data to determine which diagnostic criteria were met (Table I).
Use of asthma biologics in patients with ABPA
To quantify the clinical benefits of individual biologic classes, we summated the number of months that each of the 21 subjects was treated with a particular biologic class from January 2006 to September 2022 (Table II). Eleven subjects were treated with only 1 biologic class during the follow-up period. However, 10 subjects were treated with more than 1 biologic class during the study follow-up period. For all 10 subjects treated with multiple biologic classes, the therapies were used sequentially and not concomitantly except for only 2 months where 2 biologics were used concurrently. These 2 overlapping months as well as the following 2 months were removed from subsequent analyses. The number of patient-months for each of the biologic classes studied was as follows: 161 patient-months for anti-IgE (omalizumab), 290 patient-months for anti–IL-5/IL-5Ra (mepolizumab and benralizumab), and 390 patient-months for anti–IL-4Ra therapy (dupilumab) (Table II). We did not find any patients with ABPA treated with either reslizumab or tezepelumab; hence, they were not included in the analysis.
Table II.
Research subjects and the number of months of follow-up while receiving each biologic therapy
| Subject | No. of months on each biologic class |
|||
|---|---|---|---|---|
| Anti-IgE | Anti–IL-5/IL-5Ra | Anti–IL-4Ra | All biologics | |
| 1 | 114 | 21 | 42 | 177 |
| 2 | 18 | 29 | 42 | 89 |
| 3 | 2 | 12 | 31 | 45 |
| 4 | 0 | 18 | 5 | 23 |
| 5 | 0 | 9 | 36 | 45 |
| 6 | 0 | 7 | 43 | 50 |
| 7 | 0 | 26 | 32 | 58 |
| 8 | 0 | 17 | 58 | 75 |
| 9 | 11 | 0 | 29 | 40 |
| 10 | 16 | 55 | 0 | 71 |
| 11 | 0 | 0 | 1 | 1 |
| 12 | 0 | 0 | 8 | 8 |
| 13 | 0 | 0 | 3 | 3 |
| 14 | 0 | 0 | 16 | 16 |
| 15 | 0 | 0 | 17 | 17 |
| 16 | 0 | 0 | 4 | 4 |
| 17 | 0 | 0 | 2 | 2 |
| 18 | 0 | 0 | 21 | 21 |
| 19 | 0 | 14 | 0 | 14 |
| 20 | 0 | 11 | 0 | 11 |
| 21 | 0 | 71 | 0 | 71 |
| Patient-months on each biologic | 161 | 290 | 390 | 845 |
This table shows all 21 research subjects with ABPA included in the analysis and the number of months that each subject was treated with a particular biologic class. Subjects 1 to 10 were treated sequentially with more than 1 biologic class during the study follow-up period. Subjects 11 to 21 were treated with only 1 biologic class during the follow-up period. The bottom row shows the number of patient-months for each of the biologic classes studied.
Study outcomes: Respiratory exacerbations, daily OCS treatment, and antifungal use
To measure the effectiveness of each biologic, we measured 3 outcomes: respiratory exacerbations, duration of OCS use, and duration of antifungal use. We enumerated the months with greater than 1 respiratory exacerbation, months of daily use of OCS, and months of antifungal use for each of the biologics (Table III). During the 161 patient-months of anti-IgE therapy, we found 14 months (8.7%) with at least 1 exacerbation, 87 months (54%) with daily OCS use, and 51 months (31%) with antifungal use. This result showed that on average, more than half the time the patients were on omalizumab, they were concomitantly on OCSs. During 290 patient-months of anti–IL-5/IL-5Ra therapies, we found 22 months (7.6%) with 1 or more exacerbations, 50 months (17.2%) with daily OCS use, and 24 months (8.3%) with antifungal use. Dupilumab had the greatest patient-months compared with the other biologics. During 390 patient-months of anti–IL-4Ra therapy, we found 5 months (1.3%) with at least 1 exacerbation, 30 months (7.7%) with daily OCS use, and 4 months (1%) with antifungal use. Table III also presents this analysis by patient-days in addition to patient-months.
Table III.
Contingencies of study outcomes and biologic class treatments
| Outcomes | Biologic classes |
||
|---|---|---|---|
| Anti-IgE | Anti–IL-5/IL-5Ra | Anti–IL-4Ra | |
| At least 1 exacerbation, pt.-mo. (%) | 14 (8.7) | 22 (7.6) | 5 (1.3) |
| Daily OCS use, pt.-mo. (%) | 87 (54) | 50 (17.2) | 30 (7.7) |
| Antifungal use, pt.-mo. (%) | 51 (31.7) | 24 (8.3) | 4 (1) |
| Total pt.-mo. | 161 | 290 | 390 |
| Daily OCS use, pt.-days (%) | 2,263 (42.7) | 1,011 (11.1) | 568 (4.1) |
| Antifungal use, pt.-days (%) | 1,163 (21.9) | 366 (4.1) | 82 (0.6) |
| Total pt.-days | 5293 | 8916 | 13997 |
Rows represent outcome measures, and columns represent biologic classes. Cells show the number of patient-months (top) and patient-days (bottom) of outcome variables while on each biologic class therapy.
pt.-mo., Patient-months.
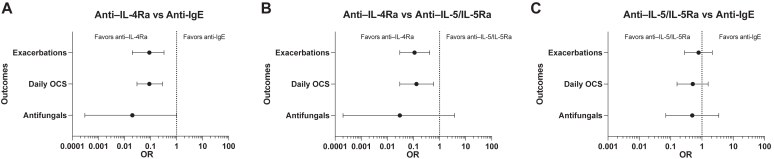
Table IV presents the ORs, 95% CI, and P values of pair-wise comparisons of biologic classes for each outcome variable. For the first outcome of respiratory exacerbations, anti–IL-4Ra therapy was associated with significantly fewer months with exacerbations compared with anti-IgE (OR, 0.09; 95% CI, 0.02-0.33; P = .0003) (Fig 1, A) and anti–IL-5/IL-5Ra (OR, 0.11; 95% CI, 0.03-0.42; P = .0011) (Fig 1, B) therapies. However, there was no significant difference between the number of months with exacerbations between anti-IgE and anti–IL-5/IL-5Ra therapies (OR, 0.79; 95% CI, 0.31-1.96; P = .6059) (Fig 1, C). For the second outcome of daily OCS, anti–IL-4Ra was associated with significantly less daily OCS use compared with anti-IgE (OR, 0.09; 95% CI, 0.03-0.29; P < .0001) (Fig 1, A) and anti–IL-5/IL-5Ra (OR, 0.18; 95% CI, 0.06-0.55; P = .0029) (Fig 1, B) therapies. However, anti–IL-5/IL-5Ra therapies were not significantly different in daily OCS use than anti-IgE therapy (OR, 0.51; 95% CI, 0.17-1.49; P = .2172) (Fig 1, C). We then quantified the OCS dose per month for each of the biologic classes and found that the average use of oral corticosteroids in milligram of prednisone or equivalent was 236.2 for anti-IgE therapy, 57.7 for anti–IL-5/IL-5Ra therapies, and 6.1 for anti–IL-4Ra therapy. For the third outcome of antifungal use, anti–IL-4Ra was associated with significantly less antifungal use than anti-IgE (OR, 0.02; 95% CI, 0.00-0.99; P = .0496) (Fig 1, A). Although there was a trend to lower use of antifungal associated with anti–IL-4Ra therapy than with anti–IL-5/IL-5Ra, this difference did not reach statistical significance given a large variability in the CI (OR, 0.04; 95% CI, 0.06-2.72; P = .1325) (Fig 1, B). Lastly, for the outcome of antifungal use, anti–IL-5/IL-5Ra therapies were comparable to anti-IgE therapy (OR, 0.49; 95% CI, 0.08-3.19; P = .4563) (Fig 1, C).
Table IV.
Pair-wise comparisons of biologic classes for each outcome variable
| OR | 95% CI | P value | |
|---|---|---|---|
| Exacerbations | |||
| IL-4Ra vs IgE | 0.09 | 0.02-0.33 | .0003 |
| IL-4Ra vs IL-5/IL-5Ra | 0.11 | 0.03-0.42 | .0011 |
| IL-5/IL-5Ra vs IgE | 0.79 | 0.31-1.96 | .6059 |
| Daily OCS | |||
| IL-4Ra vs IgE | 0.09 | 0.03-0.29 | <.0001 |
| IL-4Ra vs IL-5/IL-5Ra | 0.18 | 0.06-0.55 | .0029 |
| IL-5/IL-5Ra vs IgE | 0.51 | 0.17-1.49 | .2172 |
| Antifungals | |||
| IL-4Ra vs IgE | 0.02 | 0.00-0.99 | .0496 |
| IL-4Ra vs IL-5/IL-5Ra | 0.04 | 0.00-2.72 | .1325 |
| IL-5/IL-5Ra vs IgE | 0.49 | 0.08-3.19 | .4563 |
Fig 1.
Forrest plots of ORs of comparisons among biologic classes for each outcome under longitudinal repeated-measure models. A, Comparison of anti–IL-4Ra therapy to anti-IgE therapy for each outcome of exacerbations, daily OCS use, and antifungal use. B, Comparison of anti–IL-4Ra to anti–IL-5/IL-5Ra therapies for each outcome. C, Comparison of anti–IL-5/IL-5Ra therapies to anti-IgE therapy for each outcome. Green dots represent the OR estimates of the comparison, and the error bars represent 95% CI.
Dosing of anti-IgE therapy
The optimal 4-week dosing period of omalizumab to achieve clinical results in patients with asthma is 0.016 mg of drug per kg of body weight per IU/mL of pretreatment IgE.12 Given that we saw worse clinical response with anti-IgE in comparison to anti–IL-4Ra and anti–IL-5/IL-5Ra therapies, we wanted to explore whether underdosing of omalizumab could be the reason. Five subjects in our cohort were treated with an anti-IgE biologic. Table V summarizes the dosing of study subjects treated with omalizumab. Only 1 subject is treated with doses above 0.016 mg/kg/IU/4 wk. The other 4 subjects had a suboptimal dosing of this biologic, potentially explaining the poor clinical outcomes with omalizumab. Patients with very high levels of IgE, like the ones seen in ABPA, would require higher doses of omalizumab beyond what is recommended by the manufacturer.
Table V.
Omalizumab dosing per kg of body weight per IU/mL of pretreatment IgE
| Subject | Dose (mg) (every 2 wk) | Pretreatment IgE (IU/mL) | Body weight (kg) | Dose (mg/kg/IgE) q2 wk | Dose (mg/kg/IgE) q4 wk |
|---|---|---|---|---|---|
| 1 | 375 | 1,961 | 67.0 | 0.0029 | 0.006 |
| 2 | 375 | 477 | 109.0 | 0.0072 | 0.014 |
| 3 | 375 | 2,000 | 58.2 | 0.0032 | 0.006 |
| 9 | 300 | 818 | 57.2 | 0.0064 | 0.013 |
| 10 | 225 | 334 | 76.2 | 0.0088 | 0.018 |
Subjects treated sequentially with more than 1 biologic therapy
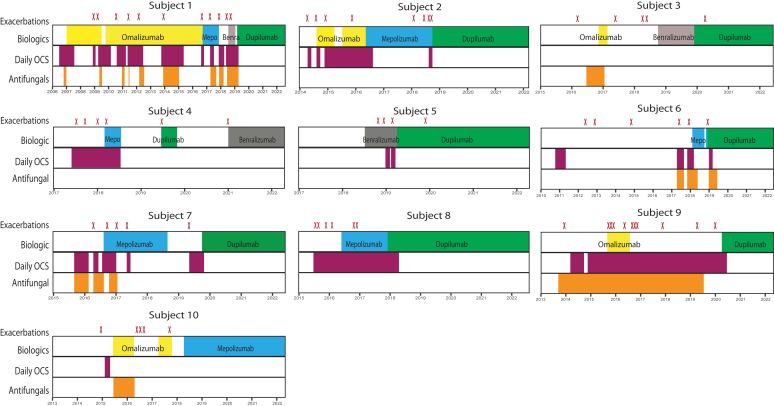
Eleven of the 21 subjects were treated with only 1 biologic, with 8 initially treated with anti–IL-4Ra and 2 with anti–IL-5/IL-5Ra therapy. Of the 21 subjects, 10 patients (48%) had been treated sequentially with 2 or more biologics (Table II). Fig 2 shows the 10 subjects who were included in this subgroup analysis. Each box represents 1 patient’s longitudinal timeline across the duration of the follow-up. Each red “X” on top of the boxes represents 1 exacerbation, the top row shows the duration of treatment for an individual biologic, the middle row shows the duration of OCS treatment, and the bottom row shows the duration of antifungal therapy for each patient. One subject was treated sequentially with 4 different biologics, 3 subjects with 3 biologics, and 6 subjects with 2 biologics. We defined treatment failure as more than 1 exacerbation or inability to discontinue daily OCS while on a specific biologic therapy. Subject 1 failed treatment with omalizumab (anti-IgE), mepolizumab (anti–IL-5), and benralizumab (anti–IL-5Ra) before finally achieving clinical control on dupilumab (anti–IL-4Ra), avoiding antifungals and OCSs as well as staying exacerbation-free for almost 3 years. Subject 2 failed omalizumab and mepolizumab but achieved clinical control on dupilumab. Subject 5 failed benralizumab, subjects 7 and 8 failed mepolizumab, and subject 9 failed omalizumab before all being well-controlled on dupilumab.
Fig 2.
Subjects treated sequentially with more than 1 biologic. Each box represents 1 subject’s longitudinal timeline across the duration of the study follow-up. Each red “X” on top of the boxes represents 1 exacerbation. The top row of each box shows the individual biologic duration of treatment, the middle row shows duration of daily OCS treatment, and the bottom row shows the duration of antifungal therapy for each subject. Omalizumab in yellow, mepolizumab (Mepo) in blue, benralizumab (Benra) in gray, dupilumab in green, daily OCS treatment in purple, and antifungal treatment in orange. Subject 2 graph is adapted with permission from Lamothe et al.22
Treatment failure occurred in 4 of 5 with omalizumab, 5 of 7 with mepolizumab, 2 of 4 with benralizumab, and none of 9 with dupilumab. Five of the 9 patients on dupilumab did not have exacerbations while receiving treatment with this biologic. For the 4 patients who had 1 exacerbation while on dupilumab, these exacerbations occurred within the first few months of the treatment (1, 1, 3, and 6 months) likely before achieving the maximal therapeutic effect of anti–IL-4Ra therapy. No patient while receiving dupilumab had more than 1 exacerbation. Every patient who received dupilumab was able to successfully discontinue OCS within the first 3 months of therapy or did not require OCS, while receiving this therapy. Eight of 9 patients receiving dupilumab did not require antifungals, and 1 patient stopped antifungals shortly after starting dupilumab.
Discussion
ABPA is a disease most commonly affecting patients with asthma or CF and is characterized by an abnormal cellular and humoral immune response leading to T2 inflammation on aspergillus colonization in the airways. The first line of treatment for ABPA is OCSs and antifungals. However, prolonged OCS and antifungal use can often become ineffective at controlling the disease and is associated with significant side effects and drug-drug interactions. Daily or repetitive use (during exacerbations) of OCSs can lead to multiple complications including the development of osteoporosis, diabetes, weight gain, mood changes, and increased risk for infections.6 Antifungals commonly used against Aspergillus include itraconazole, voriconazole, and isavuconazonium. All are substrates of cytochrome P450 3A4, and itraconazole is a direct enzyme inhibitor.29 This leads to many potential drug-drug interactions with commonly used medications including corticosteroids that are concomitantly used in patients with ABPA. Moreover, the prolonged or repeated antifungal use has public health implications due to the development of resistant fungal strains. Because Aspergillus is ubiquitous, repeat respiratory inoculations are common; thus, controlling aberrant T2 pathogenesis appears to be an important mainstay. Therefore, finding better treatments for ABPA is important to better control symptoms, reduce exacerbations, and lower the use of therapies with substantial side effects.
Because the diagnosis of ABPA is commonly associated with asthma, these patients are often started on a biologic therapy for severe asthma. Although robust, randomized placebo-controlled clinical trials assessing the safety and efficacy of asthma biologics in patients with ABPA are lacking, there are some encouraging studies showing potential benefits targeting IgE (omalizumab),11,13 IL-5 (mepolizumab),14 IL-5RA (benralizumab),15 IL-4RA (dupilumab),16, 17, 18, 19, 20, 21, 22 and TSLP (tezepelumab).23,24 Some cases report that dupilumab was effective in treating ABPA when other biologics had failed.18,21,22 However, there are no head-to-head comparisons of asthma biologics evaluating the effectiveness of treating patients with ABPA.
In this retrospective study of patients with ABPA treated with asthma biologics, we found that dupilumab was associated with decreased use of OCSs and antifungals, as well as fewer exacerbations (Fig 1 and Table IV) compared with other biologics targeting IgE or the IL-5 pathway. In addition, of the 10 patients who had been treated sequentially with more than 1 biologic, we found that dupilumab was often effective even after other biologics had failed in controlling exacerbations or corticosteroid/antifungal use (Fig 2).
As a chronic therapy to maintain asthma control, there are no randomized controlled trials of direct comparisons of anti-IgE, anti–IL-5/IL-5Ra, anti–IL-4Ra, and anti-TSLP biologics. However, a recent large multicenter retrospective study showed that patients with severe asthma on dupilumab had significantly fewer exacerbations than patients on anti–IL-5 therapies.30 Whether dupilumab is a more effective approach for all T2 asthma or whether it is superior compared with other biologics for ABPA only is not clear.
Dupilumab may specifically benefit patients with ABPA by blocking both IL-4 and IL-13 cytokine pathways. IL-13 is important for airway mucus generation by upregulating the gene MUC5AC,31 and because Aspergillus is ubiquitous, it is possible that the organism gets trapped in airway mucus in patients with ABPA, leading to local fungal antigen-specific B-cell activation in a T2-driven milieu. Therefore, inhibition of IL-13 likely benefits patients with ABPA. IL-4 is required for IgE class switching. Hence, IL-4 blocking therapies can reduce IgE production by inhibition of local mucosal naive-like B-cell differentiation into IgE plasma cells.32,33 We believe that dupilumab disrupts the initial pathogenic mechanism of IgE-mediated immune cell activation. How long-term dupilumab use modifies ABPA disease may be revealed through transcriptional and epigenetic profiles of immune cells. Additional prospective studies will be needed to assess whether dupilumab or other biologics can also prevent the progression of bronchiectasis if started early in the disease process.
Because ABPA is an allergic condition that has IgE at the center of its pathogenesis, the use of omalizumab seemed appropriate for disease control. Although some studies have shown benefit, omalizumab was inferior to anti–IL-5/IL-5Ra and anti–IL-4 therapies in our study. Omalizumab blocks free IgE and a mathematical model of pharmacokinetics was consistent with this drug decreasing IgE production.34 However, to our knowledge, there is no biological evidence that omalizumab inhibits IgE secretion from plasma cells or the generation of IgE plasma cells. Therefore, inhibition of the IgE downstream components of this pathogenic cascade may be insufficient to control the disease. Moreover, the doses recommended by the manufacturer for omalizumab might be insufficient to block enough IgE to see an optimal clinical effect12,35 because patients with ABPA have significant elevation of IgE (Table V). On the contrary, dupilumab through inhibition of IL-4 prevents IgE plasma cell formation and therefore reduces systemic IgE levels, suggesting that upstream inhibition could be more effective in controlling this disease. Interestingly, we see effects of clinical improvement with dupilumab even when serum levels of IgE are only modestly reduced. Therefore, an alternative explanation is that dupilumab could further lead to decrease of IgE in the mucosal sites, inhibition of the IL-4 pathway, which suppresses T2 inflammation, as well as inhibition of the IL-13 pathway, which decreases mucus hyperproduction.
In addition to high IgE levels, high mucosal and systemic eosinophils are commonly present in patients with ABPA. When activated, eosinophils play pathogenic roles in T2- mediated conditions, and IL-5 is the main cytokine involved in eosinophilic activation and proliferation. Single inhibitors of IL-5/IL-5Ra targets were not as effective as the IL-4Ra antagonist. Interestingly, polymorphisms in the genes for IL-4 and IL-13, but not for IL-5, have been linked to a higher risk of developing ABPA.36 Perhaps the exaggerated allergic response to a specific fungal antigen together with increased mucus production are the main drivers of pathogenesis, whereas overactive eosinophils play more secondary roles.
Limitations of our study include a small sample size, the retrospective and observational review, and enrollment from a single center. Despite a small sample size, we showed significant differences among the biologics for our outcome measures of exacerbations, OCS use, and antifungal use. Moreover, patients with sequential biologic use functioned as their own controls, increasing the power of our study. Finally, our cohort had a good distribution of age, sex, and race/ethnicity, providing application to a broad patient population of asthma and ABPA despite its small sample size. We also included 7 patients with CF, which represents 33% of the subjects in this study. This is important because patients with CF have often been excluded from studies that have evaluated the use of asthma biologics in patients with ABPA and those that have included them have shown equivocal results.13
We did not have any patients on reslizumab or tezepelumab, 2 other FDA-approved biologics for treating asthma. Reslizumab is administered intravenously, which makes it less desirable for many patients and thus it has little clinical utilization relative to other biologic therapies.37 Tezepelumab is the newest asthma biologic approved by the FDA in December of 2021 and targets the upstream alarmin TSLP.38 Although TSLP could play a role in ABPA pathogenesis, further studies are needed to evaluate its potential role in treating ABPA. Because ABPA is a rare condition, it is challenging to enroll a large number of patients from a single center; and so, most studies have been limited to case reports, case series, and small cohorts. Therefore, a multicenter collaboration would be needed to enroll a larger number of patients with ABPA in prospective studies. A randomized clinical trial of dupilumab assessing effectiveness in patients with ABPA is underway (ClinicalTrials.gov ID NCT04442269).39 However, this trial is comparing dupilumab to placebo and not to other biologics. Head-to-head comparisons of different biologics are costly, and manufacturers are often reluctant to pursue these studies. Thus, for now, we will need to rely on retrospective studies to guide clinical practice.
In conclusion, this study finds that inhibition of IL-4 and IL-13 pathways may be more effective in ABPA compared with anti-IgE and anti–IL-5/IL-5Ra therapies, and that this could also be generalizable to allergic bronchopulmonary mycosis. Therefore, we recommend considering treating patients with severe asthma and ABPA with dupilumab initially to decrease exacerbations and OCS and antifungal use.
Disclosure statement
This study was supported by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (grant nos. P01AI125180, U54CA260563, R01AI172254, and U01AI141993) and NIH/National Heart, Lung, and Blood Institute (grant no. T32HL116271).
This work does not necessarily represent the views of the US government or the Department of Veterans Affairs.
Disclosure of potential conflict of interest: P. A. Lamothe is supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (grant no. T32HL116271) and NIH/National Institute of Allergy and Infectious Diseases (NIAID) (grant no. 3P01AI125180-08S1). M. C. Runnstrom is supported by NIH/NHLBI (grant no. T32HL116271). N. Smirnova is supported by NIH/NHLBI (grant no. T32HL116271) and the National Center for Advancing Translational Sciences of the NIH (grant no. UL1TR002378). C. Swenson receives compensation and consulting fees from Insmed, Inc, which are unrelated to this article. F. E.-H. Lee is supported by NIH/NIAID (grant nos. P01AI125180, U54CA260563, R01AI121252, R01AI172254, and U01AI141993) and research grants from Genentech and the Gates Foundation; receives royalties from BLI, Inc, for Plasma cell survival media; receives consulting fees from Be Bio Pharma; received honoraria for presentations at University of Pennsylvania, University of Cincinnati, and Gerontological Advanced Practice Nurses Association; has patents on plasma cell survival media and MENSA (media of elaborated newly synthesized antibodies); and is the founder and owner of MicroB-plex, Inc. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Agarwal R., Chakrabarti A., Shah A., Gupta D., Meis J.F., Guleria R., et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850–873. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. 2009;135:805–826. doi: 10.1378/chest.08-2586. [DOI] [PubMed] [Google Scholar]
- 3.Asano K., Ueki S., Tamari M., Imoto Y., Fujieda S., Taniguchi M. Adult-onset eosinophilic airway diseases. Allergy. 2020;75:3087–3099. doi: 10.1111/all.14620. [DOI] [PubMed] [Google Scholar]
- 4.Patterson T.F., Thompson G.R., III, Denning D.W., Fishman J.A., Hadley S., Herbrecht R., et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal R., Garg M., Aggarwal A.N., Saikia B., Gupta D., Chakrabarti A. Serologic allergic bronchopulmonary aspergillosis (ABPA-S): long-term outcomes. Respir Med. 2012;106:942–947. doi: 10.1016/j.rmed.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Price D.B., Trudo F., Voorham J., Xu X., Kerkhof M., Ling Zhi Jie J., et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi: 10.2147/JAA.S176026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brusselle G.G., Koppelman G.H. Biologic therapies for severe asthma. N Engl J Med. 2022;386:157–171. doi: 10.1056/NEJMra2032506. [DOI] [PubMed] [Google Scholar]
- 8.Lamothe P.A., Capric V., Lee F.E. Viral infections causing asthma exacerbations in the age of biologics and the COVID-19 pandemic. Curr Opin Pulm Med. 2024;30:287–293. doi: 10.1097/MCP.0000000000001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuruvilla M.E., Lee F.E., Lee G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219–233. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Runnstrom M., Pitner H., Xu J., Lee F.E., Kuruvilla M. Utilizing predictive inflammatory markers for guiding the use of biologicals in severe asthma. J Inflamm Res. 2022;15:241–249. doi: 10.2147/JIR.S269297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voskamp A.L., Gillman A., Symons K., Sandrini A., Rolland J.M., O’Hehir R.E., et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2015;3:192–199. doi: 10.1016/j.jaip.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Busse W., Corren J., Lanier B.Q., McAlary M., Fowler-Taylor A., Cioppa G.D., et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 13.Jat K.R., Walia D.K., Khairwa A. Anti-IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD010288.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Schleich F., Vaia E.S., Pilette C., Vandenplas O., Halloy J.L., Michils A., et al. Mepolizumab for allergic bronchopulmonary aspergillosis: report of 20 cases from the Belgian Severe Asthma Registry and review of the literature. J Allergy Clin Immunol Pract. 2020;8:2412–2413.e2. doi: 10.1016/j.jaip.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Tomomatsu K., Sugino Y., Okada N., Tanaka J., Oguma T., Asano K. Rapid clearance of mepolizumab-resistant bronchial mucus plugs in allergic bronchopulmonary aspergillosis with benralizumab treatment. Allergol Int. 2020;69:636–638. doi: 10.1016/j.alit.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Ali M., Green O. Dupilumab: a new contestant to corticosteroid in allergic bronchopulmonary aspergillosis. Oxf Med Case Rep. 2021;2021 doi: 10.1093/omcr/omaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eldaabossi S.A.M., Awad A., Anshasi N. Mepolizumab and dupilumab as a replacement to systemic glucocorticoids for the treatment of chronic eosinophilic pneumonia and allergic bronchopulmonary aspergillosis - case series, Almoosa specialist hospital. Respir Med Case Rep. 2021;34 doi: 10.1016/j.rmcr.2021.101520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikura S., Saraya T., Yoshida Y., Oda M., Ishida M., Honda K., et al. Successful treatment of mepolizumab- and prednisolone-resistant allergic bronchopulmonary aspergillosis with dupilumab. Intern Med. 2021;60:2839–2842. doi: 10.2169/internalmedicine.6679-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura T., Okano T., Naito M., Tsuji C., Iwanaka S., Sakakura Y., et al. Complete withdrawal of glucocorticoids after dupilumab therapy in allergic bronchopulmonary aspergillosis: a case report. World J Clin Cases. 2021;9:6922–6928. doi: 10.12998/wjcc.v9.i23.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Veer T., Dallinga M.A., van der Valk J.P.M., Kappen J.H., In‘t Veen J., van der Eerden M.M., et al. Reduced exacerbation frequency and prednisone dose in patients with ABPA and asthma treated with dupilumab. Clin Transl Allergy. 2021;11 doi: 10.1002/clt2.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramonell R.P., Lee F.E., Swenson C., Kuruvilla M. Dupilumab treatment for allergic bronchopulmonary aspergillosis: a case series. J Allergy Clin Immunol Pract. 2020;8:742–743. doi: 10.1016/j.jaip.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamothe P.A., Runnstrom M., Smirnova N., Flores F.C., Shepherd A., Park J., et al. Allergic bronchopulmonary aspergillosis in identical twins: effectiveness of dupilumab. J Allergy Clin Immunol Pract. 2023;11:1556–1558.e2. doi: 10.1016/j.jaip.2022.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuno O. Allergic bronchopulmonary aspergillosis successfully treated with tezepelumab. J Allergy Clin Immunol Pract. 2023;11:2589–2591. doi: 10.1016/j.jaip.2023.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Ogata H., Sha K., Kotetsu Y., Enokizu-Ogawa A., Katahira K., Ishimatsu A., et al. Tezepelumab treatment for allergic bronchopulmonary aspergillosis. Respirol Case Rep. 2023;11 doi: 10.1002/rcr2.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darragh K., Akuthota P. Corticosteroid-sparing effect of biologics in patients with allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 2024;132:650–652. doi: 10.1016/j.anai.2024.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Asano K., Hebisawa A., Ishiguro T., Takayanagi N., Nakamura Y., Suzuki J., et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2021;147:1261–1268.e5. doi: 10.1016/j.jaci.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Saxena P., Choudhary H., Muthu V., Sehgal I.S., Dhooria S., Prasad K.T., et al. Which are the optimal criteria for the diagnosis of allergic bronchopulmonary aspergillosis? A latent class analysis. J Allergy Clin Immunol Pract. 2021;9:328–335.e1. doi: 10.1016/j.jaip.2020.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal R., Saxena P., Muthu V., Sehgal I.S., Dhooria S., Prasad K.T., et al. Evaluation of simpler criteria for diagnosing allergic bronchopulmonary aspergillosis complicating asthma. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.861866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellmann R., Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45:737–779. doi: 10.1007/s15010-017-1042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearney C.M., Sangani R., Shankar D., O’Connor G.T., Law A.C., Walkey A.J., et al. Comparative effectiveness of mepolizumab, benralizumab and dupilumab among patients with difficult-to-control asthma. Ann Am Thorac Soc. 2024;21:866–874. doi: 10.1513/AnnalsATS.202306-566OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzaki I., Kawano S., Komiya K., Tanabe T., Akaba T., Asano K., et al. Inhibition of IL-13-induced periostin in airway epithelium attenuates cellular protein expression of MUC5AC. Respirology. 2017;22:93–100. doi: 10.1111/resp.12873. [DOI] [PubMed] [Google Scholar]
- 32.Corrado A., Ramonell R.P., Woodruff M.C., Tipton C., Wise S., Levy J., et al. Extrafollicular IgD+ B cells generate IgE antibody secreting cells in the nasal mucosa. Mucosal Immunol. 2021;14:1144–1159. doi: 10.1038/s41385-021-00410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramonell R.P., Brown M., Woodruff M.C., Levy J.M., Wise S.K., DelGaudio J., et al. Single-cell analysis of human nasal mucosal IgE antibody secreting cells reveals a newly minted phenotype. Mucosal Immunol. 2023;16:287–301. doi: 10.1016/j.mucimm.2023.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe P.J., Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma: PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–320. doi: 10.1111/j.1365-2125.2011.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slavin R.G., Ferioli C., Tannenbaum S.J., Martin C., Blogg M., Lowe P.J. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107–113.e3. doi: 10.1016/j.jaci.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 36.Overton N.L., Denning D.W., Bowyer P., Simpson A. Genetic susceptibility to allergic bronchopulmonary aspergillosis in asthma: a genetic association study. Allergy Asthma Clin Immunol. 2016;12:47. doi: 10.1186/s13223-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corren J., Weinstein S., Janka L., Zangrilli J., Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799–810. doi: 10.1016/j.chest.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Menzies-Gow A., Corren J., Bourdin A., Chupp G., Israel E., Wechsler M.E., et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384:1800–1809. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]
- 39.ClinicalTrials.gov Investigating treatment with dupilumab in patients with allergic bronchopulmonary aspergillosis (ABPA) (LIBERTY ABPA AIRED) https://clinicaltrials.gov/study/NCT04442269 Available from: