Highlights
-
•
Natural killer (NK) cell activity (NKA) recovered significantly on postoperative day30 (POD30) in colorectal cancer (CRC) patients with advanced stage or with one or more preoperative high-risk factors after radical surgery of CRC tumor.
-
•
A higher postoperative increment of circulating CD56 bright NK cell percentage is significantly associated with better clinical outcomes as well as circulating higher CD56 bright NK cell/CD56 dim NK cell ratio on POD30 in CRC patients.
-
•
A higher recovery of NKA on POD30 in CRC patients with advanced stage had better clinical outcome.
-
•
These findings provide the possible application of NK cell/NKA analyses for risk stratification and development of treatment strategies in CRC patients.
Keywords: CD56bright natural killer cell, Colorectal cancer, Prognosis, NK cell activity, surgery
Abstract
Introduction
Natural killer (NK) cell activity (NKA) is downregulated in patients with colorectal cancer (CRC), and its dysfunction is possibly associated with increased risk of recurrence. However, its role in prognosis of CRC remains unclear. Prior research has shown that surgical stress can suppress NKA. This study explores the relationship between NK cell/NKA and clinicopathological factors during the perioperative period in patients with CRC.
Methods
We prospectively enrolled 45 patients with CRC. Venous blood samples were collected preoperatively and on postoperative day 3 (POD3) and 30 (POD30). NKA was assessed by measuring the plasma levels of NK cell-secreted IFN-γ.
Results
NKA was significantly reduced on POD3 compared with baseline levels before surgery but showed significant recovery by POD30. NKA on POD30 was considerably higher in patients with advanced disease stages or one or more high-risk preoperative factors. Additionally, a higher NKA recovery in patients with advanced stage exhibited improved recurrence-free survival (RFS) and progression-free survival (PFS) (hazards ratio (HR): 0.2442). Furthermore, an increased percentage of CD56bright NK cells and a higher CD56bright/CD56dim NK cell ratio postoperatively on POD30 were associated with better RFS/PFS (HR: 0.2732, P = 0.0433 and HR: 0.2193, P = 0.024, respectively).
Conclusions
Our findings indicate that a notable postoperative increase in CD56bright NK cells on POD30, both in percentage and ratio, correlates with a more favorable prognosis in CRC patients. Additionally, higher recovery rates of NKA in patients with advanced stages may offer potential applications in risk stratification and the development of treatment strategies for CRC.
Introduction
Colorectal cancer (CRC) is the third-leading cause of cancer-related deaths worldwide [1]. According to the American Cancer Society's Cancer Facts & Figures 2021, the five-year relative survival rate for CRC is 65 %, though this rate varies depending on cancer stage. Surgical resection remains the primary treatment approach. Several factors are associated with poor CRC prognosis post-surgery, including poorly differentiated histology, lymphovascular and perineural invasion, T4 stage, and elevated levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9). These factors are closely linked to increased risks of recurrence, metastasis, and lower survival rates in patients with CRC [[2.], [3.], [4.], [5.]]. Recent studies have also emphasized the profound influence of patients’ inflammatory and nutritional statuses on cancer outcomes, with relevant biomarkers including the Royal Marsden Hospital (RMH) score, pan-immune-inflammation value (PIV), and neutrophil-to-lymphocyte ratio (NLR) [[6.], [7.], [8.]]. Moreover, emerging therapies in CRC have led to the identification of predictive and prognostic markers for therapy response and adverse effects, such as upfront chemotherapy in liver transplant patients with liver metastases of CRC and various side effects (e.g., peripheral neuropathy, headache, hypertransaminasemia, hearing loss) in patients undergoing immune checkpoint inhibitor therapy [[9.], [10.], [11.], [12.]]. Additionally, immunosuppression has been associated with poor outcomes in cancer patients [13,14]. Notably, natural killer (NK) cell activity (NKA) is generally lower in CRC patients than in healthy individuals and tends to decrease further during the postoperative period [15]. However, the relationship between postoperative NKA downregulation, high-risk factors, and prognosis in CRC remains unclear.
NK cells are critical components of the innate immune system, characterized by their cytotoxic abilities. They play a dual role: directly eliminating infected or malignant cells and modulating adaptive immune responses through cytokine secretion [16,17]. The two major subsets of human NK cells, CD56dimCD16+ and CD56brightCD16−, are distinguished by the relative expression levels of CD56 and CD16 [18,19]. CD56dimCD16+ NK cells are the major circulating subset, accounting for approximately 90 % of all NK cells in the peripheral blood, whereas CD56brightCD16− NK cells predominate in tissues and secondary lymphoid organs [20]. Studies have demonstrated that CD56dimCD16+ NK cells exert cytolytic activity via perforin and granzyme production, while CD56brighCD16− NK cells are responsible for secreting cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor-α [[20.], [21.], [22.]].
NK cells produce IFN-γ to regulate immune responses [23], with studies showing that IFN-γ from NK cells can inhibit tumor growth and enhance cytolysis and apoptosis of tumor cells [[24.], [25.], [26], [27.]]. Additionally, IFN-γ has been implicated in suppressing metastasis [28,29]. Beyond their direct cytotoxic effects, NK cells also indirectly suppress cancer progression through IFN-γ-mediated regulation of other innate and adaptive immune cells, such as macrophages and T cells [30,31]. Consequently, measuring IFN-γ secretion offers valuable insights into the immune system's response during tumor progression.
In this study, we assessed the levels of IFN-γ secreted by NK cells (as NKA) using the NK VUE ELISA kit. We also analyzed NK cell subsets in patients with CRC during the preoperative and postoperative periods to evaluate the impacts of surgical intervention and preoperative risk factors on NKA and NK cell profiles following tumor resection.
Materials and methods
Patient selection
This study was conducted in accordance with the Declaration of Helsinki and received approval from the Institutional Review Board of Chang Gung Memorial Hospital, Linkou, Taiwan (Approval Number: 201900981B0). The inclusion criteria were as follows: (1) histologically confirmed CRC; (2) undergoing bowel resection for primary colorectal adenocarcinoma; (3) diagnosed with stage I–IV CRC. Patients with autoimmune diseases, inflammatory conditions, severe hematological disorders, or major organ failure were excluded. We prospectively enrolled 45 patients from the referral center, all of whom underwent bowel resection for primary colorectal adenocarcinoma. Data collected included clinicopathological characteristics and measurement variables such as preoperative parameters (age, sex, CEA, and CA19–9, NLR [8]) and postoperative pathological features (histological grade, angiolymphatic invasion, perineural invasion, and final clinical staging). Additionally, healthy subjects were recruited to compare NKA between patients with CRC and healthy controls.
Whole blood processing
Blood was drawn from each patient at three distinct times: before surgery (baseline, n = 45), 3 days after surgery (postoperative day (POD) 3, n = 43), and approximately 30 days post-surgery (POD30, n = 45).
Whole blood was collected in heparinized vacuum blood collection tubes (10 mL per tube). An aliquot of 1 mL of whole blood was transferred to a vacutainer tube containing Promoca™ for NK cell stimulation. The remaining blood was used to isolate peripheral blood mononuclear cells (PBMCs) via Ficoll density gradient centrifugation. The isolated PBMCs were then stored at -80 °C for further analysis.
NKA assay
The level of IFN-γ secretion by NK cells was quantified using the NK VUE ELISA kit (NKMAX, Korea) following the manufacturer's instructions. Briefly, 1 mL of whole blood was incubated in a vacutainer tube containing Promoca™ at 37 °C for 22 h After incubation, the samples were centrifuged, and the plasma was collected and stored at -80 °C. For the assay, cryopreserved plasma samples were thawed and centrifuged at 11,500 × g for 1 min at room temperature. The supernatants were then transferred to ELISA wells and incubated for 1 h at room temperature. The wells were washed, and 100 µl of detection solution containing biotin conjugate and streptavidin horseradish peroxidase was added, followed by an incubation for 1.5 h at room temperature. Tetramethylbenzidine solution was then added and incubated for 30 min, followed by a final wash. A stop solution was added, and the absorbance was measured at 450 nm to determine the concentration of IFN-γ secreted by the NK cells.
Flow cytometry analysis
PBMCs were isolated from whole blood and analyzed using flow cytometry. Briefly, cryopreserved PBMCs were thawed in a 37 °C water bath and transferred to a 15 mL centrifuge tube containing 5 mL of PBS. Then, PBMCs were incubated at 37 °C for 5 min, followed by centrifugation at 300 × g for 10 min. The supernatant was discarded, and the PBMCs were resuspended in 1 mL of PBS. After incubating for an additional hour at 37 °C, the cells were centrifuged again at 300 × g for 10 min. PBMCs were then resuspended and stained with directly conjugated monoclonal antibodies (mAbs) including CD45, CD3, CD56, and CD16 (BD Biosciences) for 1 h according to the manufacturer's instructions. Stained PBMCs were analyzed on a BD Fortessa flow cytometer (BD Biosciences, Becton Dickinson, Franklin Lakes, NJ, USA). NK cells were identified using the phenotype CD45+CD3−CD16+/−CD56+. Flow cytometry data were processed using BD FACSDiva and FlowJo™ software. The gating strategy was consistent with our prior studies on NK cell phenotypes in post-surgical glioblastoma patients [32].
Clinical outcome assessment
The primary clinical endpoints were recurrence-free survival (RFS) for stage I–III patients and progression-free survival (PFS) for stage IV patients. RFS was defined as the time interval between the date of surgery and the occurrence of disease recurrence or death, whichever came first. PFS was defined as the period from surgery to disease progression or death, whichever occurred first.
Statistical analysis
Comparisons between two distinct groups were performed using the two-tailed Mann–Whitney U test. For paired samples, comparisons at specified time points were conducted using the two-tailed Wilcoxon matched-pairs signed-rank test. Correlations between perioperative changes in NKA and NK cell subsets were assessed using Pearson's product-moment correlation coefficient (r). Changes in the percentage of each NK cell subset during the perioperative period were measured for their association with RFS and PFS. Prognostic cutoff points for associating NK cell subset/NKA values with survival were established to optimize the significance of splits in Kaplan-Meier plots [33].
Kaplan–Meier analyses and log-rank tests were used to investigate and compare survival rates within patient subgroups. Hazard ratios (HRs) were calculated using Cox regression analysis and are presented with their 95 % confidence intervals (CIs). Univariate Cox regression analysis evaluated the relationship between baseline factors and survival rate. Variables with P values < 0.05 were included in the multivariable Cox regression model. All statistical tests were two-sided, and a P value of <0.05 was considered statistically significant. Notations for significance are as follows: * indicates P < 0.05; ** indicates P < 0.01; *** indicates P < 0.001.
Results
Demographic characteristics
The demographic variables of the patients—including sex, age, histologic grade, angiolymphatic invasion, perineural invasion, CEA and CA19–9 levels, and TNM stage—are summarized in Table 1. Patients with advanced stages showed higher pathologic N stages and increased angiolymphatic invasion. We divided our patient population into two groups based on the presence (high risk) or absence (low risk) of specific clinicopathological risk factors, that included poorly differentiated histology, lymphovascular invasion, perineural invasion, T4 stage, and preoperative CEA levels >5 ng/mL and CA19–9 levels >37 U/mL.
Table 1.
Demographic and baseline clinicopathologic characteristics.
| Total | Stage I/II | Stage III/IV | p-value | Low risk group | High risk group | p-value | |
|---|---|---|---|---|---|---|---|
| N(%) | N(%) | N(%) | N(%) | N(%) | |||
| Total | 45 | 23 | 22 | 15 | 30 | ||
| Age(median years, 64; 95 % CI, 61–67) | 0.051 | 0.205 | |||||
| < 65 y/o | 24(53) | 9(39) | 15(68) | 6(40) | 18(60) | ||
| > = 65 y/o | 21(47) | 14(61) | 7(32) | 9(60) | 12(40) | ||
| Gender | 0.1 | 0.673 | |||||
| Female | 22(49) | 14(61) | 8(36) | 8(53) | 14(47) | ||
| male | 23(51) | 9(39 | 14(64) | 7(47) | 16(53) | ||
| Histology grade | 0.274 | 0.138 | |||||
| low | 41(91) | 22(96) | 19(86) | 15(100) | 26(87) | ||
| High | 4(9) | 1(4) | 3(14) | 0(0) | 4(13) | ||
| pathologic T stage* | 0.515 | 0.001 | |||||
| T1 | 4(9) | 3(13) | 1(5) | 3(20) | 1(3) | ||
| T2 | 4(9) | 3(13) | 1(5) | 4(27) | 0(0) | ||
| T3 | 29(64) | 13(57) | 16(72) | 8(53) | 21(72) | ||
| T4 | 8(18) | 4(17) | 4(18) | 0(0) | 8(27) | ||
| pathologic N stage* | < 0.001 | 0.105 | |||||
| N0 | 23(51) | 23(100) | 0(0) | 11(73) | 12(40) | ||
| N1 | 15(33) | 0(0) | 15(68) | 3(20) | 12(40) | ||
| N2 | 7(16) | 0(0) | 7(32) | 1(7) | 6(20) | ||
| M stage* | <0.015 | 0.094 | |||||
| M0 | 40(89) | 23(100) | 17(77) | 15(100) | 25(83) | ||
| M1 | 5(11) | 0(0) | 5(23) | 0(0) | 5(17) | ||
| Angiolymphatic invasion | < 0.001 | 0.001 | |||||
| absent | 31(69) | 22(96) | 9(41) | 15(100) | 16(53) | ||
| present | 14(31) | 1(4) | 13(59) | 0(0) | 14(47) | ||
| Perineural invasion(PNI) | 0.425 | 0.011 | |||||
| absent | 35(78) | 19(83) | 16(73) | 15(100) | 20(67) | ||
| present | 10(22) | 4(17) | 6(27) | 0(0) | 10(33) | ||
| Preop CEA | 0.053 | <0.001 | |||||
| <= 5 ng/ml | 25(56) | 16(70) | 9(41) | 15(100) | 10(33) | ||
| > 5 ng/ml | 20(44) | 7(30) | 13(59) | 0(0) | 20(67) | ||
| Preop CA199 | 0.053 | 0.018 | |||||
| <=37U/ML | 36(80) | 21(91) | 15(68) | 15(100) | 21(70) | ||
| >37U/ML | 9(20) | 2(9) | 8(32) | 0(0) | 9(30) | ||
| Preop N/L ratio | 0.666 | 12(80) | 22(73) | 0.624 | |||
| <3 | 34(76) | 18(78) | 16(73) | 3(20) | 8(27) | ||
| >=3 | 11(24) | 5(22) | 6(27) | ||||
| Blood drawing | |||||||
| Baseline | 45 | 23 | 22 | 15 | 30 | ||
| POD3 | 43 | 22 | 21 | 15 | 28 | ||
| POD30 | 45 | 23 | 22 | 15 | 30 |
P values were derived by using Chi-square test.
CI, confidence interval; CEA, carcinoembryonic antigen; CA19–9, carbohydrate antigen 19–9; AJCC, the American Joint Committee Cancer; POD, postoperative day; N/L ration, Neutrophil/Lymphocyte ration
AJCC (American Joint Committee Cancer) TNM stage, 7th edition
NKA at the preoperative period, POD3, and POD30
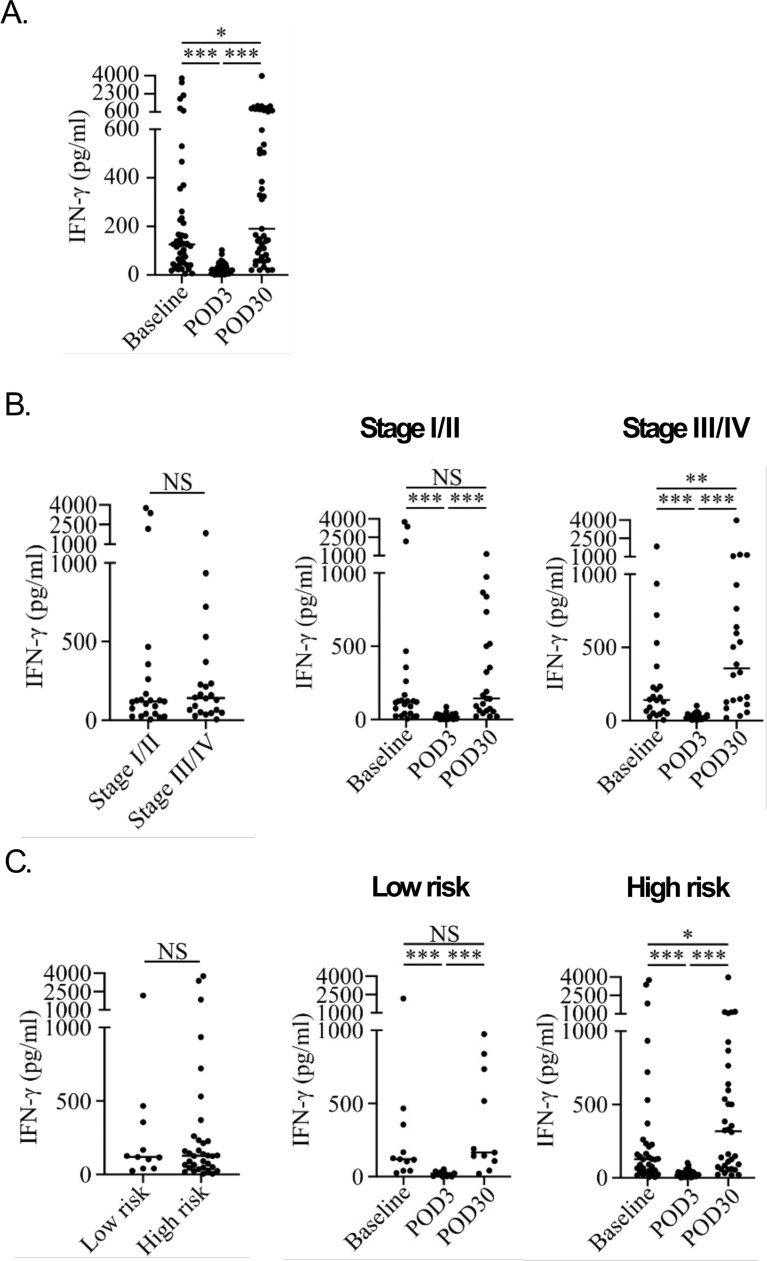
As illustrated in Supplementary Figure 1, CRC patients exhibited significantly lower NKA than healthy subjects (21.8 pg/mL vs. 874.0 pg/mL, p = 0.0013), indicating a severe impairment of NKA in CRC patients. To assess the impact of surgery on NKA, we measured NKA levels before surgery (baseline) and on POD3 and POD30, as shown in Fig. 1A. NKA was significantly reduced on POD3 (median: 20.9 pg/mL, 95 % CI: 16.1–30.2) compared to the baseline (median: 125.8 pg/mL, 95 % CI: 75.23–163.4), with a median difference of -92.1 pg/mL (95 % CI: -163.4 to -40.24). Conversely, NKA showed a marked recovery on POD30 (median: 190.1 pg/mL, 95 % CI: 126.5–503.4), significantly higher than the baseline and POD3 levels. The median differences in NKA on POD30 compared to POD3 and the baseline were 160.7 pg/mL (95 % CI: 72.83–491.1) and 40.96 pg/mL (95 % CI: -1.4 to 238.4), respectively. The average follow-up duration was 27.5 months (standard deviation: 6.5 months, range: 9.6–34.8 months).
Fig. 1.
NKA expression in the perioperative period based on stage and risk factors. (A) NKA was downregulated after surgery on POD3 and recovered on POD30. The NK VUE ELISA assay was used to measure NKA levels in the peripheral blood of patients with CRC. (B) NKA was upregulated at the recovery stage (POD30) when compared to baseline in patients with stage III/IV CRC. (C) NKA was significantly upregulated on POD30 in patients with high-risk factors. (Data were statistically analyzed using the two-tailed Mann–Whitney U test and two-tailed Wilcoxon matched-pairs signed-rank test and are presented as a scatter plot with the median. *P < 0.05, **P < 0.01, ***P < 0.001); NKA: natural killer cell activity; CRC: colorectal cancer.
Higher recovery of NKA in patients with advanced tumor stages and preoperative high-risk factors
Patients were classified into early (stage I/II) and advanced (stage III/IV) stages according to the American Joint Committee on Cancer (AJCC) staging system. At baseline, no significant differences in NKA were observed between the two groups (stage I/II, median: 118.9 pg/mL, 95 % CI: 41.1–167.2; stage III/IV, median: 141.5 pg/mL, 95 % CI: 50.4–234) as shown in Fig. 2A. However, NKA was significantly higher on POD30 (median: 356.5 pg/mL, 95 % CI: 126.5–764.1) than at baseline (median: 141.5 pg/mL, 95 % CI: 50.4–234) in patients with stage III/IV CRC (baseline, median: 118.9 pg/mL, 95 % CI: 41.1–167.2; POD30, median: 143.7 pg/mL, 95 % CI: 61.6–500.3) (Fig. 2B and C, Supplementary Table 1). NKA was dramatically decreased on POD3 in patients with either stage I/II (median: 18.3 pg/mL, 95 % CI: 3.9–33.1) or stage III/IV (median: 21.01 pg/mL, 95 % CI: 17–44.9) CRC (Fig. 1B, Supplementary Table 1).
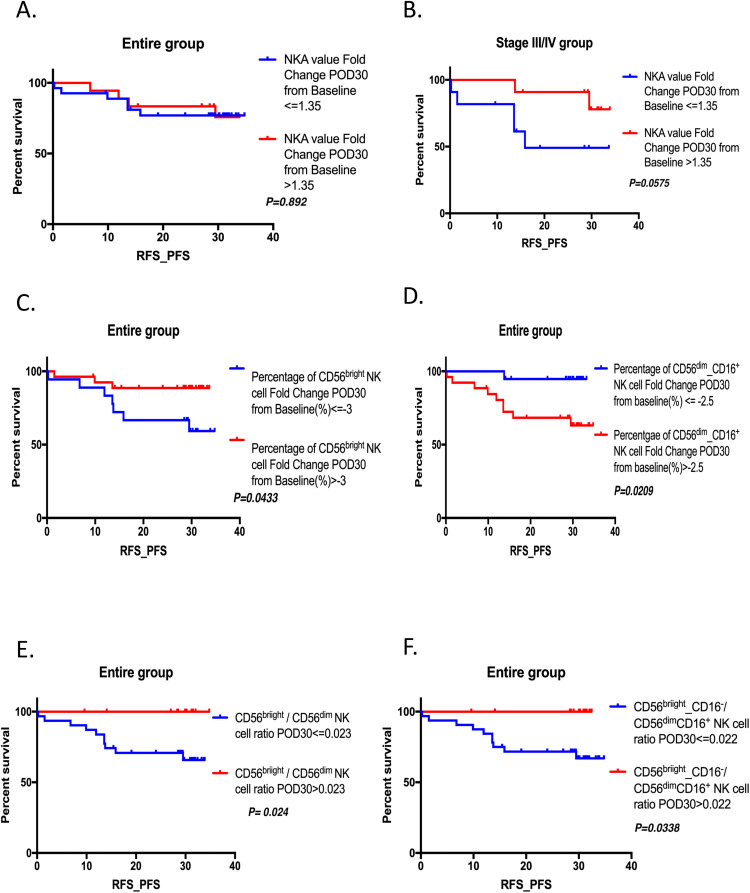
Fig. 2.
Relationship between perioperative change of circulating lymphocyte subsets with varying immunophenotypes and recurrence-free survival (RFS)/progression‐free survival (PFS) in the entire group. (A) RFS/PFS for NKA recovery on POD30 in the entire group. (B) RFS/PFS for NKA recovery on POD30 in the stage III/IV group. (C) RFS/PFS for postoperative increment of percentage of CD56bright NK cell on POD30 in the entire group. (D) RFS/PFS for postoperative increment of percentage of CD56 dim_ CD16+ NK cell on POD30 in the entire group. (E) RFS/PFS for postoperative circulating CD56bright NK cell/CD56dim NK cell ratio in the entire group. (F) RFS/PFS for postoperative circulating CD56bright_CD16− NK cell/CD56 dim_ CD16+ cell ratio in the entire group.
Further investigation into whether clinicopathological risk factors were correlated with the postoperative recovery of NKA on POD30 revealed that although there was no statistical difference in NKA between low-risk and high-risk groups at baseline (low risk, median: 118.9 pg/mL, 95 % CI: 40.6–466.8; high risk, median: 127.8 pg/mL, 95 % CI: 65.4–213.5), NKA on POD30 was significantly higher (median: 317.7 pg/mL, 95 % CI: 92.2–537.2) compared to baseline in high-risk patients (Fig. 1C, Supplementary Table 1). In contrast, in low-risk patients, NKA showed no significant change from baseline to POD30 (median: 165 pg/mL, 95 % CI: 41.4–838.4) (Fig. 1C, Supplementary Table 1). Regardless of risk status, NKA was markedly decreased on POD3 compared with baseline (low-risk, median: 22.46 pg/mL, 95 % CI: 2.3–36.4; high-risk, median: 20.3 pg/mL, 95 % CI: 16.1–35.6) (Fig. 1A and B, Supplementary Table 1). During the perioperative period, postoperative increments in circulating CD56dim NK cells were significantly positively correlated with increments in CD56+ NK cells (r: 0.75, P = 0.00) but negatively with increments in CD56bright NK cells (r: -0.304, P = 0.043). However, postoperative recovery of NKA was not associated with postoperative increments in circulating NK cell subsets.
Association between clinicopathologic factors and perioperative change of NKA and circulating NK cell subset
Patients with a higher postoperative increase in the percentage of CD56bright NK cells were younger. Additionally, those with a greater postoperative increase in the percentage of CD56bright/CD16− NK cells had lower preoperative CA19–9 levels. Although patients with a higher histological grade exhibited a higher postoperative circulating CD56bright NK cell/CD56dim NK cell ratio, the sample size is too small to draw definitive conclusions; thus, further patient enrollment is necessary to confirm these findings (Table 2).
Table 2.
Association between clinicopathologic factors and Perioperative change of NKA and Circulating NK Cell subset.
P values were derived by using Chi-square test; NK cell, Nature Killer cell; NKA, Nk cell activity; POD, postoperaive day
Association between RFS/PFS and perioperative change of NKA and circulating NK cell subset
In the entire group, postoperative NKA recovery was not significantly associated with RFS or PFS in patients with CRC (Fig. 2A). However, in patients with stage III/IV disease, those with higher postoperative NKA recovery on POD30 demonstrated improved RFS and PFS (Fig. 2B, HR: 0.2442). Functional NK cell subsets contribute to NKA, including CD56bright/CD16− and CD56dim/CD16+ cells. Therefore, we analyzed the association between perioperative changes in the percentage or postoperative ratio of circulating functional NK cell subsets and RFS/PFS. A higher postoperative increase in the percentage of CD56bright NK cells on POD30 was associated with better RFS/PFS (Fig. 2C, HR: 0.2732, P = 0.0433). Conversely, a higher postoperative increase in the percentage of CD56 dim/CD16+ NK cells was linked to worse survival outcomes (Fig. 2D, HR: 7.752, P = 0.0209). Patients with a higher postoperative circulating CD56bright NK cell/CD56dim NK cell ratio on POD30 also experienced better RFS/PFS (Fig. 2E, HR: 0.2193, P = 0.024), similar to the CD56bright_CD16− NK cells / CD56 dim_ CD16+ NK cells ratio group (Fig. 2F, HR: 0.233, P = 0.0338).
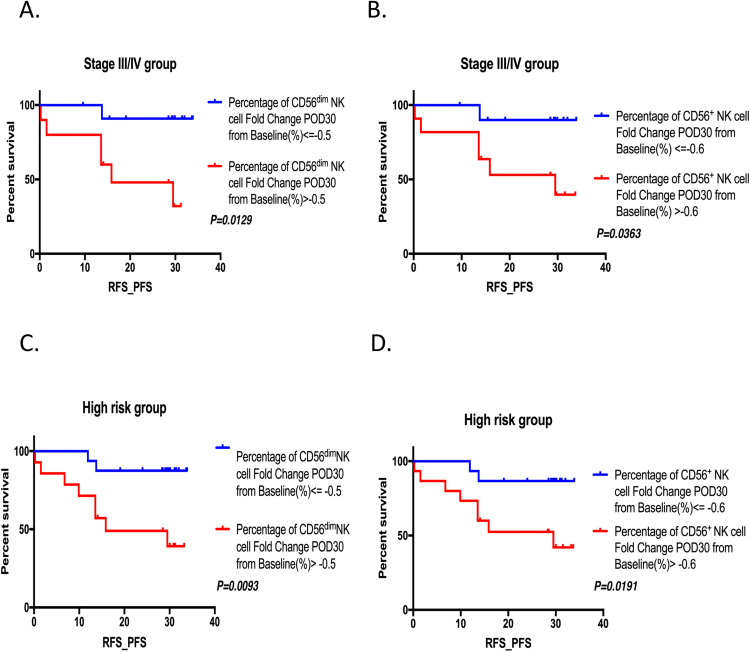
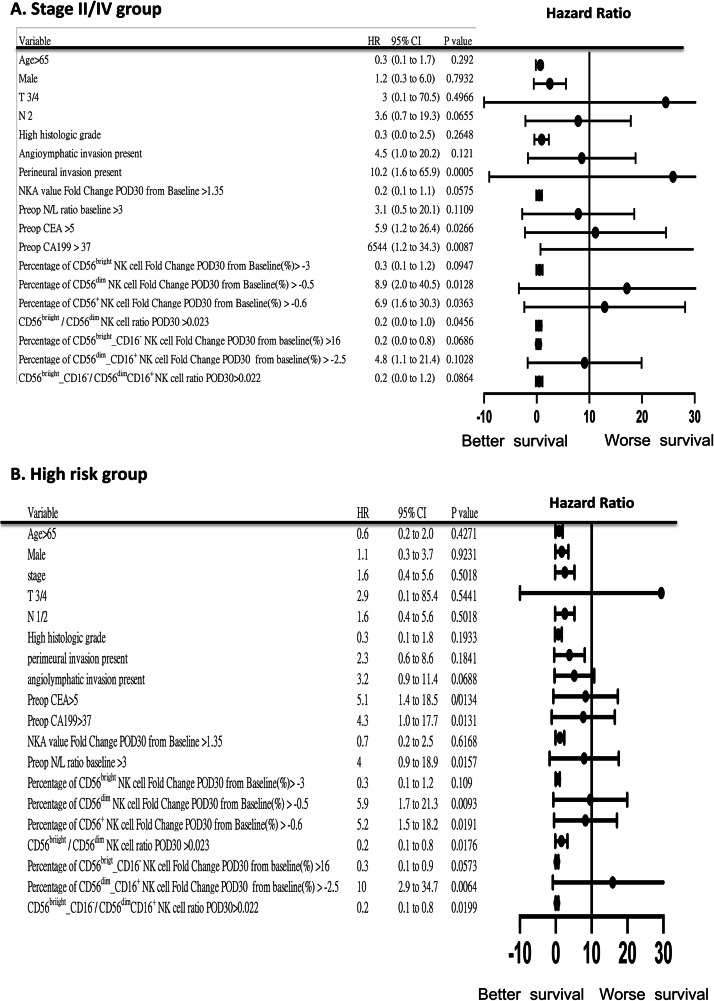
Compared with the entire group, higher postoperative increments in the percentage of CD56dim NK and CD56+ NK cells on POD30 were significantly associated with poorer RFS/PFS in patients with advanced-stage CRC (Fig. 3A, HR: 8.945, P = 0.0129 and Fig. 3B, HR: 6.868, P = 0.0363) and in those with preoperative high-risk factors (Fig. 3C, HR: 5.897, P = 0.0093 and Fig. 3D, HR: 5.192, P = 0.0191). Further multivariable analysis of clinicopathological and NK cell subset parameters identified no independent prognostic markers for RFS/PFS (Supplementary Table 2).
Fig. 3.
Relationship between perioperative change of circulating lymphocyte subsets with varying immunophenotypes and recurrence-free survival (RFS)/progression‐free survival (PFS) in the stage II/IV group and high-risk group. (A) RFS/PFS for postoperative increments of percentage of CD56dim NK cell on POD30 in stage III/IV group. (B) RFS/PFS for postoperative increments of percentage of CD56+ NK cell on POD30 in stage III/IV group. (C) RFS/PFS for postoperative increments of percentage of CD56dim NK cell on POD30 in preoperative high-risk group (D) RFS/PFS for postoperative increments of percentage of CD56+ NK cell on POD30 in preoperative high-risk group.
Subgroup analysis of RFS/PFS in the entire group and groups stratified by preoperative risk and stage
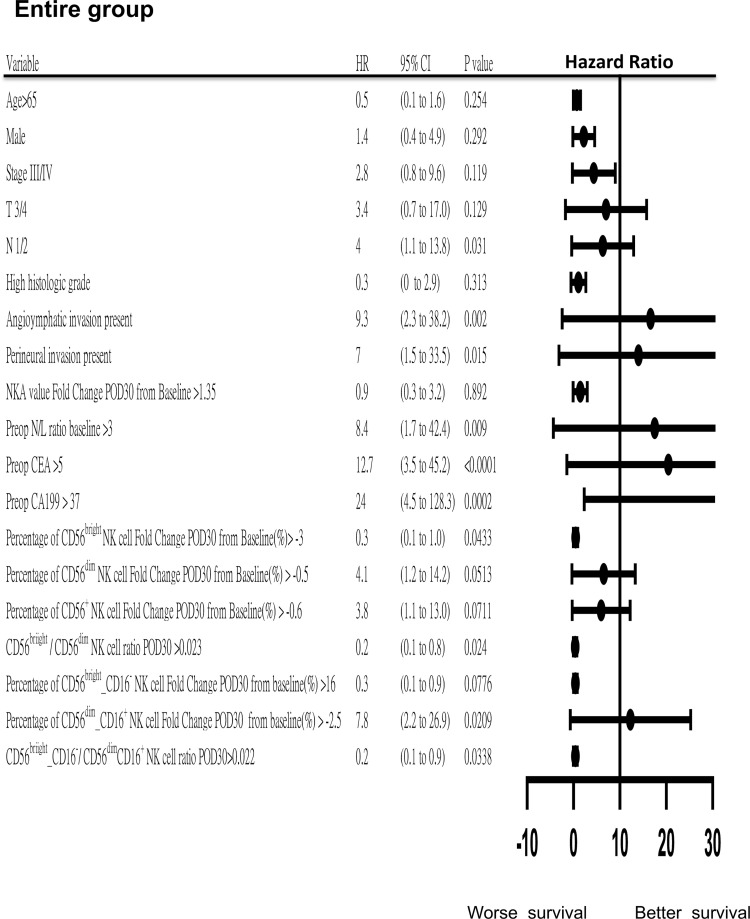
A higher postoperative circulating CD56bright NK cell/CD56dim NK cell ratio on POD30 was associated with improved RFS and PFS across the entire group (Fig. 4), as well as in patients with stage III/IV disease and those classified as preoperative high-risk (Fig. 5A and B). This trend was similarly observed in the CD56bright_CD16− NK cell/CD56dim_CD16+ cell group. Additionally, higher postoperative NKA recovery was linked to better RFS/PFS in the stage III/IV group (Fig. 5A). Other clinical factors such as T stage, N stage, preoperative levels of CEA and CA19–9, angiolymphatic invasion, perineural invasion, and preoperative NLR also showed significant associations with RFS/PFS in the whole group (Fig. 4).
Fig. 4.
Forest plot of hazard ratios (HRs) with 95 % confidence intervals (CI) for recurrence-free survival (RFS)/ progression‐free survival (PFS) in the entire group.
Fig. 5.
Forest plot of hazard ratios (HRs) with 95 % confidence intervals (CI) for recurrence-free survival (RFS)/ progression‐free survival (PFS) in the stage III/IV group and high-risk group, (A) Stage III/IV groups. (B) high-risk group.
Discussion
This study assessed changes in NKA before and after tumor resection and examined the association between preoperative risk factors and NKA in patients with CRC. We measured the levels of IFN-γ secreted by NK cells in patients with CRC preoperatively and postoperatively. We found that NKA was significantly reduced on POD3 and recovered to at least baseline levels by POD30. Notably, on POD30, NKA was significantly higher compared to preoperative levels, particularly in patients diagnosed with advanced-stage disease or those with one or more preoperative high-risk factors. Additionally, a greater recovery of NKA in these patients was associated with improved RFS and PFS. A higher CD56bright NK cell/CD56dim NK cell ratio on POD30 was also significantly correlated with better RFS/PFS in patients with CRC. These findings suggest that the degree of NKA upregulation following surgery-induced suppression is linked to tumor stage and preoperative high-risk characteristics, highlighting the importance of postoperative CD56bright NK cells and NKA in the clinical outcomes of patients with CRC after curative surgery and providing a comprehensive overview of the immune response.
Numerous preclinical and clinical studies have reported that surgical manipulation can enhance metastatic disease and shorten overall survival [[34.], [35.], [36.], [37], [38.]]. In contrast, NK cell-secreted IFN-γ has been shown to play a crucial role in anti-metastasis [[39.], [40.], [41.]], promoting the accumulation, activation, and cytotoxic ability of NK cells to eradicate metastatic cancer cells. Additionally, depletion of IFN-γ significantly enhances metastasis in a mouse model [39,41]. NK cells are also essential for eliminating circulating tumor cells [42]. Furthermore, evidence suggests that surgery-induced NK cell dysfunction is strongly linked to postoperative metastasis and recurrence [43,44]. Perioperative measures to prevent surgery-suppressed NKA can reduce postoperative metastatic disease and recurrence [43,45]. These findings underscore the indispensable roles of IFN-γ and NK cells in preventing postoperative metastasis. In our study, we confirmed that NKA was markedly suppressed in patients with CRC three days after surgical resection, likely due to surgical stress. However, NKA recovered by POD30 and even increased significantly compared to baseline levels. The NK VUE ELISA, an alternative assay, proved to be a powerful and convenient tool for measuring NKA in peripheral blood [46]. Unlike traditional methods such as tumor killing and degranulation assays, its high-throughput, time-efficient, and reproducible characteristics make it well-suited for clinical practice.
Some clinicopathological risk factors are associated with poor prognosis, recurrence, metastasis, and decreased survival in cancer patients. Clinicians often consider these risk factors when making treatment decisions. In this study, we evaluated commonly used risk factors, such as poorly differentiated histology, lymphovascular invasion, perineural invasion, T4 stage, and elevated tumor markers (CEA and CA19–9), to explore their relationship with NKA during the perioperative period of tumor resection. Our results indicated that preoperative high-risk factors correlated with NKA recovery on POD30, with NKA increasing on POD30 compared to baseline in patients with high-risk factors. Similarly, tumor stage—whether early or advanced—was associated with NKA recovery on POD30, with a significant increase in NKA on POD30 compared to baseline observed in patients with advanced tumor stages. Consequently, NKA may serve as an adjunct marker for determining postoperative therapy in clinical practice, though this requires further evaluation.
Our findings demonstrated that NKA was suppressed on POD3 and returned to at least baseline levels by POD30, regardless of tumor stage or risk factors. Patients with advanced tumor stages showed notable NKA recovery on POD30 compared to the preoperative baseline levels. Additionally, patients who exhibited higher NKA recovery on POD30 had better RFS and PFS. This suggests that NKA is crucial for managing relatively advanced CRC tumors. The P-value was not significant, possibly due to inadequate follow-up time and a limited number of patients. Another factor is that IFN-γ, while a key contributor to NKA, is not the sole mediator; other cytokines such as TNF-α, IL-2, IL-12, IL-15, and IL-18 also play roles in inducing NK ’cell cytotoxicity [47]. Therefore, it is necessary to utilize a convenient method such as the whole blood NK cytotoxicity assay for clinical laboratory research, such as the flow cytometry-based overnight whole blood NK cytotoxicity assay [48].
CD56bright NK cells are now recognized as more than just a minor subpopulation among total NK cells. Due to their ability to produce various cytokines, they play a critical role in early immune responses and in shaping the adaptive immune response through IFN-γ and act as regulatory NK cells through interleukin-10 (IL-10) [49]. In comparison with CD56dim NK cells, human CD56bright NK cells uniquely contribute to the innate immune response as the primary producers of immunoregulatory cytokines such as IFN-γ, thereby regulating NKA [50]. Our findings indicate that a higher CD56bright NK cell/CD56dim NK cell ratio and increased percentages of circulating CD56dim NK cells on POD30 were significantly associated with improved RFS and PFS in patients with CRC. Conversely, higher postoperative increments of circulating CD56dim NK and CD56+ NK cells on POD30 were significantly associated with poorer RFS/PFS. These results underscore the prognostic importance of CD56bright NK cells and their role in regulating NKA.
This study has several limitations. First, the small number of cases limited further exploration of potential confounders associated with NKA recovery after CRC surgery. Second, despite the clinical significance of NKA recovery on POD30 in patients with advanced tumor stages and high-risk factors, the underlying mechanisms remain poorly understood. Therefore, prospective studies with larger sample sizes and the inclusion of healthy controls are warranted to elucidate these findings further.
Conclusion
Our results reveal that a higher postoperative increase in the percentage/ratio of CD56bright NK cells on POD30 in CRC patients correlates with better clinical outcomes and enhanced recovery of NKA in those with advanced-stage disease. These findings not only offer potential implications for the use of postoperative NKA and circulating NK cell subset analyses in patient risk stratification and the development of treatment strategies but also provide a foundation for CRC research. This includes molecular classification to guide precision therapy by interpreting NKA data and analysis of NK cell subsets. Further research is necessary to confirm the role and function of recovered NK cells following the surgical removal of CRC tumors and to investigate potential confounding factors that may affect NKA recovery.
CRediT authorship contribution statement
Jeng-Fu You: Writing – review & editing, Writing – original draft, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Cheng-Chi Lee: Resources, Methodology, Investigation, Conceptualization. Yun-Shien Lee: Resources, Methodology, Investigation, Formal analysis, Data curation. Yih-Jong Chern: Resources, Investigation, Data curation. Chun-Kai Liao: Software, Resources, Formal analysis, Data curation. Hung-Chih Hsu: Writing – review & editing, Writing – original draft, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgements
We acknowledge the Chang Gung Memorial Hospital and University for their support in this research. This work was supported by grants from Chang Gung Memorial Hospital (CMRPG3J1531 to Jeng-Fu You).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102198.
Appendix. Supplementary materials
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834. May-Jun. [DOI] [PubMed] [Google Scholar]
- 2.Ueno H., Mochizuki H., Hashiguchi Y., Ishiguro M., Kajiwara Y., Sato T., Shimazaki H., Hase K., Talbot I.C. Histological grading of colorectal cancer: a simple and objective method. Ann. Surg. 2008;247:811–818. doi: 10.1097/SLA.0b013e318167580f. [DOI] [PubMed] [Google Scholar]
- 3.Akagi Y., Adachi Y., Ohchi T., Kinugasa T., Shirouzu K. Prognostic impact of lymphatic invasion of colorectal cancer: a single-center analysis of 1,616 patients over 24 years. Anticancer Res. 2013;33:2965–2970. [PubMed] [Google Scholar]
- 4.Cienfuegos J.A., Martinez P., Baixauli J., Beorlegui C., Rosenstone S., Sola J.J., Rodriguez J., Hernandez-Lizoain J.L. Perineural Invasion is a Major Prognostic and Predictive Factor of Response to Adjuvant Chemotherapy in Stage I-II Colon Cancer. Ann. Surg. Oncol. 2017;24:1077–1084. doi: 10.1245/s10434-016-5561-0. [DOI] [PubMed] [Google Scholar]
- 5.Li Destri G., Rubino A.S., Latino R., Giannone F., Lanteri R., Scilletta B., Di Cataldo A. Preoperative carcinoembryonic antigen and prognosis of colorectal cancer. An independent prognostic factor still reliable. Int. Surg. 2015;100:617–625. doi: 10.9738/INTSURG-D-14-00100.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahin TK, Rizzo A, Aksoy S, Guven DC. Prognostic Significance of the Royal Marsden Hospital (RMH) Score in Patients with Cancer: A Systematic Review and Meta-Analysis. Cancers. (Basel) 2024;16(10):1835. doi: 10.3390/cancers16101835. May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guven DC, Sahin TK, Erul E, Kilickap S, Gambichler T, Aksoy S. The association between the pan-immune-inflammation value and cancer prognosis: a systematic review and meta-analysis. Cancers. (Basel) 2022;14(11):2675. doi: 10.3390/cancers14112675. May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misiewicz A, Fashionable Dymicka-Piekarska V. but What is their real clinical usefulness? nlr, lmr, and plr as a promising indicator in colorectal cancer prognosis: a systematic review. J. Inflamm. Res. 2023;16:69–81. doi: 10.2147/JIR.S391932. Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandi G, Ricci AD, Rizzo A, Zanfi C, Tavolari S, Palloni A, De Lorenzo S, Ravaioli M, Cescon M. Is post-transplant chemotherapy feasible in liver transplantation for colorectal cancer liver metastases? Cancer Commun. (Lond) 2020;40(9):461–464. doi: 10.1002/cac2.12072. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzo A, Santoni M, Mollica V, Logullo F, Rosellini M, Marchetti A, Faloppi L, Battelli N, Massari F. Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: the MOUSEION-02 study. Expert. Opin. Drug Metab. Toxicol. 2021;17(12):1455–1466. doi: 10.1080/17425255.2021.2029405. Dec. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo A, Mollica V, Tateo V, Tassinari E, Marchetti A, Rosellini M, De Luca R, Santoni M, Massari F. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol. Immunther. 2023;72(6):1381–1394. doi: 10.1007/s00262-023-03366-x. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guven DC, Erul E, Kaygusuz Y, Akagunduz B, Kilickap S, De Luca R, Rizzo A. Immune checkpoint inhibitor-related hearing loss: a systematic review and analysis of individual patient data. Support. Care Cancer. 2023;31(12):624. doi: 10.1007/s00520-023-08083-w. Oct 11. [DOI] [PubMed] [Google Scholar]
- 13.Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E., Lichtor T., Decker W.K., Whelan R.L., Kumara H., Signori E., Honoki K., Georgakilas A.G., Amin A., Helferich W.G., Boosani C.S., Guha G., Ciriolo M.R., Chen S., Mohammed S.I., Azmi A.S., Keith W.N., Bilsland A., Bhakta D., Halicka D., Fujii H., Aquilano K., Ashraf S.S., Nowsheen S., Yang X., Choi B.K., Kwon B.S. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. Suppl. [DOI] [PubMed] [Google Scholar]
- 14.Papaioannou N.E., Beniata O.V., Vitsos P., Tsitsilonis O., Samara P. Harnessing the immune system to improve cancer therapy. Ann. Transl. Med. 2016;4:261. doi: 10.21037/atm.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angka L., Martel A.B., Kilgour M., Jeong A., Sadiq M., de Souza C.T., Baker L., Kennedy M.A., Kekre N., Auer R.C. Natural killer cell ifngamma secretion is profoundly suppressed following colorectal cancer surgery. Ann. Surg. Oncol. 2018;25:3747–3754. doi: 10.1245/s10434-018-6691-3. [DOI] [PubMed] [Google Scholar]
- 16.Prager I., Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019;105:1319–1329. doi: 10.1002/JLB.MR0718-269R. [DOI] [PubMed] [Google Scholar]
- 17.Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., Yokoyama W.M., Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science (1979) 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper M.A., Fehniger T.A., Turner S.C., Chen K.S., Ghaheri B.A., Ghayur T., Carson W.E., Caligiuri M.A. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 19.Cooper M.A., Fehniger T.A., Caligiuri M.A. The biology of human natural killer-cell subsets. Trends. Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 20.Caligiuri M.A. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poli A., Michel T., Theresine M., Andres E., Hentges F., Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalbeth N., Gundle R., Davies R.J., Lee Y.C., McMichael A.J., Callan M.F. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J. Immunol. 2004;173:6418–6426. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 23.Burke J.D., Young H.A. IFN-gamma: a cytokine at the right time, is in the right place. Semin. Immunol. 2019;43 doi: 10.1016/j.smim.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R., Jaw J.J., Stutzman N.C., Zou Z., Sun P.D. Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1. J. Leukoc. Biol. 2012;91:299–309. doi: 10.1189/jlb.0611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui F., Qu D., Sun R., Zhang M., Nan K. NK cell-produced IFN-gamma regulates cell growth and apoptosis of colorectal cancer by regulating IL-15. Exp. Ther. Med. 2020;19:1400–1406. doi: 10.3892/etm.2019.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kundu M., Roy A., Pahan K. Selective neutralization of IL-12 p40 monomer induces death in prostate cancer cells via IL-12-IFN-gamma. Proc. Natl. Acad. Sci. u S. a. 2017;114:11482–11487. doi: 10.1073/pnas.1705536114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao Q., Tang H. Interferon-gamma and Smac mimetics synergize to induce apoptosis of lung cancer cells in a TNFalpha-independent manner. Cancer Cell Int. 2018;18:84. doi: 10.1186/s12935-018-0579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal D., Vijayan D., Putz E.M., Aguilera A.R., Markey K.A., Straube J., Kazakoff S., Nutt S.L., Takeda K., Hill G.R., Waddell N., Smyth M.J. Interleukin-12 from CD103(+) Batf3-dependent dendritic cells required for nk-cell suppression of metastasis. Cancer Immunol. Res. 2017;5:1098–1108. doi: 10.1158/2326-6066.CIR-17-0341. [DOI] [PubMed] [Google Scholar]
- 29.Glasner A., Levi A., Enk J., Isaacson B., Viukov S., Orlanski S., Scope A., Neuman T., Enk C.D., Hanna J.H., Sexl V., Jonjic S., Seliger B., Zitvogel L., Mandelboim O. NKp46 receptor-mediated interferon-gamma production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity. 2018;48:107–119. doi: 10.1016/j.immuni.2017.12.007. e104. [DOI] [PubMed] [Google Scholar]
- 30.Bhat P., Leggatt G., Waterhouse N., Frazer I.H. Interferon-gamma derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death. Dis. 2017;8:e2836. doi: 10.1038/cddis.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baer C., Squadrito M.L., Laoui D., Thompson D., Hansen S.K., Kiialainen A., Hoves S., Ries C.H., Ooi C.H., De Palma M. Suppression of microRNA activity amplifies IFN-gamma-induced macrophage activation and promotes anti-tumour immunity. Nat. Cell Biol. 2016;18:790–802. doi: 10.1038/ncb3371. [DOI] [PubMed] [Google Scholar]
- 32.Lee CC, You JF, Wang YC, Lan SW, Wei KC, Chen KT, Huang YC, Wu TE, Huang AP. Gross total resection promotes subsequent recovery and further enhancement of impaired natural killer cell activity in glioblastoma patients. Brain Sci. 2022;12(9):1144. doi: 10.3390/brainsci12091144. Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. Epub 2012 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi K., Takagi Y., Aoki S., Futamura M., Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann. Surg. 2000;232:58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuchiya Y., Sawada S., Yoshioka I., Ohashi Y., Matsuo M., Harimaya Y., Tsukada K., Saiki I. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547–555. doi: 10.1067/msy.2003.141. [DOI] [PubMed] [Google Scholar]
- 36.Glasner A., Avraham R., Rosenne E., Benish M., Zmora O., Shemer S., Meiboom H., Ben-Eliyahu S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J. Immunol. 2010;184:2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 37.Lerut T., Moons J., Coosemans W., Van Raemdonck D., De Leyn P., Decaluwe H., Decker G., Nafteux P. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann. Surg. 2009;250:798–807. doi: 10.1097/SLA.0b013e3181bdd5a8. [DOI] [PubMed] [Google Scholar]
- 38.Eberhardt J.M., Kiran R.P., Lavery I.C. The impact of anastomotic leak and intra-abdominal abscess on cancer-related outcomes after resection for colorectal cancer: a case control study. Dis. Colon Rectum. 2009;52:380–386. doi: 10.1007/DCR.0b013e31819ad488. [DOI] [PubMed] [Google Scholar]
- 39.Lin Q., Rong L., Jia X., Li R., Yu B., Hu J., Luo X., Badea S.R., Xu C., Fu G., Lai K., Lee M.C., Zhang B., Gong H., Zhou N., Chen X.L., Lin S.H., Fu G., Huang J.D. IFN-gamma-dependent NK cell activation is essential to metastasis suppression by engineered Salmonella. Nat. Commun. 2021;12:2537. doi: 10.1038/s41467-021-22755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda K., Nakayama M., Sakaki M., Hayakawa Y., Imawari M., Ogasawara K., Okumura K., Smyth M.J. IFN-gamma production by lung NK cells is critical for the natural resistance to pulmonary metastasis of B16 melanoma in mice. J. Leukoc. Biol. 2011;90:777–785. doi: 10.1189/jlb.0411208. [DOI] [PubMed] [Google Scholar]
- 41.Dyck L., Lynch L. New Job for NK Cells: Architects of the Tumor Microenvironment. Immunity. 2018;48:9–11. doi: 10.1016/j.immuni.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Brodbeck T., Nehmann N., Bethge A., Wedemann G., Schumacher U. Perforin-dependent direct cytotoxicity in natural killer cells induces considerable knockdown of spontaneous lung metastases and computer modelling-proven tumor cell dormancy in a HT29 human colon cancer xenograft mouse model. Mol. Cancer. 2014;13:244. doi: 10.1186/1476-4598-13-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tai L.H., de Souza C.T., Belanger S., Ly L., Alkayyal A.A., Zhang J., Rintoul J.L., Ananth A.A., Lam T., Breitbach C.J., Falls T.J., Kirn D.H., Bell J.C., Makrigiannis A.P., Auer R.A. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013;73:97–107. doi: 10.1158/0008-5472.CAN-12-1993. [DOI] [PubMed] [Google Scholar]
- 44.Market M., Tennakoon G., Auer R.C. Postoperative Natural killer cell dysfunction: the prime suspect in the case of metastasis following curative cancer surgery. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tai L.H., Zhang J., Auer R.C. Preventing surgery-induced NK cell dysfunction and cancer metastases with influenza vaccination. Oncoimmunology. 2013;2:e26618. doi: 10.4161/onci.26618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S.B., Cha J., Kim I.K., Yoon J.C., Lee H.J., Park S.W., Cho S., Youn D.Y., Lee H., Lee C.H., Lee J.M., Lee K.Y., Kim J. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem. Biophys. Res. Commun. 2014;445:584–590. doi: 10.1016/j.bbrc.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 47.Ghazvinian Z, Abdolahi S, Tokhanbigli S, Tarzemani S, Piccin A, Reza Zali M, Verdi J, Baghaei K. Contribution of natural killer cells in innate immunity against colorectal cancer. Front. Oncol. 2023 Jan 4;12 doi: 10.3389/fonc.2022.1077053. PMID: 36686835; PMCID: PMC9846259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Phan MT, Kweon S, Yu H, Park J, Kim KH, Hwang I, Han S, Kwon MJ, Cho D. A Flow cytometry-based whole blood natural killer cell cytotoxicity assay using overnight cytokine activation. Front. Immunol. 2020;11:1851. doi: 10.3389/fimmu.2020.01851. Aug 14PMID: 32922399; PMCID: PMC7457041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. doi: 10.1111/j.1365-2567.2008.03027.x. AprPMID: 19278419; PMCID: PMC2673358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. May 15PMID: 11342442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.