Abstract
Actively avoiding danger is necessary for survival. Most research on active avoidance has focused on the behavioral and neurobiological processes when individuals learn to avoid alone, within a solitary context. Therefore, little is known about how social context affects active avoidance. Using a modified version of the platform-mediated avoidance task in rats, we investigated whether the presence of a social partner attenuates conditioned freezing and enhances avoidance compared to avoidance in a solitary context. Rats spent a similar amount of time avoiding during either context; however, rats trained in the social context exhibited greater freezing as well as lower rates of darting and food seeking compared to rats trained in the solitary context. In addition, we observed higher levels of avoidance in females compared to males in the solitary context, but this sex difference was not present in rats trained in the social context. To gain greater mechanistic insight, we optogenetically inactivated glutamatergic projection neurons in the anterior cingulate cortex (ACC) following avoidance training in either context. After avoidance was learned in a social context, photoinactivation of ACC reduced expression of avoidance during a test when the social partner was absent, but not when the partner was present. Our findings suggest a novel contribution of the ACC in avoidance that is learned with a social partner, which has translational implications for understanding ACC dysfunction in those suffering from trauma-related disorders.
Keywords: Prefrontal cortex, Fear, Sex differences, Optogenetics
Highlights
-
•
Learning to actively avoid danger in a social context led to increased freezing and decreased darting and food seeking.
-
•
Females increased avoidance and decreased food seeking compared to males when avoiding alone, but not with a partner.
-
•
Optogenetic inactivation of the anterior cingulate cortex reduced avoidance in a social context when the partner was absent.
1. Introduction
The ability to assess danger and respond appropriately is critical for an individual's well-being. Active avoidance is a commonly used strategy to appropriately evade danger but can become maladaptive when avoidance is excessive and impedes the ability to complete daily activities. Excessive avoidance is a hallmark feature in those suffering from anxiety disorders (American Psychiatric Association, 2013). Preclinical studies have investigated the behavioral and neural mechanisms of active avoidance using the platform-mediated avoidance (PMA) task. During this task, rats are trained to avoid a tone-signaled footshock by stepping onto a safe platform, which conflicts with lever-pressing for a sucrose reward (Bravo-Rivera et al., 2014; Diehl et al., 2019). However, prior research using the PMA task has only been conducted when animals learn alone, within a solitary context, leaving much unknown about how a social context may alter avoidance behaviors and their underlying neural mechanisms.
Previous rodent studies have focused on learning about danger from a social partner using various tasks. During social transmission of conditioned fear, a naïve rat that interacts with a fear-conditioned cagemate will subsequently express greater freezing to the conditioned stimulus compared to another naïve rat that interacted with a naïve cagemate (Brandl et al., 2022; Bruchey et al., 2010; Jones and Monfils, 2016). Learning about danger from a social partner has also been demonstrated using observational learning, in which an observer rodent witnesses a demonstrator rodent undergo fear conditioning and is later tested for the same fear responses. Enhanced fear learning via observation has been reported during fear expression (Allsop et al., 2018; Jeon et al., 2010), fear extinction (Brill-Maoz and Maroun, 2016; Gorkiewicz et al., 2023), and expression of shuttle avoidance (Del Russo, 1975; John et al., 1968; Presley and Riopelle, 1959). Altogether, these studies show that fear and avoidance can be learned from a social partner; however, it remains unknown whether avoidance learning is enhanced in a different social context when rodents learn simultaneously compared to when avoidance is learned alone.
The neural circuits regulating active avoidance have been previously characterized in male subjects that learn alone (for review, see Diehl et al., 2019). The prelimbic cortex (PL) is key for avoidance behavior in PMA learned in a solitary context (Diehl et al., 2018). PL signals both the basolateral amygdala (BLA) and the ventral striatum (VS) to bidirectionally control avoidance, allowing the animal to make appropriate decisions while facing competing motivational drives (Diehl et al., 2020). Prior studies have reported that activity in the anterior cingulate cortex (ACC) is correlated with active avoidance in rabbits during a wheel running task (Freeman et al., 1996; Gabriel, 1990). More recent studies demonstrate that the ACC plays a key role for social transmission of fear and observational fear conditioning (for review, see Burgos-Robles et al., 2019; Debiec and Olsson, 2017; Olsson and Phelps, 2007). Despite these advances uncovering the neural substrates of social fear learning, there are no studies investigating the neural substrates of active avoidance acquired within a social context.
The current study had two main goals. First, we were interested in whether avoidance learning is enhanced in a social versus solitary context. To do this, we modified the PMA task so that two rats could perform the task together while maintaining access to their own lever, food dish, and platform. Second, we were interested in whether activity in the ACC is necessary for the expression of active avoidance in this social context. To test this, we optogenetically inactivated ACC neural activity during the tone to determine if avoidance expression is impaired.
2. Materials and methods
2.1. Subjects
173 Adult male and female Sprague Dawley (n = 81 females, n = 92 males from 26 litters) rats were bred in-house from rats purchased from a commercial vendor (Charles River Laboratories, Wilmington, MA) and were 3–5 months old, weighing at least 215 g (females) and 300 g (males) at the start of experiments. Subjects were same-sex housed in groups of 2–3 rats per cage and maintained on a 12 h reverse light cycle (lights off at 0830 h) and handled as previously described (Diehl et al., 2018). All experiments were completed between 0900 and 1800 h during the active dark cycle of the rats, with rats undergoing training sessions at the same time of day across all training and test sessions.
Rats were restricted to 16–18 g/day of standard laboratory rat chow to maintain 85% of their target weight and trained to lever-press for sucrose pellets (BioServ, Flemington, NJ) on a variable interval schedule of reinforcement (VI-30 s). Rats were trained to a criterion of >10 presses/min prior to surgical and/or behavioral procedures. All procedures were approved by the Institutional Animal Care and Use Committee of Kansas State University in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals.
2.2. Surgery
For optogenetic experiments, rats were anesthetized under isoflurane and bilaterally infused with 0.5–0.6 μL of a viral vector (flow rate: 0.05–0.06 μL/min) in the ACC (+1.0 mm AP; ±0.50 mm ML; −2.0 mm DV to bregma, at a 0°angle; this region overlaps with anatomical regions A24a and A24b) (Paxinos and Watson, 2014). The syringe remained in place for an additional 10 min to reduce backflow. Optical fibers (6 mm length; 0.22 NA; 200 nm core from RWD life sciences, Dover, DE) targeted the ACC (+1.0 mm AP, ±3.0 mm ML, −3.0 mm DV, at a 15° angle) and were anchored to the skull with black cement (C&B-Metabond, Parkell, Brentwood, NY; Ortho Acrylic, Lang Dental, Wheeling, IL). Rats were administered an analgesic (Meloxicam, 1 mg/kg or Flunixin, 1–2 mg/kg) subcutaneously, and triple antibiotic was applied around the surgical incision. Rats recovered for a minimum of 3 weeks prior to behavioral training to allow for sufficient viral expression (at least 6 weeks before first laser test). Supplementary Fig. 3 shows the spread of viral expression and location of optical fiber probes across all ArchT-eYFP rats.
2.3. Viruses
The adeno-associated viruses (AAVs; serotype 5) were obtained from the University of North Carolina Vector Core (Chapel Hill, NC). Viral titers were 4 × 1012 particles/mL for archaerhodopsin (AAV5:CaMKIIαeArchT3.0-eYFP), and 3 × 1012 particles/mL for enhanced yellow fluorescent protein (eYFP) control (AAV5:CaMKIIαeYFP). Rats expressing eYFP control were used to control for changes due to laser-induced heating of tissue (Stujenske et al., 2015). The CaMKIIα promoter was used to enable transgene expression favoring pyramidal neurons (Liu and Jones, 1996) in cortical regions (Jones et al., 1994; Van den Oever et al., 2013; Warthen et al., 2016). Viruses were housed in a −80 °C freezer until the day of infusion.
2.4. Behavior
For solitary PMA, rats were trained as previously described (Bravo-Rivera et al., 2014; Diehl et al., 2018). Briefly, rats were first trained to lever-press for sucrose pellets on a VI-30 schedule prior to PMA training. Rats were then conditioned with a pure tone (30 s, 4 kHz, 75 dB) co-terminating with a scrambled footshock (2 s, 0.4 mA) and given 9 tone-shock pairings per day with an average inter-trial interval (ITI) of 3 min. An acrylic square platform (14.0 cm each side, 0.33 cm tall) located in the opposite corner of the food dish allowed rats to avoid shock. The platform was fixed to the floor and present during all training stages. Rats were trained for 10 days with 9 tone-shock pairings per day. A VI-30 schedule was maintained across all training and test sessions.
For social partner PMA, rats were conditioned with the same tone, footshock, and ITI parameters as solitary PMA, but in the presence of another rat. Rats were separated by a transparent, perforated acrylic barrier which allowed rats access to their own lever, food dish, and platform. Rats were paired with partners that were either previously trained in PMA (Trained Partners) or were naïve to the task (Learner Rat) prior to training. Partners were same-sex and non-cagemates. Rats underwent PMA training with their partner across all daily sessions. After 10 days of social partner PMA, rats underwent an additional session in the absence of their partner on Day 11 (9 tone-shock presentations).
Following 10 days of PMA training, rats in the solitary optogenetic group underwent a test of avoidance expression (2 tones presented without shock) (for details, see section 2.5 Laser Delivery and Diehl et al., 2018; Diehl et al., 2020). Rats in the social partner optogenetic group underwent two expression tests, one in the presence and one in the absence of their partner. Expression tests were counterbalanced to prevent any order effects.
2.5. Laser delivery
ACC neurons were bilaterally illuminated using a DPSS green laser (532 nm, constant, 10–12 mW at the optical fiber tip; OptoEngine, Midvale, UT). The laser was activated at tone onset during Tone 1 of the test and persisted throughout the 30 s tone presentation. Light passed through a shutter/coupler (200 nm, SRS, Stanford, CA), patch cord (200 nm core, ThorLabs, Newton NJ), rotary joint (200 nm core, 1x2, Doric Lenses, Quebec City, Canada), dual patch cord (0.22 NA, 200 nm core, ThorLabs or RWD life sciences), and optical fibers targeting ACC. Rats were familiarized with dummy patch cords prior to tests.
2.6. Open field task
Locomotion was automatically assessed (ANY-Maze, Stoelting Co, Wood Dale, IL) in an open field arena (90 cm diameter) during 30 s laser off and 30 s laser on time periods. A 6 min acclimation period preceded laser illumination. Speed and distance traveled were used to assess locomotion, and time in the center was used to assess anxiety-like behaviors.
2.7. Pressing test
Lever-pressing was assessed with a VI-30 schedule and began with a 60 s acclimation period, followed by 7 Laser On (30 s) trials and 6 Laser Off (60 s) intervals. The number of lever presses was compared during Laser Off and Laser On periods using a paired-t-test.
2.8. Histology
After experiments, rats were deeply anesthetized with sodium pentobarbital (450 mg/kg i. p.) and transcardially perfused with 0.9 % saline followed by a 10 % formalin solution. Brains were removed and stored in 30 % sucrose for cryoprotection for at least 72 h before sectioning. Coronal sections were cut (40 μm), mounted on slides and analyzed for viral expression and optical fiber placement.
2.9. Data collection and analysis
Behavior was recorded with digital video cameras and quantified using ANY-Maze software (Stoelting, Wood Dale, IL) or manually handscored by experimenters blind to conditions. The number of shocks avoided was calculated as the rat spending at least 1.75 s on the platform during the 2 s shock period of each tone presentation. Shock reactivity was calculated using the maximum speed of each rat during exposure to the first shock on Day 1 of PMA. Darting bouts, which are characterized by a rapid movement across the chamber reaching a velocity greater than 23.5 cm/s (Gruene et al., 2015) and lasting a maximum of 1 s, were calculated during the tone periods outside of the shock (i.e., the first 28 s of each tone presentation).
Multilevel regressions were performed on each behavioral measure of interest to assess significant differences in several behaviors observed during PMA. Multilevel regressions are powerful statistical models that can account for multiple behaviors (i.e. avoidance, freezing, pressing) that may not be normally distributed (e.g., data with a binomial or Poisson distribution), and account for fixed and random effects (Bolker, 2015). Multilevel regressions have the ability to report the probability that a behavior is likely to occur based on observations of the data (Sommet and Morselli, 2017) and can better accommodate potential issues with complex datasets compared to ANOVAs, such as dealing with data that is not normally distributed and/or missing/excluded data points (Bolker, 2015). For each behavior of interest, we used training day (10), group type (social/solitary), and sex as fixed effect variables and individual variation and trial (9 tone-shock presentations) as random effects for each regression model. For percentage of time spent on the platform and time spent freezing during the 30 s tone, multilevel beta regressions were performed to account for individual differences and proportion data (time spent avoiding or freezing during the 30 s tone) being bounded by zero and one (Bolker, 2015). For number of shocks avoided, number of darting bouts during the tone, and number of ITI presses (30 s prior to tone onset), multilevel negative binomial regressions (similar to Poisson regression for count data, but accounting for overdispersion in the data; see Gardner et al., 1995; Payne et al., 2017) were performed to account for individual differences and positively skewed count data. To investigate effects of Partner Type (Learner Rat vs. Trained Partner), additional models were run on the social partner PMA data, with Partner Type added as a predictor variable. Parameter estimates for each of these models are available in Supplementary Tables S1–S10. All analyses for experiments in Fig. 1, Fig. 2 were conducted in R (version 4.2.1), using the lme4 library, version 1.1–30 (Bates et al., 2014). The emmeans library, version 1.8.0 (Lenth et al., 2022) was used to calculate post-hoc Tukey tests and estimated marginal means from each model (all reported means in the Supplementary Tables are model estimates).
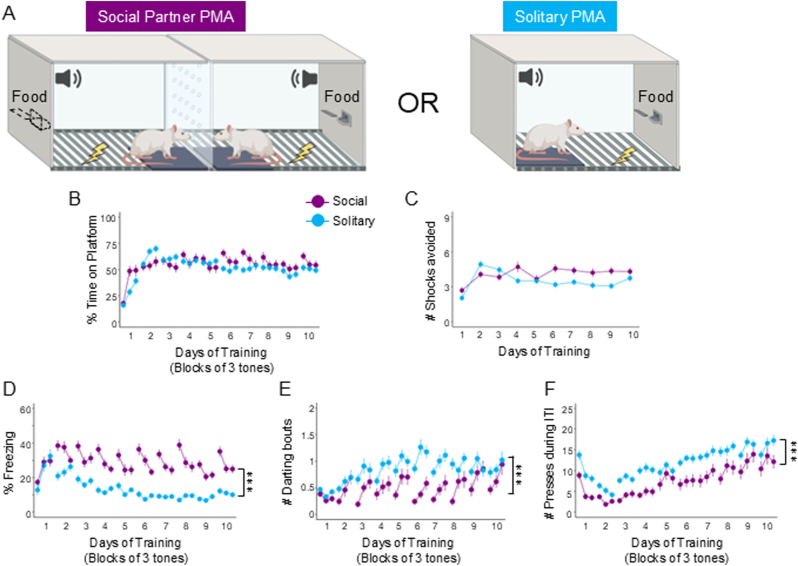
Fig. 1.
Social Partner platform-mediated avoidance (PMA) increases freezing and decreases darting and food-seeking compared to Solitary PMA. A. Schematic of PMA within a social (left, purple, n = 42) or solitary (blue, n = 59) context. B. Percentage of time spent on the platform during the tone, C. Number of shocks avoided per day, D. Percentage of time freezing during the tone, E. Number of darting bouts during the tone, and F. Number of lever presses during the ITI. There was no significant difference in time on platform (z = −0.868, p = 0.385) or number of shocks avoided (z = −0.552, p = 0.581). There was a significant increase in freezing (z = 6.229, p < 0.001) and a significant decrease in darting (z = −4.786, p < 0.001) and ITI pressing (z = −6.034, p < 0.001) in social compared to solitary rats. Data reported are main effects of the regression models (see Supplementary Tables S1–S5). Data shown across 10 days of training (trials shown in blocks of 3) and as mean ± SEM; ∗∗∗p < 0.001.
Fig. 2.
Social partner PMA reduces behavioral sex differences that are observed during solitary PMA. A. Percentage of time on platform during the tone, B. Number of shocks avoided and shock reactivity (inset), as measured by the maximum velocity of each rat during Tone 1 on the first day of training, C. Percentage of time freezing during the tone, D. Number of darting bouts during the tone, and E. Number of lever presses during the ITI in female (n = 21, salmon) and male (n = 21, teal) rats trained in Social Partner PMA. During social partner PMA training, females pressed significantly less than males (z = −3.112, p = 0.002), but there were no sex differences in time on platform (z = 1.638, p = 0.102), number of shocks avoided (z = 1.590, p = 0.112), freezing (z = −1.045, p = 0.296), or darting (z = −0.886, p = 0.375). F. Percentage of time on platform during the tone, G. Number of shocks avoided and shock reactivity (inset), H. Percentage of time freezing during the tone, I. Number of darting bouts during the tone, and J. Number of presses during the ITI in female (n = 27, salmon) and male (n = 32, teal) rats trained in solitary PMA. During solitary PMA training, females spent significantly more time on the platform (z = 2.791 p = 0.005), avoided significantly more shocks (z = 2.949, p = 0.003), and pressed significantly less (z = −3.044, p = 0.002) compared to males, but there were no sex differences in freezing (z = 1.311, p = 0.190) or darting (z = 0.401, p = 0.688). Data reported are post-hoc Tukey tests on the regression models. Data shown across 10 days of training (trials shown in blocks of 3) and as mean ± SEM; ∗∗p < 0.01.
For analyzing effects of optogenetic manipulations on behavior, repeated-measures ANOVA, followed by post-hoc Tukey tests, or Student's two-tailed t-tests were used where appropriate using Prism (Graphpad, La Jolla, CA), or JMP (SAS, Cary, NC) software. Some of the data points in the solitary PMA group were lost for behavioral measures during acquisition, and during the laser test (latency to avoid and suppression of bar pressing). Aspects of operant box schematics were created with Biorender.com.
3. Results
3.1. Platform-mediated avoidance (PMA) in a social context increases freezing and decreases darting and lever-pressing compared to PMA in a solitary context
Previous studies of PMA under solitary conditions have shown that freezing during the tone decreases and avoidance increases as rats progress through training (Bravo-Rivera et al., 2014; Martínez-Rivera et al., 2020). To investigate how the presence of another rat may affect avoidance acquisition, several behaviors across PMA training were compared in social or solitary contexts. One group of rats trained in social partner PMA (n = 42: females n = 21, males n = 21) underwent training simultaneously with another rat (Fig. 1A, left), and another group of rats trained in solitary PMA (n = 59: females n = 27, males n = 32) learned avoidance alone (Fig. 1A, right). Across 10 days of training, both social (purple) and solitary (blue) groups showed similar levels of avoidance, as measured by the percentage of time spent on the platform during the tone (Fig. 1B) and the average number of shocks avoided each day (Fig. 1C). A multilevel regression showed no significant effect of Group Type for time on platform (social vs. solitary; z = −0.868, p = 0.385) or number of shocks avoided (z = −0.552, p = 0.581).
Interestingly, rats trained in social partner PMA showed greater freezing compared to rats trained in solitary PMA (Fig. 1D). A multilevel regression showed a significant effect of Group Type predicting time freezing (z = 6.229, p < 0.001). In addition, solitary rats exhibited significantly more darting bouts during the tone (Fig. 1E, z = −4.786, p < 0.001) and pressed significantly more than social rats (Fig. 1F, z = −6.304, p < 0.001). In sum, social partner PMA training enhanced freezing responses while decreasing food-seeking and darting, with no effect on avoidance, compared to solitary PMA training.
3.2. Solitary PMA reveals behavioral sex differences that are not present during social partner PMA
The majority of PMA studies have used only male rats (Bravo-Rivera et al., 2014, 2015; Diehl et al., 2018, 2020; López-Moraga et al., 2024; Martínez-Rivera et al., 2018, 2020; Rodriguez-Romaguera et al., 2016). However, recent PMA studies have included both sexes (Gabriel et al., 2022; Gongwer et al., 2023; Halcomb et al., 2023; Landin and Chandler, 2022). To investigate sex differences in PMA between social and solitary contexts, we compared behaviors between male (n = 53: solitary n = 21, social n = 32) and female (n = 48: solitary n = 21, social n = 27) rats across both contexts. Data in Fig. 2 is the same data shown in Fig. 1 but separated by sex. Post-hoc Tukey tests on the previous regression models showed that males (teal) and females (salmon) trained in social partner PMA exhibited no significant differences in avoidance (Fig. 2A; z = 1.638, p = 0.102), number of shocks avoided (Fig. 2B; z = 1.590, p = 0.112), freezing (Fig. 2C; z = −1.045, p = 0.296), or darting (Fig. 2D; z = −0.886, p = 0.375). However, social males showed significantly increased pressing compared to social females (Fig. 2E; z = −3.112, p = 0.002).
During solitary PMA, females avoided significantly more than males, as measured by time on platform (Fig. 2F; z = 2.791, p = 0.005) and number of shocks avoided (Fig. 2G; z = 2.947, p = 0.003). This effect was not due to shock reactivity, as measured by maximum velocity during the first shock (Fig. 2G, inset, t-test, t(47) = 1.465, p = 0.150). Post-hoc Tukey tests on the regression model showed no significant differences between solitary males and females in freezing (Fig. 2H; z = 1.311, p = 0.190) or darting (Fig. 2I; z = 0.401, p = 0.688), but males pressed more than females (Fig. 2J, z = −3.044, p = 0.002). Altogether, these results suggest that any sex differences present during solitary PMA are suppressed during social partner PMA.
We next investigated whether training context affected males or females differently using contrast tests on the previous regression models (Supplementary Fig. 1). Post-hoc Tukey tests identified significantly greater freezing in social compared to solitary females (z = 6.097, p < 0.001) and greater freezing in social compared to solitary males (Supplementary Figs. 1C and H, z = 8.684, p < 0.001). We also observed significantly greater darting in solitary compared to social females (z = −4.423, p < 0.001) and greater darting in solitary compared to social males (z = −3.179, p = 0.002; Supplementary Figs. 1D and I). Finally, significantly greater pressing was found in solitary compared to social females (z = −4.069, p < 0.001) and males (Supplementary Figs. 1E and J, z = −3.623, p < 0.001). Collectively, social partner PMA training enhanced freezing and reduced darting and food seeking, regardless of sex.
3.3. Previous PMA experience of a social partner does not affect acquisition but increases fear and avoidance in the absence of the partner
Previous studies have reported that prior experience of a social partner can affect learning during social fear conditioning by proxy (Bruchey et al., 2010) and social fear extinction tasks (Gorkiewicz et al., 2023). We were therefore interested in whether prior PMA experience of a social partner would affect avoidance learning in a PMA-naïve rat. Therefore, all rats that underwent the social partner PMA task were paired with either a partner that had previously undergone PMA (Learner Rat with a Trained Partner, n = 20) or a partner that was also naïve to the task at the start of PMA (Learner Rat with another Learner Rat, n = 22; data for both Learner Rat partners were combined for analysis, see Supplementary Fig. 2A) and compared the same behaviors across the 10 days of training. Our regression models found no significant effect of Partner Type for time on platform (Supplementary Fig. 2B; z = −1.451, p = 0.147), number of shocks avoided (Supplementary Fig. 2C; z = −0.845, p = 0.398), freezing (Supplementary Fig. 2D; z = −0.699, p = 0.485), or pressing (Supplementary Fig. 2F; z = 0.278, p = 0.781). There was a significant main effect of Partner Type on darting (Supplementary Fig. 2E; z = 2.448, p = 0.014), with more darting bouts in Learner Rats with a Trained Partner. Overall, rats learn social partner PMA at a similar rate, regardless of the partner's prior avoidance experience.
We were next interested in whether the partner's absence would alter behavior after PMA training (see Day 11 in Supplementary Fig. 2A). Using contrast comparisons on the above regression models adjusted to examine only Day 10 and Day 11, we found that Learner Rats with either partner type spent more time on the platform (Supplementary Fig. 2B, Trained Partner z = −3.221, p = 0.001; Learner Rat z = −3.446, p < 0.001), more time freezing (Supplementary Fig. 2D, Trained Partner z = −7.061, p < 0.001; Learner Rat z = −8.029, p < 0.001), and darting bouts (Supplementary Fig. 2E, Trained Partner z = −2.133, p = 0.033; Learner Rat z = −3.513, p < 0.001) in the absence of the partner. Learner Rats paired with another Learner Rat, however, avoided more shocks (Supplementary Fig. 2C, z = −2.324, p = 0.020). Taken together, we observed elevated fear and avoidance responses when the partner was removed, following social partner PMA training.
3.4. Photoinactivation of ACC impairs avoidance expression that is learned within a social context only when the partner is absent
Previous studies have linked ACC activity with social learning (Apps et al., 2016; Burgos-Robles et al., 2019; Debiec and Olsson, 2017) and other forms of active avoidance (Freeman et al., 1996; Gabriel, 1990). We therefore reasoned that ACC activity would be necessary for the expression of avoidance that is learned in a social context. To assess this, we used an optogenetic approach to test if photoinactivation of ACC neurons would impair avoidance following social partner PMA training. Following viral infusion of Archaerhodopsin (ArchT-eYFP) targeting ACC glutamatergic projection neurons and surgical placement of optical probes, the virus was allowed to express for 4–6 weeks. Rats were then trained in PMA over 10 days as previously described (Diehl et al., 2018, 2020), but in the presence of a social partner (Fig. 3A; see Supplementary Figs. 3A–D for behavioral acquisition data). Histological analysis confirmed that expression of ArchT-eYFP was largely confined to the ACC (including anatomical areas Cg1 and Cg2) with minimal spread to secondary motor cortex (M2; lateral to Cg1), or prelimbic cortex (PL; anterior to Cg2 and ventral to Cg1; see Supplementary Fig. 4).
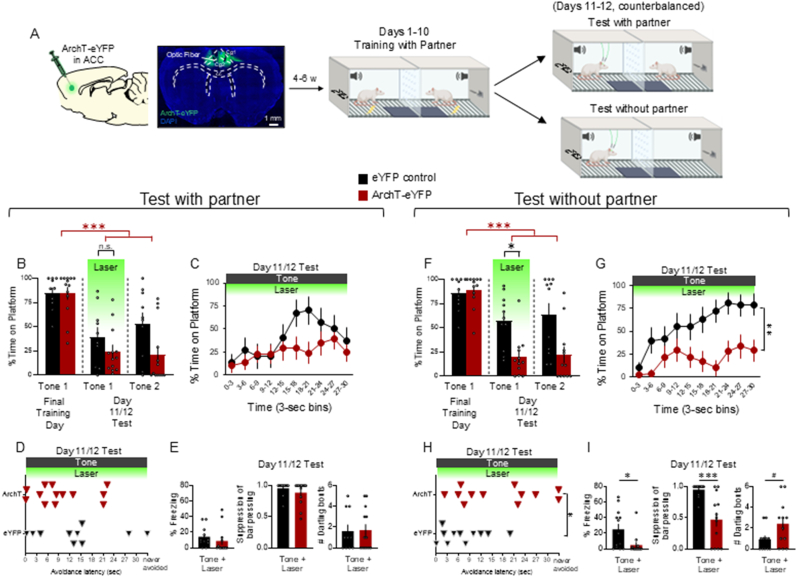
Fig. 3.
Photoinactivation of ACC projection neurons during social partner PMA impairs avoidance at test when the partner is absent. A. Schematic of virus infusion, micrograph of AAV expression, followed by avoidance training and tests. At Test with partner and without partner (Days 11/12), 532 nm light was delivered to ACC during the entire 30-s tone presentation (Tone 1). B. Percentage of time on platform at final training day and Test (Day 11/12, Tone 1 with laser ON and Tone 2 with laser OFF) for ArchT-eYFP rats (n = 13, red) and eYFP control rats (n = 10, black) when the partner was present. There was a significant main effect of AAV (F(1,21) = 6.870, p = 0.016) in the percentage of time spent on the platform between ArchT-eYFP and eYFP control rats but no significant difference between groups during Tone 1 of test (post-hoc Tukey, p = 0.209). Within the ArchT-eYFP group, there was a significant decrease in time on platform during Tone 1 and Tone 2 of test compared to the last training day (post-hoc Tukey tests, all p's < 0.001, red stars). I In the eYFP control group, there was also a significant decrease in time on platform between Tone 1 of the last training day and Tone 1 of test (post-hoc Tukey, p = 0.022). C. Percentage of time on platform in 3 s bins (Tone 1 at Test) during Test w/partner. D. Latency of avoidance for each rat during Test w/partner. E. Percentage of freezing (left), suppression of bar pressing (middle), and number of darting bouts (right) during Tone 1 at Test w/partner in all eYFP control (black) and all ArchT-eYFP (red) rats. F. Percentage time on platform at final training day and Test (Day 11/12, Tone 1 with laser ON and Tone 2 with laser OFF) for ArchT-eYFP (n = 12, red) and eYFP control rats (n = 11, black) when the partner was absent. There was a significant main effect of trial (repeated measures ANOVA, F(2,42) = 23.220, p < 0.001), a significant main effect of AAV (F(1,21) = 18.550, p < 0.001), and an interaction between trial and AAV (F(2,42) = 4.877, p = 0.013) for time on platform between ArchT-eYFP and eYFP control rats. Post-hoc Tukey tests revealed a significant decrease in time on platform between the ArchT-eYFP and eYFP control groups during Tone 1 of test (Laser ON) (p = 0.0016). Within the ArchT-eYFP group, we also observed a significant decrease in time on platform during Tone 1 and Tone 2 of test compared to the last training day (post-hoc Tukey tests, all p's < 0.001) and no significant difference within the eYFP control group across trials (all p's > 0.05). G. Percentage of time on platform in 3 s bins (Tone 1 at Test) during Test w/o partner revealed a significant reduction in the timecourse of avoidance in ArchT-eYFP rats compared to eYFP controls (repeated measures ANOVA, main effect of AAV, F (1,21) = 13.82, p = 0.0013). H. Latency of avoidance for each rat during Test w/o partner revealed a significant decrease in the latency to avoid for all ArchT-eYFP compared to eYFP control rats (t(21) = 2.555, p = 0.018). I. Percentage of freezing (left), suppression of bar pressing (middle), and number of darting bouts (right) during the Tone + Laser trial revealed a significant decrease in freezing in ArchT-eYFP rats (t(21) = 2.593, p = 0.016), a significantly lower suppression of bar pressing in ArchT-eYFP rats (t(21) = 4.275, p < 0.001) and a trend for increased darting in ArchT-eYFP rats (t(21) = 2.072, p = 0.051). All data are shown as mean ± SEM; #p < 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Following 10 days of social partner PMA (Fig. 3A, middle), rats underwent two avoidance expression tests: one in the presence of their partner and another in the absence of their partner (Fig. 3A, top and bottom right, respectively). Each test included two tone presentations with no shock (similar to Diehl et al., 2018; Diehl et al., 2020), and the order of each test was counterbalanced across rat partners. Green light was illuminated concurrently with the first 30 s tone (Laser ON), but not during the second tone (Laser OFF). During the test with the partner present, there was a significant main effect of trial (repeated measures ANOVA, F(2,42) = 23.63, p < 0.001) and a significant main effect of AAV (F(1,21) = 6.870, p = 0.016) in the percentage of time spent on the platform between ArchT-eYFP (n = 13) and eYFP control (n = 10) rats. Post-hoc Tukey tests revealed no difference between ArchT-eYFP and eYFP controls during Tone 1 of test (Laser ON) (Fig. 3B; p = 0.209). Within the ArchT-eYFP group, we did observe a significant decrease in time on platform during Tone 1 and Tone 2 of the test compared to Tone 1 of the last training day (post-hoc Tukey tests, all p's < 0.001, red stars with brackets). However, within the eYFP control group, we also observed a significant decrease in time on platform between Tone 1 of the last training day and Tone 1 of test (post-hoc Tukey test, p = 0.022). When comparing the timecourse of avoidance, as measured in 3 s bins of the tone period (Laser ON), there was no significant difference between ArchT-eYFP and eYFP control rats (Fig. 3C; repeated measures ANOVA: F(1,21) = 1.903, p = 0.182), nor when comparing avoidance latency during Tone 1 of the test (Fig. 3D; t(21) = 1.068, p = 0.298). Finally, photoinactivation had no effect on freezing (t(21) = 0.870, p = 0.394) suppression of bar pressing (t(21) = 1.057, p = 0.302), nor on darting bouts (t(21) = 0.913) in the presence of the partner (Fig. 3E).
During the test with partner absent, there was a significant main effect of trial (repeated measures ANOVA, F(2,42) = 23.220, p < 0.001), a significant main effect of AAV (F(1,21) = 18.550, p < 0.001), and interaction between trial and AAV (F(2,42) = 4.877, p = 0.013) in the percentage of time spent on the platform between ArchT-eYFP (n = 12) and eYFP control (n = 11) rats. Post-hoc Tukey tests revealed a significant decrease in time on platform between the ArchT-eYFP and eYFP control groups during Tone 1 of test (Laser ON) (Fig. 3F; p = 0.0016), and importantly, there was no significant difference within the eYFP control group across trials (all p's > 0.05). Within the ArchT-eYFP group, we also observed a significant decrease in time on platform during Tone 1 and Tone 2 of test compared to Tone 1 of the last training day (post-hoc Tukey tests, all p's < 0.001, red stars with brackets). When assessing the timecourse of avoidance, ACC photoinactivation significantly reduced avoidance throughout the tone in ArchT-eYFP rats (Fig. 3G, F(1,21) = 13.82, p = 0.001)). In addition, ACC photoinactivation increased avoidance latency in the ArchT-eYFP rats (Fig. 3H, t(21) = 2.555, p = 0.018). Finally, ACC photoinactivation in the absence of the partner significantly decreased both freezing (t(21) = 2.593, p = 0.016) and suppression of bar pressing (t(21) = 4.275, p < 0.001), with a trend toward increased darting bouts (t(21) = 2.072, p = 0.051) (Fig. 3I). Taken together, photoinactivation of ACC projection neurons reduced both avoidance and fear responses in the absence, but not in the presence, of the social partner.
3.5. Photoinactivation of ACC has no effect on expression of avoidance in a solitary context
To determine whether activity in ACC may also be necessary for avoidance in a solitary context, we photoinactivated ACC neurons during an expression test following solitary PMA training. Similar to the experiment in Fig. 3, rats were infused with ArchT-eYFP (n = 22) or eYFP control virus (n = 26) and subsequently underwent solitary PMA training (Fig. 4A; see Supplementary Figs. 3E–H for behavioral acquisition of PMA). A 2-way repeated measures ANOVA comparing the percentage of time spent on the platform between ArchT-eYFP and eYFP control rats showed no main effect of AAV (F(1,46) = 1.500, p = 0.227) but a significant main effect of trial (F(1,46) = 12.830, p < 0.001). Post-hoc Tukey tests showed no significant difference in avoidance between the ArchT-eYFP and eYFP control groups during Tone 1 of test (Laser ON) (Fig. 4B; p = 0.783). Within the ArchT-eYFP group, we did observe a significant decrease in time on platform during Tone 1 and Tone 2 of test compared to the last training day (post-hoc Tukey tests, all p's < 0.05, red star with brackets). However, within the eYFP control group, we also observed a significant decrease in time on platform between Tone 1 of the last training day and Tone 1 of test (post-hoc Tukey test, p = 0.025). There was also no effect on the timecourse of avoidance (Fig. 4C; repeated measures ANOVA, F(1,46) = 3.082, p = 0.086), nor on avoidance latency (Fig. 4D; t(46) = 0.758, p = 0.452). Finally, there were no significant differences in freezing (t(46) = 0.316, p = 0.753) or suppression of bar pressing (t(40) = 1.268, p = 0.212), although we did observe a minor trend toward increased darting bouts (t(32) = 1.969, p = 0.058) (Fig. 4E).
Fig. 4.
Photoinactivation of ACC projection neurons during solitary PMA has no effect on expression of avoidance. A. Schematic of virus infusion, micrograph of AAV expression, followed by avoidance training and test. At Test, 532 nm light was delivered to ACC during the entire 30-s tone presentation (Tone 1). B. Percentage of time on platform on the final training day and Test (Day 11, Tone 1 with laser ON and Tone 2 with laser OFF) for ArchT-eYFP (n = 22, red) and eYFP control rats (n = 26, black). There was no main effect of AAV (F(1,46) = 1.500, p = 0.227) but a significant main effect of trial (F(1,46) = 12.830, p < 0.001). Post-hoc Tukey tests showed no significant difference in time on platform between the ArchT-eYFP and eYFP control groups during Tone 1 of test (Laser ON; p = 0.783). Within the ArchT-eYFP group, there was a decrease in time on platform during Tone 1 and Tone 2 of test compared to Tone 1 of the last training day (post-hoc Tukey tests, all p's < 0.05, red star). Within the eYFP control group, there was a decrease in time on platform between Tone 1 of the last training day and Tone 1 of test (post-hoc Tukey test, p = 0.025). C. Percentage of time on platform in 3 s bins during Test. D. Latency of avoidance for each rat during Test. E. Percentage of freezing (left) suppression of bar pressing (middle), and number of darting bouts (right) during the Tone + Laser trial revealed a trend in increased darting in ArchT-eYFP rats (t(32) = 1.969, p = 0.058). Data shown as mean ± SEM; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; #p > 0.05.
Because we observed sex differences in avoidance during solitary PMA (Fig. 2F–G), we were interested in whether there were any sex differences in avoidance during photoinhibition of ACC during avoidance in a solitary context. Both males (orange) and females (yellow) showed a decrease in time on platform between Tone 1 of the final training day and Tone 1 of test (Supplementary Fig. 5; Post-Hoc Tukey tests on the repeated measures ANOVA, males p < 0.014 [orange star with bracket], females p < 0.004 [yellow stars with brackets]). This effect persisted at Tone 2 of test (Laser OFF) in females (p < 0.007) but not in males (p = 1.000). Finally, in a subset of our ArchT-eYFP and eYFP control rats, we confirmed that ACC photoinactivation had no effect on spontaneous bar-pressing (t-test, t(24) = 0.343, p = 0.738; ArchT-eYFP n = 11, M = 4.7, eYFP n = 13, M = 4.8), locomotion, as measured by distance traveled in an open field (t(41) = −1.22, p = 0.232; ArchT-eYFP n = 17, M = 1.1 m, eYFP n = 24, M = 0.97 m), nor on anxiety-like behaviors, as both groups spent similar amounts of time in the center of the open field (t(41) = −1.35, p = 0.184; ArchT-eYFP n = 17, M = 3.3 s, eYFP n = 24, M = 2.0 s).
4. Discussion
We have developed a behavioral task to investigate active avoidance within a social context. We found that freezing was enhanced while darting and food seeking were suppressed in social compared to solitary contexts, demonstrating that active avoidance does not always reduce fear responses (Cain, 2019; Diehl et al., 2019; LeDoux et al., 2017). During solitary training, females avoided more than males, whereas males showed greater food seeking than females. Overall, sex differences were more prominent when rats learned PMA alone. Thus, a social context of aversive learning appears to suppress sex differences, causing female rats to “act more like” male rats. When investigating the role of ACC in avoidance, ACC activity modulated the expression of avoidance, only when it was learned in a social context and the partner was not present. Below, we highlight some unexpected findings from our study along with possible explanations and future research directions.
The surprising finding that freezing increased during social partner PMA compared to solitary PMA (Fig. 1C) suggests that being in the presence of a fearful rat can enhance fear responses when avoiding danger. It also suggests that rats associate their partner with danger. This agrees with studies showing that rats can associate another rat with a shock (Dawud et al., 2021), and witnessing another rat that is freezing to a conditioned stimulus (CS) can also become a signal for danger, in addition to the CS itself (Cruz et al., 2020). It is also possible that rats increased freezing during social partner PMA due to a lack of detecting any movement from their partner, similar to previous studies showing that social transmission of fear can be propagated by silence caused by the cessation of movement (Pereira et al., 2012). In the current study, it is likely that rats perceived their partner as a negative stimulus, which led to increased freezing, rather than a positive stimulus, which would have produced a buffering effect and led to less freezing. This agrees with studies showing that partner presence during a traumatic event does not reduce fear responses, but rather a partner present after a traumatic event does reduce fear responses (Gorkiewicz et al., 2023). Future studies using social partner PMA can address this question by testing if the presentation of a partner after solitary PMA training would reduce freezing and promote extinction of avoidance.
We also observed decreased food seeking in the social context compared to the solitary context (Fig. 1F), suggesting again that rats are more afraid and may be less motivated to forage for food when another rat is present. Rats may also be pressing less because they are spending more time investigating their partner for sensory cues to learn about PMA. During other forms of social learning, rodents use auditory, visual (Paraouty et al., 2020) and olfactory cues (Contestabile et al., 2021; Sanchez-Andrade and Kendrick, 2009) to gather context information. In the current study, rats may observe their partner avoid, causing them to also avoid, or they may hear an alarm call emitted by their partner. Although visual attention and vocalizations were not assessed in the current study, future studies should assess whether social partner PMA relies on such sensory cues in addition to behavioral synchrony between partners.
During social partner PMA, females avoided similarly to males (Fig. 2A), which was lower than avoidance levels observed in solitary females (Supplementary Fig. 1A). Previous studies have shown that female rats avoid foraging in large open areas by themselves (Zambetti et al., 2019) and show greater defensive responses (Blanchard et al., 1991) compared to male rats, which could explain why females spend more time on the platform than males in the solitary context. In addition, males pressed more than females, regardless of training condition (Fig. 2D & H), but this difference was less robust in the social context. This might be due to the possibility that males show riskier behaviors (Ishii et al., 2018; Jolles et al., 2015; Orsini et al., 2016) and could explain why males pressed more than females under both PMA contexts. All social rats avoided more and displayed enhanced freezing when they were alone than with their partner (Supplementary Figs. 2B–C), which might reflect increased fear. Although this disagrees with studies showing that female rodents benefit more from social buffering compared to male rodents (Barnett, 2007; Ishii et al., 2016; Modlinska and Pisula, 2020), the presence of a social partner during PMA training did not induce a sex-dependent change in freezing. Overall, this supports the idea that avoiding danger in a social context suppresses sex differences observed when learning to avoid alone.
Studies investigating social learning have pointed to the anterior cingulate cortex (ACC) as a key region across many species (Burgos-Robles et al., 2019). Previous research on observational fear has demonstrated that ACC integrates information about social cues and aversive stimuli, which are mediated by connections between the ACC and basolateral amygdala (BLA) (Allsop et al., 2018). In addition, prior studies have reported ACC correlates of active avoidance in rabbits performing the wheel running task (Freeman et al., 1996; Gabriel, 1990), and other research has also shown that the ACC is involved in other types of avoidance tasks (Holloway and McIntyre, 2011; Liu et al., 2009; Malin et al., 2007). The ACC is a highly interconnected cortical brain region, projecting densely to the PL (Conde et al., 1995; Jones et al., 2005) and BLA (Bissière et al., 2008; Cassell and Wright, 1986; Gabbott et al., 2005), making this region a possible candidate structure to regulate active avoidance. Since the ACC processes social information, these neurons may signal the PL information about the social partner since PL is a prominent regulator of avoidance in PMA (Diehl et al., 2018, 2020). The ACC also comprises part of the value processing network together with the BLA, integrating information about observed emotion and social interactions (Apps et al., 2016; Burgos-Robles et al., 2019). Therefore, it is possible that ACC neurons may also signal the BLA during social partner PMA, thereby allowing rats to use social information to avoid danger.
We found that during social partner PMA, ACC photoinactivation impaired avoidance only when the social partner was removed. This suggests that the ACC is recruited to recall the memory of avoidance when it is learned in a social context, but ACC activity may not be required when other cues from the partner are still present and can facilitate the avoidance response. Future studies using electrophysiological recordings could determine ACC correlates of behavior during PMA and how ACC activity differs with and without the partner's presence in the social context.
We unexpectedly observed that some eYFP controls showed reduced avoidance during the expression test compared to avoidance observed on the final training day (Fig. 3, Fig. 4B). To rule out effects of possible tissue damage due to laser heat, laser illumination was maintained between 10 and 12 mW (irradiance: 79–96 mW/mm2) at the tip of the optical fiber, a range which should not produce any phototoxic effects (Senova et al., 2017). However, it is possible that the mere introduction of photons altered biophysical properties within neurons (Bernard, 2020) or that rats experienced “optoception,” in which subjects perceive photo-manipulation of their own brain tissue (Luis-Islas et al., 2022). Although most of these studies have been performed in neurons expressing channelrhodopsin using blue light (473 nm), future studies should confirm if similar effects might also occur with neurons expressing ArchT using green light (532 nm). Finally, there is also a possibility that the behavior of the social partner may have altered the behavior of the rat that was receiving laser illumination, thus influencing their behavior. Nevertheless, we observed a significant decrease in avoidance between ArchT-eYFP and eYFP controls when the partner was absent in social rats. In addition, we observed within subject impairments in avoidance, supporting the notion that ACC contributes to avoidance behavior. However, these results should be treated with caution, and further experiments are needed to confirm if ACC activity is necessary for active avoidance.
Excessive avoidance is a key symptom in several neuropsychiatric illnesses including PTSD (Asmundson et al., 2004; Breslau, 2001), OCD (McGuire et al., 2012), social anxiety disorder (SAD) (Wake et al., 2021), depression (Trew, 2011), and autism spectrum disorder (ASD) (Madipakkam et al., 2017). The solitary PMA task has previously been used as a model to study extinction-based treatment for PTSD and OCD (Martínez-Rivera et al., 2020; Rodriguez-Romaguera et al., 2016). Therefore, modifying the PMA task to include a social context could be used as an animal model of behavior to study other anxiety disorders such as SAD, in which social context is a key factor. The human homolog of the rat ACC, Brodmann areas 24a/b (Burgos-Robles et al., 2019), has also been implicated in these disorders (OCD: Kosová et al., 2023; Lee et al., 2023), (SAD: Jamieson et al., 2023), (depression: Alexander et al., 2023), (ASD: Bartolotti et al., 2020). Interestingly, a meta-analysis reported that the ACC was one of seven regions with disrupted functional connectivity across different anxiety disorders (Rezaei et al., 2023). Therefore, the findings of the current study are consistent with clinical studies that the ACC is an area of interest for developing treatments to resolve symptoms of these neuropsychiatric disorders.
CRediT authorship contribution statement
Shannon Ruble: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation. Karissa Payne: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis. Cassandra Kramer: Writing – review & editing, Visualization, Investigation, Data curation. Lexe West: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Data curation. Halle Ness: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Data curation. Greg Erickson: Supervision, Methodology, Investigation, Conceptualization. Alyssa Scott: Investigation. Maria M. Diehl: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
This study was supported by NIH grants #P20-GM103418 and #P20-GM113109 (subawards to MMD), and the department of Psychological Sciences, College of Arts & Sciences, and the Vice President of Research at Kansas State University.
Declaration of competing interest
None.
Acknowledgements
We thank Drs. Fabricio Do Monte, Anthony Burgos-Robles, Debra Bangasser, and Gabriel Gasque for helpful comments on the manuscript. We also thank Tessa Maze, Jenna Thompson, Kelly Krehbiel, Ashvini Wickramasundara, Allison Moser, Allison Drouhard, Emma Wrampe, Charlotte Kettler, and Ivy Auletti for technical assistance with animal training, Dr. Rodolfo Flores for technical assistance with data processing, and Dr. Michael Young for helpful comments on data analyses.
Handling Editor: Prof R Lawrence Reagan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2024.100702.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Alexander L., Hawkins P.C.T., Evans J.W., Mehta M.A., Zarate C.A. Preliminary evidence that ketamine alters anterior cingulate resting-state functional connectivity in depressed individuals. Transl. Psychiatr. 2023;13(1):371. doi: 10.1038/s41398-023-02674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop S.A., Wichmann R., Mills F., Burgos-Robles A., Chang C.J., Felix-Ortiz A.C., Tye K.M. Corticoamygdala transfer of socially derived information gates observational learning. Cell. 2018;173(6):1329–1342.e1318. doi: 10.1016/j.cell.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders : DSM-5. [Google Scholar]
- Apps M.A., Rushworth M.F., Chang S.W. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron. 2016;90(4):692–707. doi: 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson G.J., Stapleton J.A., Taylor S. Are avoidance and numbing distinct PTSD symptom clusters? J. Trauma Stress. 2004;17(6):467–475. doi: 10.1007/s10960-004-5795-7. [DOI] [PubMed] [Google Scholar]
- Barnett S.A. AldineTransaction; 2007. The Rat : a Study in Behavior. [Google Scholar]
- Bartolotti J., Sweeney J.A., Mosconi M.W. Functional brain abnormalities associated with comorbid anxiety in autism spectrum disorder. Dev. Psychopathol. 2020;32(4):1273–1286. doi: 10.1017/S0954579420000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B.M., Walker S. 1.1–5. 2014. lme4: linear mixed-effects models using Eigen and S4. (R Package Version). [Google Scholar]
- Bernard C. Optogenetics: keep interpretations light. eNeuro. 2020;7(2) doi: 10.1523/ENEURO.0091-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissière S., Plachta N., Hoyer D., McAllister K.H., Olpe H.R., Grace A.A., Cryan J.F. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol. Psychiatr. 2008;63(9):821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D.C., Shepherd J.K., De Padua Carobrez A., Blanchard R.J. Sex effects in defensive behavior: baseline differences and drug interactions. Neurosci. Biobehav. Rev. 1991;15(4):461–468. doi: 10.1016/s0149-7634(05)80132-0. [DOI] [PubMed] [Google Scholar]
- Bolker B.M. In: Ecological Statistics: Contemporary Theory and Application. 1 ed. Fox G.A., Negrete-Yankelevich S., Sosa V.J., editors. Oxford University Press; 2015. Linear and generalized linear mixed models; pp. 309–333. [Google Scholar]
- Brandl H.B., Pruessner J.C., Farine D.R. The social transmission of stress in animal collectives. Proc. Biol. Sci. 2022;289(1974) doi: 10.1098/rspb.2021.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Rivera C., Roman-Ortiz C., Brignoni-Perez E., Sotres-Bayon F., Quirk G.J. Neural structures mediating expression and extinction of platform-mediated avoidance. J. Neurosci. 2014;34(29):9736–9742. doi: 10.1523/JNEUROSCI.0191-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Rivera C., Roman-Ortiz C., Montesinos-Cartagena M., Quirk G.J. Persistent active avoidance correlates with activity in prelimbic cortex and ventral striatum. Front. Behav. Neurosci. 2015;9:184. doi: 10.3389/fnbeh.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. Outcomes of posttraumatic stress disorder. J. Clin. Psychiatr. 2001;62(Suppl. 17):55–59. (NOT IN FILE) [PubMed] [Google Scholar]
- Brill-Maoz N., Maroun M. Extinction of fear is facilitated by social presence: synergism with prefrontal oxytocin. Psychoneuroendocrinology. 2016;66:75–81. doi: 10.1016/j.psyneuen.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Bruchey A.K., Jones C.E., Monfils M.H. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav. Brain Res. 2010;214(1):80–84. doi: 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A., Gothard K.M., Monfils M.H., Morozov A., Vicentic A. Conserved features of anterior cingulate networks support observational learning across species. Neurosci. Biobehav. Rev. 2019;107:215–228. doi: 10.1016/j.neubiorev.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C.K. Avoidance problems reconsidered. Curr. Opinion Behav. Sci. 2019;26:9–17. doi: 10.1016/j.cobeha.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell M.D., Wright D.J. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res. Bull. 1986;17(3):321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- Conde F., Maire-Lepoivre E., Audinat E., Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J. Comp. Neurol. 1995;352(4):567–593. doi: 10.1002/cne.903520407. (NOT IN FILE) [DOI] [PubMed] [Google Scholar]
- Contestabile A., Casarotto G., Girard B., Tzanoulinou S., Bellone C. Deconstructing the contribution of sensory cues in social approach. Eur. J. Neurosci. 2021;53(9):3199–3211. doi: 10.1111/ejn.15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Heinemans M., Márquez C., Moita M.A. Freezing displayed by others is a learned cue of danger resulting from Co-experiencing own freezing and shock. Curr. Biol. 2020;30(6):1128–1135.e1126. doi: 10.1016/j.cub.2020.01.025. [DOI] [PubMed] [Google Scholar]
- Dawud L.M., Loetz E.C., Lloyd B., Beam R., Tran S., Cowie K., Bland S.T. A novel social fear conditioning procedure alters social behavior and mTOR signaling in differentially housed adolescent rats. Dev. Psychobiol. 2021;63(1):74–87. doi: 10.1002/dev.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J., Olsson A. Social fear learning: from animal models to human function. Trends Cognit. Sci. 2017;21(7):546–555. doi: 10.1016/j.tics.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Russo J.E. Observational learning of discriminative avoidance in hooded rats. Anim. Learn. Behav. 1975;3(1):76–80. doi: 10.3758/BF03209103. [DOI] [Google Scholar]
- Diehl M.M., Bravo-Rivera C., Rodriguez-Romaguera J., Pagan-Rivera P.A., Burgos-Robles A., Roman-Ortiz C., Quirk G.J. Active avoidance requires inhibitory signaling in the rodent prelimbic prefrontal cortex. Elife. 2018;7 doi: 10.7554/eLife.34657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M.M., Bravo-Rivera C., Quirk G.J. The study of active avoidance: a platform for discussion. Neurosci. Biobehav. Rev. 2019;107:229–237. doi: 10.1016/j.neubiorev.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M.M., Iravedra-Garcia J.M., Morán-Sierra J., Rojas-Bowe G., Gonzalez-Diaz F.N., Valentín-Valentín V.P., Quirk G.J. Divergent projections of the prelimbic cortex bidirectionally regulate active avoidance. Elife. 2020;9 doi: 10.7554/eLife.59281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J.H., Cuppernell C., Flannery K., Gabriel M. Context-specific multi-site cingulate cortical, limbic thalamic, and hippocampal neuronal activity during concurrent discriminative approach and avoidance training in rabbits. J. Neurosci. 1996;16(4):1538–1549. doi: 10.1523/JNEUROSCI.16-04-01538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott P.L., Warner T.A., Jays P.R., Salway P., Busby S.J. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492(2):145–177. doi: 10.1002/cne.20738. (NOT IN FILE) [DOI] [PubMed] [Google Scholar]
- Gabriel M. U.S. Gov't, Non-P.H.S; 1990. Functions of Anterior and Posterior Cingulate Cortex during Avoidance Learning in Rabbits [Research Support. [PubMed] [Google Scholar]
- Gabriel C.J., Zeidler Z., Jin B., Guo C., Goodpaster C.M., Kashay A.Q., DeNardo L.A. BehaviorDEPOT is a simple, flexible tool for automated behavioral detection based on markerless pose tracking. Elife. 2022;11 doi: 10.7554/eLife.74314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner W., Mulvey E.P., Shaw E.C. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol. Bull. 1995;118(3):392–404. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- Gongwer M.W., Klune C.B., Couto J., Jin B., Enos A.S., Chen R., DeNardo L.A. Brain-wide projections and differential encoding of prefrontal neuronal classes underlying learned and innate threat avoidance. J. Neurosci. 2023 doi: 10.1523/JNEUROSCI.0697-23.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorkiewicz T., Danielewski K., Andraka K., Kondrakiewicz K., Meyza K., Kaminski J., Knapska E. Social buffering diminishes fear response but does not equal improved fear extinction. Cerebr. Cortex. 2023;33(8):5007–5024. doi: 10.1093/cercor/bhac395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene T.M., Flick K., Stefano A., Shea S.D., Shansky R.M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4 doi: 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcomb C.J., Philipp T.R., Dhillon P.S., Cox J.H., Aguilar-Alvarez R., Vanderhoof S.O., Jasnow A.M. Sex divergent behavioral responses in platform-mediated avoidance and glucocorticoid receptor blockade. Psychoneuroendocrinol. 2023;159 doi: 10.1016/j.psyneuen.2023.106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway C.M., McIntyre C.K. Post-training disruption of Arc protein expression in the anterior cingulate cortex impairs long-term memory for inhibitory avoidance training. Neurobiol. Learn. Mem. 2011;95(4):425–432. doi: 10.1016/j.nlm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Ishii A., Kiyokawa Y., Takeuchi Y., Mori Y. Social buffering ameliorates conditioned fear responses in female rats. Horm. Behav. 2016;81:53–58. doi: 10.1016/j.yhbeh.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Ishii H., Onodera M., Ohara S., Tsutsui K.I., Iijima T. Sex differences in risk preference and c-fos expression in paraventricular thalamic nucleus of rats during gambling task. Front. Behav. Neurosci. 2018;12:68. doi: 10.3389/fnbeh.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A.J., Harrison B.J., Delahoy R., Schmaal L., Felmingham K.L., Phillips L., Davey C.G. A brain model of altered self-appraisal in social anxiety disorder. Transl. Psychiatr. 2023;13(1):344. doi: 10.1038/s41398-023-02644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D., Kim S., Chetana M., Jo D., Ruley H.E., Lin S.Y., Rabah D., Kinet J.P., Shin H.S. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 2010 Apr;13(4):482–488. doi: 10.1038/nn.2504. Epub 2010 Feb 28. PMID: 20190743; PMCID: PMC2958925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John E.R., Chesler P., Bartlett F., Victor I. Observation learning in cats. Sci. 1968;159(3822):1489–1491. doi: 10.1126/science.159.3822.1489. [DOI] [PubMed] [Google Scholar]
- Jolles J.W., Boogert N.J., van den Bos R. Sex differences in risk-taking and associative learning in rats. R. Soc. Open Sci. 2015;2(11) doi: 10.1098/rsos.150485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.E., Monfils M.H. Dominance status predicts social fear transmission in laboratory rats. Anim. Cognit. 2016;19(6):1051–1069. doi: 10.1007/s10071-016-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.F., Groenewegen H.J., Witter M.P. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neurosci. 2005;133(1):193–207. doi: 10.1016/j.neuroscience.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Jones E.G., Huntley G.W., Benson D.L. Alpha calcium/calmodulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: comparison with GAD-67 expression. J Neurosci. 1994 Feb;14(2):611–629. doi: 10.1523/JNEUROSCI.14-02-00611.1994. PMID: 8301355; PMCID: PMC6576801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosová E., Pajuelo D., Greguš D., Brunovský M., Stopková P., Fajnerová I., Horáček J. Glutamatergic abnormalities in the pregenual anterior cingulate cortex in obsessive-compulsive disorder using magnetic resonance spectroscopy: a controlled study. Psychiatr. Re.s Neuroimag. 2023;335 doi: 10.1016/j.pscychresns.2023.111721. [DOI] [PubMed] [Google Scholar]
- Landin J.D., Chandler L.J. Adolescent alcohol exposure alters threat avoidance in adulthood. Front. Behav. Neurosci. 2022;16 doi: 10.3389/fnbeh.2022.1098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E., Moscarello J., Sears R., Campese V. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol. Psychiatr. 2017;22(1):24–36. doi: 10.1038/mp.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Kim K.M., Lim M.H. Theta and gamma activity differences in obsessive-compulsive disorder and panic disorder: insights from resting-state EEG with eLORETA. Brain Sci. 2023;13(10) doi: 10.3390/brainsci13101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R.V., Buerkner P., Herve M., Jung M., Love J., Miguez F. Emmeans: estimated marginal means, aka least-squares means. 2022;1.8. 1–1 [Google Scholar]
- Liu F., Zheng X.L., Li B.M. The anterior cingulate cortex is involved in retrieval of long-term/long-lasting but not short-term memory for step-through inhibitory avoidance in rats. Neurosci. Lett. 2009;460(2):175–179. doi: 10.1016/j.neulet.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Liu X.B., Jones E.G. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc. Natl. Acad. Sci. U.S.A. 1996 Jul 9;93(14):7332–7336. doi: 10.1073/pnas.93.14.7332. PMID: 8692993; PMCID: PMC38984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Moraga A., Luyten L., Beckers T. A history of avoidance does not impact extinction learning in male rats. NPJ Sci. Learn. 2024;9(1):11. doi: 10.1038/s41539-024-00223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis-Islas J., Luna M., Floran B., Gutierrez R. Optoception: perception of optogenetic brain perturbations. eNeuro. 2022;9(3) doi: 10.1523/ENEURO.0216-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madipakkam A.R., Rothkirch M., Dziobek I., Sterzer P. Unconscious avoidance of eye contact in autism spectrum disorder. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-13945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin E.L., Ibrahim D.Y., Tu J.W., McGaugh J.L. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiol. Learn. Mem. 2007;87(2):295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Rivera F.J., Bravo-Rivera C., Velázquez-Díaz C.D., Montesinos-Cartagena M., Quirk G.J. Prefrontal circuits signaling active avoidance retrieval and extinction. Psychopharmacology (Berl) 2018 doi: 10.1007/s00213-018-5012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Rivera F.J., Sánchez-Navarro M.J., Huertas-Pérez C.I., Greenberg B.D., Rasmussen S.A., Quirk G.J. Prolonged avoidance training exacerbates OCD-like behaviors in a rodent model. Transl. Psychiatr. 2020;10(1):212. doi: 10.1038/s41398-020-00892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J.F., Storch E.A., Lewin A.B., Price L.H., Rasmussen S.A., Goodman W.K. The role of avoidance in the phenomenology of obsessive-compulsive disorder. Compr. Psychiatr. 2012;53(2):187–194. doi: 10.1016/j.comppsych.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Modlinska K., Pisula W. The Norway rat, from an obnoxious pest to a laboratory pet. Elife. 2020;9 doi: 10.7554/eLife.50651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A., Phelps E.A. Social learning of fear. Nat. Neurosci. 2007;10(9):1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Orsini C.A., Willis M.L., Gilbert R.J., Bizon J.L., Setlow B. Sex differences in a rat model of risky decision making. Behav. Neurosci. 2016;130(1):50–61. doi: 10.1037/bne0000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraouty N., Charbonneau J.A., Sanes D.H. Social learning exploits the available auditory or visual cues. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-71005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. seventh ed. Elsevier/AP, Academic Press is an imprint of Elsevier; 2014. Paxino's and Watson's the Rat Brain in Stereotaxic Coordinates. [still image] [Google Scholar]
- Payne E.H., Hardin J.W., Egede L.E., Ramakrishnan V., Selassie A., Gebregziabher M. Approaches for dealing with various sources of overdispersion in modeling count data: scale adjustment versus modeling. Stat. Methods Med. Res. 2017;26(4):1802–1823. doi: 10.1177/0962280215588569. [DOI] [PubMed] [Google Scholar]
- Pereira A.G., Cruz A., Lima S.Q., Moita M.A. Silence resulting from the cessation of movement signals danger. Curr. Biol. 2012;22(16):R627–R628. doi: 10.1016/j.cub.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Presley W.J., Riopelle A.J. Observational learning of an avoidance response. J. Genet. Psychol. 1959;95:251–254. doi: 10.1080/00221325.1959.10534265. [DOI] [PubMed] [Google Scholar]
- Rezaei S., Gharepapagh E., Rashidi F., Cattarinussi G., Sanjari Moghaddam H., Di Camillo F., Delvecchio G. Machine learning applied to functional magnetic resonance imaging in anxiety disorders. J. Affect. Disord. 2023;342:54–62. doi: 10.1016/j.jad.2023.09.006. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J., Greenberg B.D., Rasmussen S.A., Quirk G.J. An avoidance-based rodent model of exposure with response prevention therapy for obsessive-compulsive disorder. Biol. Psychiatr. 2016;80(7):534–540. doi: 10.1016/j.biopsych.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Andrade G., Kendrick K.M. The main olfactory system and social learning in mammals. Behav. Brain Res. 2009;200(2):323–335. doi: 10.1016/j.bbr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Senova S., Scisniak I., Chiang C.C., Doignon I., Palfi S., Chaillet A., Pain F. Experimental assessment of the safety and potential efficacy of high irradiance photostimulation of brain tissues. Sci. Rep. 2017;7 doi: 10.1038/srep43997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommet N., Morselli D. Keep calm and learn multilevel logistic modeling: a simplified three-step procedure using stata, R, Mplus, and SPSS. Int. Rev. Soc. Psychol. 2017 doi: 10.5334/irsp.90. [DOI] [Google Scholar]
- Stujenske J.M., Spellman T., Gordon J.A. Modeling the spatiotemporal dynamics of light and heat propagation for in vivo optogenetics. Cell Rep. 2015;12(3):525–534. doi: 10.1016/j.celrep.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew J.L. Exploring the roles of approach and avoidance in depression: an integrative model. Clin. Psychol. Rev. 2011;31(7):1156–1168. doi: 10.1016/j.cpr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Van den Oever M.C., Rotaru D.C., Heinsbroek J.A., Gouwenberg Y., Deisseroth K., Stuber G.D., Mansvelder H.D., Smit A.B. Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. J. Neurosci. 2013 Nov 13;33(46):18225–18233. doi: 10.1523/JNEUROSCI.2412-13.2013. PMID: 24227731; PMCID: PMC3828471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake S., van Reekum C.M., Dodd H. The effect of social anxiety on the acquisition and extinction of low-cost avoidance. Behav. Res. Ther. 2021;146 doi: 10.1016/j.brat.2021.103967. [DOI] [PubMed] [Google Scholar]
- Warthen D.M., Lambeth P.S., Ottolini M., Shi Y., Barker B.S., Gaykema R.P., Newmyer B.A., Joy-Gaba J., Ohmura Y., Perez-Reyes E., Güler A.D., Patel M.K., Scott M.M. Activation of pyramidal neurons in mouse medial prefrontal cortex enhances food-seeking behavior while reducing impulsivity in the absence of an effect on food intake. Front. Behav. Neurosci. 2016 Mar 30;10:63. doi: 10.3389/fnbeh.2016.00063. PMID: 27065827; PMCID: PMC4813092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti P.R., Schuessler B.P., Kim J.J. Sex differences in foraging rats to naturalistic aerial predator stimuli. iSci. 2019;16:442–452. doi: 10.1016/j.isci.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.